Abstract
Colorectal cancer (CRC) is a leading cause of cancer-related death, partly due to a lack of reliable biomarkers for early diagnosis. To improve the outcome of CRC, it is critical to provide diagnosis at an early stage using promising sensitive/specific marker(s). Using immunohistochemistry and histopathology, IL-38 expression was determined in tissue arrays of CRC with different TNM status and depth of tumour invasion. Data were compared to IL-38 in adjacent non-cancer tissue and correlated with demographic information, including survival. A substantial reduction of IL-38 was detected in the CRC tissue compared to adjacent non-cancer colonic tissue. IL-38 correlated with the extent of tumour differentiation (P < 0.0001); CRC location in the left side of the colon (P < 0.05), and smaller tumour size (≤ 5 cm; P < 0.05). Receiver operating characteristic (ROC) curve analysis demonstrated both high specificity and high sensitivity of IL-38 for the diagnosis of CRC [area under the curve (AUC) = 0.89)]. By sub-group analysis, AUC of IL-38 for the diagnosis of CRC was higher in poorly differentiated, right-sided CRC or tumour size > 5 cm (all AUC > 0.9). Significantly, longer survival was observed for the IL-38high versus the IL-38low groups in CRC patients (P = 0.04). Survival was also longer for IL-38high patients with lymph node metastasis (P = 0.01) and TNM stage III-IV (P = 0.02). Multivariate analysis demonstrated that IL-38 (P = 0.05) and tumour invasion depth (P = 0.04) were independent factors for survival. High IL38 in CRC is an independent prognostic factor for the longer survival of CRC patients. IL-38 signalling may constitute a therapeutic target in CRC.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02440-7) contains supplementary material, which is available to authorized users.
Keywords: IL-38, Colorectal cancer, Prognosis, Potential target
Introduction
Colorectal cancer (CRC) is one of the leading causes of cancer-related death in the world [1], partly due to a lack of reliable biomarkers for early diagnosis, resulting in many CRC patients only receiving palliative care due to diagnosis at a later stage. CRC mainly affects older adults, but there is a rising incidence in people who are younger [1].
CRC-associated mortality has declined substantially over recent decades [2], mainly due to cancer screening programmes, the preoperative and postoperative standardization of management, improved surgical techniques and the availability of more-effective precision medicine [3]. The overall survival rate for CRC patients is 64%, but the survival rate of CRC varies from as high as 91% at a localized stage, to as low as 14% in metastatic CRC patients. Therefore, it is fundamentally important for medical practitioners to access a reliable and sensitive prognostic marker for detection of CRC at an early stage to improve the outcomes of CRC.
Host immunity, particularly involving the checkpoint molecules programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), plays a critical role during the development of many cancers, which has provided a foundation for precision medicine in the form of a potent therapeutic target [4]. Furthermore, carcinogenesis of CRC has been extensively studied [5], e.g., CRC subtypes and their related genetic mutations.
The precise underlying mechanism of regulation of host immunity against CRC remains to be explored, despite decades of extensive research, including Th17 mediated cancer immunobiology [6]. Cytokine expression has been clearly associated with the modulation of colorectal cancer [7]. The concept that tumour growth is determined by the balance between inflammatory networks that may either promote tumorigenesis non-specifically or promote specific anti-tumour activity has been proposed [7]. Defining which cytokines may influence patient survival in CRC is of importance, given both the prognostic value of determining levels of expression, but also because manipulation of the immune environment has enormous therapeutic potential.
IL-38, originally identified as IL-1 family 10 (IL-1F10), is an anti-inflammatory cytokine [8]. Circulating IL-38 has been shown to correlate with the relapse and remission of systemic lupus erythematosus (SLE), supporting its anti-inflammatory role during disease progression of autoimmunity [9]. The anti-inflammatory action of IL-38 is consistent with our previous findings, showing that IL-38 is inversely correlated with the severity of gestational diabetes mellitus [10]. More recently, the relationship between IL-38 and lung adenocarcinoma has indicated that IL-38 may be related to the production of PD-L1 by lung cancer cells [11]. Furthermore, high levels of tissue IL-38 have been shown to correlate with poor differentiation and reduced 5-year survival rate in lung cancer. However, it remains to be investigated whether IL-38 is also related to CRC differentiation and the survival rate of CRC patients.
In the current experiments, we generated CRC tissue array blocks from patients with extensive clinical data, including sex, age, tumour position, tumour size, lymph node metastasis, tumour differentiation, tumour invasion depth and TNM score. Expression of IL-38 was quantified using histopathology/immunohistochemistry and the Wilcoxon signed-rank test/Mann–Whitney U test was used to correlate IL-38 with a range of clinical pathological features in the CRC patient cohort. IL-38 was found to be a specific and sensitive biomarker in predicting survival rate among these CRC patients, using Cox proportional hazards regression analysis (univariate and multivariate). Our data support the view that IL-38 (an anti-inflammatory cytokine) contributes to the suppression of the development of gastrointestinal cancers, which is concordant with data showing that inflammatory mediated Th17 activity (a pro-inflammatory cytokine) is closely related to the development of CRC [5]. Finally, our current findings may be useful for diagnosis, as well as defining a potential therapeutic target for precision medicine.
Materials and methods
Demography of CRC patients and samples
Wax embedded tissues were obtained from colorectostomy for primary CRC patients (n = 198) and matched with non-cancer tissues (n = 157) from the adjacent histopathologically non-cancerous tissue. Thus, for all measurements made, each tumour was compared to its own adjacent histopathologically normal tissue, from the same side of the colon. It is well known that the most common colorectal cancer is sporadic colon cancer arising from mutations within the APC/WNT pathway [12]; whereas the incidence of mucinous adenocarcinomas usually associated with mismatch repair gene mutations is rather small. Thus, we focused on the typical form of adenocarcinoma and excluded mucinous adenocarcinomas in the current study [13]. Complete clinical information was collected for all of the colorectostomy patients; none had prior chemotherapy. Tissue was obtained from Shanghai Tongren Hospital, China between 2013 and 2017. Among 198 CRC patients, 125 were males and ages ranged from 24 to 94 years.
Among the 198 CRC patients, 80 had follow-up until their death or their most recent contact (November 2017), 52 patients were still alive and 28 had died. The longest survival period for a CRC patient was 53 months. To facilitate uniform staining for quantitation, several tissue array blocks were generated, each consisting of the tissues from different patient blocks. The CRC tissue samples exhibited a range of different levels of differentiation, invasion and metastasis, with control matched non-cancer colon tissues from the same patients. The tissues within the pathology blocks were obtained from the patents at surgery. The treatment regimens among all these CRC patients were similar.
Immunohistochemistry
Immunohistochemistry was performed as described previously [14, 15]. Briefly, sections (4 µm) from the tissue microarray were labelled with rabbit anti-human IL-38 antibody (Ab180898, Abcam, Cambridge, UK). HRP-conjugated secondary antibody (Beijing Sequoia Jinqiao Biological Technology) was applied. The specific target was visualized with a DAB detection kit and counterstained with haematoxylin. IL-38 expression was quantified using ImagePro Plus 9.1, as described [10, 16].
Histopathology and immunohistochemistry were performed in the Department of Pathology, Tongren Hospital, Shanghai Jiaotong University School of Medicine. The image analysis of these histopathology blocks and immunohistochemistry blocks was also performed in the Tongren Hospital, Shanghai, China. The data analysis, statistical analysis and manuscript writing were performed in The Discipline of Pathology, School of Medical Sciences, The University of Sydney.
Statistical analysis
Statistical analysis was performed as described previously [17], using SPSS 24.0 statistical software package. Comparison between two paired groups was performed via the Wilcoxon signed-rank test. Comparison between the two unpaired groups was performed via the Mann–Whitney U test. Comparisons among multiple groups were performed using the Kruskal–Wallis H test. The low and high cut-off values for IL-38 expression within the tumours were defined by the median value of the CRC tissue IL-38 expression, quantitated in image units. The overall survival was defined as the number of patient’s days elapsed between surgery and the date of their last follow-up or death. Survival curves were plotted by the Kaplan–Meier method and compared by the log-rank test. Cox’s proportional hazards model was used to identify the prognostic factors that influenced survival. Results were considered as statistically significant at P < 0.05.
Results
Demographic information for the patients
Demographic data from the 198 primary CRC patients and the 157 matched control tissues are listed in Table 1. However, actual numbers for some comparisons were slightly lower due to incomplete clinical data for a small number of patients, i.e., for the left- or right-sided CRC comparison the numbers were 135 and 60, respectively. The number of tumours whose differentiation was well, moderate or poor were 5, 153 or 37, respectively, based on the new criteria of histological grading of CRC [18]. The number of tumours whose size was smaller or larger than 5 cm was 152 or 45, respectively. Selection of the tumour size cut-off of 5 cm is well recognised as being of prognostic value in CRC [19].
Table 1.
Clinicopathological characteristics of patients with CRC
| Characteristics | No. of patients with CRC (non-cancer) |
|---|---|
| Cancer vs non-cancer | |
| Unpaired | 198 (157) |
| Paired | 157 (157) |
| Gender | |
| Male | 125 (98) |
| Female | 73 (59) |
| Age (years) | |
| < 70 | 104 (80) |
| ≥ 70 | 94 (77) |
| Position | |
| Right-sided | 60 (49) |
| Left-sided | 135 (107) |
| Size (diameter, cm) | |
| ≤ 5 | 152 (126) |
| > 5 | 45 (30) |
| Lymph node metastasis | |
| No | 119 (93) |
| Yes | 79 (64) |
| Differentiation | |
| Well | 5 (4) |
| Moderate | 153 (122) |
| Poor | 37 (29) |
| Invasion depth | |
| T1 | 9 (9) |
| T2 | 25 (17) |
| T3 | 34 (28) |
| T4 | 128 (103) |
| TNM | |
| I | 30 (22) |
| II | 86 (71) |
| III | 69 (55) |
| IV | 11 (9) |
Association between IL-38 and clinicopathological characteristics in CRC patients
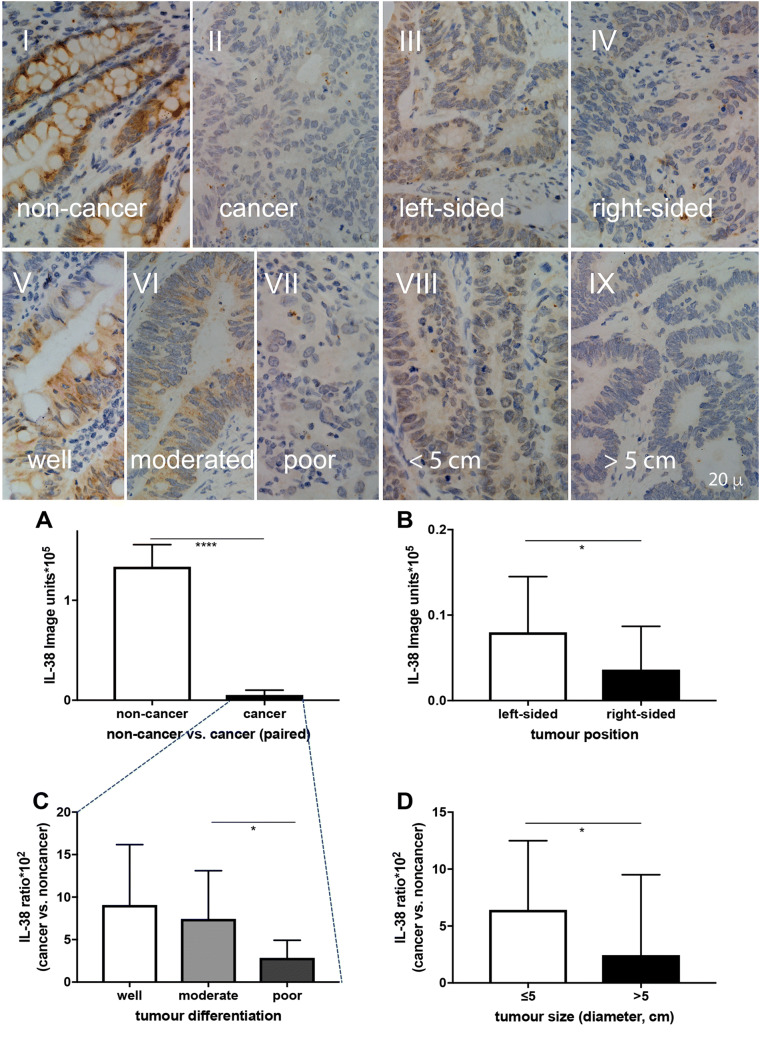
Colonic IL-38 was detected in the adjacent non-cancer part of the colon, mainly localised in the cytoplasm in both colonic epithelial cells and goblet cells (Fig. 1I). In contrast, there was weaker IL-38 staining in the CRC tissues (Fig. 1II), that was diffusely distributed within cytoplasm and on the cell membrane of the cancer cells. Quantitative analysis (Table 2) demonstrated that IL-38 expression was 95% reduced in the CRC tissues compared to that of the non-cancer colon tissue (Fig. 1a; P < 0.001). CRC arising in the right-sided colon is reported to carry a poorer prognosis compared to that from the left-side [20]. Our data show that CRC IL-38 was 2.2-fold higher in the left- (Fig. 1III) compared to the right-sided colon (Fig. 1IV) (Fig. 1b; P < 0.05). IL-38 was more than 60% reduced in the poorly differentiated CRC compared to that of the moderate differentiated CRC (Fig. 1c; P < 0.05). The representative microphotographs are illustrated in Fig. 1V, VI, VII. Finally, colonic IL-38 within CRC tumours > 5 cm was almost 50% reduced compared to that within tumours ≤ 5 cm (Fig. 1d; P < 0.05), as shown in the corresponding representative microphotographs (Fig. 1VIII, IX).
Fig. 1.
Comparison of IL-38 expression between paired non-cancer vs cancer tissue samples in each patient (a) using Wilcoxon signed-rank test. Comparison of IL-38 expression between colonic position (b), differentiation (c) and size (d) using Mann–Whitney U test. IL-38 expression has been quantitated in arbitrary image units (a, b) and has been expressed as the ratio of IL-38 expression in paired cancer vs non-cancer tissue for each patient (c, d). Representative images of IL-38 expression are illustrated in the microphotographs: non-cancer (I), cancer (II), and CRC from left-sided (III), right-sided colon (IV), CRC differentiation at well (V), moderate (VI), poor (VII) levels, and tumour size ≤ 5 cm (VIII) and > 5 cm (IX). *P < 0.05; ****P < 0.0001. The bar represents 20 µm
Table 2.
Association between IL-38 production and clinicopathological characteristics in patients with CRC
| Characteristics | IL-38 (units × 105) | P value | IL-38 ratio (cancer vs. non-cancer × 102) | P value | ||
|---|---|---|---|---|---|---|
| N | Median | N | Median | |||
| Cancer vs non-cancer (paired) | ||||||
| Cancer | 157 | 0.0533 | < 0.0001 | |||
| Non-cancer | 157 | 1.3360 | ||||
| Cancer vs non-cancer (unpaired) | ||||||
| Cancer | 198 | 0.0572 | < 0.0001 | |||
| Non-cancer | 157 | 1.3360 | ||||
| Gender | ||||||
| Male | 125 | 0.0555 | NS | 98 | 6.0845 | NS |
| Female | 73 | 0.0652 | 59 | 4.9309 | ||
| Age (years) | ||||||
| < 70 | 104 | 0.0547 | NS | 80 | 5.0523 | NS |
| ≥ 70 | 94 | 0.0650 | 77 | 6.1020 | ||
| Position | ||||||
| Right-sided | 60 | 0.0362 | 0.05 | 49 | 4.7711 | NS |
| Left-sided | 135 | 0.0800 | 107 | 6.0233 | ||
| Size (diameter, cm) | ||||||
| ≤ 5 | 152 | 0.0676 | NS | 126 | 6.4133 | 0.03 |
| > 5 | 45 | 0.0299 | 30 | 2.4473 | ||
| Lymph node metastasis | ||||||
| No | 119 | 0.0844 | NS | 93 | 7.0598 | NS |
| Yes | 79 | 0.0380 | 64 | 4.5315 | ||
| Differentiation | ||||||
| Well | 5 | 0.1103 | NS | 4 | 9.0806 |
All:0.02 H/M: NS H/L: NS M/L:0.02 |
| Moderate | 153 | 0.0787 | 122 | 7.4385 | ||
| Poor | 37 | 0.0284 | 29 | 2.8655 | ||
| Invasion depth | ||||||
| T1 | 9 | 0.0427 | NS | 9 | 4.3749 | NS |
| T2 | 25 | 0.1731 | 17 | 11.7530 | ||
| T3 | 34 | 0.0332 | 28 | 2.9147 | ||
| T4 | 128 | 0.0548 | 103 | 6.1020 | ||
| TNM | ||||||
| I | 30 | 0.1670 | NS | 22 | 14.9808 | NS |
| II | 86 | 0.0767 | 71 | 5.6415 | ||
| III | 69 | 0.0442 | 55 | 4.7249 | ||
| IV | 11 | 0.0324 | 9 | 3.1851 | ||
P values for Wilcoxon signed-rank test and Mann–Whitney U test. IL-38 ratio: divide the expression of IL-38 in tumours by the paired adjacent tissues
Association between IL-38 and ROC curves in CRC patients
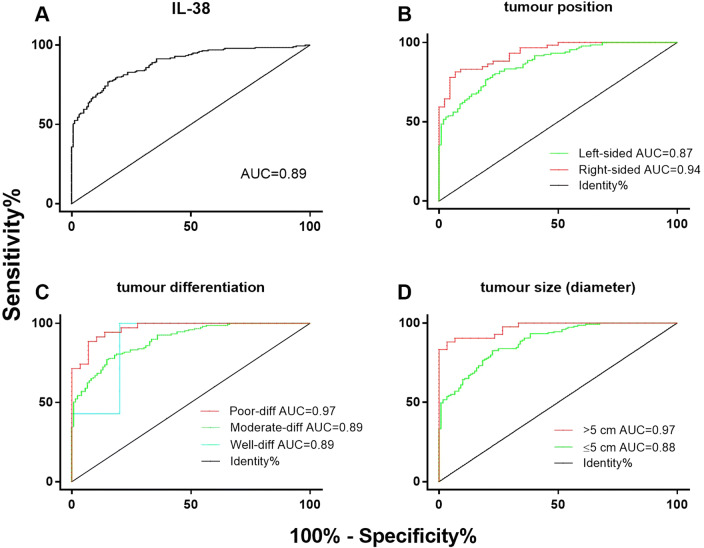
ROC curves were used to confirm the specificity and sensitively for colonic IL-38 expression in the prediction of CRC outcomes. The ROC curve yielded a cut-off of 0.33 IL-38 Image units × 105, which corresponded with a strong Youden index = 0.60 [17]. The capacity of IL-38 expression to predict CRC yielded an AUC of the ROC curve (AUROC) of 0.89 in the cancer and non-cancer tissues (Fig. 2a), consistent with IL-38 being a reliable and sensitive biomarker for differentiating between CRC and non-cancer colonic tissue. Similarly, the AUROC of IL-38 expression for tumours within the right-sided colon (AUC = 0.94, Fig. 2b), compared to CRC in the left-sided colon (AUC = 0.87, Fig. 2b), supports the conclusion that IL-38 expression was an excellent biomarker for differentiating between CRC arising in the right- versus left-sided colon. Furthermore, the AUROC of IL-38 expression for tumour differentiation was determined, showing that IL-38 expression was an excellent, very good or very good prediction biomarker for poorly- (AUC = 0.97, Fig. 2c), moderately- (AUC = 0.89, Fig. 2c) or well-differentiated CRC (AUC = 0.89, Fig. 2c), respectively. Notably, the AUC data obtained for the well-differentiated CRC shows large steps, due to the small number of samples available for analysis (n = 5). Finally, in relation to tumour size the AUROC of IL-38 expression was AUC = 0.97 (Fig. 2d) or AUC = 0.88 (Fig. 2d) from the CRC > 5 cm or ≤ 5 cm in tumour size, also supporting the conclusion that AUROC of IL-38 expression was reliable.
Fig. 2.
The specificity vs sensitivity of CRC diagnosis determined by IL-38 expression, determined using ROC curves. Area under the curve (AUC), IL-38 (a): AUC = 0.89. Sub-group analysis based on tumour position: b CRC diagnosis in patients with left-sided CRC = 0.87, right-sided CRC = 0.94; tumour differentiation c: well-differentiation = 0.89, moderate-differentiation = 0.89, poor-differentiation = 0.97; tumour size (diameter) d: > 5 cm = 0.97, ≤ 5 cm = 0.88
Association IL-38 and survival curves in patients with CRC
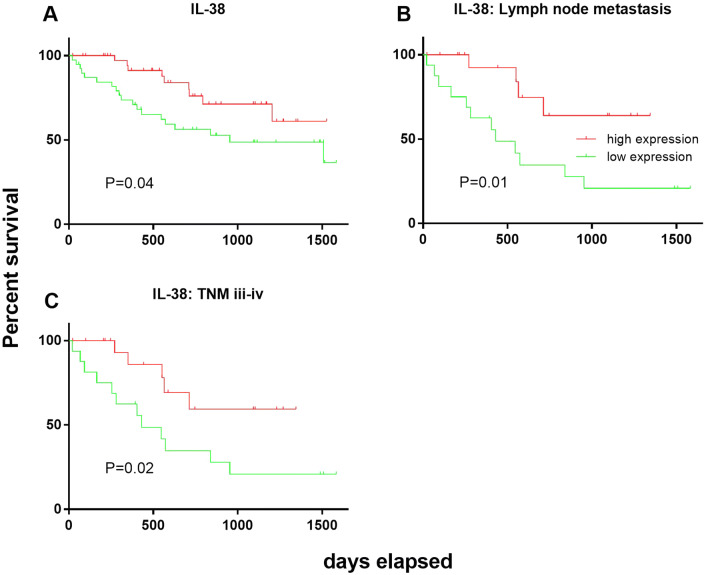
Using the log-rank test, the associations between IL-38 expression and postoperative survival of CRC patients was assessed, based on a defined cut-off of IL-38 levels, obtained from the median of CRC tissue image unit, i.e., high versus low. Our data demonstrate that the overall survival rate among the CRC patients within the IL-38 high group was significantly longer than those CRC patients within the low CRC IL-38 expression group (P = 0.04; Fig. 3a).
Fig. 3.
Kaplan–Meier survival curves for patients with either a high or low level of expression of IL-38. The cut-off for IL-38 expression was derived by the median of the expression of IL-38 in CRC tissue, quantitated in image units. Correlation between production of IL-38 in CRC (a). Sub-group analysis of survival for patients with lymph node metastasis (b) and TNM stage III–IV (c), P values from statistical analysis used the log-rank test
We further stratified patients into two sub-groups, those with lymph node metastases at surgery and patients that were TNM stage III–IV at surgery. Patients who expressed a high level of IL-38 had a significantly higher survival rate compared to those patients with low CRC IL-38 expression, in both the lymph node metastasis at surgery sub-group (P = 0.01, Fig. 3c) and the TNM stage III-IV at surgery subgroup (P = 0.02, Fig. 3d).
Univariate and multivariate analyses of the relationship between survival of CRC patients and IL-38
Univariate analysis was applied to determine whether a range of factors (IL-38, sex, age, CRC position, size, metastasis, differentiation, invasion depth, and TNM) contributes to the prediction of survival rate (Table 3). Univariate and multivariate analyses were undertaken for determining CRC survival rate, as described previously [17]. IL-38 expression (HR 0.45; 95% CI 0.20–0.99; P = 0.05), lymph node metastasis (HR 2.39; 95% CI 1.13–5.07; P = 0.02), invasion depth (HR 2.87; 95% CI 1.32–6.25; P = 0.008) and TNM (HR 2.28; 95% CI 1.37–3.80; P = 0.001) were found to be good predictors for survival of patients with CRC, by univariate analysis. More importantly, multivariate analysis demonstrated that IL-38 expression (HR 0.43; 95% CI 0.19–0.98; P = 0.05) and tumour invasion depth (HR 2.30; 95% CI 1.03–5.13; P = 0.04) were independent and reliable biomarkers for predicting survival rate among these CRC patients (Table 3). However, using multivariate analysis, other factors, including sex, age, CRC localisation, size, lymph node metastasis, differentiation, and TNM were not significant in predicting survival rate among these CRC patients.
Table 3.
Univariate and multivariate analysis of IL-38 and clinicopathological factors affecting survival of patients with CRC
| Characteristics | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| IL-38 | ||||
| High/low | 0.45 (0.20–0.99) | 0.05 | 0.43 (0.19–0.98) | 0.05 |
| Gender | ||||
| Male/female | 1.03 (0.46–2.28) | NS | ||
| Age (years) | ||||
| < 70/≥ 70 | 1.73 (0.80–3.76) | NS | ||
| Position | ||||
| Right-sided/left-sided | 1.21 (0.53–2.78) | NS | ||
| Size (diameter, cm) | ||||
| ≤ 5/> 5 | 1.53 (0.65–3.60) | NS | ||
| Lymph node metastasis | ||||
| No/yes | 2.39 (1.13–5.07) | 0.02 | 0.87 (0.18–4.17) | NS |
| Differentiation | ||||
| Well/moderate/poor | 2.30 (0.96–5.54) | NS | ||
| Invasion depth | ||||
| T1/T2/T3/T4 | 2.87 (1.32–6.25) | 0.008 | 2.30 (1.03–5.13) | 0.04 |
| TNM | ||||
| I/II/III/IV | 2.28 (1.37–3.80) | 0.001 | 1.96 (0.62–6.13) | NS |
P values for Cox proportional hazards regression analysis
HR hazard ratio, CI confidence interval
Further sub-group analysis based on age and sex was undertaken. For the analysis, an age cut-off of 70 years was selected, based on the study by Brenner et al. that showed that the median age at CRC diagnosis in developed countries is approximately 70 years [21]. No significant difference in colonic IL-38 expression was detected between young and old (< 70 vs ≥ 70 years) (SFig 1A), by sex (male vs female) (SFig 1B), and by TNM (I–IV) status (SFig 1C) in the CRC patients.
Discussion
This study shows that colonic IL-38 expression is substantially reduced in CRC compared to that of matched non-CRC colonic tissue, and has demonstrated a direct correlation between the level of IL-38 expression and the extent of differentiation of CRC. Importantly, higher expression of IL-38 was also found to be an independent predictor of an improved prognosis in the CRC patients, when analysed by multivariate analysis.
The observation that colonic IL-38 expression is ~ 95% lower in CRC compared to non-CRC colonic tissue, coupled with the direct correlation between differentiation of CRC, and colonic tumour expression of IL-38, suggests that IL-38 expression may be beneficial for maintaining homeostasis of intestinal mucosa in the normal colorectal micro-environment. Furthermore, our data suggest that a low level of IL-38 expression within CRC may contribute to tumour growth and spread. This concept is supported by others, who have shown that IL-38 is an anti-inflammatory cytokine, contributing to the maintenance of local host immunity [22]. Increased levels of inflammation are likely to be conducive to both the development of, and more rapid growth and metastasis of tumours [23]. The anti-inflammatory role of IL-38 is in agreement with our previous findings that IL-38 is protective in placental responses to gestational diabetes mellitus [10]. Thus, our current finding of suppressed/reduced colonic IL-38 in CRC may be consistent with dysregulated intestinal mucosal immunity in the micro-environment during the development of colon cancer [24].
Our speculation is supported by other data showing that down-regulated IL-38 is detected in a number of auto-immune diseases, including rheumatoid arthritis [25], systemic lupus erythematosus [26], primary Sjogren’s Syndrome [27] and inflammatory bowel disease [28]. However, it is still unclear why IL-38 is reduced/suppressed in CRC, but we hypothesise that reduced CRC IL-38 expression may be due to compromised host immunity following unknown stimuli, e.g., genetic, environmental, and possible infectious factors [29]. Oncogenic genetic mutations within the cancer cells may also contribute to altered expression.
Consequently, we propose that dysregulated host immunity in the colon compromises immune-regulatory mechanisms, resulting in a higher level of uncontrolled inflammation, which may enhance colonic epithelial cell malignant transformation, and ultimately the development of CRC [30]. Additionally, excessive inflammation may contribute to more rapid tumour growth and spread. This mechanism is consistent with the findings that a close correlation exists between chronic inflammatory status and the development of gastrointestinal cancer [23].
Importantly, our study has only examined IL-38 expression within CRC and adjacent non-cancer tissue at the time of surgery. We acknowledge that we do not know if reduced colonic IL-38 within normal colonic tissue renders the colon more susceptible to the development of CRC, which constitutes another important point for our future study.
IL-38 shares receptors with IL-36α, β, and γ, and may inhibit the activity of these IL-36 isoforms by competing with their signalling pathway [31]. It is well known that the IL-36 isoforms are pro-inflammatory cytokines, which contribute to activation and proliferation of leucocytes, including macrophages, dendritic cells and lymphocytes [32]. It is unknown why reduced colonic IL-38 expression within the CRC correlates with a worse outcome for CRC, particularly the observed direct correlation with CRC cellular differentiation. The precise underlying mechanism of IL-38 activity within CRC, however, remains unclear, which will be clarified in our future experiments.
There is a substantially different status of biological markers, genes and prognosis between left- and right-sided CRC [33]. In addition, it has been demonstrated that clinical symptoms from the right-sided CRC patients present at a relatively late stage, compare to that of the left-sided colon, partially due to anatomic/physiological mechanisms, which results in a diagnosis of right-sided colon cancer later than left-sided colon cancer [34]. This is in agreement with our findings that colonic IL-38 was almost 50% lower in the right-sided CRC compared to that of the left, supporting our speculation above that IL-38 is a protective biological factor during the development of CRC. Furthermore, substantially reduced colonic IL-38 was observed in the CRC tumour size > 5 cm, compared to that of ≤ 5 cm, which is also consistent with the poorer prognosis of larger size primary CRC [35]. This is in line with the findings from others, showing that tumour size and infiltration depth correlates with the survival rate of the postoperative patients [36]. Thus, these data above could be useful information for the development of personalised precision medicine in the management of CRC.
The ROC curves demonstrated that the level of IL-38 expression was highly specific and sensitive for the prediction of several clinical parameters, specifically tumour location within the colon, the extent of tumour differentiation and the size of the tumour. These data support our finding from multi-variate analysis that the level of expression of IL-38 is an independent predictor of prognosis and survival. Our survival curve data have shown that higher CRC IL-38 expression is associated with a better prognosis and longer survival.
Our data also show that the level of IL-38 expression is an important determinant for survival in patients with advanced CRC. Within two sub-groups with advanced disease (patients with mesenteric metastases and patients who were TNM (III–IV) at surgery), both demonstrated that high levels of IL-38 expression resulted in a significant survival benefit, suggesting that IL-38 seems to be a reliable and consistent independent factor in predicting the prognosis of CRC.
In contrast to our own data, a recent study of lung adenocarcinoma showed a correlation between high IL-38 expression and poor differentiation of lung adenocarcinoma [11]. Moreover, high IL-38 expression was also found by these authors to positively correlate with high TNM and correlate with a shorter disease-free survival. However, these data were only obtained in PD-L1 negative tumours, where T cell activity is presumably not suppressed by the tumour. Although the level of IL-38 expression was found to influence survival in both lung adenocarcinoma and in this study on CRC, the explanation for why the effect was opposite in the two tumour types is unknown. Notably, our study demonstrated a high level of constitutive expression of IL-38 in non-cancer colonic tissue, while no data are available for IL-38 constitutive expression in normal lung tissue. The high level of expression of IL-38 within the colon may be consistent with the high level of immune surveillance required within the gut wall. There is an almost completely different micro-environment within the gastrointestinal mucosal surface compared to the lung, despite both organs being classified as mucosal organs [37]. The substantially different micro-environment between colon and lung would certainly contribute to differences in disease progression and/or pathogenesis, mainly due to host mucosal defences and flora [37].
No significant difference in IL-38 expression was observed between young versus old patients or between males and females. The failure to observe a difference was probably due to the age range of the CRC patients being mainly concentrated in 62–78 years old range, and the small numbers of younger patients in our study (< 60 years n = 38/198). Additionally, most females within our study were post-menopausal (< 55 years n = 6/73), which probably has negated the known benefit of estrogen in reducing the incidence of CRC in women of fertile age [38].
It is well known that there is a close correlation between TNM and survival in CRC patients, which has been well reviewed by Rodgers et al. [39]. However, we observed that there was no significant difference in survival in CRC based on TNM stage in the current study, using multivariate analysis. Notably, one of the elements of TNM, namely invasion depth, did positively correlate with survival by multivariate analysis. Our explanation of the data is that statistical power was compromised because there were not sufficient numbers of CRC patients at TNM I stage, due to rather late diagnosis, nor at TNM stage IV. To overcome this problem, we are currently identifying more TNM I and IV stage samples for our future study.
Our pilot study has demonstrated that IL-38 expression within pre-cancerous colorectal tissue is slightly lower than that of non-cancerous tissue (data not shown). The complete analysis of these data will be undertaken in our future experiments.
In conclusion, our data demonstrate that IL-38 is a reliable prediction factor for prognosis of CRC, based on multivariate analysis, and constitutes a strong and reliable predictor of survival post-surgery. IL-38 expression exhibits high sensitivity and specificity. Furthermore, our data also suggest that IL-38 may serve as a therapeutic target in post-surgery management of CRC patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge support from the staff of the Department of Pathology, Tongren Hospital, Shanghai Jiao University School of Medicine, and staff of the Discipline of Pathology, Sydney Medical School, The University of Sydney.
Abbreviations
- AUC
Area under the curve
- AUROC
AUC of the ROC curve
- CRC
Colorectal cancer
- CTLA-4
Cytotoxic T-lymphocyte-associated protein 4
- DCs
Dendritic cells
- IL
Interleukin
- MAPK
Mitogen-activated protein kinase
- NFkB
Nuclear factor kappa B
- PD-1
Programmed cell death protein 1
- ROC
Receiver operating characteristic
- TNM
Tumour, node and metastasis
Author contributions
FC: performed the experiment, analysed the data, and wrote the manuscript. FZ and ZT: performed histopathology. BH, SB and KT: designed the experiment and critically reviewed the manuscript. SB, and KT: provided financial support for the experiment.
Funding
Shanghai Jiaotong University Medical Professional Cross Fund (Kun Tao) and the Joint Research Initiative Grant from Shanghai Jiaotong University, China (Kun Tao and Shisan Bao). The Natural Science Foundation of Shanghai, China 16ZR1432600 (Kun Tao), School of Medical Sciences, The University of Sydney Small Equipment Grant (Shisan Bao) and SJTU Research Project grant, The University of Sydney 2019 (Shisan Bao) are acknowledged.
Compliance with ethical standards
Conflict of interest
The authors declare there is no conflict of interest.
Ethical approval and ethical standards
Our current experiment has been approved by the Human Ethics Committee of Tongren Hospital, Shanghai Jiaotong University School of Medicine for the use of the tissues and the associated deidentified clinical data (ZH2018ZDA33). The guidelines of the Declaration of Helsinki on Medical Research involving human subjects has been strictly adhered to.
Informed consent
The oral consent for surgery included consent for the tissues to be used for diagnostic and research purposes in a deidentified manner. A written explanation of the surgical procedures and the potential research use of the tissues was provided to all patients prior to surgery. All of the patients were adults who were older than 16 years.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shisan Bao, Email: bob.bao@sydney.edu.au.
Kun Tao, Email: taokun@shtrhospital.com.
References
- 1.Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Speizer FE, Willett WC. A prospective study of family history and the risk of colorectal cancer. N Engl J Med. 1994;331(25):1669–1674. doi: 10.1056/NEJM199412223312501. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics. CA Cancer J Clin. 2014;64(2):104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 3.Welch HG, Robertson DJ. Colorectal cancer on the decline-why screening can’t explain it all. N Engl J Med. 2016;374(17):1605–1607. doi: 10.1056/NEJMp1600448. [DOI] [PubMed] [Google Scholar]
- 4.Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86. doi: 10.3389/fonc.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. 2017;17(4):268. doi: 10.1038/nrc.2017.24. [DOI] [PubMed] [Google Scholar]
- 6.De Robertis M, Poeta ML, Signori E, Fazio VM. Current understanding and clinical utility of miRNAs regulation of colon cancer stem cells. Semin Cancer Biol. 2018 doi: 10.1016/j.semcancer.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Mager LF, Wasmer MH, Rau TT, Krebs P. Cytokine-induced modulation of colorectal cancer. Front Oncol. 2016;6:96. doi: 10.3389/fonc.2016.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinarello C, Arend W, Sims J, Smith D, Blumberg H, O’Neill L, Goldbach-Mansky R, Pizarro T, Hoffman H, Bufler P, Nold M, Ghezzi P, Mantovani A, Garlanda C, Boraschi D, Rubartelli A, Netea M, van der Meer J, Joosten L, Mandrup-Poulsen T, Donath M, Lewis E, Pfeilschifter J, Martin M, Kracht M, Muehl H, Novick D, Lukic M, Conti B, Solinger A, Kelk P, van de Veerdonk F, Gabel C. IL-1 family nomenclature. Nat Immunol. 2010;11(11):973. doi: 10.1038/ni1110-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeuchi Y, Seki T, Kobayashi N, Sano K, Shigemura T, Shimojo H, Matsumoto K, Agematsu K. Analysis of serum IL-38 in juvenile-onset systemic lupus erythematosus. Mod Rheumatol. 2018 doi: 10.1080/14397595.2018.1436118. [DOI] [PubMed] [Google Scholar]
- 10.Yu Z, Liu J, Zhang R, Huang X, Sun T, Wu Y, Hambly BD, Bao S. IL-37 and 38 signalling in gestational diabetes. J Reprod Immunol. 2017;124:8–14. doi: 10.1016/j.jri.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Takada K, Okamoto T, Tominaga M, Teraishi K, Akamine T, Takamori S, Katsura M, Toyokawa G, Shoji F, Okamoto M, Oda Y, Hoshino T, Maehara Y. Clinical implications of the novel cytokine IL-38 expressed in lung adenocarcinoma: possible association with PD-L1 expression. PLoS One. 2017;12(7):e0181598. doi: 10.1371/journal.pone.0181598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novellasdemunt L, Antas P, Li VS. Targeting Wnt signaling in colorectal cancer. A review in the theme: cell signaling: proteins, pathways and mechanisms. Am J Physiol Cell Physiol. 2015;309(8):C511–C521. doi: 10.1152/ajpcell.00117.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu W, Dong X, Mai M, Seelan RS, Taniguchi K, Krishnadath KK, Halling KC, Cunningham JM, Boardman LA, Qian C, Christensen E, Schmidt SS, Roche PC, Smith DI, Thibodeau SN. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating beta-catenin/TCF signalling. Nat Genet. 2000;26(2):146–147. doi: 10.1038/79859. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Zhu C, Li F, Wang Y, Bao R, Cao Z, Xiang X, Yan L, Lin L, Zhao G, Xie Q, Bao S, Wang H. Correlation between hepatic human males absent on the first (hMOF) and viral persistence in chronic hepatitis B patients. Cell Biosci. 2018;8:14. doi: 10.1186/s13578-018-0215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou T, Sun Y, Li M, Ding Y, Yin R, Li Z, Xie Q, Bao S, Cai W. Enhancer of zeste homolog 2-catalysed H3K27 trimethylation plays a key role in acute-on-chronic liver failure via TNF-mediated pathway. Cell Death Dis. 2018;9(6):590. doi: 10.1038/s41419-018-0670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chami B, Yeung A, Buckland M, Liu H, Fong GM, Tao K, Bao S. CXCR3 plays a critical role for host protection against Salmonellosis. Sci Rep. 2017;7(1):10181. doi: 10.1038/s41598-017-09150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao Z, Li Z, Wang H, Liu Y, Xu Y, Mo R, Ren P, Chen L, Lu J, Li H, Zhuang Y, Liu Y, Wang X, Zhao G, Tang W, Xiang X, Cai W, Liu L, Bao S, Xie Q. Algorithm of Golgi protein 73 and liver stiffness accurately diagnoses significant fibrosis in chronic HBV infection. Liver Int. 2017;37(11):1612–1621. doi: 10.1111/liv.13536. [DOI] [PubMed] [Google Scholar]
- 18.Ueno H, Kajiwara Y, Shimazaki H, Shinto E, Hashiguchi Y, Nakanishi K, Maekawa K, Katsurada Y, Nakamura T, Mochizuki H, Yamamoto J, Hase K. New criteria for histologic grading of colorectal cancer. Am J Surg Pathol. 2012;36(2):193–201. doi: 10.1097/PAS.0b013e318235edee. [DOI] [PubMed] [Google Scholar]
- 19.Chen CH, Hsieh MC, Hsiao PK, Lin EK, Lu YJ, Wu SY. A critical reappraisal for the value of tumor size as a prognostic variable in rectal adenocarcinoma. J Cancer. 2017;8(10):1927–1934. doi: 10.7150/jca.17930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghidini M, Petrelli F, Tomasello G. Right versus left colon cancer: resectable and metastatic disease. Curr Treat Options Oncol. 2018;19(6):31. doi: 10.1007/s11864-018-0544-y. [DOI] [PubMed] [Google Scholar]
- 21.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383(9927):1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 22.van de Veerdonk FL, de Graaf DM, Joosten LA, Dinarello CA. Biology of IL-38 and its role in disease. Immunol Rev. 2018;281(1):191–196. doi: 10.1111/imr.12612. [DOI] [PubMed] [Google Scholar]
- 23.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140(6):1807–1816. doi: 10.1053/j.gastro.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 24.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 25.Takenaka SI, Kaieda S, Kawayama T, Matsuoka M, Kaku Y, Kinoshita T, Sakazaki Y, Okamoto M, Tominaga M, Kanesaki K, Chiba A, Miyake S, Ida H, Hoshino T. IL-38: a new factor in rheumatoid arthritis. Biochem Biophys Rep. 2015;4:386–391. doi: 10.1016/j.bbrep.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudloff I, Godsell J, Nold-Petry CA, Harris J, Hoi A, Morand EF, Nold MF. Brief report: interleukin-38 exerts antiinflammatory functions and is associated with disease activity in systemic lupus erythematosus. Arthritis Rheumatol. 2015;67(12):3219–3225. doi: 10.1002/art.39328. [DOI] [PubMed] [Google Scholar]
- 27.Ciccia F, Accardo-Palumbo A, Alessandro R, Alessandri C, Priori R, Guggino G, Raimondo S, Carubbi F, Valesini G, Giacomelli R, Rizzo A, Triolo G. Interleukin-36alpha axis is modulated in patients with primary Sjogren’s syndrome. Clin Exp Immunol. 2015;181(2):230–238. doi: 10.1111/cei.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boutet MA, Bart G, Penhoat M, Amiaud J, Brulin B, Charrier C, Morel F, Lecron JC, Rolli-Derkinderen M, Bourreille A, Vigne S, Gabay C, Palmer G, Le Goff B, Blanchard F. Distinct expression of interleukin (IL)-36alpha, beta and gamma, their antagonist IL-36Ra and IL-38 in psoriasis, rheumatoid arthritis and Crohn’s disease. Clin Exp Immunol. 2016;184(2):159–173. doi: 10.1111/cei.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ummarino D. Experimental arthritis: IL-38 promotes anti-inflammatory effects. Nat Rev Rheumatol. 2017;13(5):260. doi: 10.1038/nrrheum.2017.55. [DOI] [PubMed] [Google Scholar]
- 30.Grivennikov SI. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol. 2013;35(2):229–244. doi: 10.1007/s00281-012-0352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Catalan-Dibene J, McIntyre LL, Zlotnik A. Interleukin 30 to interleukin 40. J Interferon Cytokine Res. 2018;38(10):423–439. doi: 10.1089/jir.2018.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vigne S, Palmer G, Martin P, Lamacchia C, Strebel D, Rodriguez E, Olleros ML, Vesin D, Garcia I, Ronchi F, Sallusto F, Sims JE, Gabay C. IL-36 signaling amplifies Th1 responses by enhancing proliferation and Th1 polarization of naive CD4 + T cells. Blood. 2012;120(17):3478–3487. doi: 10.1182/blood-2012-06-439026. [DOI] [PubMed] [Google Scholar]
- 33.Lee MS, Menter DG, Kopetz S. Right versus left colon cancer biology: integrating the consensus molecular subtypes. J Natl Compr Cancer Netw. 2017;15(3):411–419. doi: 10.6004/jnccn.2017.0038. [DOI] [PubMed] [Google Scholar]
- 34.Venook AP. Right-sided vs left-sided colorectal cancer. Clin Adv Hematol Oncol. 2017;15(1):22–24. [PubMed] [Google Scholar]
- 35.Kato T, Alonso S, Muto Y, Perucho M, Rikiyama T. Tumor size is an independent risk predictor for metachronous colorectal cancer. Oncotarget. 2016;7(14):17896–17904. doi: 10.18632/oncotarget.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saha S, Kanaan MN, Shaik M, Abadeer B, Korant A, Krishnamoorthy M, Kaushal S, Singh TT, Arora ML, Wiese D. Tumor size as a prognostic factor for patients with colon cancer undergoing sentinel lymph node mapping and conventional surgery. J Clin Oncol. 2013 doi: 10.1200/jco.2013.31.4_suppl.546. [DOI] [Google Scholar]
- 37.Marsland BJ, Trompette A, Gollwitzer ES. The gut-lung axis in respiratory disease. Ann Am Thorac Soc. 2015;12(Suppl 2):S150–S156. doi: 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- 38.Lin JH, Giovannucci E. Sex hormones and colorectal cancer: what have we learned so far? J Natl Cancer Inst. 2010;102(23):1746–1747. doi: 10.1093/jnci/djq444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogers AC, Winter DC, Heeney A, Gibbons D, Lugli A, Puppa G, Sheahan K. Systematic review and meta-analysis of the impact of tumour budding in colorectal cancer. Br J Cancer. 2016;115(7):831–840. doi: 10.1038/bjc.2016.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.