Abstract
Sensory nerves sensitive to capsaicin are afferent nerve fibers which contain TRPV1 channels. Activation of these channels induces release of neuropeptides which regulate local blood flow and immune response. Inactivation of sensory neurons either with high-dose capsaicin treatment or local ablation of vagal sensory nerve activity markedly increases metastasis of breast carcinoma formed by 4T1 derivative cells. These cancer cells also induce an extensive systemic inflammatory response. Further findings have documented that lack of local sensory neuromediators alters phenotype of cancer cells within primary tumor leading to overgrowth of metastatic subsets. This might be due to decreases in local and systemic immune response to growing tumor. Specifically, Substance P, one of the most abundant sensory neuropeptides, enhances anti-tumoral immune response evoked by radiotherapy under in vivo conditions. These findings further suggest that activation of TRPV1 channels on sensory neurons may induce an anti-tumoral immune response. We are testing this hypothesis. Our initial results as reported here demonstrate anti-inflammatory consequences of low-dose systemic capsaicin treatment. In conclusion, sensory nerve fibers sensitive to capsaicin have important roles in defense against metastatic breast carcinoma; hence, controlled activation of these neural pathways might be effective in cancer therapy. Specifically, activation of sensory fibers of left vagus nerve using a perineuronal stimulation may inhibit metastasis of breast carcinoma. Likewise, pharmacological modulators of TRPV1 channels may induce anti-tumoral immune response. Exact players of this newly explored defense system are, however, only partly validated, and further studies are required.
Keywords: Metastasis, Breast cancer, Vagus, Immune, Capsaicin, CITIM 2019
Introduction
Cancer survival decreases markedly in patients experiencing various psychosocial stresses [1]. Stress by altering neuroendocrine regulations on immune functions may contribute to cancer mortality [2]. The autonomous nervous system (ANS) plays a significant role in stress-induced activation of neuroimmune pathways [3–5]. The ANS consists of efferent fibers that innervate specific target tissues such as heart smooth muscle, exocrine and endocrine glands, metabolic tissues, and immune cells. Afferent (sensory) nerve fibers of ANS convey information from peripheral tissues to the central nervous system [6]. The ANS includes sympathetic as well as parasympathetic branches of the nervous system, and each of these systems contributes differently to neuronal regulation of immunological responses to stress. The sympathetic nervous system innervates lymphoid organs through noradrenergic efferent fibers. The cholinergic parasympathetic system, through the vagus nerve, is involved in anti-inflammatory reflex. Parasympathetic efferent fibers end in the sympathetic celiac ganglion just proximal to lymphoid organs [7, 8]. Growing evidence suggests that a specific subset of sensory nerves sensitive to capsaicin have diverse and profound effects on the immune response to a wide range of diseases including cancer [9–15]. These sensory nerve fibers are largely unmyelinated and include TRPV1 channels that have both sensory and effector functions. Activation of these nerve endings release vasoactive peptides such as Substance P, vasoactive intestinal polypeptide, and calcitonin gene-related peptide which can all modulate local blood flow and the activity of immune cells [16–19]. Effector function of the sensory nerve fibers is due to the release of these vasoactive peptides following axon reflexes that occur when action potentials generated in the nerve endings of unmyelinated sensory fibers are conducted retrogradely to the terminal endings within the target tissue [20]. Based on these previous findings, we have evaluated the specific role of TRPV1 containing sensory nerve fibers in breast cancer metastasis in a series of studies.
Systemic inactivation of sensory fibers
Initially, a neurotoxic dose of capsaicin was used to inactivate sensory nerve fibers [21]. Systemic inactivation of sensory nerve fibers enhanced metastasis of 4T1 murine breast carcinoma cells [22]. Marked increases in lung and heart metastasis were observed while growth of primary tumor in the mammary fat pad was unaffected demonstrating changes in metastatic potential of cancer cells as well as the metastatic niche occurred. The observed effects were dose-dependent and correlated with the degree of denervation determined by an eye-wiping test [22].
Neurotoxic effects of capsaicin are reversible in adult mice [23], and metastatic potential returned to baseline levels when sensory nerves were allowed to regenerate for 3 weeks after capsaicin treatment. These initial findings could not be due to direct effects of capsaicin which clears within 48 h of injection [24], long before the injection of tumor cells.
Denervation of vagal sensory nerve fibers
Sensory nerves innervate lung and heart tissue extensively. A significant part of the sensory innervation of lung tissue is supplied by the vagus nerve and vagal sensory fibers innervate the entire respiratory tract from trachea to lung parenchyma [25, 26]. We previously found that unilateral cervical left vagotomy markedly increased lung metastasis of breast carcinoma [27]. Depending on the vagal branch studied, 70–90% of the vagal nerve fibers are sensory (afferent fibers) [28, 29]. We subsequently examined the effects of specific inactivation of vagal afferent fibers on lung metastasis using perineural capsaicin treatment and are reporting the results here for the first time.
Perineural treatment with capsaicin inactivates nociceptive afferent nerves selectively, inducing long-lasting regional thermal and chemical analgesia without affecting non-nociceptive sensory nerves, autonomic efferents, and motor nerves [30]. As seen in Fig. 1, inactivation of sensory fibers in the left vagus markedly enhanced lung metastasis further proving the protective role of sensory nerve fibers against the formation of lung metastasis. These results also demonstrate that activation of afferent fibers in left vagus nerve using a perineuronal stimulation may have therapeutic potential in metastatic breast carcinoma. Perineuronal stimulation also activates vagal efferent pathways. Activation of vagal efferents increases cholinergic parasympathetic activity and the cholinergic anti-inflammatory pathway which may also prevent inflammation-related metastasis as demonstrated in our models [31–33]. On the other hand, this approach may not be suitable for all types of cancer and modifications might be required since increased parasympathetic cholinergic fibers were shown to promote prostate cancer invasion and metastasis via activation of stromal type 1 muscarinic receptors [34].
Fig. 1.
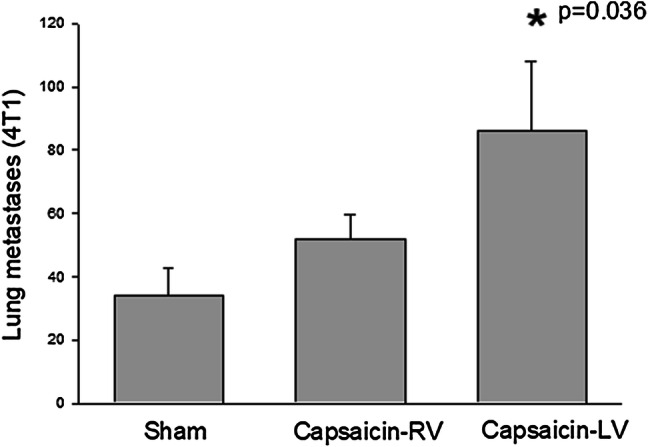
Effects of perineural capsaicin treatment of vagus nerve on breast cancer metastasis. We here applied perivagal capsaicin to inactivate vagal sensory nerve fibers. Female Balb-c mice were used. The cervical vagi (right (RV) or left (LV)) were exposed and freed from the carotid artery for a distance of 2–3 mm following a midline incision in the neck. Parafilm was placed around the nerve, and a cotton wool soaked in capsaicin (1%) or vehicle was placed around each vagus nerve for 30 min. One week after perivagal capsaicin application, 4T1 breast carcinoma cells (100,000 cells/mouse/0.1 ml) were injected into the right upper mammary pad. Four weeks after injection of 4T1 cells, lung tissues were removed and macroscopic metastasis were determined as described previously [27]. Student’s t test was used for statistical analysis. Animals: Sham-sham operated
Changes in phenotype of primary tumors in the absence of sensory neuromediators
Possible mechanisms of denervation-induced enhancement of metastasis could be approached by two main hypotheses. First, increased metastasis might be due to lack of local effects of mediators of sensory nerve fibers on primary tumor cells. These mediators are also likely to influence cells at the metastatic loci since, compared to most other tissues, lung and heart are abundantly innervated by sensory nerve fibers [35]. Secondly, changes in local and systemic immune response in the absence of local sensory nerve activity might decrease clearance of cancer cells. These possibilities were evaluated using different experimental approaches.
In order to determine whether lack of local sensory factors alter the phenotype of cancer cells, microarray technology was used to determine denervation-induced changes in gene expression of primary tumors [36]. We also selected myocardial metastases for culture (named as 4THM), because they are very rare and seemed to be specifically induced by sensory nerve inactivation as previously reported [22]. We identified a small cohort of genes including caspase-7, ADAM10 (a disintegrin and metalloprotease), and Elk-3 which were significantly downregulated in 4T1 primary tumors developed after sensory nerve denervation with high-dose capsaicin treatment. A majority of these genes were also downregulated in primary tumors of heart metastatic cells (4THM) formed in mice that have functional sensory nerves. 4THM cells, when inoculated orthotopically, induced more metastasis compared to 4T1 cells in untreated mice. These results demonstrated that more aggressive subsets of breast cancer cells gain a survival advantage and dominate within the primary tumors [36]. Hence, the effector function of sensory nerve fibers participates in host defense against cancer. Further functional studies demonstrated that ADAM-10, one of the genes markedly downregulated in both 4THM tumors and 4T1 tumors of mice that were sensory denervated, might function as a tumor suppressor gene. Specifically, protease activity of ADAM-10 resulted in bioactive fragments of Substance P creating anti-tumorigenic peptides [36]. This also explains why the loss of Substance P following sensory nerve denervation enhanced metastasis, since Substance P in the presence of appropriate peptidases is converted to anti-tumorigenic peptides [37].
Substance P enhances anti-tumoral effects of radiotherapy
Substance P, one of the major immunoreactive peptides found in sensory nerve fibers, mediates effector functions such as local increases in blood flow and extravasation of immune cells [36, 38]. Besides possible direct anti-tumoral effects of Substance P fragments, Substance P may also participate in the formation of an anti-tumoral immune response. Specifically, Substance P enhances lymphokine-activated killer cell cytotoxicity as well as NK cell cytotoxicity, augmenting IL-12 production by macrophages [39]. Similarly, Substance P, acting through Neurokinin 1 receptors (NK1R) found on dendritic cells, promotes potent type 1 immunity, IL-12 secretion, and dendritic cell maturation that collectively enhance the efficiency of dendritic cell vaccines as previously reported [40]. Hence, protective effects of sensory nerve fibers may be mimicked by Substance P-treatment. To explore this possibility, continuous treatment with Substance P containing pumps was used. The possible therapeutic value of Substance P as an adjuvant treatment was also examined, and results were reported. Specifically, Substance P was also combined with radiotherapy, which is commonly used in breast cancer treatment, especially in early stages [41]. Ionizing radiation increases the effectiveness of antitumor immune responses even at distant sites from radiation exposure [42]. Besides direct cytotoxic effects, radiation also induces immunogenic death inducing abscopal effect, which appears to be largely dependent on the immune status of the host [43]. Because host immune response can be enhanced by Substance P, Substance P may increase radiotherapy-induced immunological death. We, therefore, examined the effects of Substance P on highly aggressive brain metastatic subset of 4T1 breast carcinoma cells (denoted as 4TBM). In accordance with the previous findings, we found that continuous exposure to a relatively low dose of Substance P enhances therapeutic efficacy of radiotherapy inducing complete response in 50% of the mice [10]. Underlying mechanisms seemed to be largely mediated by Substance P-induced alterations in immune response to growing tumors. Specifically, Substance P-treatment decreased the number of tumor-infiltrating myeloid-derived suppressor cells as well as the release of TNF-α while enhancing IFN-γ secretion from leukocytes. Another striking feature of Substance P-treatment was prevention of tumor-induced degeneration of sensory nerve endings. Substance P also altered the non-immune microenvironment through activating NK1R (Neurokinin 1 Receptor). Substance P-treatment reversed the effects of radiotherapy, decreasing the secretion of angiogenic factor VEGF [44] from both cancer-associated fibroblasts and tumor explants in a NK1R-dependent manner [10]. Substance P alone, however, was ineffective in decreasing tumor growth and metastasis [10] demonstrating that other sensory neuromediators are involved in anti-tumoral responses.
Effects of TRPV1 activation on metastatic breast cancer
We have recently tried another approach to enhance sensory nerve function during tumor growth. Capsaicin at low doses activates sensory nerve fibers through TRPV1 channels and induces neuropeptide release. We used capsaicin as a TRPV1 agonist which was also shown to alter cancer immune response following intratumoral injection [45]. Capsaicin, therefore, may inhibit cancer growth and metastasis by activating sensory nerve fibers as well as by effecting on anti-tumoral immune response. We examined the systemic effects of low (1 mg/kg ip) and relatively high doses of capsaicin (10 mg/kg ip) in mice injected with 4TBM metastatic breast carcinoma cells which were previously described [46]. Treatments were started two days after orthotopic injection of 4TBM cells. Although the neurotoxic dose of capsaicin is above 50 mg/kg in balb-c mice [22], tumor-bearing animals could not tolerate 10 mg/kg capsaicin. Mice developed hypothermia and started to die after three injections. Remaining mice were followed for 12 more days, and the immune response of leukocytes obtained from spleen and draining lymph nodes was determined. Surprisingly, in the low-dose group, mice also started to die after the fifth injection of 1 mg/kg capsaicin; thus, the dose of capsaicin for the remaining injections was decreased to 0.5 mg/kg and given every other day for three times. The mice were sacrificed 15 days after initial treatment. Cumulative dose of capsaicin and changes in cytokine response of stimulated leucocyte culture of tumor-bearing animals were determined as previously described [10] and are reported below.
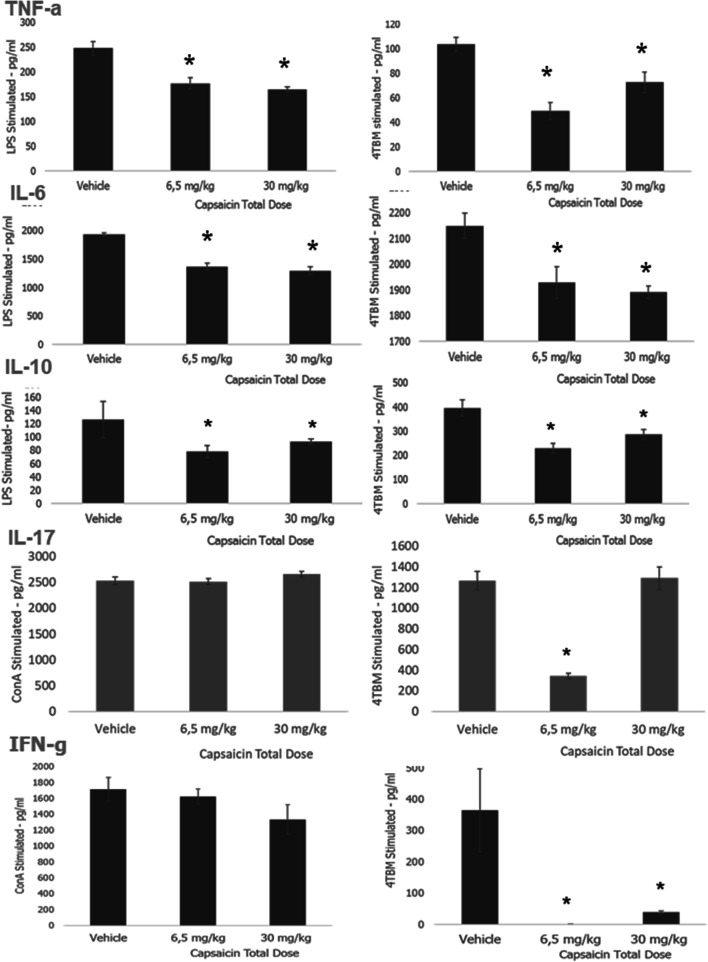
As seen in Fig. 2, systemic capsaicin treatment significantly decreased the secretion of TNF-α and IL-6 from leucocytes challenged with LPS or irradiated tumor cells. Both TNF-α and IL-6 are involved in excessive inflammation, tumor progression, and metastasis [47]. Relatively low doses of capsaicin decreased IL-17 secretion in response to stimulation with irradiated tumor cells but not to Concanavalin A (Fig. 2), which may further contribute to an anti-inflammatory microenvironment [48]. Capsaicin also induced moderate but significant decreases in IL-10 response which has both tumor-promoting and inhibiting activity (Fig. 2) [49].
Fig. 2.
Capsaicin-induced changes in cytokine response of leucocyte culture. Capsaicin treatment was performed as described above. Female Balb-c mice were used. TNF-α levels were measured 16–20 h after stimulation. Levels of other cytokines were determined 36–40 h after stimulation. Leucocytes were stimulated with LPS, Con-A, or irradiated tumor cells (4TBM). Levels of the secreted cytokines were measured using ELISA. Statistical analysis was performed using ANOVA followed by Dunnett’s Test *p < 0.05 compared to vehicle-treated group
IFN-γ response to Concanavalin-A stimulation was considerably higher compared to stimulation with irradiated tumor cells as seen in Fig. 2. Capsaicin treatment suppressed the IFN-γ response only to irradiated tumor cells. IFN-γ induces inflammation as well as cytotoxic anti-tumoral immunity; hence, consequences of this effect might be both tumorigenic and anti-tumorigenic depending on the milieu [50]. These findings demonstrated that relatively low doses of systemic capsaicin treatment may inhibit excessive inflammation. Although systemic capsaicin treatment could not be tolerated by tumor-bearing mice, which was unexpected and might be independent of TRPV1 activation, different TRPV1 agonists or modulators might be tolerated better and induce similar favorable immunological responses. We are in the process of testing the effects of these agents such as olvanil [26].
In conclusion, our previous studies as well as our new findings reported here demonstrated that specific subset of sensory nerve fibers which are sensitive to capsaicin have important roles in defense against metastatic breast carcinoma. Exact players of this newly explored defense system are only partly validated. Our findings expose the neuroimmune pathways guided by sensory nerve mediators as potential performers and suggest potential for targeting these pathways in cancer therapy. We, however, do not know how activation of sensory nerve fibers will affect the sympathetic response since preclinical animal studies indicated that inhibiting sympathetic activity, specifically β-adrenergic blockade, can reduce the immune-suppressive and metastasis-promoting effects of stress and surgery in several tumor models [5, 51–58]. It was shown that loss of sensory fibers leads to sympathetic sprouting and increases noradrenaline in tissues that are denervated [59, 60]. Determining whether increased sensory activity decreases noradrenergic response requires further studies. It was also shown that stimulation of sympathetic ganglia by Substance P, main neuromediator of most sensory fibers, increases renal sympathetic nerve activity, blood pressure, and heart rate [61, 62]. Further detailed studies are required to determine the effects of increased afferent nerve activity on systemic sympathetic response to assure there are no undesirable effects.
Abbreviations
- ADAM10
A disintegrin and metalloprotease 10
- ANS
Autonomous nervous system
- NK1R
Neurokinin 1 receptors
Funding
Newly described results on TRPV1 channels and on perivagal capsaicin treatment were from studies supported by funds from The Scientific and Technological Research Council of Turkey (TÜBİTAK), Project Nos: 115S943 and 104S492, respectively.
Compliance with ethical standards
Conflict of interest
The author declares that she has no conflict of interest.
Ethical approval and ethical standards
All animal experimentation was performed following the guidelines of an accredited animal care committee of Akdeniz University. Ethical approval from the Akdeniz University Ethics Committee was given to Nuray Erin (Protocol No. 70904504). All protocols described in Figs. 1 and 2 were approved and performed under the supervision of the Akdeniz University Institutional Animal Care and Use Committee.
Animal source
Wild-type (WT) female BALB/c mice were purchased from Kobay Research Animal Laboratory, Ankara Turkey.
Cell line authentication
4T1 cells were a gift from Dr. Danny Welch who was a faculty member of the Gittlen Cancer Center, Hershey Medical School, Hershey PA in 2001. The 4TBM cell line, established by Nuray Erin, was derived from brain metastasis of the 4T1 cells. The 4TBM cell line, kept in liquid nitrogen, forms primary tumors when injected into the mammary pad of BALB/c mice even after 50 passages.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Sixth International Conference on Cancer Immunotherapy and Immunomonitoring (CITIM 2019), held in Tbilisi, Georgia, 29th April–2nd May 2019. It is part of a series of CITIM 2019 papers in Cancer Immunology, Immunotherapy.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Spiegel D. Effects of psychotherapy on cancer survival. Nat Rev Cancer. 2002;2(5):383–389. doi: 10.1038/nrc800. [DOI] [PubMed] [Google Scholar]
- 2.Spiegel D, Sephton SE, Terr AI, Stites DP. Effects of psychosocial treatment in prolonging cancer survival may be mediated by neuroimmune pathways. Ann NY Acad Sci. 1998;840:674–683. doi: 10.1111/j.1749-6632.1998.tb09606.x. [DOI] [PubMed] [Google Scholar]
- 3.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5(3):243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 4.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11(9):625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozinovski S, Vlahos R, Anthony D, McQualter J, Anderson G, Irving L, Steinfort D. COPD and squamous cell lung cancer: aberrant inflammation and immunity is the common link. Br J Pharmacol. 2016;173(4):635–648. doi: 10.1111/bph.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catala M, Kubis N. Gross anatomy and development of the peripheral nervous system. Handb Clin Neurol. 2013;115:29–41. doi: 10.1016/B978-0-444-52902-2.00003-5. [DOI] [PubMed] [Google Scholar]
- 7.Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity. J Exp Med. 2012;209(6):1057–1068. doi: 10.1084/jem.20120571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavlov VA, Tracey KJ. Neural circuitry and immunity. Immunol Res. 2015;63(1–3):38–57. doi: 10.1007/s12026-015-8718-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Malley D. Neuroimmune cross talk in the gut. Neuroendocrine and neuroimmune pathways contribute to the pathophysiology of irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2016;311(5):G934–G941. doi: 10.1152/ajpgi.00272.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erin N, Korcum AF, Tanriover G, Kale S, Demir N, Koksoy S. Activation of neuroimmune pathways increases therapeutic effects of radiotherapy on poorly differentiated breast carcinoma. Brain Behav Immun. 2015;48:174–185. doi: 10.1016/j.bbi.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 11.Bonaz B, Sinniger V, Pellissier S. The vagus nerve in the neuro-immune axis: implications in the pathology of the gastrointestinal tract. Front Immunol. 2017;8:1452. doi: 10.3389/fimmu.2017.01452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bassi GS, Dias DPM, Franchin M, Talbot J, Reis DG, Menezes GB, Castania JA, Garcia-Cairasco N, Resstel LBM, Salgado HC, Cunha FQ, Cunha TM, Ulloa L, Kanashiro A. Modulation of experimental arthritis by vagal sensory and central brain stimulation. Brain Behav Immun. 2017;64:330–343. doi: 10.1016/j.bbi.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erin N, Duymus O, Ozturk S, Demir N. Activation of vagus nerve by semapimod alters substance P levels and decreases breast cancer metastasis. Regul Pept. 2012;179(1–3):101–108. doi: 10.1016/j.regpep.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Erin N, Ercan F, Yegen BC, Arbak S, Okar I, Oktay S. Role of capsaicin-sensitive nerves in gastric and hepatic injury induced by cold-restraint stress. Dig Dis Sci. 2000;45(9):1889–1899. doi: 10.1023/a:1005597220334. [DOI] [PubMed] [Google Scholar]
- 15.Ercan F, Oktay S, Erin N. Role of afferent neurons in stress induced degenerative changes of the bladder. J Urol. 2001;165(1):235–239. doi: 10.1097/00005392-200101000-00070. [DOI] [PubMed] [Google Scholar]
- 16.Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24(3):739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- 17.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve–an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52(4):595–638. [PubMed] [Google Scholar]
- 18.Miller AH, Spencer RL, Pearce BD, Pisell TL, Azrieli Y, Tanapat P, Moday H, Rhee R, McEwen BS. Glucocorticoid receptors are differentially expressed in the cells and tissues of the immune system. Cell Immunol. 1998;186(1):45–54. doi: 10.1006/cimm.1998.1293. [DOI] [PubMed] [Google Scholar]
- 19.Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol. 2004;5(6):575–581. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- 20.Namer B, Pfeffer S, Handwerker HO, Schmelz M, Bickel A. Axon reflex flare and quantitative sudomotor axon reflex contribute in the diagnosis of small fiber neuropathy. Muscle Nerve. 2013;47(3):357–363. doi: 10.1002/mus.23543. [DOI] [PubMed] [Google Scholar]
- 21.Hiura A. Neuroanatomical effects of capsaicin on the primary afferent neurons. Arch Histol Cytol. 2000;63(3):199–215. doi: 10.1679/aohc.63.199. [DOI] [PubMed] [Google Scholar]
- 22.Erin N, Boyer PJ, Bonneau RH, Clawson GA, Welch DR. Capsaicin-mediated denervation of sensory neurons promotes mammary tumor metastasis to lung and heart. Anticancer Res. 2004;24(2B):1003–1009. [PubMed] [Google Scholar]
- 23.Buck SH, Burks TF. The neuropharmacology of capsaicin: review of some recent observations. Pharmacol Rev. 1986;38(3):179–226. [PubMed] [Google Scholar]
- 24.Saria A, Skofitsch G, Lembeck F. Distribution of capsaicin in rat tissues after systemic administration. J Pharm Pharmacol. 1982;34(4):273–275. doi: 10.1111/j.2042-7158.1982.tb04245.x. [DOI] [PubMed] [Google Scholar]
- 25.Carr MJ, Undem BJ. Bronchopulmonary afferent nerves. Respirology. 2003;8(3):291–301. doi: 10.1046/j.1440-1843.2003.00473.x. [DOI] [PubMed] [Google Scholar]
- 26.Chu KM, Ngan MP, Wai MK, Yeung CK, Andrews PL, Percie du Sert N, Rudd JA. Olvanil: a non-pungent TRPV1 activator has anti-emetic properties in the ferret. Neuropharmacology. 2010;58(2):383–391. doi: 10.1016/j.neuropharm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Erin N, Akdas Barkan G, Harms JF, Clawson GA. Vagotomy enhances experimental metastases of 4THMpc breast cancer cells and alters substance P level. Regul Pept. 2008;151(1–3):35–42. doi: 10.1016/j.regpep.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85(1–3):1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 29.Prechtl JC, Powley TL. The fiber composition of the abdominal vagus of the rat. Anat Embryol (Berl) 1990;181(2):101–115. doi: 10.1007/bf00198950. [DOI] [PubMed] [Google Scholar]
- 30.Oszlacs O, Jancso G, Kis G, Dux M, Santha P. Perineural capsaicin induces the uptake and transganglionic transport of choleratoxin B subunit by nociceptive C-fiber primary afferent neurons. Neuroscience. 2015;311:243–252. doi: 10.1016/j.neuroscience.2015.10.042. [DOI] [PubMed] [Google Scholar]
- 31.Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J Intern Med. 2011;269(1):45–53. doi: 10.1111/j.1365-2796.2010.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erin N, Tanriover G, Curry A, Akman M, Duymus O, Gorczynski R. CD200fc enhances anti-tumoral immune response and inhibits visceral metastasis of breast carcinoma. Oncotarget. 2018;9(27):19147–19158. doi: 10.18632/oncotarget.24931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erin N, Podnos A, Tanriover G, Duymus O, Cote E, Khatri I, Gorczynski RM. Bidirectional effect of CD200 on breast cancer development and metastasis, with ultimate outcome determined by tumor aggressiveness and a cancer-induced inflammatory response. Oncogene. 2015;34(29):3860–3870. doi: 10.1038/onc.2014.317. [DOI] [PubMed] [Google Scholar]
- 34.Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341(6142):1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 35.Lundberg JM, Franco-Cereceda A, Hua X, Hokfelt T, Fischer JA. Co-existence of substance P and calcitonin gene-related peptide-like immunoreactivities in sensory nerves in relation to cardiovascular and bronchoconstrictor effects of capsaicin. Eur J Pharmacol. 1985;108(3):315–319. doi: 10.1016/0014-2999(85)90456-X. [DOI] [PubMed] [Google Scholar]
- 36.Erin N, Zhao W, Bylander J, Chase G, Clawson G. Capsaicin-induced inactivation of sensory neurons promotes a more aggressive gene expression phenotype in breast cancer cells. Breast Cancer Res Treat. 2006;99(3):351–364. doi: 10.1007/s10549-006-9219-7. [DOI] [PubMed] [Google Scholar]
- 37.MacKinnon AC, Waters C, Jodrell D, Haslett C, Sethi T. Bombesin and substance P analogues differentially regulate G-protein coupling to the bombesin receptor. Direct evidence for biased agonism. J Biol Chem. 2001;276(30):28083–28091. doi: 10.1074/jbc.M009772200. [DOI] [PubMed] [Google Scholar]
- 38.Szolcsanyi J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides. 2004;38(6):377–384. doi: 10.1016/j.npep.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Croitoru K, Ernst PB, Bienenstock J, Padol I, Stanisz AM. Selective modulation of the natural killer activity of murine intestinal intraepithelial leucocytes by the neuropeptide substance P. Immunology. 1990;71(2):196–201. [PMC free article] [PubMed] [Google Scholar]
- 40.Janelsins BM, Sumpter TL, Tkacheva OA, Rojas-Canales DM, Erdos G, Mathers AR, Shufesky WJ, Storkus WJ, Falo LD, Jr, Morelli AE, Larregina AT. Neurokinin-1 receptor agonists bias therapeutic dendritic cells to induce type 1 immunity by licensing host dendritic cells to produce IL-12. Blood. 2013;121(15):2923–2933. doi: 10.1182/blood-2012-07-446054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elsamany S, Abdullah S. Triple-negative breast cancer: future prospects in diagnosis and management. Med Oncol. 2014;31(2):834. doi: 10.1007/s12032-013-0834-y. [DOI] [PubMed] [Google Scholar]
- 42.Nesslinger NJ, Sahota RA, Stone B, Johnson K, Chima N, King C, Rasmussen D, Bishop D, Rennie PS, Gleave M, Blood P, Pai H, Ludgate C, Nelson BH. Standard treatments induce antigen-specific immune responses in prostate cancer. Clin Cancer Res. 2007;13(5):1493–1502. doi: 10.1158/1078-0432.CCR-06-1772. [DOI] [PubMed] [Google Scholar]
- 43.Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst. 1979;63(5):1229–1235. [PubMed] [Google Scholar]
- 44.Bakker JL, Meijers-Heijboer H, Verheul H. Novel strategies towards the use of anti-angiogenic agents in breast cancer. Eur J Pharmacol. 2013;717(1–3):36–39. doi: 10.1016/j.ejphar.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 45.Ghosh AK, Basu S. Tumor macrophages as a target for Capsaicin mediated immunotherapy. Cancer Lett. 2012;324(1):91–97. doi: 10.1016/j.canlet.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Erin N, Kale S, Tanriover G, Koksoy S, Duymus O, Korcum AF. Differential characteristics of heart, liver, and brain metastatic subsets of murine breast carcinoma. Breast Cancer Res Treat. 2013;139(3):677–689. doi: 10.1007/s10549-013-2584-0. [DOI] [PubMed] [Google Scholar]
- 47.Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beringer A, Noack M, Miossec P. IL-17 in chronic inflammation: from discovery to targeting. Trends Mol Med. 2016;22(3):230–241. doi: 10.1016/j.molmed.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Geginat J, Larghi P, Paroni M, Nizzoli G, Penatti A, Pagani M, Gagliani N, Meroni P, Abrignani S, Flavell RA. The light and the dark sides of Interleukin-10 in immune-mediated diseases and cancer. Cytokine Growth Factor Rev. 2016;30:87–93. doi: 10.1016/j.cytogfr.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Castro F, Cardoso AP, Goncalves RM, Serre K, Oliveira MJ. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front Immunol. 2018;9:847. doi: 10.3389/fimmu.2018.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ben-Eliyahu S, Shakhar G, Page GG, Stefanski V, Shakhar K. Suppression of NK cell activity and of resistance to metastasis by stress: a role for adrenal catecholamines and beta-adrenoceptors. NeuroImmunoModulation. 2000;8(3):154–164. doi: 10.1159/000054276. [DOI] [PubMed] [Google Scholar]
- 52.Lee JW, Shahzad MM, Lin YG, Armaiz-Pena G, Mangala LS, Han HD, Kim HS, Nam EJ, Jennings NB, Halder J, Nick AM, Stone RL, Lu C, Lutgendorf SK, Cole SW, Lokshin AE, Sood AK. Surgical stress promotes tumor growth in ovarian carcinoma. Clin Cancer Res. 2009;15(8):2695–2702. doi: 10.1158/1078-0432.CCR-08-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Melamed R, Rosenne E, Shakhar K, Schwartz Y, Abudarham N, Ben-Eliyahu S. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun. 2005;19(2):114–126. doi: 10.1016/j.bbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Roche-Nagle G, Connolly EM, Eng M, Bouchier-Hayes DJ, Harmey JH. Antimetastatic activity of a cyclooxygenase-2 inhibitor. Br J Cancer. 2004;91(2):359–365. doi: 10.1038/sj.bjc.6601967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shakhar G, Ben-Eliyahu S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J Immunol. 1998;160(7):3251–3258. [PubMed] [Google Scholar]
- 56.Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas BD, Wu L, Sood AK, Cole SW. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70(18):7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, Li Y, Gershenson DM, Lutgendorf S, Cole SW. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12(2):369–375. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yakar I, Melamed R, Shakhar G, Shakhar K, Rosenne E, Abudarham N, Page GG, Ben-Eliyahu S. Prostaglandin e(2) suppresses NK activity in vivo and promotes postoperative tumor metastasis in rats. Ann Surg Oncol. 2003;10(4):469–479. doi: 10.1245/aso.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 59.Hill CE, Vidovic M. The role of competition in the refinement of the projections of sympathetic neurons to the rat eye during development. Int J Dev Neurosci. 1989;7(5):539–551. doi: 10.1016/0736-5748(89)90013-0. [DOI] [PubMed] [Google Scholar]
- 60.Luthman J, Stromberg I, Brodin E, Jonsson G. Capsaicin treatment to developing rats induces increase of noradrenaline levels in the iris without affecting the adrenergic terminal density. Int J Dev Neurosci. 1989;7(6):613–622. doi: 10.1016/0736-5748(89)90020-8. [DOI] [PubMed] [Google Scholar]
- 61.Hancock JC, Lindsay GW. Pressor and tachycardic responses to intravenous substance P in anesthetized rats. Peptides. 1995;16(8):1439–1445. doi: 10.1016/0196-9781(95)02049-7. [DOI] [PubMed] [Google Scholar]
- 62.Hancock JC, Lindsay GW. Enhanced ganglionic responses to substance P in spontaneously hypertensive rats. Peptides. 2000;21(4):535–541. doi: 10.1016/s0196-9781(00)00170-4. [DOI] [PubMed] [Google Scholar]