Abstract
The safety of anti-programmed cell death 1 (PD-1) antibody for patients with preexisting interstitial lung disease (ILD) remains unknown. The aim of this study was to evaluate the dependence of preexisting ILD on anti-PD-1 antibody-induced pneumonitis in non-small cell lung cancer (NSCLC) patients. We retrospectively reviewed the association of preexisting ILD with the incidence, radiographic pattern, and outcome of pneumonitis in NSCLC patients receiving anti-PD-1 antibody. A total of 331 patients were included in this study. Of these patients, 17 had preexisting ILD. The incidence of pneumonitis was higher among the patients with preexisting ILD than among those without preexisting ILD (29% vs. 10%, P = 0.027). The distributions of the CT appearances at the onset of anti-PD-1 antibody-induced pneumonitis were as follows: for the patients with preexisting ILD, two patients (40%) had diffuse alveolar damage (DAD), one patient each with organizing pneumonia-like (OP), hypersensitivity pneumonitis (HP), and other patterns (20% each); for the patients without preexisting ILD, 19 patients (61%) had OP, 8 (26%) had HP, 3 (10%) had DAD, and 1 (3.2%) had other patterns. The median onset time from the initiation of anti-PD-1 antibody treatment until the development of pneumonitis was 1.3 months (range 0.3–2.1 months) for the patients with preexisting ILD and 2.3 months (range 0.2–14.6 months) for the patients without preexisting ILD. Careful attention to the development of pneumonitis is needed, especially within the first 3 months after the start of anti-PD-1 antibody treatment, when using anti-PD-1 antibody to treat patients with preexisting ILD.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02431-8) contains supplementary material, which is available to authorized users.
Keywords: Non-small cell lung cancer, Immune checkpoint inhibitor, Pneumonitis, Interstitial lung disease, Anti-PD-1 antibody, Immune-related adverse event
Introduction
Interstitial lung disease (ILD) is characterized by damage to the lung parenchyma due to chronic inflammation and progressive fibrosis [1]. ILD is associated with an increased risk of lung cancer [2, 3]. Preexisting ILD has been reported to exist in approximately 15% of patients with lung cancer at the time of initial diagnosis [4, 5] and is associated with a poor prognosis. Preexisting ILD is also a risk factor for antitumor drug-induced pneumonitis. Several studies have reported that the incidence and mortality of pneumonitis were higher in patients with preexisting ILD than in those without preexisting ILD [6, 7]. The prognosis of drug-induced pneumonitis was related to the short interval to the onset of pneumonitis and the computed tomography (CT) phenotypical appearance of pneumonitis; thus, the appearance of a diffuse alveolar damage (DAD) pattern can indicate a fatal outcome [8, 9]. In clinical practice, systemic anticancer therapy is carefully performed in patients with lung cancer with preexisting ILD. Therefore, additional treatment options that are safer for advanced non-small cell lung cancer (NSCLC) patients with preexisting ILD are needed [10].
The development of immune checkpoint inhibitors (ICIs) represents an important advance in the management of lung cancer, and ICIs have become a standard treatment for patients with advanced NSCLC. The blockade of immune checkpoint pathways by anti-programmed cell death 1 (PD-1)/programmed death ligand 1 (PD-L1) antibodies such as nivolumab, pembrolizumab, and atezolizumab prevents the inhibition of interactions between PD-1 and PD-L1/L2, enhances T-cell activation, and stimulates antitumor immune responses. However, T-cell activation can lead to the development of autoimmune manifestations, referred to as immune-related adverse events (irAEs). Most irAEs are well manageable and reversible by counteracting lymphocyte activation with corticosteroid therapy [11, 12], but some irAEs are irreversible despite treatment with immunosuppressive therapy and can result in life-threatening side effects.
Among the reported irAEs, pneumonitis developed in approximately 10% of the patients and resulted in potentially serious toxicity, especially among NSCLC patients [13]. Most cases of ICI-induced pneumonitis are low grade and improve with immunosuppressive therapy, similar to other irAEs, but severe pneumonitis can easily lead to fatal respiratory failure [14]. In clinical trials with ICIs in patients with advanced NSCLC, some pneumonitis-related deaths have been reported [15, 16]. A previous study reported that the toxicity grade of pneumonitis was associated with the radiologic CT pattern [13], and ICI-related pneumonitis can have various radiologic CT patterns [17, 18].
In a previous study, we has demonstrated that the efficacy of anti-PD-1 antibody and the value of PD-L1 expression as a biomarker were comparable between advanced NSCLC patients with and without preexisting ILD [19]. The purpose of this study was to investigate the clinical characteristics, radiographic appearance, onset time, and outcome of pneumonitis in patients with preexisting ILD treated with anti-PD-1 antibody.
Methods
Patients
The medical records of NSCLC patients treated with anti-PD-1 antibody (e.g., nivolumab and pembrolizumab) at the National Cancer Center, Tokyo, Japan between December 1, 2015, and May 31, 2018, were retrospectively reviewed. The end of the follow-up period was July 31, 2018.
Evaluation of chest CT findings of pneumonitis
A retrospective radiology review of serial chest CT scans of all the patients was independently performed by two pulmonologists (Ryota Shibaki and Shuji Murakami) and one radiologist (Masahiko Kusumoto), who had no knowledge of the patients’ outcomes. The chest CT images were reconstructed to a 1–5 mm slice thickness. Preexisting ILD was diagnosed by pre-treatment CT, according to the official guidelines of the American Thoracic Society, the European Respiratory Society, the Japanese Respiratory Society, and the Latin American Thoracic Society association [20]. We excluded collagen vascular disease–ILD and occupational lung disease based on medical history, work history, physical findings, and CT reports. The CT phenotypical appearance of anti-PD-1 antibody-induced pneumonitis was classified by referring to the Japanese respiratory society classification of drug-induced lung injuries, as organizing pneumonia-like (OP), hypersensitivity pneumonitis (HP), DAD, or others [21]. Most cases of preexisting ILD and immune-related pneumonitis were diagnosed only by CT.
The toxicity grades for pneumonitis were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) based on the patients’ medical records. The onset time of pneumonitis, treatment for pneumonitis, admission, and clinical course was obtained from the medical records.
Statistical analysis
The cumulative incidence of pneumonitis was estimated using the Kaplan–Meier method. A difference in cumulative incidence between patients with and those without preexisting ILD was assessed using the log-rank test, and the hazard ratio (HR) and 95% confidence interval (CI) were estimated using the Cox proportion hazard model. Patients who were lost to follow-up, those still alive at the cutoff date, or those who were dead were censored. A univariate analysis for the development of drug-induced pneumonitis was performed using Cox proportional hazard regression models for smoking status, sex, tumor histologic type, and the presence of preexisting ILD. These covariates, other than preexisting ILD, were adopted based on the results of recent trials suggesting that they might affect the development of drug-induced pneumonitis [22–24].
Dichotomous variables such as the baseline characteristics were shown as numbers and percentages. A comparison between groups divided according to the presence of preexisting ILD or the radiographic pattern of pneumonitis was performed using the Fisher’s exact test for categorical data. All P values were based on a two-sided hypothesis, and values less than 0.05 were considered statistically significant. All the statistical analyses were performed using JMP Pro software, version 13.0.0 (SAS Institute, Cary, NC, USA).
Results
Patients’ characteristics
Three hundred and thirty-one patients with advanced or recurrent NSCLC received anti-PD-1 antibody. Table 1 shows the baseline characteristics. Of those, 17 patients had preexisting ILD (Fig. 1). The proportions of patients who were 75 years old or older (41% vs. 10%; P = 0.001), male (94% vs. 64%; P = 0.006), had squamous cell carcinoma (47% vs. 19%; P = 0.012), and had received anti-PD-1 antibody as a first-line treatment (29% vs. 11%; P = 0.049) were significantly higher among the patients with preexisting ILD than among the patients without preexisting ILD.
Table 1.
Baseline characteristics
| All, n (%) | Preexisting ILD | p | ||
|---|---|---|---|---|
| Yes, n (%) | No, n (%) | |||
| Patients | 331 | 17 | 314 | |
| Age | ||||
| Median (range) | 62 (30–84) | 66 (33–83) | 62 (30–84) | |
| ≥ 75 years | 38 (11) | 7 (41) | 31 (10) | 0.001 |
| Sex | 0.006 | |||
| Male | 216 (65) | 16 (94) | 200 (64) | |
| ECOG PS | 0.15 | |||
| 0–1 | 289 (87) | 13 (76) | 276 (88) | |
| ≥ 2 | 42 (13) | 4 (24) | 38 (12) | |
| Smoking status | 0.084 | |||
| Never-smoker | 74 (23) | 1 (6) | 73 (24) | |
| Current or former smoker | 249 (77) | 15 (94) | 234 (76) | |
| Histology | 0.012 | |||
| Squamous | 69 (21) | 8 (47) | 61 (19) | |
| Nonsquamous | 262 (79) | 9 (53) | 253 (81) | |
| Clinical stage | 0.34 | |||
| III/IV | 248 (75) | 14 (82) | 234 (75) | |
| Recurrence | 83 (25) | 3 (18) | 80 (25) | |
| EGFR mutated | 57 (23) | 1 (11) | 56 (23) | 0.35 |
| PD-L1 22C3 TPS subgroups | 0.61 | |||
| < 1% | 48 (23) | 3 (23) | 45 (23) | |
| ≥ 1% | 161 (77) | 10 (77) | 151 (77) | |
| Prior curable operation | 76 (23) | 3 (18) | 73 (23) | 0.42 |
| Thoracic radiotherapy | 125 (38) | 5 (29) | 120 (38) | 0.32 |
| Treatment line | 0.046 | |||
| 1 | 41 (12) | 5 (29) | 36 (11) | |
| 2– | 290 (83) | 12 (71) | 278 (89) | |
| Drug | 0.43 | |||
| Nivolumab | 248 (75) | 12 (71) | 236 (75) | |
| Pembrolizumab | 83 (25) | 5 (29) | 78 (25) | |
ILD, interstitial lung disease; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; PD-L1, programmed death ligand 1; TPS, tumor proportion score
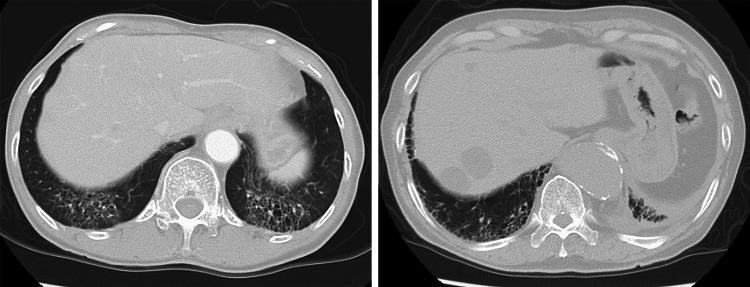
Fig. 1.
Computed tomography scans of the chest in patients with preexisting interstitial lung disease reveal subpleural distribution, honeycomb cysts, bronchiectasis, and ground glass abnormality
Among the 331 patients who received anti-PD-1 antibody, 36 patients (11%) developed pneumonitis. The incidence of pneumonitis was significantly higher among the patients with preexisting ILD than among those without preexisting ILD (29% vs. 9.9%; P = 0.027).
Radiographic patterns of pneumonitis
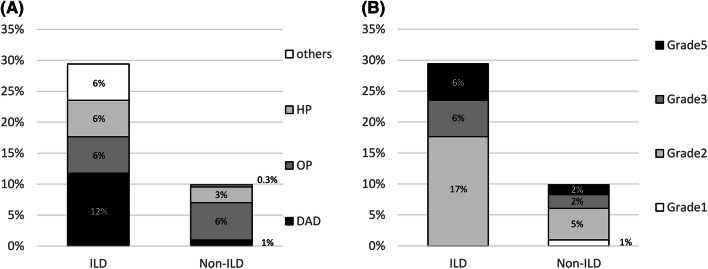
Among all the patients who developed pneumonitis, the overall distribution of the radiographic patterns of pneumonitis on chest CT was as follows: OP was the most common pattern, seen in 20 patients (56%), followed by the HP pattern in 9 patients (25%), the DAD pattern in 5 patients (14%), and other patterns in 2 patients (5.5%) (Fig. 2). Among the patients with preexisting ILD, the distribution of the radiographic patterns of pneumonitis was as follows: DAD was the most common pattern, seen in two patients (40%), followed by OP, HP, and other patterns in one patient each (20% each). Among the patients without preexisting ILD, the distribution of the radiographic patterns of pneumonitis on chest CT was as follows: OP was the most common pattern, seen in 19 patients (61%), followed by the HP pattern in 8 patients (26%), the DAD pattern in 3 patients (10%), and others in one patient (3.2%). The incidence of pneumonitis according to the radiographic pattern differed significantly between the patients with and those without preexisting ILD (P = 0.005) (Fig. 3a). The incidence of the DAD pattern of pneumonitis was significantly higher in the patients with preexisting ILD than that in patients without preexisting ILD (12% vs. 1.0%; P = 0.023). However, the incidence of the OP pattern of pneumonitis was not significantly different between the two groups (5.9% vs. 6.1%; P = 1.00).
Fig. 2.
Radiographic pattern of anti-programmed cell death 1-induced pneumonitis
Fig. 3.
Comparison of a the distribution of the radiographic pattern and b the grade distribution of pneumonitis in the patients with and without preexisting interstitial lung disease. DAD, diffuse alveolar damage; HP, hypersensitivity pneumonitis; OP, organizing pneumonia; ILD, interstitial lung disease
Among all the patients who developed pneumonitis, the grade distribution of pneumonitis was as follows: grade 1 in 3 patients (8.3%), grade 2 in 19 patients (53%), grade 3 in 8 patients (22%), and grade 5 in 6 patients (17%). Among the patients with vs. those without preexisting ILD, the grade distribution of pneumonitis was grade 1 in 0 patients (0.0%) vs. 3 (9.7%) patients, grade 2 in 3 patients (60%) vs. 16 patients (52%), grade 3 in 1 patient (20%) vs. 7 patients (23%), and grade 5 in 1 patient (20%) vs. 5 patients (16%), respectively (Fig. 3b). The incidence of the CTCAE grade ≥ 3 of pneumonitis was not significantly different between the two groups (40% vs. 39%; P = 0.96). The incidence of the CTCAE grade 5 of pneumonitis was not significantly different between the two groups (20% vs. 16%; P = 0.83).
Cumulative incidence of pneumonitis
During the follow-up period, the median onset time from anti-PD-1 antibody initiation to the development of pneumonitis was 1.3 months (range 0.3–2.1) and 2.3 months (range 0.2–14.6) in patients with and those without preexisting ILD, respectively. The incidence of pneumonitis at 1 month was 12% (95% CI 3.1–28) in the patients with preexisting ILD and 3.2% (95% CI 1.7–5.9) in patients without preexisting ILD. At 3 months, they were 34% (95% CI 15–60) and 6.4% (95% CI 4.1–9.8), respectively (Fig. 4). The cumulative incidence of pneumonitis using the Kaplan–Meier method was overwhelmingly higher in the patients with preexisting ILD than in the patients without ILD (HR 4.4; 95% CI 1.5–10; P = 0.01). A Cox proportional hazard regression analysis showed that only the presence of preexisting ILD was significantly associated with the incidence of drug-induced pneumonitis (Table 2).
Fig. 4.

Cumulative incidence of pneumonitis among advanced non-small cell lung cancer patients receiving anti-PD-1 antibody. Patients who were lost to follow-up, those still alive at the cutoff date, or death were censored. Incidence rate indicates the rate of cumulative event at each time point among all patients who developed pneumonitis. Among the 331 patients, 295 patients were censored; 28 patients were lost to follow-up, 131 patients were alive, and 136 patients were dead. ILD, interstitial lung disease; PD-1, programmed cell death 1
Table 2.
Cox proportional hazard regression analysis
| Univariate analysis | ||
|---|---|---|
| HR (95% CI) | p | |
| The incidence of drug-induced pneumonitis | ||
| Preexisting ILD (±) | 4.4 (1.5–10) | 0.01 |
| Smoking status (current or former/never) | 1.4 (0.64–3.9) | 0.39 |
| Sex (male/female) | 1.3 (0.64–2.7) | 0.50 |
| Histologic type (Sq/non-Sq) | 1.8 (0.85–3.6) | 0.12 |
HR, hazard ratio; ILD interstitial lung disease; Sq, squamous cell carcinoma
Treatment for pneumonitis and follow-up
The details of the treatment regimens for pneumonitis, CTCAE grade, and clinical courses are shown in Electronic Supplementary Tables 1 and 2. The majority of patients (29/36; 81%) received steroid therapy as a treatment for pneumonitis. One patient (2.8%) also received infliximab in addition to corticosteroids. Five patients (14%) exhibited an improved clinical and radiographic appearance without immunosuppression therapy. Anti-PD-1 antibody therapy was restarted in two patients (one with grade 1 and one with grade 2) who developed an OP pattern of pneumonitis. The two patients with retreatment did not develop recurrent pneumonitis.
Discussion
The overall incidence of pneumonitis was 11% among all the patients receiving anti-PD-1 antibody. The incidence of 29% in patients with preexisting ILD was significantly higher than the incidence of 9.9% in patients without preexisting ILD. Preexisting ILD was significantly associated with the development of pneumonitis (HR 4.4; 95% CI 1.5–10). Pneumonitis in patients with preexisting ILD developed at an earlier time, compared with that in patients without preexisting ILD. Among the patients with preexisting ILD, the onset time of pneumonitis occurred within 3 months of the start of anti-PD-1 antibody treatment. Most of the pneumonitis patients were successfully treated with steroid therapy.
Preexisting ILD is a consistent risk factor for the development of pneumonitis due to molecular targeted drugs and conventional chemotherapy [22, 25]. Previous epidemiological data regarding the risk factors for drug-induced pneumonitis are useful for determining the proper indications for these anticancer drugs. In a previous study, abnormal chest CT findings including preexisting ILD, pulmonary emphysema, and pulmonary infection were identified as a risk factor for the occurrence of pneumonitis during treatment with nivolumab [26]. We also found that the incidence of pneumonitis was significantly higher in patients with preexisting ILD than in those without preexisting ILD. This finding indicates that preexisting ILD may be a risk factor for pneumonitis even in patients receiving anti-PD-1 antibody, consistent with the findings for patients receiving molecular targeted therapy or chemotherapy. This result might reflect that chronic inflammation of the lungs would be a risk factor for the development of pneumonitis in patients receiving ICIs.
Patient prognosis is greatly dependent on the radiographic pattern on chest CT images observed at the onset of pneumonitis. Studies on molecular targeted therapy or conventional chemotherapy indicate that radiographic patterns are associated with the severity of pneumonitis, and the presentation of DAD patterns on chest CT images is thought to be strongly correlated with a high mortality. Therefore, the identification of a DAD pattern at the time of the development of pneumonitis is important. In anti-PD-1 antibody-induced pneumonitis, the OP radiographic pattern of pneumonitis on chest CT is reportedly the most frequently observed [13]. In our study, the OP pattern was also the most common radiographic pattern (56%), consistent with previous reports regarding the radiographic pattern of pneumonitis in patients with various kinds of cancer who were treated with ICIs [13]. On the other hand, in patients with preexisting ILD, the DAD pattern was the most common pattern (40%), which was also similar to the results of previous studies [27]. This difference in radiographic patterns between patients with and those without preexisting ILD can likely be explained by the chronic inflammation burden of preexisting ILD, resulting in the development of the DAD pattern of pneumonitis.
The onset time of irAEs after the start of ICIs treatment can vary greatly. The onset time of immune-related pneumonitis was reported to range from 0.5 to 21.3 months [13, 28, 29], which is similar to those of other irAEs. Indeed, the onset of pneumonitis in patients without preexisting ILD varied widely in the present study. In comparison, the onset of pneumonitis in patients with preexisting ILD occurred relatively early within the first 3 months of treatment. The early onset of pneumonitis in patients with preexisting ILD may be associated with chronic inflammation. We suggest that patients with preexisting ILD who are treated with anti-PD-1 antibody should be frequently examined for the possible development of pneumonitis, particularly during the first 3 months after the start of anti-PD-1 antibody treatment.
The treatment for drug-induced pneumonitis includes the termination of any drug suspected to cause pneumonitis. In addition, patients with drug-induced pneumonitis should be treated with immunosuppressive therapy; however, response to steroid therapy varies with each anticancer treatment [22, 25]. Even among patients with drug-induced pneumonitis who receive appropriate therapy, some cases may prove fatal. Our study reported that the mortality rate due to pneumonitis was 17% among all the patients who received anti-PD-1 antibody and 20% among patients with preexisting ILD. Of note, the mortality rate due to pneumonitis induced by anti-PD-1 antibody was similar in patients with and without preexisting ILD. Previous studies on ICIs also reported that the mortality rate due to pneumonitis ranged from 0 to 20% [24, 28, 30]. The mortality rate due to pneumonitis for patients receiving ICIs may be lower than those receiving molecular targeted therapy or conventional chemotherapy [22, 25]. This result may be due to the difference in the pathological mechanism of pneumonitis between ICIs and other drugs. The mechanism of pneumonitis development following anticancer treatment include direct pulmonary toxicity and indirect effects through enhancement of inflammatory reactions [31]. Most molecular targeted drugs or conventional chemotherapy causes direct damage to alveolar epithelial cells, airway epithelial cells, and capillaries leading to irreversible fibrosis. On the other hand, ICI treatment may result in T-cell hyperactivation causing reversible immune reaction in lung tissue [11]. These findings support the notion that pneumonitis related to anti-PD-1 antibody may be because of a manageable toxicity owing to immunosuppressive therapy.
This study had several limitations. First, this study was conducted based on a retrospective review of medical records. The presence of preexisting ILD and the development and pattern of drug-induced pneumonitis were radiologically confirmed in most of the patients, and bronchoalveolar lavage or a lung biopsy was rarely conducted. Second, because the use of anti-PD-1 antibody in patients with severe preexisting ILD was likely avoided, the incidence rate of pneumonitis in the patients with preexisting ILD could be inaccurate.
In conclusion, anti-PD-1 antibody-induced pneumonitis differed in its frequency and time of onset between patients with and without preexisting ILD. Most patients with anti-PD-1 antibody-induced pneumonitis were responsive to steroid therapy. These results suggest the importance of increased surveillance in patients with preexisting ILD for early diagnosis and treatment of pneumonitis. Future prospective studies are needed to show that anti-PD-1 antibody is safe and tolerable in patients with preexisting ILD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Mr. Takeharu Yamanaka for statistical support.
Abbreviations
- CI
Confidence interval
- CT
Computed tomography
- CTCAE
Common Terminology Criteria for Adverse Events
- DAD
Diffuse alveolar damage
- HP
Hypersensitivity pneumonitis
- HR
Hazard ratio
- ICIs
Immune checkpoint inhibitors
- ILD
Interstitial lung disease
- irAEs
Immune-related adverse events
- NSCLC
Non-small cell lung cancer
- OP
Organizing pneumonia-like
- PD-1
Programmed cell death 1
- PD-L1
Programmed death ligand 1
Author contributions
RS: collection of clinical data, data quality control, statistical data analysis, interpretation of results, and writing of the manuscript; SM: interpretation of results, and participating in writing the manuscript; YM, TY, YG, SK, HH, YF, Nobuyuki Y, Noboru Y, and YO: manuscript writing and editing; MK: collection of clinical data, and interpretation of results. All authors have approved the manuscript’s final version.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interest
Shuji Murakami has served on speakers’ bureaus for Taiho Pharmaceutical, Ono Pharmaceutical. Yasushi Goto has had consulting/advisory roles for Taiho Pharmaceutical; served on speakers’ bureaus for Taiho Pharmaceutical, Ono Pharmaceutical, Bristol-Myers Squibb, Merck Sharp and Dohme (MSD); and received research funding from Taiho Pharmaceutical, Bristol-Myers Squibb, and Ono Pharmaceutical. Shintaro Kanda has received research funding from Ono Pharmaceutical; and received honoraria from Ono Pharmaceutical, Bristol-Myers Squibb. Hidehito Horinouchi has received research funding from MSD, Bristol-Myers Squibb, Ono Pharmaceutical, Taiho Pharmaceutical. Yutaka Fujiwara has received research funding from MSD; and served on speakers’ bureaus for MSD, Taiho Pharmaceutical, Bristol-Myers Squibb, and Ono Pharmaceutical. Nobuyuki Yamamoto has had consulting/advisory roles for Taiho Pharmaceutical; served on speakers’ bureaus for Ono Pharmaceutical, Bristol-Myers Squibb, MSD; and received honoraria from Ono Pharmaceutical, and MSD. Noboru Yamamoto has received research funding from Taiho Pharmaceutical, Bristol-Myers Squibb, and Ono Pharmaceutical; and served on speakers’ bureaus for Bristol-Myers Squibb, Ono Pharmaceutical. Yuichiro Ohe has received research funding from Taiho Pharmaceutical, MSD; and received honoraria from Taiho Pharmaceutical, MSD. All remaining authors have declared no conflicts of interest.
Ethical approval
This study was approved by the Institutional Review Board of the National Cancer Center Hospital, Tokyo, Japan (study approval no. 2015-355) and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was not obtained from each patient, because this retrospective analysis of existing data did not require any interaction with patients and did not intervene in their treatment.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.King TE., Jr Clinical advances in the diagnosis and therapy of the interstitial lung diseases. Am J Respir Crit Care Med. 2005;172:268–279. doi: 10.1164/rccm.200503-483OE. [DOI] [PubMed] [Google Scholar]
- 2.Le Jeune I, Gribbin J, West J, Smith C, Cullinan P, Hubbard R. The incidence of cancer in patients with idiopathic pulmonary fibrosis and sarcoidosis in the UK. Respir Med. 2007;101:2534–2540. doi: 10.1016/j.rmed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Hubbard R, Venn A, Lewis S, Britton J. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med. 2000;161:5–8. doi: 10.1164/ajrccm.161.1.9906062. [DOI] [PubMed] [Google Scholar]
- 4.Omori T, Tajiri M, Baba T, et al. Pulmonary resection for lung cancer in patients with idiopathic interstitial pneumonia. Ann Thorac Surg. 2015;100:954–960. doi: 10.1016/j.athoracsur.2015.03.094. [DOI] [PubMed] [Google Scholar]
- 5.Fujimoto D, Kato R, Morimoto T, et al. Characteristics and prognostic impact of pneumonitis during systemic anti-cancer therapy in patients with advanced non-small-cell lung cancer. PLoS ONE. 2016;11:e0168465. doi: 10.1371/journal.pone.0168465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenmotsu H, Naito T, Kimura M, et al. The risk of cytotoxic chemotherapy-related exacerbation of interstitial lung disease with lung cancer. J Thorac Oncol. 2011;6:1242–1246. doi: 10.1097/JTO.0b013e318216ee6b. [DOI] [PubMed] [Google Scholar]
- 7.Enomoto Y, Inui N, Kato T, Baba T, Karayama M, Nakamura Y, Ogura T, Suda T. Low forced vital capacity predicts cytotoxic chemotherapy-associated acute exacerbation of interstitial lung disease in patients with lung cancer. Lung Cancer. 2016;96:63–67. doi: 10.1016/j.lungcan.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Inoue A, Saijo Y, Maemondo M, et al. Severe acute interstitial pneumonia and gefitinib. Lancet. 2003;361:137–139. doi: 10.1016/s0140-6736(03)12190-3. [DOI] [PubMed] [Google Scholar]
- 9.Sakai F, Noma S, Kurihara Y, Yamada H, Azuma A, Kudoh S, Ichikawa Y. Leflunomide-related lung injury in patients with rheumatoid arthritis: imaging features. Mod Rheumatol. 2005;15:173–179. doi: 10.1007/s10165-005-0387-9. [DOI] [PubMed] [Google Scholar]
- 10.Fujimoto D, Morimoto T, Ito J, et al. A pilot trial of nivolumab treatment for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia. Lung Cancer. 2017;111:1–5. doi: 10.1016/j.lungcan.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785–792. doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 13.Nishino M, Ramaiya NH, Awad MM, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. 2016;22:6051–6060. doi: 10.1158/1078-0432.CCR-16-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33:2004–2012. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 17.Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;373:287–288. doi: 10.1056/NEJMc1506004. [DOI] [PubMed] [Google Scholar]
- 18.Nishino M, Chambers ES, Chong CR, et al. Anti-PD-1 inhibitor-related pneumonitis in non-small cell lung cancer. Cancer Immunol Res. 2016;4:289–293. doi: 10.1158/2326-6066.CIR-15-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibaki R, Murakami S, Matsumoto Y, et al. Tumor expression and usefulness as a biomarker of programmed death ligand 1 in advanced non-small cell lung cancer patients with preexisting interstitial lung disease. Med Oncol. 2019;36:49. doi: 10.1007/s12032-019-1274-0. [DOI] [PubMed] [Google Scholar]
- 20.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 21.Kubo K, Azuma A, Kanazawa M, et al. Consensus statement for the diagnosis and treatment of drug-induced lung injuries. Respir Investig. 2013;51:260–277. doi: 10.1016/j.resinv.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Ando M, Okamoto I, Yamamoto N, Takeda K, Tamura K, Seto T, Ariyoshi Y, Fukuoka M. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2006;24:2549–2556. doi: 10.1200/JCO.2005.04.9866. [DOI] [PubMed] [Google Scholar]
- 23.Mizushima Y, Kobayashi M. Clinical characteristics of synchronous multiple lung cancer associated with idiopathic pulmonary fibrosis. A review of Japanese cases. Chest. 1995;108:1272–1277. doi: 10.1378/chest.108.5.1272. [DOI] [PubMed] [Google Scholar]
- 24.Suresh K, Voong KR, Shankar B, et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. 2018;13:1930–1939. doi: 10.1016/j.jtho.2018.08.2035. [DOI] [PubMed] [Google Scholar]
- 25.Kudoh S, Kato H, Nishiwaki Y, et al. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med. 2008;177:1348–1357. doi: 10.1164/rccm.200710-1501OC. [DOI] [PubMed] [Google Scholar]
- 26.Kenmotsu H, Sakai F, Kato T, et al. Nivolumab-induced interstitial lung disease (ILD) in Japanese patients with non-small cell lung cancer: a study on risk factors using interim results of post-marketing all-case surveillance. J Clin Oncol. 2017;35:9078. doi: 10.1200/JCO.2017.35.15_suppl.9078. [DOI] [Google Scholar]
- 27.Kanai O, Kim YH, Demura Y, et al. Efficacy and safety of nivolumab in non-small cell lung cancer with preexisting interstitial lung disease. Thorac Cancer. 2018;9:847–855. doi: 10.1111/1759-7714.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho JY, Kim J, Lee JS, et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer. 2018;125:150–156. doi: 10.1016/j.lungcan.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Cooper JA, Jr, White DA, Matthay RA. Drug-induced pulmonary disease. Part 1: cytotoxic drugs. Am Rev Respir Dis. 1986;133:321–340. doi: 10.1164/arrd.1986.133.2.321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.