Abstract
Despite significant therapeutic improvements chronic lymphocytic leukemia (CLL) remains an incurable disease and there is a persistent pursuit of new treatment alternatives. Lurbinectedin, a selective inhibitor of active transcription of protein-coding genes, is currently in phase II/III clinical trials for solid tumors such as small-cell lung cancer (SCLC). In this study, we aimed to evaluate the activity of Lurbinectedin on circulating mononuclear cells from CLL patients and to determine whether Lurbinectedin could affect the cross-talk between B-CLL cells and the tumor microenvironment. We found that Lurbinectedin induced a dose- and time-dependent death in all cell types evaluated, with B cells, monocytes and monocytic myeloid derived suppressor cells (Mo-MDSC) being the most susceptible populations. At sub-apoptotic doses, Lurbinectedin decreased the expression of CCR7 in B-CLL cells and impaired their migration towards CCL19 and CCL21. Furthermore, low concentrations of Lurbinectedin stimulated the synthesis of pro-IL1β in monocytes and nurse-like cells, without inducing the inflammasome activation. Altogether, these results indicate that Lurbinectedin might have antitumor activity in CLL due to its direct action on leukemic cells in combination with its effects on the tumor microenvironment. Our findings encourage further investigation of Lurbinectedin as a potential therapy for CLL.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02513-y) contains supplementary material, which is available to authorized users.
Keywords: Lurbinectedin, Chronic lymphocytic leukemia (CLL), Tumor microenvironment, CCR7, IL1β
Introduction
Chronic Lymphocytic Leukemia (CLL) is a disease characterized by the accumulation of clonal CD5+ B lymphocytes in peripheral blood, lymph nodes, bone marrow and spleen [1]. It is the most common leukemia in adults in the Western world [2]. Despite advances over the last 15 years in CLL therapy, including chemoimmunotherapy and the development of target drugs, CLL is still an incurable disease. Patients often relapse after treatment and many times develop resistance or transformation to more aggressive forms of leukemia [3]. Numerous studies have shown the fundamental role the tumor microenvironment plays, not only in CLL development and progression but also in treatment failure [4–7]. Currently, it is well known that the leukemic clone proliferates mainly in the lymph nodes and, to a lesser extent, in the bone marrow and spleen, within histological structures called proliferation centers or pseudo-follicles [8]. In these survival niches, accumulations of proliferating leukemic cells can be observed in contact with activated T lymphocytes, stromal cells and nurse-like cells (NLC, the tumor-associated macrophages (TAM) of CLL) [8]. Lymphocyte migration from the blood to the lymphoid tissues is a finely regulated process that has been proposed as a potential therapeutic target in this pathology [9]. In fact, Ibrutinib, one of the most promising treatments currently available for CLL, is a BTK (Bruton’s tyrosine kinase) inhibitor that interferes with the migration and adhesion of B-CLL cells [10]. Thus, investigations into novel treatments that affect both the leukemic clone and its interactions with the tumor microenvironment are imperative.
Lurbinectedin (also known as PM01183), a Trabectedin analogue, is a selective inhibitor of the active transcription of protein-coding genes [11]. The mechanism of action involves the arrest of the elongating RNA polymerase II and its degradation by the ubiquitin/proteasome machinery. Subsequently, the recruitment of DNA repair factors, including XPF nuclease, generates an accumulation of double-strand breaks that leads to apoptosis [12]. The fact that Lurbinectedin interferes with the action of the RNA polymerase II is very appealing because it has been reported that Fludarabine, one of the most widely used chemotherapy in CLL, kills the leukemic cells through its action on this polymerase [13, 14]. Moreover, there is evidence suggesting that Lurbinectedin, in addition to its direct activity on tumor cells, can modify the tumor microenvironment by affecting myeloid cells [15, 16]. Belgiovine et al. [15] demonstrated in a murine model of fibrosarcoma that Lurbinectedin decreases the number of circulating monocytes and TAMs, inhibiting angiogenesis and restricting tumor growth, even when cancer cells are resistant to the direct effect of the drug. Furthermore, Kuroda et al. [16] show that Mo-MDSC could also be a target of this drug. In this work, we investigated the in vitro activity of Lurbinectedin on leukemic B cells and non-malignant leukocytes from CLL patients and evaluated its regulatory effects at sub-apoptotic concentrations on the tumor microenvironment.
Material and methods
Reagents and antibodies
RPMI 1640, penicillin and streptomycin were purchased from GIBCO (New Zealand). Fetal bovine serum (FBS) was obtained from Natocor (Argentina). The Ficoll-Paque Plus and Dextran 500 used for cell separation were purchased from GE Healthcare (Germany). MACS CD14 cell isolation kit was from Miltenyi Biotec (Germany). DMSO and ATP were acquired from Sigma-Aldrich (USA). Annexin-V-FITC, PE, FITC or PECy5-conjugated mAbs anti-CCR7 (clone 3D12), anti-CXCR4 (clone 12G5), anti-CD56 (clone B159), anti-HLA-DR (clone G46-6), anti-CD3 (HIT3a), anti-CD4 (OKT4), anti-CD8 (HIT8a), anti-phosphorylated Akt (clone M89-61), anti-cleaved PARP (clone F21-852), control Abs with irrelevant specificities (isotype control) and human IL-1β ELISA Set II, were obtained from BD Biosciences (USA). PECy5-anti-CD19 (clone J3-119) and anti-CD3 (clone UCHT1) antibodies were purchased from Beckman Coulter (USA). PE-anti-CD14 (clone HCD14) mAb was purchased from BioLegend (USA). Mouse monoclonal anti-IL-1β antibody (clone AS10) was acquired from LifeSpan BioScience (USA). The following secondary antibodies were obtained from Jackson Immunoresearch Laboratories (USA): DyLight 549 conjugated F(ab’)2 anti-mouse IgG and peroxidase conjugated anti-rabbit IgG or anti-mouse IgG. CCL19 and CCL21 chemokines were from PeproTech (México). FAM-FLICA Caspase-1 Assay Kit was procured from ImmunoChemistry Technologies (USA). Phosphorylated Extracellular signal-regulated kinase 1/2 (P-Erk 1/2) (clone 12D4) was obtained from Santa Cruz Biotechnology (USA). Antibodies used as load control that recognize β-actin and tubulin were acquired from Cell Signaling (USA). Lurbinectedin was gently provided by PharmaMar S.A (Spain), it was dissolved at a concentration of 1 mM in DMSO and stored aliquoted at − 70 °C. The aliquots were thawed immediately before use and diluted in culture medium. ABT-199 was purchased from MedKoo Biosciences, Inc (USA).
CLL patients and healthy donor samples
CLL samples were obtained from two different hospitals from Buenos Aires, Argentina: Sanatorio Julio Méndez and Hospital General de Agudos Dr. Teodoro Álvarez. CLL was diagnosed according to standard clinical and laboratory criteria. At the time of analysis, all patients were free from clinically relevant infectious complications and were either untreated or had not received antineoplastic treatment for a period of at least 6 months.
Healthy donor samples were obtained from the blood bank of the Fundación Hemocentro Buenos Aires.
Cell isolation procedures and culture
Peripheral blood leukocytes from healthy donors and CLL patients were separated as previously described in detail in [17]. Cells were cultured at a concentration of 3 × 106/ml and treated for different periods with clinically relevant doses of Lurbinectedin. Monocytes were isolated from PBMC from healthy donors or CLL patients using MACS CD14 cell isolation kit according to manufacturer’s instructions. NLC were obtained by culturing PBMC from CLL patients (5 × 106 cells/ml) or co-culturing purified HD monocytes with B-CLL cells for 14 days as previously reported [7]. Neutrophils were obtained from CLL patient blood samples by Ficoll-Paque centrifugation followed by dextran sedimentation as was previously described in detail in [17].
To determine the sensitivity of proliferating T lymphocytes to Lurbinectedin, PBMC from CLL patients were incubated in a 48-well culture plate with immobilized anti-CD3 mAb to induce T cell proliferation, or isotype control (0.3 µg/ml). Lurbinectedin 1 nM or 3 nM was added 48 h later and cells were incubated for 3 more days. Finally, cells were collected and stained with mAbs specific for CD4 (PECy5) and for CD8 (PE). T cell death was evaluated by flow cytometry.
Flow cytometry analysis
Annexin V staining was used to evaluate the percentage of cell death induced by Lurbinectedin. First, cell populations were identified using specific surface markers and then suspensions were incubated for 20 min at room temperature with Annexin V-FITC to discriminate viable and dead cells. Flow cytometry analysis was performed immediately after incubation. In addition, cell death was corroborated by flow cytometric alterations of light-scattering properties. For surface antigen staining, cells were incubated for 30 min at 4 °C with the corresponding antibodies diluted in PBS supplemented with 0.5% BSA. Cells were twice washed before acquisition on a FACScalibur cytometer (Becton Dickinson, USA) Data were analyzed with FlowJo 8 software (Facs software, Tree Star, Inc).
Western blot
Purified B-CLL cells were exposed to Lurbinectedin or vehicle for the indicated time, then were washed with cold PBS and protein extracts were prepared following standard procedures in RIPA buffer in the presence of protease and phosphatase inhibitors. After quantification with the Micro-BCA Protein Assay Kit (Thermo Scientific), 15–25 µg of protein were separated by SDS-PAGE and transferred to PVDF membranes. Membranes were then incubated with specific primary antibodies overnight. Next, membranes were washed and incubated with HRP-conjugated secondary antibodies. Finally, enhanced chemiluminescence (ECL) was used to develop the western bolt. Actin or Tubulin were used as total load controls. The analysis of the results was carried out with the ImageJ software (NIH, USA).
Chemokine receptor evaluation
Given that leukocyte isolation can transiently reduce chemokine receptor expression [18], PBMC from CLL patients were incubated overnight at 37 °C to allow complete re-expression of CCR7 and CXCR4. Cells were then treated with Lurbinectedin (1 or 3 nM) or vehicle. After 24 h, CCR7 and CXCR4 expression on CD19+ cells were evaluated by flow cytometry.
Chemotaxis assay
Chemotaxis response toward CCL19 and CCL21 was determined using Transwell plates with 5 µm pore polycarbonate membranes (Costar, Corning Incorporated). Briefly, CLL PBMC were treated with Lurbinectedin (1 or 3 nM) for 24 h in a 48-well microplate. Next, cells were washed, counted and diluted so that 70 µl of medium contains 0.5 × 106 cells. That volume was placed in the upper chamber of a Transwell plate. Aliquots of 200 µl of medium alone (control) or medium with CCL19 or CCL21 was placed in the lower chamber. Cells were incubated at 37 °C for 2 h. Next, migrated cells in the lower chamber were collected and stained with anti-CD19 antibody. Live CD19+ cells were quantified by flow cytometry as previously described [19]. Migration index was calculated by subtracting the number of CD19+ cells that migrated spontaneously (control without chemokine) from the number of CD19+ cells that migrated in the presence of the chemokines and normalizing to the input [20].
Analysis of IL-1β secretion by ELISA
IL-1β release was evaluated in monocytes purified from CLL PBMC or NLC differentiated from CLL monocytes. Cells were treated with Lurbinectedin 3 nM or vehicle for 24 h. In some experiments, ATP 2 mM was added for the last 90 min of culture. IL-1β levels in culture supernatants were quantified by ELISA following the manufacturer’s instructions (BD-Biosciences).
Confocal microscopy
Assessment of intracellular Pro-IL-1β/IL-1β
NLC differentiated over microscope slides were treated with Lurbinectedin 3 nM or vehicle for 24 h. Then, cells were fixed with 4% PFA for 30 min, blocked with PBS-glycine (0.1 M) for 15 min, permeabilized with acetone at − 20 °C for 7 min, rehydrated with PBS and blocked with PBS supplemented with 5% goat serum overnight at 4 °C. Subsequently, NLC were incubated with the primary antibody in blocking buffer for 1 h at room temperature, washed and incubated with the secondary antibody conjugated with Alexa 549 for 1 h at room temperature. Finally, cells were washed and mounted with AcquaPolymount mounting medium for microscopy examination.
Assessment of activated Caspase-1
Caspase-1 activity was evaluated using the FAM-FLICA commercial kit. NLC were obtained by co-culturing HD monocytes with CLL B-cells for 14 days over microscope slides. Once NLC were fully differentiated, non-adherent cells were discarded and adherent cells were treated for 24 h with 3 nM Lurbinectedin. When indicated, 2 mM ATP was added during the last hour of treatment. Finally, staining and fixation were done according to the manufacturer's instructions.
For the acquisition of images, the FluoView FV1000 confocal microscope (Olympus, Tokyo, Japan) equipped with an oil immersion objective Plapon 60×/1.42 was used. Images were analyzed using Fiji or Olympus FV10-ASW programs.
Total RNA preparation, cDNA synthesis and qRT-PCR
Total RNA was extracted from 2 × 106 purified monocytes from CLL PBMC or NLC (differentiated from HD monocytes co-culture with B-CLL cells) using TRIzol reagent according to the manufacturer’s instructions. cDNA synthesis and qRT-PCR were performed as previously described in detail in [21]. Primers were purchased from Ruralex-Fagos (Buenos Aires, Argentina): GAPDH Fw 5´ GAGTCAACGGATTTGGTCGT 3´, GAPDH Rv 5´ TTGATTTTGGAGGGATCTCG 3´, pro-IL-1β Fw 5´ CTGAACTGCACGCTCCGGG 3´ and pro-IL-1β Rv 5´ GCTTATCATCTTTCAACACGCAGG 3´.
Statistical analysis
Statistical significance was determined using the following tests: Wilcoxon signed-rank test, Friedman test followed by the Dunn’s multiple comparison post-test, Mann–Whitney test or two-ways ANOVA followed by Tukey multiple comparison post-test. Data were analyzed using GraphPad Prism software version 7. In all cases, p < 0.05 was considered statistically significant.
Results
B-CLL cells are sensitive to Lurbinectedin
In an effort to evaluate CLL B-cells sensitivity to Lurbinectedin PBMC were isolated from CLL patient’s samples and then treated with different drug concentrations for 24–72 h. Cell death was determined by flow cytometry using Annexin V staining and anti-CD19 antibody to select the population of interest (Fig. 1a). Dot plot analysis is depicted in Supplementary Fig. 1. We found that the cytotoxic effect of Lurbinectedin on leukemic B cells was dose- and time-dependent. According to available clinical data, the maximum serum concentration of Lurbinectedin is approximately 188.8–196.0 nM [22]. This concentration drops rapidly in the first hours after administration due to the distribution of the drug to different tissues, to a concentration of approximately 10–15 nM. Complete elimination of Lurbinectedin requires 9.6 days [23]. Taking these findings into account, our results indicate that B-CLL cells are highly sensitive to Lurbinectedin at clinically relevant concentrations.
Fig. 1.
B cells are sensitive to Lurbinectedin. a CLL PBMC were incubated with Lurbinectedin 1, 3, 10, 30 nM or vehicle for 24, 48 and 72 h. Cells were stained with anti-CD19-PECy5 mAb and Annexin V-FITC. Shown are the individual values and the mean ± SEM from 10 samples. b The experiment on (a) was repeated using HD PBMC. Shown are the individual values and the mean ± SEM from 13 samples after 24 h of treatment. Statistical analysis was performed using Friedman test followed by the Dunn’s multiple comparison post-test. c, d B-CLL cells were treated with Lurbinectedin 10 nM for 24 h. c Then, γ-H2AX and PARP fragmentation were evaluated by western blot. d Bands on the immunoblots were quantified. Results are shown as the mean ± SEM of the ratio γ-H2AX/tubulin and PARP/tubulin. Statistical analysis was performed using Wilcoxon’s matched-pairs signed-rank test. Lur Lurbinectedin; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
To determine if the sensitivity of leukemic B cells to Lurbinectedin is due to their malignant cell condition, the experiment was repeated using PBMC from healthy donors (HD). Results depicted in Fig. 1b and Supplementary Fig. 2a show that Lurbinectedin induced comparable levels of cytotoxicity on CD19+ cells from HD and CLL patients. Lurbinectedin-induced apoptosis on normal B cells was corroborated by evaluating caspase-3 cleavage (Supplementary Fig. 2b). Together these results show that B lymphocytes, regardless of their malignant condition, are sensitive to Lurbinectedin. This result might be considered when Lurbinectedin is used in other types of cancer.
To evaluate if the mechanism of action of Lurbinectedin in B-CLL cells is the same as previously described in cell lines, we investigated the phosphorylation of the histone γH2AX. This, in turn, would allow us to identify the induction of DNA double-strand breaks. We also analyzed the cleavage of PARP to determine levels of apoptosis. We noted an increase in both markers (Fig. 1c, d) after treatment with 10 nM Lurbinectedin for 24 h, indicating that the mechanism of cell death induced by Lurbinectedin in B-CLL cells involves the induction of DNA damage and the apoptotic program.
Lurbinectedin affects the viability of non-malignant leukocytes
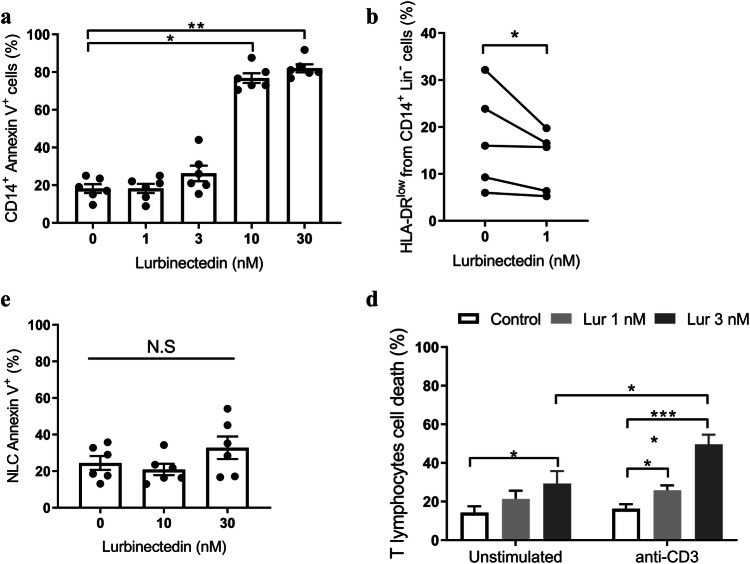
Next, we evaluated the cytotoxic effect of Lurbinectedin in leukocyte populations that were shown to affect the CLL environment. We found that monocytes and neutrophils show sensitivity to Lurbinectedin comparable to B cells (Fig. 2a and Supplementary Fig. 3 respectively). Regarding Mo-MDSC, which were identified as CD14+HLA-DRlowLin− [24, 25], we corroborated that their number in circulation is increased in CLL patients compared to age-matched HD (Supplementary Fig. 4). Of note, Mo-MDSC was the most susceptible leukocyte population to Lurbinectedin in vitro where doses as low as 1 nM significantly decreased its percentages (Fig. 2b). On the other hand, NLC were surprisingly resistant to Lurbinectedin (Fig. 2c). Finally, we assessed the sensitivity of unstimulated and proliferating T lymphocytes to Lurbinectedin. To that aim, T cells were activated or not through their TCR and exposed to Lurbinectedin for 72 h. We found that the induction of proliferation significantly increased T cell susceptibility to Lurbinectedin (Fig. 2d). This is an interesting finding considering that the most relevant contribution of T lymphocytes to CLL cell survival occurs in the proliferation centers, where activated CD4+ T lymphocytes promote the leukemic progression [26].
Fig. 2.
Effects of Lurbinectedin on tumor-microenvironment cells. a Monocytes were treated with different concentrations of Lurbinectedin for 24 h. Cell death was determined by Annexin V-FITC staining and flow cytometry analysis. The individual values and the mean ± SEM are shown, n = 6. b The sensitivity of Mo-MDSC to Lurbinectedin was determined by incubating PBMC with or without Lurbinectedin 1 nM for 24 h. Cells were stained with anti-CD3 PECy5, anti-CD19 PECy5, anti-CD56 PeCy5, anti-CD14-PE and anti-HLA-DR-FITC antibodies. Mo-MDSC population was selected as Lin− (CD3−, CD19−, CD56−), CD14+ and HLA-DRlow. c NLC were incubated with vehicle, Lurbinectedin 10 or 30 nM for 24 h. Then, cells were labeled with Annexin V-FITC and analyzed by flow cytometry. The individual values and the mean ± SEM are shown, n = 6. d CLL PBMC were plated over immobilized anti-CD3 antibody (anti-CD3i) or isotype control. After 48 h of incubation, Lurbinectedin or vehicle were added for another 72 h of culture. Finally, cells were labeled with anti-CD4-PECy5 and anti-CD8-PE antibodies and analyzed by flow cytometry. The percentage of total non-viable T lymphocytes after 5 days of culture is shown (mean ± SEM), n = 11. Statistical significance was determined by the Friedman test followed by Dunn’s post-test of multiple comparisons (a, c and d) or Wilcoxon signed-rank test (b). Lur Lurbinectedin, N.S no statistically significant difference, *p < 0.05; **p < 0.01; ****p < 0.0001
Effects of Lurbinectedin on B-CLL cells in the presence of a supportive microenvironment
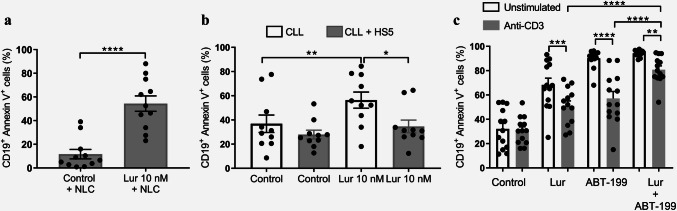
As we mentioned before, interaction with a supportive microenvironment is essential for B-CLL cell survival and therapy resistance. Among relevant interactions with B-CLL cells are those of NLC, stromal cells and activated T lymphocytes [27]. Therefore, we next evaluated if the co-culture of leukemic B cells with any of these cell types could interfere with the cytotoxic activity of Lurbinectedin on B-CLL cells. As shown in Fig. 3a, B-CLL cells exposed to 10 nM of Lurbinectedin for 24 h in the presence of NLC were as sensitive as B-CLL cells cultured without NLC (Fig. 1a), indicating that signals from NLC do not impair Lurbinectedin activity. By contrast, co-culture with HS5 (a stromal cell line) diminished the cytotoxic activity of Lurbinectedin on B-CLL cells (Fig. 3b).
Fig. 3.
Sensitivity of B-CLL cells to Lurbinectedin in the presence of microenvironmental stimuli. a NLC cells were differentiated as previously described. Next, Lurbinectedin (10 nM) was added to the co-culture for 24 h and the percentage of CD19+ cell death was determined by flow cytometry. The individual values and the mean ± SEM are shown (n = 11). b B-CLL cells were co-cultured with HS5 stromal cell line for 72 h. Then, Lurbinectedin 10 nM or vehicle was added for 24 h and CD19+ cell death was assessed by flow cytometry. The individual values and the mean ± SEM are shown (n = 10). c CLL PBMC were plated in wells with or without immobilized anti-CD3 antibody and after 48 h of incubation Lurbinectedin 3 nM was added. Then, cells were incubated for another 48 h before the addition of 100 nM ABT-199 for the last 24 h. Cell death was evaluated by flow cytometry. The results show the individual values and the mean ± SEM, n = 13. Statistical analysis was performed by the Friedman test followed by Dunn’s post-test of multiple comparisons. Lur Lurbinectedin, *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
Our group recently reported that the BCL-2 inhibitor ABT-199 (Venetoclax), a novel target agent for CLL, is markedly less toxic towards leukemic cells in the presence of activated T lymphocytes [28]. Therefore, we questioned if Lurbinectedin was able to overcome this resistance in vitro. To that aim, we cultured PBMC from CLL samples with or without immobilized anti-CD3 antibodies and added 3 nM Lurbinectedin on day 2. After an additional 48 h of incubation, cells were exposed to 100 nM ABT-199 for 24 h and B-CLL cell death was subsequently evaluated. As shown in Fig. 3c activated T lymphocytes reduced the cytotoxic activity of both ABT-199 and Lurbinectedin on leukemic cells. Nevertheless, the combination of both drugs partially overcame resistance induced by activated T Lymphocytes as the cytotoxic effect induced by both drugs together on B-CLL cells was higher than that induced by each drug alone.
Lurbinectedin decreases CCR7 expression on B-CLL cells and their migration towards CLL19 and CCL21
The most important interactions between the tumor microenvironment and the leukemic clone are located in the lymph nodes and the bone marrow. Thus, impairing mechanisms responsible for leukemic cell migration to these organs could be an interesting approach in the fight against CLL. Therefore, we decided to evaluate if Lurbinectedin treatment affects the expression of the chemokine receptors CCR7 and CXCR4, two of the most relevant receptors implicated in B-CLL cell migration [29, 30]. We found that sub-apoptotic doses of Lurbinectedin (3 nM for 24 h) induced a significant decrease in CCR7 expression without modifying that of CXCR4 (Fig. 4a, b). Next, we performed an in vitro migration assay in transwell plates using CCL19 or CCL21, two specific chemokines recognized by CCR7 and observed a reduction in B-CLL cell migration after Lurbinectedin treatment for both chemoattractants (Fig. 4c, d). Finally, we confirmed our results by observing a reduction in the phosphorylation of Erk 1/2 and Akt, key steps in the signaling pathways triggered by these chemokines (Fig. 4e–h).
Fig. 4.
Lurbinectedin downregulates the expression of CCR7 on B-CLL cells and inhibits the migration towards CCL19 and CCL21. CLL PBMC were cultured for 24 h at 37 °C. Then, Lurbinectedin 1 nM, 3 nM or vehicle were added for 24 h. After that, cells were labeled with anti-CD19-PECy5 and anti-CCR7-PE (a) or anti-CXCR4-PE (b) antibodies and analyzed by flow cytometry. Figure shows the individual values and the mean ± SEM, n = 14. c, d The effect of Lurbinectedin over CLL CD19+ cell migration was measured by a migration assay on transwell plates (for more details on the procedure see the methods section). The results show individual values and mean ± SEM of the migration index to CCL19 (n = 7) and CCL21 (n = 6). The statistical analysis was performed using Friedman test followed by Dunn’s post-test. e CLL B lymphocytes were treated with Lurbinectedin 3 nM or vehicle for 24 h. Cells were stimulated with the chemokines and whole protein extracts were obtained. Cell extracts were then analyzed by Western blot using antibodies directed toward P-Erk 1/2. Figure shows a representative example of the bands obtained. f Quantification of the experiment described on (e) as mean ± SEM of the intensity ratio of the P-Erk vs actin, n = 8. Statistical analysis was performed by two-ways ANOVA followed by Tukey multiple comparison post-test. g CLL B lymphocytes were treated with Lurbinectedin 3 nM or vehicle for 24 h. Cells were stimulated with the chemokines and stained for P-Akt as was described in materials and methods. Results showed representative histograms and the corresponding MFI. Isotype control is depicted in grey. h Mean ± SEM of the MFI of p-Akt staining are shown (n = 9). The statistical analysis was performed by a two-ways ANOVA followed by Tukey’s post-test of multiple comparisons. Lur Lurbinectedin, MFI mean fluorescence intensity, N.S no statistically significant difference; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
Lurbinectedin increases IL-1β production in monocytes and NLC
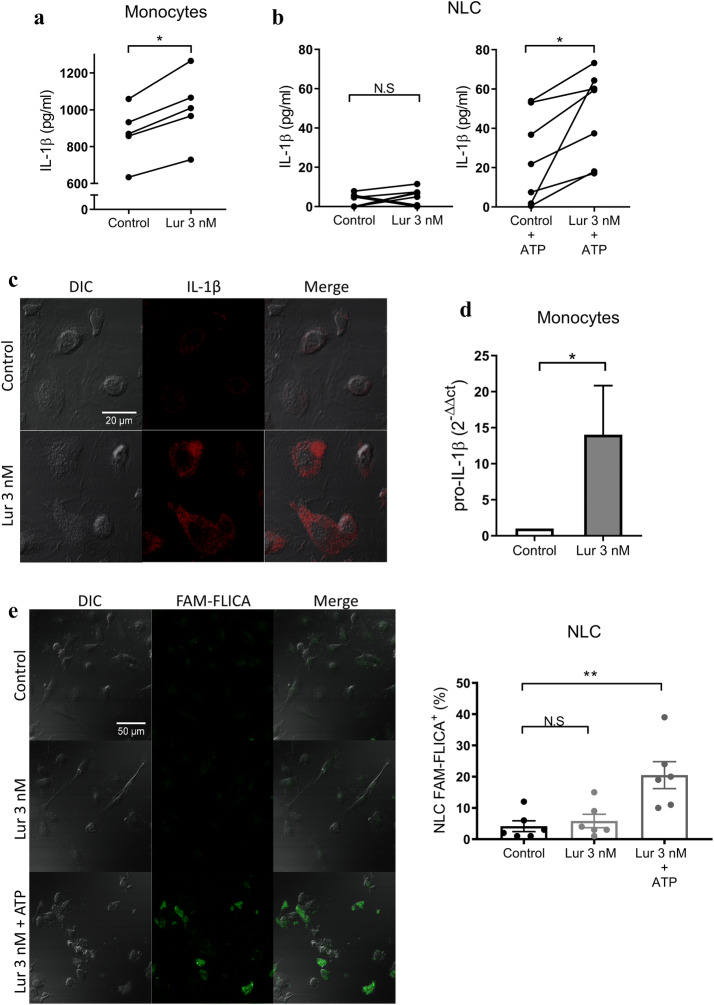
Certain antineoplastic drugs, e.g. 5-Fluorouracil and Gemcitabine induce the assembly of the inflammasome, which results in the secretion of the proinflammatory cytokine IL-1β [31, 32]. IL-1β is not secreted through the conventional endoplasmic reticulum–Golgi route of protein secretion but requires the processing of pro-IL-1β by caspase 1 within the inflammasome platform [33]. Considering that increased plasma levels of IL-1β in CLL patients is associated with longer survival [34], we wondered if Lurbinectedin could affect the production of IL-1β in myeloid cells. To address this, we first quantified the levels of IL-1β released into the supernatant when purified monocytes from CLL patients were exposed to a sub-apoptotic dose of Lurbinectedin (3 nM). Results in Fig. 5a shows that, after 24 h in culture, Lurbinectedin increased IL-1β secretion by monocytes. By contrast, when the experiment was repeated with NLC differentiated from monocytes in vitro we did not observe IL-1β secretion unless we added ATP to induce the activation of the inflammasome (Fig. 5b). This could be explained by the fact that monocytes but not macrophages have a constitutively active inflammasome which allows the release of IL-1β to the extracellular medium [35]. These results suggest that Lurbinectedin could increase the production of IL-1β in monocytes and NLC but does not induce the assembly of the inflammasome which is a necessary step for the processing and release of this cytokine. In fact, the incubation of NLC with Lurbinectedin (3 nM for 24 h) induced the accumulation of pro-IL-1β/IL-1β in the cytoplasm as can be observed by confocal microscopy (Fig. 5c). We also corroborated that Lurbinectedin does not promote the inflammasome assembly by evaluating the activation of caspase 1 employing the fluorescent probe FAM-Flica that forms irreversible covalent bonds specifically with active caspase 1 enzyme. As shown in Fig. 5e, incubation of NLC with 3 nM Lurbinectedin did not induce caspase 1 activation unless ATP was added. Finally, we found that Lurbinectedin significantly increased the levels of mRNA of pro-IL1β (Fig. 5d). Taken together, our results indicate that Lurbinectedin induces the synthesis of IL-1β in myeloid cells, but does not activate the inflammasome, as it has been reported for 5-Fluorouracil and Gemcitabine.
Fig. 5.
Lurbinectedin increases IL-1β production by myeloid cells. a Monocytes were purified from CLL samples and treated with 3 nM Lurbinectedin or vehicle for 24 h. IL-1β secretion was quantified by ELISA. The results are shown as individual values associating, in each sample, the concentration of the control and treated condition, n = 5. b NLC were treated with Lurbinectedin 3 nM for 24 h. In the indicated cases 2 mM ATP was added for the last 90 min of culture. The concentration of IL-1β secreted was quantified by ELISA. The results are expressed as individual values associating, in each sample, the control and treated condition, n = 7. c NLCs were differentiated over sterile microscope coverslips. Then, NLC were treated for 24 h with Lurbinectedin 3 nM and labeled with anti-IL-1β antibody. Images of a representative experiment are shown (n = 3). d Purified HD monocytes were treated for 24 h with Lurbinectedin 3 nM or vehicle and then pro-IL1β mRNA level was evaluated by qRT-PCR. Results were normalized to GAPDH human gene and were represented as relative units (2−ΔΔct). The mean ± SEM of 6 experiments is shown. e NLC were differentiated from HD monocytes co-cultured with B-CLL cells over sterile coverslips for 14 days. Leukemic cells were removed and NLC were treated with 3 nM Lurbinectedin for 24 h. For the positive control, 2 mM ATP was added for 1 h. FAM-FLICA staining was carried out according to the fabricant indications. Representative images of confocal microscopy and the quantification of the percentage of NLC positive for activated caspase-1 are shown (mean ± SEM; n = 6). a–d Statistical analysis was carried out using the Wilcoxon test or e Friedman test followed by Dunn’s post-test. Lur Lurbinectedin, N.S no statistically significant difference; *p < 0.05; **p < 0.01
Discussion
Despite advances in the last 15 years in CLL treatment, CLL remains an incurable disease. Thus, there is a need to find new alternatives that may affect both the leukemic clone and its interactions with the supportive microenvironment. In this study, we showed that Lurbinectedin, a selective inhibitor of active transcription, has a strong direct in vitro effect on leukemic B cells from CLL patients, and on non-malignant leukocytes such as monocytes, monocytic derived suppressor cells (Mo-MDSC), activated T lymphocytes and neutrophils. The relevance of these accompanying cells in CLL is well known and makes Lurbinectedin an interesting therapeutic alternative for this pathology. Circulating neutrophils, which are sensitive to Lurbinectedin, play a role in CLL, favoring the leukemic clone survival, as was recently described by our group [21, 36]. Monocytes, a population particularly sensitive to Lurbinectedin, were reported to be enriched in the circulation of CLL patients where their high absolute number positively correlates with progressive disease [37, 38]. Similarly, Mo-MDSC are also expanded in CLL patients and correlate with poor prognosis [24, 25]. Our results indicate that circulating Mo-MDSC from CLL patients were the most sensitive cell type to Lurbinectedin, supporting the findings of Kuroda et al. [16] who reported comparably sensitivity in Mo-MDSC obtained from murine spleen. Interestingly, we found that NLC, which are also differentiated from monocytes, are resistant to Lurbinectedin. Belgiovine et al. demonstrated, in a murine cancer model, that Lurbinectedin reduced the number of TAM infiltrating the tumor mass and that, even in the cases where the tumor was resistant to the cytotoxic action of Lurbinectedin, its growth was inhibited as a consequence of the reduction in TAM number. Therefore, Lurbinectedin might indirectly reduce the proportion of NLC in lymphoid organs through its pro-apoptotic effects on circulating monocytes [15]. In addition to providing survival and proliferation signals in the proliferation centers, NLC are also responsible for secreting chemokines that recruit the leukemic clone to the lymphoid tissues [7, 39]. This implies that the decrease in the NLC number could also interfere with B-CLL cell migration and homing in proliferation centers. Lurbinectedin not only could impair CLL recruitment to the lymph tissues by reducing the number of NLC but also through a direct effect on cell migration. We showed that non-apoptotic doses of Lurbinectedin are sufficient to decrease the expression of the chemokine receptor CCR7 in B-CLL cells and their migration towards CCL21 and CCL19. Ibrutinib success in CLL therapy highlights the benefits of interfering with clone trafficking. In terms of the potential use of Lurbinectedin for CLL treatment, the combination of its cytotoxic effect with the detachment of leukemic cells from the tissues could be an interesting approach. In line with our results, Lohmann et al. analyzed the effects of Trabectedin, the drug from which Lurbinectedin derives, in several CLL murine models. They reported that Trabectedin has good antitumor activity even in cases resistant to chemoimmunotherapy [40]. Furthermore, in an aggressive CLL model performed in immunocompetent mice, it was observed that, although Trabectedin slightly reduced the number of leukemic cells in the periphery, there was a marked decrease in the bone marrow compared to untreated or Fludarabine-treated mice [40]. Based on our results, we hypothesize that Trabectedin, like Lurbinectedin, decreases the recruitment of B-CLL cells to the bone marrow through its net effect on NLC and the impairment of leukemic cell migration.
In this study, we also observed that co-culture of CLL cells with activated T lymphocytes or stromal cells partially protected them from Lurbinectedin cytotoxicity. This is probably due to the survival signals delivered by accompanying cells which, as reported for other therapeutic agents, are responsible for treatment resistance. In this regard, our group has recently reported that activation of T lymphocytes fosters ABT-199 resistance in CLL [28].
Here we showed that, in the presence of activated T lymphocytes, the combination of Lurbinectedin and ABT-199 induced significantly higher levels of B-CLL cell death compared to single drug use. Altogether our results strengthen the role of the tumor microenvironment in the promotion of drug resistance and suggest the combined use of Lurbinectedin and ABT-199 in CLL therapy.
Finally, we demonstrated the capacity of non-apoptotic doses of Lurbinectedin to increase the production of IL-1β by monocytes and NLC. Unlike other antineoplastic drugs [31, 32], Lurbinectedin increased the secretion of IL-1β by directly stimulating pro-IL-1β synthesis without inducing the assembly of the inflammasome. Lurbinectedin was previously reported to decrease the release of CXCL8 and CCL2 by human monocytes [15], while, to our knowledge, its effects on IL-1β production had not been investigated. In CLL, plasma levels of IL-1β were reported increased [34] or decreased [41] compared to age-matched healthy donors. Despite this discrepancy, both studies indicate that higher concentrations of IL-1β in CLL patients are associated with good prognostic markers or increased survival, suggesting that IL-1β could play an antitumor role in CLL. IL-1β is a key cytokine in the differentiation of human CD4+ T lymphocytes towards a TH17 profile [42] which is overrepresented in CLL and correlates with longer survival [43, 44]. Therefore, the ability of Lurbinectedin to promote the synthesis of IL-1β could favor the immune response against leukemia.
In conclusion, our results indicate that Lurbinectedin might have antitumor activity in CLL due to its direct action on leukemic cells in combination with its effects on the tumor microenvironment. Our findings encourage further investigation of Lurbinectedin as a potential therapy for CLL.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors want to specially thank the patients and healthy donors who provided blood samples for this research. Also, we are indebted to Beatriz Loria, María Tejeda, Romina Pagano and Federico Fuentes for their technical assistance.
Abbreviations
- CLL
Chronic lymphocytic leukemia
- HD
Healthy donor
- Mo-MDSC
Monocytic myeloid-derived suppressor cells
- NLC
Nurse-like cells
- P-Erk½
Phosphorylated extracellular signal-regulated kinase 1/2
- SCLC
Small-cell lung cancer
- TAM
Tumor-associated macrophages
Author contributions
DR and MG designed the study. DR, AC, EP, MBA, NS and EEE performed the experiments. RFB and HF-G recruited patients and performed clinical analysis. DR, MB, PO, RG and MG analyzed the data. DR, AC and MG wrote the manuscript. All authors reviewed and approved the manuscript.
Funding
This work was supported by a Grant from FONCyT (PICT 212/2012), Ministry of Science, Technology and Innovation, Argentina.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This research was conducted in accordance with the Helsinki Declaration. The study was approved on September 11th of 2012 and revalidated on April 11th of 2018 by the Institutional Review Board of the National Academy of Medicine, Buenos Aires, Argentina.
Informed consent
Peripheral blood samples were collected from CLL patients and healthy donors after written informed consent allowing the use of their specimens and data for research and for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 2.Dighiero G, Hamblin TJ. Chronic lymphocytic leukaemia. Lancet (London, England) 2008;371:1017–1029. doi: 10.1016/S0140-6736(08)60456-0. [DOI] [PubMed] [Google Scholar]
- 3.Cramer P, Langerbeins P, Eichhorst B, Hallek M. Advances in first-line treatment of chronic lymphocytic leukemia: current recommendations on management and first-line treatment by the German CLL Study Group (GCLLSG) Eur J Haematol. 2016;96:9–18. doi: 10.1111/ejh.12678. [DOI] [PubMed] [Google Scholar]
- 4.Ghia P, Circosta P, Scielzo C, et al. Differential effects on CLL cell survival exerted by different microenvironmental elements. Curr Top Microbiol Immunol. 2005;294:135–145. doi: 10.1007/3-540-29933-5_8. [DOI] [PubMed] [Google Scholar]
- 5.Caligaris-Cappio F, Bertilaccio MTS, Scielzo C. How the microenvironment wires the natural history of chronic lymphocytic leukemia. Semin Cancer Biol. 2014;24:43–48. doi: 10.1016/j.semcancer.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Kurtova AV, Balakrishnan K, Chen R, et al. Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance. Blood. 2009;114:4441–4450. doi: 10.1182/blood-2009-07-233718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burger JA, Tsukada N, Burger M, et al. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655–2663. doi: 10.1182/blood.V96.8.2655. [DOI] [PubMed] [Google Scholar]
- 8.Caligaris-Cappio F. Role of the microenvironment in chronic lymphocytic leukaemia. Br J Haematol. 2003;123:380–388. doi: 10.1046/j.1365-2141.2003.04679.x. [DOI] [PubMed] [Google Scholar]
- 9.Redondo-Muñoz J, García-Pardo A, Teixidó J. Molecular players in hematologic tumor cell trafficking. Front Immunol. 2019 doi: 10.3389/fimmu.2019.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Rooij MFM, Kuil A, Geest CR, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119:2590–2594. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 11.Leal JFM, Martínez-Díez M, García-Hernández V, et al. PM01183, a new DNA minor groove covalent binder with potent in vitro and in vivo anti-tumour activity. Br J Pharmacol. 2010;161:1099–1110. doi: 10.1111/j.1476-5381.2010.00945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santamaría Nuñez G, Robles CMG, Giraudon C, et al. Lurbinectedin specifically triggers the degradation of phosphorylated RNA polymerase II and the formation of DNA breaks in cancer cells. Mol Cancer Ther. 2016;15:1–14. doi: 10.1158/1535-7163.MCT-16-0172. [DOI] [PubMed] [Google Scholar]
- 13.Huang P, Plunkett W. Action of 9-beta-d-arabinofuranosyl-2-fluoroadenine on RNA metabolism. Mol Pharmacol. 1991;39:449–455. [PubMed] [Google Scholar]
- 14.Huang P, Sandoval A, Van Den Neste E, et al. Inhibition of RNA transcription: a biochemical mechanism of action against chronic lymphocytic leukemia cells by fludarabine. Leukemia. 2000;14:1405–1413. doi: 10.1038/sj.leu.2401845. [DOI] [PubMed] [Google Scholar]
- 15.Belgiovine C, Bello E, Liguori M, et al. Lurbinectedin reduces tumour-associated macrophages and the inflammatory tumour microenvironment in preclinical models. Br J Cancer. 2017;117:628–638. doi: 10.1038/bjc.2017.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuroda H, Mabuchi S, Kozasa K, et al. PM01183 inhibits myeloid-derived suppressor cells in vitro and in vivo. Immunotherapy. 2017;9:805–817. doi: 10.2217/imt-2017-0046. [DOI] [PubMed] [Google Scholar]
- 17.Risnik D, Podaza E, Almejún MB, et al. Revisiting the role of interleukin-8 in chronic lymphocytic leukemia. Sci Rep. 2017;7:15714. doi: 10.1038/s41598-017-15953-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berhanu D, Mortari F, De Rosa SC, Roederer M. Optimized lymphocyte isolation methods for analysis of chemokine receptor expression. J Immunol Methods. 2003;279:199–207. doi: 10.1016/S0022-1759(03)00186-8. [DOI] [PubMed] [Google Scholar]
- 19.Colado A, Almejún MB, Podaza E, et al. The kinase inhibitors R406 and GS-9973 impair T cell functions and macrophage-mediated anti-tumor activity of rituximab in chronic lymphocytic leukemia patients. Cancer Immunol Immunother. 2017;66:461–473. doi: 10.1007/s00262-016-1946-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niemann CU, Herman SEM, Maric I, et al. Disruption of in vivo chronic lymphocytic leukemia tumor-microenvironment interactions by ibrutinib—findings from an investigator-initiated phase II study. Clin Cancer Res. 2016;22:1572–1582. doi: 10.1158/1078-0432.CCR-15-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Podaza E, Risnik D, Colado A, et al. Chronic lymphocytic leukemia cells increase neutrophils survival and promote their differentiation into CD16 high CD62L dim immunosuppressive subset. Int J Cancer. 2019;144:1128–1134. doi: 10.1002/ijc.31762. [DOI] [PubMed] [Google Scholar]
- 22.Elez ME, Tabernero J, Geary D, et al. First-in-human phase I study of Lurbinectedin (PM01183) in patients with advanced solid tumors. Clin Cancer Res. 2014;20:2205–2214. doi: 10.1158/1078-0432.CCR-13-1880. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Teruel C, Gonzalez I, Trocóniz IF, et al. Population-pharmacokinetic and covariate analysis of Lurbinectedin (PM01183), a new RNA polymerase II inhibitor, in pooled phase I/II trials in patients with cancer. Clin Pharmacokinet. 2019;58:363–374. doi: 10.1007/s40262-018-0701-2. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Zhou Y, Huang Q, Qiu L. CD14+HLA-DRlow/- expression: a novel prognostic factor in chronic lymphocytic leukemia. Oncol Lett. 2015;9:1167–1172. doi: 10.3892/ol.2014.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jitschin R, Braun M, Büttner M, et al. CLL-cells induce IDOhi CD14+HLA-DRlo myeloid-derived suppressor cells that inhibit T-cell responses and promote TRegs. Blood. 2014;124:750–760. doi: 10.1182/blood-2013-12-546416. [DOI] [PubMed] [Google Scholar]
- 26.Patten PEM, Buggins AGS, Richards J, et al. CD38 expression in chronic lymphocytic leukemia is regulated by the tumor microenvironment. Blood. 2008;111:5173–5181. doi: 10.1182/blood-2007-08-108605. [DOI] [PubMed] [Google Scholar]
- 27.Burger JA, Gribben JG. The microenvironment in chronic lymphocytic leukemia (CLL) and other B cell malignancies: insight into disease biology and new targeted therapies. Semin Cancer Biol. 2014;24:71–81. doi: 10.1016/j.semcancer.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Elías EE, Almejún MB, Colado A, et al. Autologous T-cell activation fosters ABT-199 resistance in chronic lymphocytic leukemia: rationale for a combined therapy with SYK inhibitors and anti-CD20 monoclonal antibodies. Haematologica. 2018;103:e458–e461. doi: 10.3324/HAEMATOL.2018.188680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Till KJ, Lin K, Zuzel M, Cawley JC. The chemokine receptor CCR7 and alpha4 integrin are important for migration of chronic lymphocytic leukemia cells into lymph nodes. Blood. 2002;99:2977–2984. doi: 10.1182/blood.V99.8.2977. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Giral S, Quintana NE, Cabrerizo M, et al. Chemokine receptors that mediate B cell homing to secondary lymphoid tissues are highly expressed in B cell chronic lymphocytic leukemia and non-Hodgkin lymphomas with widespread nodular dissemination. J Leukoc Biol. 2004;76:462–471. doi: 10.1189/jlb.1203652.1. [DOI] [PubMed] [Google Scholar]
- 31.Ghiringhelli F, Bruchard M, Apetoh L. Immune effects of 5-fluorouracil: ambivalence matters. Oncoimmunology. 2013;2:e23139. doi: 10.4161/onci.23139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shurin MR. Dual role of immunomodulation by anticancer chemotherapy. Nat Med. 2013;19:20–22. doi: 10.1038/nm.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan X-J, Dozmorov I, Li W, et al. Identification of outcome-correlated cytokine clusters in chronic lymphocytic leukemia. Blood. 2011;118:5201–5210. doi: 10.1182/blood-2011-03-342436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Netea MG, Nold-Petry CA, Nold MF, et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Podaza E, Sabbione F, Risnik D, et al. Neutrophils from chronic lymphocytic leukemia patients exhibit an increased capacity to release extracellular traps (NETs) Cancer Immunol Immunother. 2017 doi: 10.1007/s00262-016-1921-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herishanu Y, Kay S, Sarid N, et al. Absolute monocyte count trichotomizes chronic lymphocytic leukemia into high risk patients with immune dysregulation, disease progression and poor survival. Leuk Res. 2013;37:1222–1228. doi: 10.1016/j.leukres.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 38.Friedman DR, Sibley AB, Owzar K, et al. Relationship of blood monocytes with chronic lymphocytic leukemia aggressiveness and outcomes: a multi-institutional study. Am J Hematol. 2016;91:687–691. doi: 10.1002/ajh.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishio M, Endo T, Tsukada N, et al. Nurselike cells express BAFF and APRIL, which can promote survival of chronic lymphocytic leukemia cells via a paracrine pathway distinct from that of SDF-1alpha. Blood. 2005;106:1012–1020. doi: 10.1182/blood-2004-03-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lohmann G, Vasyutina E, Bloehdorn J, et al. Targeting transcription-coupled nucleotide excision repair overcomes resistance in chronic lymphocytic leukemia. Leukemia. 2017;31:1177–1186. doi: 10.1038/leu.2016.294. [DOI] [PubMed] [Google Scholar]
- 41.Hulkkonen J, Vilpo J, Vilpo L, et al. Interleukin-1 beta, interleukin-1 receptor antagonist and interleukin-6 plasma levels and cytokine gene polymorphisms in chronic lymphocytic leukemia: correlation with prognostic parameters. Haematologica. 2000;85:600–606. [PubMed] [Google Scholar]
- 42.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 43.Pang N, Zhang R, Li J, et al. Increased IL-10/IL-17 ratio is aggravated along with the prognosis of patients with chronic lymphocytic leukemia. Int Immunopharmacol. 2016;40:57–64. doi: 10.1016/j.intimp.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Jain P, Javdan M, Feger FK, et al. Th17 and non-Th17 interleukin-17-expressing cells in chronic lymphocytic leukemia: delineation, distribution, and clinical relevance. Haematologica. 2012;97:599–607. doi: 10.3324/haematol.2011.047316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.