Abstract
αv integrins have been identified as coreceptors for adenovirus (Ad) internalization; however, direct interactions of these molecules with Ad have not been demonstrated. We report here the expression of soluble integrin αvβ5, which retains the ability to recognize the Ad penton base as well as vitronectin, an Arg Gly Asp (RGD)-containing extracellular matrix protein. Soluble integrin αvβ5 reacted with seven different Ad serotypes (subgroups A to E) in solid-phase binding assays. The soluble integrin exhibited different levels of binding to each Ad serotype; however, binding to multiple Ad types required the presence of divalent metal cations and was inhibited by a synthetic RGD peptide, indicating that RGD and cation-binding sequences regulate Ad interactions with αvβ5. Incubation of Ad particles with soluble αvβ5 integrin also inhibited subsequent Ad internalization into epithelial cells as well as virus attachment to monocytic cells. These findings suggest that soluble αv integrins or antagonists of these coreceptors could be used to limit infection by multiple Ad types. The generation of soluble αv integrins should also permit further detailed kinetic and structural analysis of Ad interactions with its coreceptors.
Integrins are a large family of heterodimeric receptors which mediate several important cell functions, including cell adhesion, cell growth and differentiation, cell motility, wound repair (16), and phagocytosis (29). Inappropriate or reduced expression of these receptors on certain cell types in vivo is also associated with the development of tumor growth and metastasis (9) and retinal degeneration (10). The pairing of different α and β integrins subunits determines the ligand binding specificity of each receptor (20). Several different integrins recognize the Arg Gly Asp (RGD) sequence in different extracellular matrix proteins, such as fibronectin and vitronectin (28), and also require the presence of divalent metal cations for binding (21).
Integrins have also been subverted by a number of different viral (5, 22, 27, 34, 36) and bacterial (6, 17, 18) pathogens in order to gain entrance to host cells. In the majority of cases, integrins mediate the initial attachment of the pathogen to the host cell surface. However, human adenoviruses (Ad) use the vitronectin binding integrins αvβ3 and αvβ5 to promote virus internalization (3, 36) rather than virus attachment (4, 33). Ad entry into hematopoietic cells is mediated by distinct integrin receptors that facilitate both virus attachment (αMβ2) and internalization (αvβ3, αvβ5) (14).
αv integrins recognize an RGD sequence on the Ad penton base, which is conserved among several different virus serotypes (23). Recent structural studies have localized the αv integrin binding site on intact Ad serotype 2 (Ad2) particles by using cryoelectron microscopy and image reconstruction (31). The integrin binding sites, identified by the use of an RGD-specific monoclonal antibody (MAb) (designated DAV-1), are located at the apex of exposed, highly mobile loops on the Ad2 penton base protein. More recent structural studies have revealed that different Ad serotypes contain different-size RGD protrusions on the penton base protein (5a); however, it is not yet known how this influences integrin binding.
While integrins αvβ3 and αvβ5 both support Ad internalization, integrin αvβ5 selectively plays an important role in subsequent steps in virus penetration (36). Binding of the penton base protein to integrin αvβ5 at reduced pH promotes Ad permeabilization of the cell membrane. Integrin αvβ5 is expressed on human bronchial epithelial cells (25), a major site of Ad infection in vivo. In contrast, primary epithelial cells express little if any integrin αvβ3. The level of αvβ5 integrin expression on human airway epithelial cells in vivo has also been shown to be directly related to the efficiency of Ad-mediated gene transfer (11, 12).
In order to gain a better understanding of Ad interactions with its integrin coreceptors, we have produced the entire ectodomain of integrin αvβ5 as a secreted protein in insect cells using baculovirus vectors. Direct interactions of the secreted integrin with multiple Ad serotypes, as well as host cell-derived RGD ligands, were examined by solid-phase binding assays.
MATERIALS AND METHODS
Viruses, extracellular matrix proteins, and antibodies.
Ad2, Ad3, Ad4, Ad12, Ad19, and Ad37 were purchased from the American Type Culture Collection (Manassas, Va.) and propagated in A549 cells. A recombinant Ad5 vector expressing green fluorescent protein (Ad.GFP) was grown in 293 cells (15). Viruses were purified by CsCl density gradient ultracentrifugation, as previously described (8). For virus binding and internalization assays, purified Ad2 was labeled with 125NaI (IODO-GEN; Pierce) to a specific activity of 3.5 × 104 cpm/ng. Recombinant Ad2 penton base was produced in insect cells by using baculovirus, as previously described (36). Flockhouse virus (FHV), a nonenveloped insect virus, was kindly provided by A. Schneemann (Scripps Research Institute). Extracellular matrix proteins vitronectin and fibronectin were purchased from Sigma (St. Louis, Mo.). A non-function-blocking anti-αv MAb (LM142) and a function-blocking anti-αvβ5 MAb (P1F6) were kindly provided by D. Cheresh (Scripps Research Institute). MAbs directed against the αv subunit (VNR139; Chemicon), the β5 subunit (1D11;D. Cheresh), or αMβ2 (CP3; Z. Ruggeri, Scripps Research Institute) were used for immunoblotting.
Construction of baculovirus vectors encoding soluble αvFos and β5Jun integrin subunits.
A 2,941-bp cDNA encoding the entire αv integrin ectodomain (32) was PCR amplified (Pfu polymerase; Stratagene) from a plasmid [pGEM7zf(+)] encoding the full-length integrin subunit (kindly provided by D. Cheresh). An appropriate set of PCR primers (5′-GCGCGGCTAGCGACGATGGCTT, 3′-CATGGTACCTGGCTGAATGCCC) was used to create NheI and KpnI restriction endonuclease cloning sites (underlined). Following sequencing, the 2.9-kb cDNA fragment was digested with NheI and KpnI and ligated into the baculovirus transfer vector pBB4 (Invitrogen), using the same cloning sites. The 2.9-kb αv cDNA, as well as a 5′ KpnI and a 3′ stop codon and EcoRI cloning sites, was also inserted into pBB4 in frame with a 144-bp cDNA fragment encoding the Fos dimerization domain (pPIC9DraFOS) (19).
Using a similar cloning strategy, we PCR amplified a 2,154-bp cDNA fragment encoding the entire ectodomain of the β5 integrin subunit (26) (pCI/β5; D. Cheresh) with BamHI and XhoI restriction cloning sites and PCR primers (5′-AGGGGATCCACCATGCCGCGGG, 3′-ATGGTCTCGAGGTTGGGGGTGTT). Following sequencing, the 2.1-kb fragment was digested with BamHI and XhoI and ligated into the pBB4 baculovirus transfer vector with the same cloning sites. A separate construct was also generated to produce β5 linked to the Jun dimerization domain. pBB4/β5 was digested with XhoI and EcoRI, and a 144-bp fragment containing a polyglycine linker sequence and the Jun dimerization domain (pPIC9DRβJun) was digested with SalI and EcoRI and then ligated in frame with the truncated β5 cDNA in pBB4, which had been previously digested with XhoI and EcoRI.
To generate recombinant baculoviruses, Sf9 insect cells were cotransfected with 0.5 μg of wild-type baculovirus DNA (Bac’n Blue DNA; Invitrogen) and 4 μg of either pBB4/αv or pBB4/β5 or the same vectors containing the Fos and Jun forms of the integrin subunits (see Fig. 1). Following transfections, recombinant baculoviruses were isolated by plaque formation on Sf9 cells. Candidate recombinant baculoviruses were then amplified by growth on Sf9 cells, and viral DNA was isolated and analyzed on a 0.8% agarose gel and subsequently sequenced (Baculo/PCR; Invitrogen). High-titered baculovirus stocks expressing αv and β5 were generated by infection of Sf9 spinner cultures at a multiplicity of infection (MOI) of 0.3.
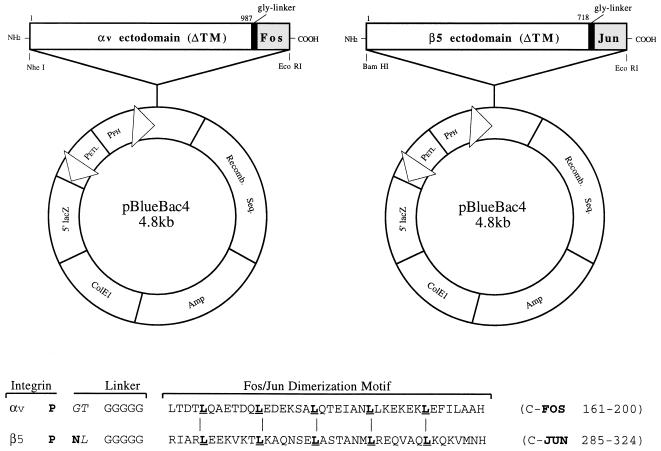
FIG. 1.
Diagram of baculovirus plasmid vectors used for the expression of soluble αvβ5. The cDNA fragments encoding the entire ectodomains of the αv and β5 integrin subunits were inserted in frame with a glycine spacer and a Fos/Jun dimerization domain (as shown in the sequence alignment) into the pBlueBac4 baculovirus transfer vector.
Immunoprecipitation, SDS-PAGE, and immunoblotting.
For immunoprecipitation studies, 50% confluent monolayers of Tn5B cells in 24-well plastic tissue culture plates were infected with various ratios of αv and β5 recombinant baculoviruses or with each baculovirus alone. Eighteen hours postinfection, the cells were cultured for an additional 24 h in serum-free insect cell medium (Ex-Cell 400; JRH Biosciences, Lenexa, Kans.) containing 50 μCi of [35S]methionine (Amersham, Bedford, Mass.) per ml. The supernatants from infected or uninfected control cells were then harvested and incubated for 90 min at 4°C with 50 μl of protein G-Sepharose beads (Pierce) containing 10 μg of MAb P1F6. After a wash, the beads were boiled for 2 min in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and electrophoresed under nonreducing conditions on a 7% SDS gel (Tris-glycine; Novex). Following electrophoresis, the gel was fixed, dried, and subjected to autoradiography. For immunoblotting, soluble integrin was electrophoresed on an 8 to 16% SDS gel and then stained with Colloidal Coomassie blue (Novex) or transferred to a polyvinylidene difluoride membrane (Immobilon P; Amersham). The membrane was blocked by incubation in 10% nonfat dry milk in TBST (50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 0.2% Tween 20). The blot was then probed with MAbs specific for the αv subunit (VNR 139) or the β5 subunit (1D11) or with a control anti-αMβ2 antibody (CP3). Following extensive washing in TBST, the blots were incubated with a goat anti-mouse immunoglobulin G antibody linked to horseradish peroxidase (Bio-Rad). After further washes, the blots were developed with an enhanced chemiluminescence system (SuperSignal; Pierce).
Purification and ligand binding properties of soluble αvβ5 integrin.
Tn5B insect cells were grown to a density of 2 × 106 cells/ml in Ex-cell 400 medium in 2-liter shake flasks and then infected at an MOI of 1.0 with recombinant baculoviruses encoding αv and β5 (1:1 ratio). Protease inhibitors (1 μg of leupeptin per ml, 5 μg of aprotinin per ml) and a final concentration of 2% fetal calf serum were added at 16 h postinfection, and the culture supernatants were harvested by centrifugation usually at 48 h postinfection, while the cells retained 90 to 100% viability. Culture supernatants were concentrated approximately 15-fold (Ultralab 2 concentrator; Pall Filtron) with a 30K membrane filter (Omega) and then adjusted to a final concentration of 0.002% sodium azide with additional protease inhibitors. The concentrated culture supernatants were incubated overnight at 4°C with constant agitation with MAb P1F6 (22 mg) covalently linked to 5 ml of CNBr-Sepharose beads. The beads were then washed five times with 50 ml of 10 mM sodium phosphate buffer, pH 7.4, containing 0.6 M NaCl and 1 mM (each) MgCl2 and CaCl2. The integrin was then eluted with 10 mM Na-acetate, pH 3.1, containing 1 mM MgCl2 and CaCl2 and immediately neutralized with 1 M Tris-HCl, pH 8.1. The eluted integrin fractions were analyzed by SDS-PAGE and for binding to the Ad2 penton base in an enzyme-linked immunosorbent assay (ELISA), as described below. Fractions containing the 155- and 100-kDa integrin heterodimer were pooled and concentrated (C-100 microconcentrator; Amicon) to approximately 300 μg/ml and stored at −80°C.
Integrin binding to Ad serotypes and extracellular matrix proteins was quantitated in an ELISA. Purified Ad serotypes, recombinant Ad2 penton base, or extracellular matrix proteins were used to coat 96-well plastic tissue culture plates (Immulon-4; Dynatech) at a concentration of 1 μg of protein/well. Nonspecific binding sites were quenched by incubation with a blocking agent (Superblock; Pierce), and then cell culture supernatants containing soluble integrin or immunoaffinity-purified integrin at 100 ng to 1 μg/well were added to the plates and incubated at 22°C for 2 h. The wells were washed with phosphate-buffered saline containing a 1:50 dilution of Blocker Blotto (Pierce)–0.2% Tween 20 and then incubated for 1 h at 22°C with 100 μl of 10 μg of LM142 anti-αv MAb per ml in blocking buffer. After further washes, the wells were incubated for 1 h with 100 μl of a 1:10,000 dilution of goat anti-mouse immunoglobulin G linked to horseradish peroxidase (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) in blocking buffer. Following additional washing, the reactions were developed by addition of substrate (ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid]; Kirkegaard and Perry Laboratories) and analyzed at 405 nm in a SpectraMax 250 ELISA plate reader (Molecular Devices, Sunnyvale, Calif.).
Ad binding, internalization, and gene delivery assays.
125I-labeled Ad2 (125 ng, 500 virus particles per cell) was preincubated for 2 h at 4°C with 70 μg/ml of a polyclonal antibody to the Ad2 fiber protein or the DAV-1 anti-penton base MAb (31) or with purified αvFos/β5Jun integrin in 10 mM Tris-HCl buffer, pH 8.1, containing 1 mM (each) MgCl2 and CaCl2. The samples were then incubated for 1 h at 4°C with 1 × 106 SW480 epithelial cells or 2 × 106 THP-1 monocytic cells in 20 mM HEPES-buffered saline (HBS), pH 7.4, containing 2% bovine serum albumin and 1 mM (each) MgCl2 and CaCl2. The cells were then warmed to 37°C for 0 to 15 min and then washed three times in cold HBS binding buffer. Cell samples maintained at 4°C were then counted to determine the level of virus attachment or were incubated with medium containing trypsin-EDTA at 37°C for 15 min to determine the amount of endocytosed virus particles. The amount of nonspecific Ad binding was determined by incubating cells with a 100-fold excess of unlabeled virus particles.
For gene delivery studies, 50 ng of Ad.GFP (MOI, 500) was incubated with 300 ng of soluble integrin in HBS binding buffer or in buffer alone for 2 h at 4°C. The samples were then added to 4 × 105 SW480, A549, or THP-1 cells and incubated further for 1 h at 4°C. The cells were then warmed to 37°C for 15 min and washed and cultured in complete Dulbecco modified Eagle medium for 48 to 72 h at 37°C. GFP expression was then quantitated by flow cytometry (FACScan II), and the data were expressed as mean fluorescence intensities.
RESULTS
Baculovirus expression of soluble integrin αvβ5.
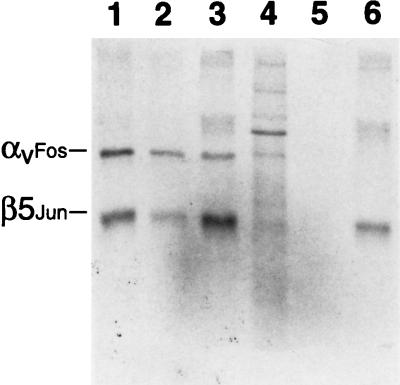
In order to study direct interactions of integrins with human Ad and host cell ligands, we used recombinant baculovirus vectors to express a soluble form of αvβ5 in Tn5B insect cells. To facilitate assembly of the integrin heterodimer (19), we inserted a glycine linker and Fos/Jun dimerization domain in frame with the C terminus of the integrin ectodomains (Fig. 1). Baculovirus transfer vectors containing these constructs were used to produce recombinant baculoviruses in Sf9 cells. Plaque-purified recombinant baculoviruses were then tested for the ability to produce a soluble αvβ5 integrin heterodimer in Tn5B insect cells by immunoprecipitation. Two proteins, of 155 and 100 kDa, similar to the expected size of the integrin ectodomains, were immunoprecipitated by a function-blocking αvβ5 MAb (P1F6) from supernatants of cells infected with both the αvFos and β5Jun baculoviruses (Fig. 2). An infection ratio of 1:1 proved optimal for integrin expression (Fig. 2, lanes 1 to 3). As expected, the integrin heterodimer was not detected in culture supernatants from cells infected with a αvFos or β5Jun baculovirus alone (lanes 5, 6). The β5Jun, but not the αvFos, integrin subunit was immunoprecipitated by MAb P1F6 (lane 6), while the αvFos integrin subunit was capable of being immunoprecipitated by MAb LM142 (data not shown).
FIG. 2.
Immunoprecipitation analysis of soluble recombinant αvβ5 integrin. 35S-labeled insect cells were infected with a 1:1 (lane 1), 10:1 (lane 2), or 1:10 (lane 3) ratio (PFU) of the αvFos and β5Jun baculoviruses, were left uninfected (lane 4), or were infected with αvFos (lane 5) or β5Jun (lane 6) baculovirus alone and then immunoprecipitated with MAb P1F6 and analyzed under nonreducing conditions on an SDS–7% polyacrylamide gel, followed by autoradiography.
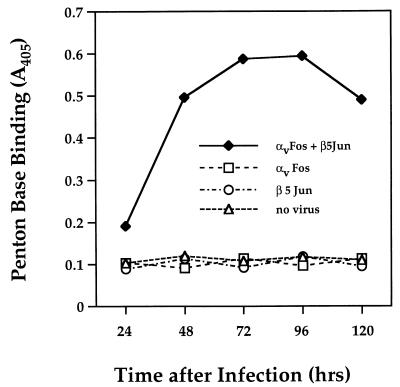
Studies were next performed to assess the functional activity of soluble integrin molecules produced in insect cells. Culture supernatants derived from cells infected with a combination of αvFos and β5Jun baculoviruses, but not those from cells infected with the αvFos or β5Jun baculovirus alone, reacted with the penton base protein in an ELISA (Fig. 3). Optimal expression occurred at 3 to 4 days postinfection and declined thereafter. These findings suggest that a functionally active integrin heterodimer was secreted from cells coinfected with αv and β5 recombinant baculoviruses.
FIG. 3.
Time course of expression and functional activity of soluble αvβ5 integrin. Culture supernatants derived from insect cells infected with a combination of the αvFos and β5Jun baculoviruses (1:1) or from cells infected with each baculovirus alone were assayed in an ELISA at various times after infection for binding to immobilized penton base.
Purification and ligand binding analysis of soluble integrin αvβ5.
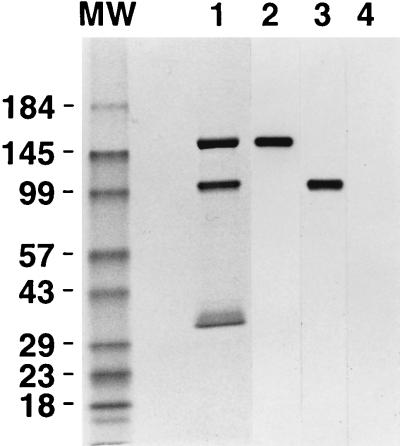
In order to study further the ligand binding properties of the Ad coreceptor, we isolated the soluble integrin by immunoaffinity chromatography. As expected, the purified integrin was comprised of two subunits with relative mobilities of 155 and 100 kDa (Fig. 4). Each of the integrin subunits was also recognized by the appropriate anti-αv or anti-β5 MAb, as detected by immunoblotting (Fig. 4). A minor protein of approximately 35 kDa, derived from proteolytic cleavage of the β5 integrin subunit, was detected in some integrin preparations. Approximately 250 to 300 μg of soluble integrin per liter of Tn5B insect cells was isolated.
FIG. 4.
SDS-PAGE and Western blot of immunoaffinity-purified αvβ5 integrin. Five micrograms of isolated αvβ5 were electrophoresed on an SDS–8 to 16% polyacrylamide gel under nonreducing conditions and then stained with Coomassie blue (lane 1) or transferred to a polyvinyidene difluoride membrane and reacted with an anti-αv (lane 2), anti-β5 (lane 3), or anti-αM (lane 4) antibody in a Western blot.
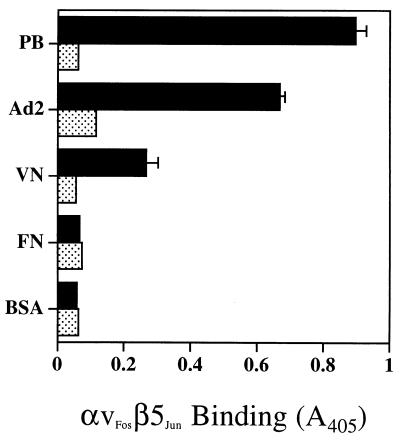
Further studies examined the functional properties of the purified αvβ5 integrin. Purified αvβ5 bound to immobilized Ad2 penton base and virus particles as well as to vitronectin but did not recognize another RGD-containing extracellular matrix protein, fibronectin (Fig. 5). Binding to Ad2 and vitronectin was blocked by 20 mM EDTA, indicating that these interactions require the presence of divalent metal cations. These results indicate that soluble recombinant integrin αvβ5 possesses the ligand binding activity of the cell-associated integrin.
FIG. 5.
Interactions of soluble αvβ5 integrin with Ad ligands or extracellular matrix proteins. ELISA plates were coated with 1 μg per well of penton base (PB), Ad2 particles, vitronectin (VN), fibronectin (FN), or bovine serum albumin (BSA). Following quenching of nonspecific binding sites, the plates were incubated with 6 ng of purified αvβ5 integrin in the presence (stippled bars) or absence (solid bars) of 20 mM EDTA. Integrin binding was detected with a non-function-blocking MAb (LM142), as described in Materials and Methods.
Association of integrin αvβ5 with different Ad serotypes.
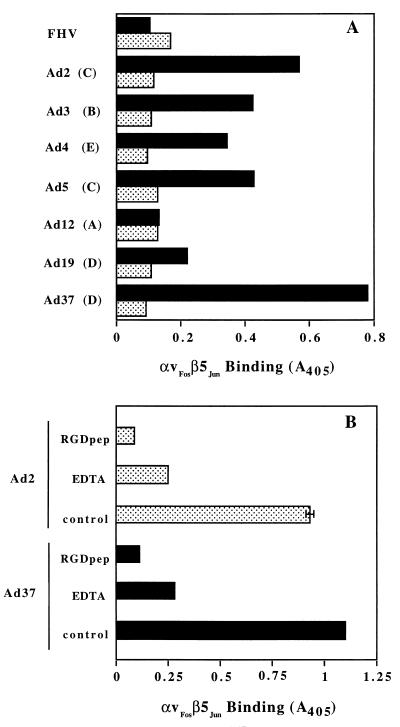
The penton base proteins of multiple Ad serotypes contain conserved RGD sequences (23), suggesting that different Ad are capable of associating with cell integrins. Competition studies with synthetic RGD peptides or function-blocking integrin MAbs also suggested that multiple Ad serotypes use integrins for infection. However, direct interactions of integrins with different Ad serotypes have not yet been examined. We therefore analyzed the association of soluble recombinant αvβ5 with seven different Ad serotypes representing five major Ad subgroups (A to E). Soluble αvβ5 exhibited significant binding to Ad serotypes belonging to subgroups B (Ad3), C (Ad2, Ad5), D (Ad19, Ad37), and E (Ad4) but did not bind to a control, nonenveloped virus, FHV (Fig. 6A). Minimal binding of αvβ5 to Ad12 was observed, consistent with the relatively short RGD surface loop on this virus (23). Binding of the soluble integrin to different Ad required the presence of divalent metal cations and was also inhibited by the presence of a synthetic RGD peptide (Fig. 6B).
FIG. 6.
Binding of soluble αvβ5 integrin to different Ad serotypes. (A) Direct interactions of soluble integrin with different Ad serotypes from subgroups A to E were quantitated in an ELISA in the presence (stippled bars) or absence (solid bars) of 20 mM EDTA. (B) Binding of soluble integrin αvβ5 to Ad2 and Ad37 was measured in the presence or absence (control) of 100 μg of a synthetic RGD peptide (RGDpep) per ml or 20 mM EDTA.
Effect of soluble integrin αvβ5 on Ad binding and internalization.
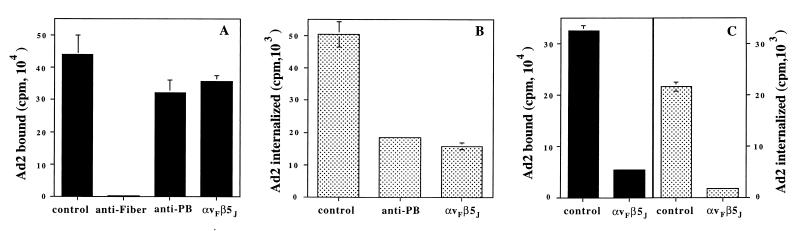
Previous studies had indicated that αv integrin-deficient epithelial cells do not support efficient Ad internalization (36) and infection (12), whereas cells expressing these receptors following transfection with αv integrin cDNA are rendered susceptible to infection, suggesting that these receptors play a crucial role in viral entry. In order to substantiate this hypothesis, we preincubated purified virions with antibodies to the Ad2 fiber or the penton base protein or with soluble αvβ5 and then assayed virus binding and entry into host cells. Ad2 binding to SW480 epithelial cells was completely inhibited by an antifiber antibody, whereas incubation of virus with the DAV-1 penton base MAb or soluble αvβ5 integrin had little effect on binding (Fig. 7A). In contrast, soluble integrin αvβ5, as well as MAb DAV-1, significantly reduced (approximately 65%) Ad2 internalization (Fig. 7B). These findings are consistent with a specific role of αv integrins in Ad internalization into epithelial cells rather than in Ad attachment. In contrast to epithelial cells, both binding and internalization of Ad to monocytic cells has been reported to be mediated by penton base interaction with cell integrins (14). We therefore examined whether soluble αvβ5 was capable of interfering with Ad interactions with THP-1 monocytic cells. Consistent with the previous report, incubation of Ad2 with soluble integrin αvβ5 blocked both virus attachment to and internalization into THP-1 monocytic cells (Fig. 7C).
FIG. 7.
Effect of antifiber or anti-penton base antibodies or soluble integrin αvβ5 on Ad2 binding and internalization. 125I-labeled Ad2 particles were preincubated with a polyclonal antifiber antibody with the DAV-1 anti-penton base MAb, or with soluble αvβ5 integrin prior to measurement of virus binding (A) and internalization into SW480 epithelial cells (B) or THP-1 monocytic cells (C).
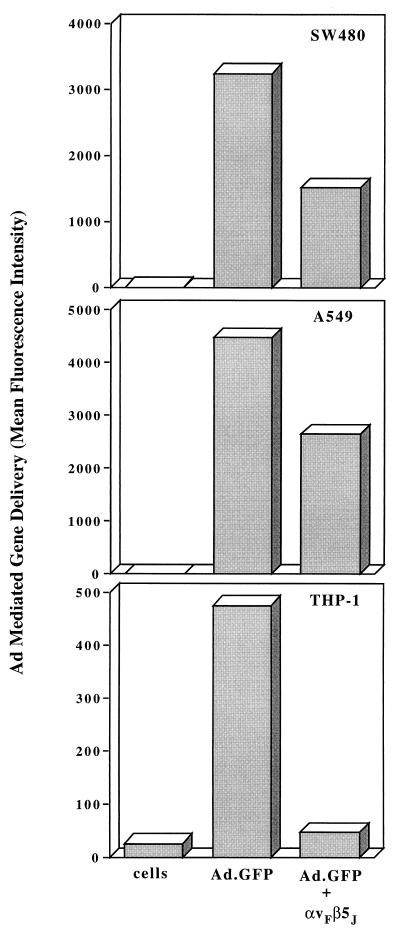
Inhibition of Ad-mediated gene delivery by soluble integrin αvβ5.
Further studies were performed to determine whether soluble integrin αvβ5 was capable of inhibiting Ad infection. For these studies we used a recombinant Ad5 vector encoding GFP (15). Incubation of Ad5.GFP with soluble αvβ5 significantly inhibited virus-mediated gene delivery to both SW480 and A549 epithelial cells (approximately 50%) and nearly abolished delivery to THP-1 monocytic cells (Fig. 8). These results further indicate that αv integrins promote Ad-mediated gene delivery as well as virus internalization.
FIG. 8.
Effect of soluble integrin αvβ5 on Ad-mediated gene delivery. A recombinant Ad vector encoding GFP (Ad.GFP) was preincubated with medium alone or with a 20-fold excess (relative to the number of penton base RGD sites) of soluble integrin αvβ5 prior to infection of SW480 cells, A549 epithelial cells, or THP-1 monocytic cells. Gene delivery was measured by flow cytometry at 48 to 72 h postinfection and expressed as mean fluorescence intensity. The data are representative of four separate experiments.
DISCUSSION
The inherent difficulties associated with isolating native αv integrins from human tissues (30) has prevented an extensive analysis of integrin structure and function. The extracellular domains of integrins αvβ6 (35) and αIIbβ3 (24) have been previously expressed in mammalian or insect cells as secreted proteins. In each case, the expressed α and β integrin subunits formed a stable heterodimer in the absence of the transmembrane or cytoplasmic domains and also retained antigenic properties and ligand binding activity. We were therefore encouraged to examine whether integrin αvβ5 could also be expressed as a soluble protein, thereby facilitating analysis of its interactions with human Ad. Unfortunately, only small amounts of the αv and β5 ectodomains were produced by baculovirus vectors in Tn5B insect cells. However, the addition of a Fos/Jun dimerization domain (Fig. 1) increased production of the soluble αvβ5 integrin approximately fourfold (data not shown). This could be due to increased association of the integrin subunits, as has been reported for other multimeric cell surface receptors (19). The soluble αv and β5 integrin subunits formed a stable heterodimeric complex (Fig. 2), and the assembled integrin was also capable of binding to the Ad penton base protein (Fig. 3) as well as its natural RGD-containing ligand, vitronectin (Fig. 5). The secreted αvβ5 integrin was also recognized by the function-blocking P1F6 MAb, which enabled isolation of the receptor by immunoaffinity chromatography (Fig. 4).
Since distinct Ad serotypes have been shown to contain a conserved integrin-binding motif (RGD) in their penton base proteins, we examined whether soluble αvβ5 integrin was capable of recognizing different Ad serotypes. While the level of integrin binding to different Ad types varied (Fig. 6A), competition studies indicated that the RGD sequence as well as the presence of divalent metal cations were required for integrin interactions with multiple Ad serotypes (Fig. 6B). The least amount of integrin binding observed was to Ad12. It is worth noting that the Ad12 penton base contains a relatively short RGD loop (23) compared to other Ad serotypes, a structural feature which could reduce integrin binding (1, 7). Previous studies have also reported that the Ad12 penton base RGD motif is capable of mediating cell adhesion and virus infection (2). However, significant differences in Ad2- and Ad12-mediated cell adhesion and infection were noted, indicating that the integrin binding epitopes on these two proteins are not functionally equivalent. One possibility which could explain reduced interactions of Ad12 with αvβ5 in solid-phase binding assays is that the Ad12 penton base has a low intrinsic affinity for integrin αvβ5. Recent structural studies have indicated that a more extended flexible RGD loop may be important for receptor interactions (1, 7). In vivo, high-affinity binding of Ad12 via the fiber receptor (4, 33) could permit subsequent low-affinity interactions of the penton base with the αvβ5 integrin. Further kinetic studies are needed to determine the precise binding affinities and stoichiometries of αv integrin interactions with different Ad penton base proteins.
The generation of a soluble integrin αvβ5 molecule also provided an opportunity to examine the requirements for this coreceptor in Ad entry and infection of epithelial or monocytic cells. Incubation of Ad2 particles with purified integrin had little effect on virus binding to epithelial cells; however, substantial inhibition of virus attachment to monocytic cells was observed (Fig. 7). We interpret this result as the ability of soluble αvβ5 to compete for Ad2 binding to cell surface αMβ2 integrins (14). The integrin also inhibited virus internalization into both cell types. These results are consistent with a previous report (14), suggesting that integrins play distinct roles in diverse cell types. Soluble integrin αvβ5 was also capable of blocking Ad-mediated gene delivery to both epithelial and monocytic cells (Fig. 8). The more pronounced inhibition of gene delivery to THP1 monocytic cells (Fig. 7B) is probably due to the fact that integrins on these cells mediate both virus attachment and virus internalization.
The findings reported here suggest that soluble integrins or specific antagonists of these molecules may represent an approach to the inhibition of infection by multiple Ad serotypes. To date, there are few antiviral agents capable of inhibiting Ad infections. Although Ad infections do not generally cause serious complications, fatal disseminated Ad infections have been noted with increasing frequency in immunocompromised and AIDS patients (13). Strategies based on preventing Ad interactions with its cellular coreceptor may provide an approach to restricting cell entry by distinct Ad serotypes.
ACKNOWLEDGMENTS
We express our gratitude to Phoebe Stewart and Charles Chiu (UCLA School of Medicine) and members of the Cheresh laboratory (Scripps Research Institute) for helpful discussions and advice. We also thank Bonnie Bradt for technical support and Catalina Hope and Joan Gausepohl for preparation of the manuscript.
This work was supported by NIH grants EY11431 and HL54352.
REFERENCES
- 1.Akke M, Liu J, Cavanagh J, Erickson H P, Palmer A G., III Pervasive conformational fluctuations on microsecond time scales in a fibronectin type III domain. Nat Struct Biol. 1998;5:55–59. doi: 10.1038/nsb0198-55. [DOI] [PubMed] [Google Scholar]
- 2.Bai M, Campisi L, Freimuth P. Vitronectin receptor antibodies inhibit infection of HeLa and A549 cells by adenovirus type 12 but not by adenovirus type 2. J Virol. 1994;68:5925–5932. doi: 10.1128/jvi.68.9.5925-5932.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai M, Harfe B, Freimuth P. Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell-rounding activity and delay virus reproduction in flat cells. J Virol. 1993;67:5198–5205. doi: 10.1128/jvi.67.9.5198-5205.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 5.Bergelson J M, Shepley M P, Chan B M C, Hemler M E, Finberg R W. Identification of the integrin VLA-2 as a receptor for echovirus I. Science. 1992;255:1718–1720. doi: 10.1126/science.1553561. [DOI] [PubMed] [Google Scholar]
- 5a.Chiu, C., et al. Submitted for publication.
- 6.Coburn J, Barthold S W, Leong J M. Diverse Lyme disease spirochetes bind integrin αIIbβ3 on human platelets. Infect Immun. 1994;62:5559–5567. doi: 10.1128/iai.62.12.5559-5567.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Copie V, Tomita Y, Akiyama S K, Aota S, Yamada K M, Venable R M, Pastor R W. Solution structure and dynamics of linked cell attachment modules of mouse. J Mol Biol. 1998;277:663–682. doi: 10.1006/jmbi.1998.1616. [DOI] [PubMed] [Google Scholar]
- 8.Everitt E, Meador S A, Levine A S. Synthesis and processing of the precursor to the major core protein of adenovirus type 2. J Virol. 1977;21:199–214. doi: 10.1128/jvi.21.1.199-214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felding-Habermann B, Mueller B M, Romerdahl C A, Cheresh D A. Involvement of integrin αv gene expression in human melanoma tumorigenicity. J Clin Investig. 1992;89:2018–2022. doi: 10.1172/JCI115811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finnemann S C, Bonilha V L, Marmorstein A D, Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires αvβ5 integrin for binding but not for internalization. Proc Natl Acad Sci USA. 1997;94:12932–12937. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman M, Su Q, Wilson J M. Gradient of RGD-dependent entry of adenoviral vector in nasal and intrapulmonary epithelia: implications for gene therapy of cystic fibrosis. Gene Ther. 1996;3:811–818. [PubMed] [Google Scholar]
- 12.Goldman M J, Wilson J M. Expression of αvβ5 integrin is necessary for efficient adenovirus-mediated gene transfer in the human airway. J Virol. 1995;69:5951–5958. doi: 10.1128/jvi.69.10.5951-5958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hierholzer J C. Adenoviruses in the immunocompromised host. Clin Microbiol Rev. 1992;5:262–274. doi: 10.1128/cmr.5.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang S, Kamata T, Takada Y, Ruggeri Z M, Nemerow G R. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J Virol. 1996;70:4502–4508. doi: 10.1128/jvi.70.7.4502-4508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang S, Stupack D G, Mathias P, Wang Y, Nemerow G. Growth arrest of Epstein-Barr virus immortalized B lymphocytes by adenovirus-delivered ribozymes. Proc Natl Acad Sci USA. 1997;94:8156–8161. doi: 10.1073/pnas.94.15.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hynes R O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 17.Isberg R R, Tran Van Nhieu G. The mechanism of phagocytic uptake promoted by invasin-integrin interaction. Trends Cell Biol. 1995;120:120–124. doi: 10.1016/s0962-8924(00)88962-x. [DOI] [PubMed] [Google Scholar]
- 18.Ishibashi Y, Claus S, Relman D A. Bordeltella pertussis filamentous hemagglutinin interacts with a leukocyte signal transduction complex and stimulates bacterial adherence to monocyte CR3 (CD11b/CD18) J Exp Med. 1994;180:1225–1233. doi: 10.1084/jem.180.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalandadze A, Galleno M, Foncerrada L, Strominger J L, Wucherpfennig K W. Expression of recombinant HLA-DR2 molecules. J Biol Chem. 1996;271:20156–20162. doi: 10.1074/jbc.271.33.20156. [DOI] [PubMed] [Google Scholar]
- 20.Kühn K, Eble J. The structural bases of integrin-ligand interactions. Trends Cell Biol. 1994;4:256–261. doi: 10.1016/0962-8924(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 21.Loftus J W, Smith J C, Ginsberg M H. Integrin-mediated cell adhesion: the extracellular face. J Biol Chem. 1994;269:25235–25238. [PubMed] [Google Scholar]
- 22.Mason P W, Rieder E, Baxt B. RGD sequence of foot-and-mouth disease virus is essential for infecting cells via the natural receptor but can be bypassed by an antibody-dependent enhancement pathway. Proc Natl Acad Sci USA. 1994;91:1932–1936. doi: 10.1073/pnas.91.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathias P, Wickham T J, Moore M, Nemerow G. Multiple adenovirus serotypes use αv integrins for infection. J Virol. 1994;68:6811–6814. doi: 10.1128/jvi.68.10.6811-6814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKay B S, Annis D S, Honda S, Christie D, Kunicki T J. Molecular requirements for assembly and function of a minimized human integrin. J Biol Chem. 1996;271:30544–30547. doi: 10.1074/jbc.271.48.30544. [DOI] [PubMed] [Google Scholar]
- 25.Mette S A, Pilewski J, Buck C A, Albelda S M. Distribution of integrin cell adhesion receptors on normal bronchial epithelial cells and lung cancer cells in vitro and in vivo. Am J Respir Cell Mol Biol. 1993;8:562–572. doi: 10.1165/ajrcmb/8.5.562. [DOI] [PubMed] [Google Scholar]
- 26.Ramaswamy H, Hemler M E. Cloning, primary structure and properties of a novel human integrin β subunit. EMBO J. 1990;9:1561–1568. doi: 10.1002/j.1460-2075.1990.tb08275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roivaninen M, Hypiä T, Pirainen L, Kalkkinen N, Stanway G, Hovi T. RGD-dependent entry of coxsackievirus A9 into host cells and its bypass after cleavage of VP1 protein by intestinal proteases. J Virol. 1991;65:4735–4740. doi: 10.1128/jvi.65.9.4735-4740.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruoslahti E, Pierschbacher M D. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 29.Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- 30.Smith J W, Vestal D J, Irwin S V, Burke T A, Cheresh D A. Purification and functional characterization of integrin αvβ5: an adhesion receptor for vitronectin. J Biol Chem. 1990;265:11008–11013. [PubMed] [Google Scholar]
- 31.Stewart P L, Chiu C, Huang S, Muir T, Zhao Y, Chait B, Mathias P, Nemerow G. Localization of an integrin binding motif (RGD) on adenovirus type 2 particles by cryo-electron microscopy. EMBO J. 1997;16:1189–1198. doi: 10.1093/emboj/16.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki S, Argraves W S, Pytela R, Arai H, Krusius T, Pierschbacher M D, Ruoslahti E. cDNA and amino acid sequences of the cell adhesion protein receptor recognizing vitronectin reveal a transmembrane domain and homologies with other adhesion protein receptors. Proc Natl Acad Sci USA. 1986;83:8614–8618. doi: 10.1073/pnas.83.22.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomko R P, Xu R, Philipson L. HCAR and MAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogel B E, Lee S-J, Hildebrand A, Craig W, Pierschbacher M D, Wong-Staal F, Ruoslahti E. A novel integrin specificity exemplified by binding of the αvβ5 integrin to the basic domain of the HIV tat protein and vitronectin. J Cell Biol. 1993;121:461–468. doi: 10.1083/jcb.121.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinacker A, Chen A, Agrez M, Cone R I, Nishimura S, Wayner E, Pytela R, Sheppard D. Role of the integrin αvβ6 in cell attachment to fibronectin. J Biol Chem. 1994;269:1–9. [PubMed] [Google Scholar]
- 36.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]