Abstract
The solvation of membrane proteins by both lipids and water makes their membrane immersion difficult to predict and the choice of a membrane mimic challenging. To characterize protein-lipid contacts and bicelle membrane mimics, we examined protein-lipid cross-relaxation of integrin αIIb and β3(A711P) transmembrane helices in isotropic phospholipid bicelles (q=0.5 and 0.7). Long-chain bicelle lipids dominated contacts with central helix segments, whereas both short- and long-chain lipids contacted the terminal turns of each helix in corroboration of the mixed bicelle model. The saturation transfer profiles from long-chain lipids directly established helix midpoints in the lipid bilayer. Lipid headgroups and water molecules engaged the sidechains of buried serine and threonine in competition with intrahelical hydrogen bonding, illustrating that polar sidechains seek the most favorable electrostatic contacts. Thus, protein-lipid cross-relaxation provides information on bicelle lipid distribution, transmembrane helix topology and polar sidechain partitioning.
Keywords: Bicelle, Cross-relaxation, Integrin, Membrane mimic, Membrane protein topology, NMR, Protein-lipid interaction
Graphical Abstract
The weak association of lipids with membrane proteins generally leads to a loss of the native lipid environment during protein purification. Nonetheless, it is often possible to reconstitute isolated membrane proteins in colloidal suspensions that provide a simplified lipid bilayer environment. Phospholipid bicelles represent an attractive membrane mimic due to their controllable size.1–4 In bicelles, long-chain lipids form a lipid bilayer whose area is controlled by the ratio of long- to short-chain lipids, the q-factor. The short-chain lipids constitute a rim surrounding the bilayer and, in accordance with the mixed bicelle model,4,5 a fraction of short-chain lipids exists in the long-chain bilayer (Figure 1a). Bicelles are especially useful for solution NMR studies of membrane proteins, for which relatively small, fast-tumbling bicelles (q<1.0) are applied.2,3 The mixed bicelle model is based on model calculations and has not been validated by direct experimental observation.4,5 Moreover, it is unknown whether this model holds in the presence of bicelle-immersed protein. For the β-barrel protein OmpX and the transmembrane (TM) α-helix of TrkA, cross-relaxation in the form of NOE signals between lipids and protein were reported only for long-chain lipids.6,7 On the other hand, molecular dynamics simulations support the mixing of short- and long-chain lipids.8 The importance and widespread use of bicelles as membrane mimic9–12 requires the thorough understanding of their structure. Here, we examined protein-lipid 1H-1H cross-relaxation for two TM α-helices in isotropic bicelles to answer these questions and to characterize the nature of isotropic bicelles in general.
Figure 1.
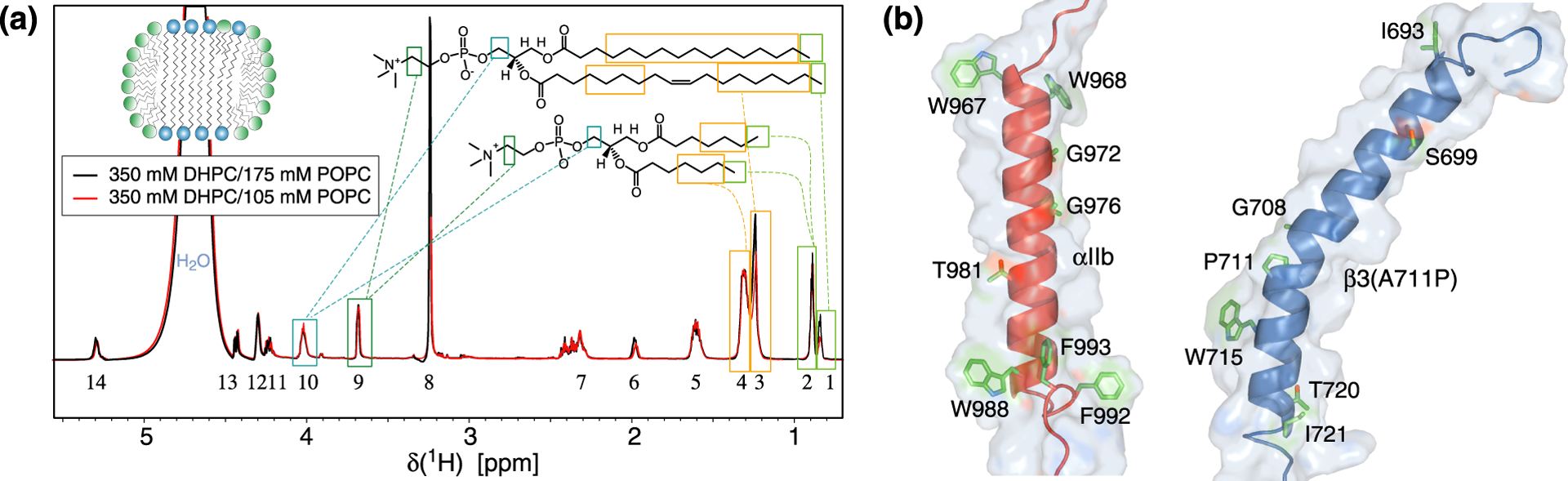
Overview of experimental system. (a) 1D 1H NMR spectrum of 1,2-dihexanoly-sn-glycero-3-phosphocholine (DHPC) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) at the indicated concentrations. Chemical shift assignments of interest to the present study are indicated by dashed lines. Lipid resonances are enumerated consecutively from right to left and referred to by their number and chemical identity, e.g., POPC(#1|CH3) for peak #1, representing the terminal methyl groups of POPC. Spectra were recorded in 25 mM NaH2PO4/Na2HPO4, pH 7.4, at 35 °C and 700 MHz. (b) Cartoon representations of the structures of integrin αIIb and β3(A711P) TM segments (PDB entries 2k1a and 2n9y, respectively).18,20 The depicted van der Waals surfaces illustrate the approachability of backbone HN nuclei. In αIIb, not only re-immersed C-terminal Phe992-Phe993 residues complicate the evaluation of its membrane immersion but also the hydrophobic Ala-Ile-Pro sequence at its N-terminus. Compared to the corresponding Gly-Pro-Asp sequence of the integrin β3 TM helix, that sequence provides enhanced protection from the paramagnetic Mn2+EDDA2− complex.17,18
In structural and functional studies of membrane proteins, it is important to define the border of the protein relative to the membrane. A bordering residue may be defined by testing its amenability to glycosylation before the membrane prevents such a reaction.13 Alternatively, paramagnetic agents covalently linked to lipids or solubilized either in the aqueous or hydrophobic phases can provide information on immersion depths of individual residues.14–16 Provided protection from paramagnetic agents is symmetrical on both lipid bilayer faces, the midpoint of a TM helix can be simply determined.17 However, it is difficult to verify this assumption because residues preceding and succeeding the TM helix may linger in the lipid headgroup region and hence render protection asymmetric (Figure 1b).18 A direct observation of protein-lipid contacts would avoid such obstacles. Accordingly, the present study further examined cross-relaxation of protein and lipid 1H nuclei to establish an approach for directly determining the bilayer immersion of TM helices.
Calorimetric measurements that capture the entropy saving arising from TM helix-helix preorientation in a common bilayer have shown that q=1.0 bicelles provide an essentially fully-fledged bilayer, whereas q=0.24 bicelles do not offer any preorientation.19 Bicelles with q=0.5 were shown to be intermediate between these limiting scenarios and, therefore, provide a significant bilayer shell for TM helices while remaining accessible to solution NMR. To evaluate cross-relaxation between protein amide and lipid protons, we eliminated dipolar contributions from carbon-bonded hydrogens by protein perdeuteration and then, in q=0.5 bicelles, quantified the ratio of H-N cross-peak intensities recorded with and without presaturation of a particular lipid resonance, termed I/I0 (Figure S1). For 1,2-dihexanoly-sn-glycero-3-phosphocholine (DHPC) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) lipids, the methylene and methyl moieties of their hydrocarbon tails resolved while the chemical shifts of other 1H nuclei coincided (Figure 1a). For model TM helices, we employed the TM segments of the integrin receptor αIIb and β3 subunits. With 24 and 29 lipid-immersed helical residues for αIIb and β3, respectively, small and large helix tilts arise relative to the bilayer normal (Figure 1b).17,18 In αIIb, the non-helical Phe992-Phe993 residues immersed back into the membrane at the C-terminus of the TM helix (Figure 1b). Moreover, to break the point symmetry at the center of the β3 TM helix, a proline-mediated helix kink was introduced via the β3(A711P) substitution (Figure 1b).17,20
For αIIb, three principal peptide/DHPC/POPC arrangements appear possible (Figure 2c); model I predicts no contacts to DHPC, model II represents contacts to DHPC at the bicelle rim and model III predicts interactions with interspersed but not rim DHPC. To assess relative populations of these models, cross-relaxation between either CH2 or CH3 of lipid tails and backbone HN nuclei was evaluated. For both αIIb and β3(A711P) TM segments, lipid tail CH2-HN saturation transfer was comparable between DHPC and POPC at their helix termini, whereas POPC dominated transfers for the central helix turns (Figure 2a–b). Accordingly, at the helix termini, DHPC and POPC contacted the peptides at similar frequencies, while long-chain lipids dominantly contacted the helix core. For αIIb, this result indicates model I not to be prevalent. Conversely, model III, where DHPC interspersed into the POPC bilayer, has to be populated significantly in order to observe a congruence of long- and short-chain lipid tail CH2-HN transfers around the αIIb helix termini. For arrangement II, contrary to model prediction, saturation transfer from the termini of short-chain lipids, DHPC(#2|CH3), to HN is not larger than the corresponding transfer from long-chain lipid termini, POPC(#1|CH3) (Figure 3a). Moreover, the relative I/I0 increase at the helix center for DHPC(#4|CH2)-HN transfers confirms model II not to be prevalent. Altogether, the dominance of model III supports the mixed bicelle model.
Figure 2.
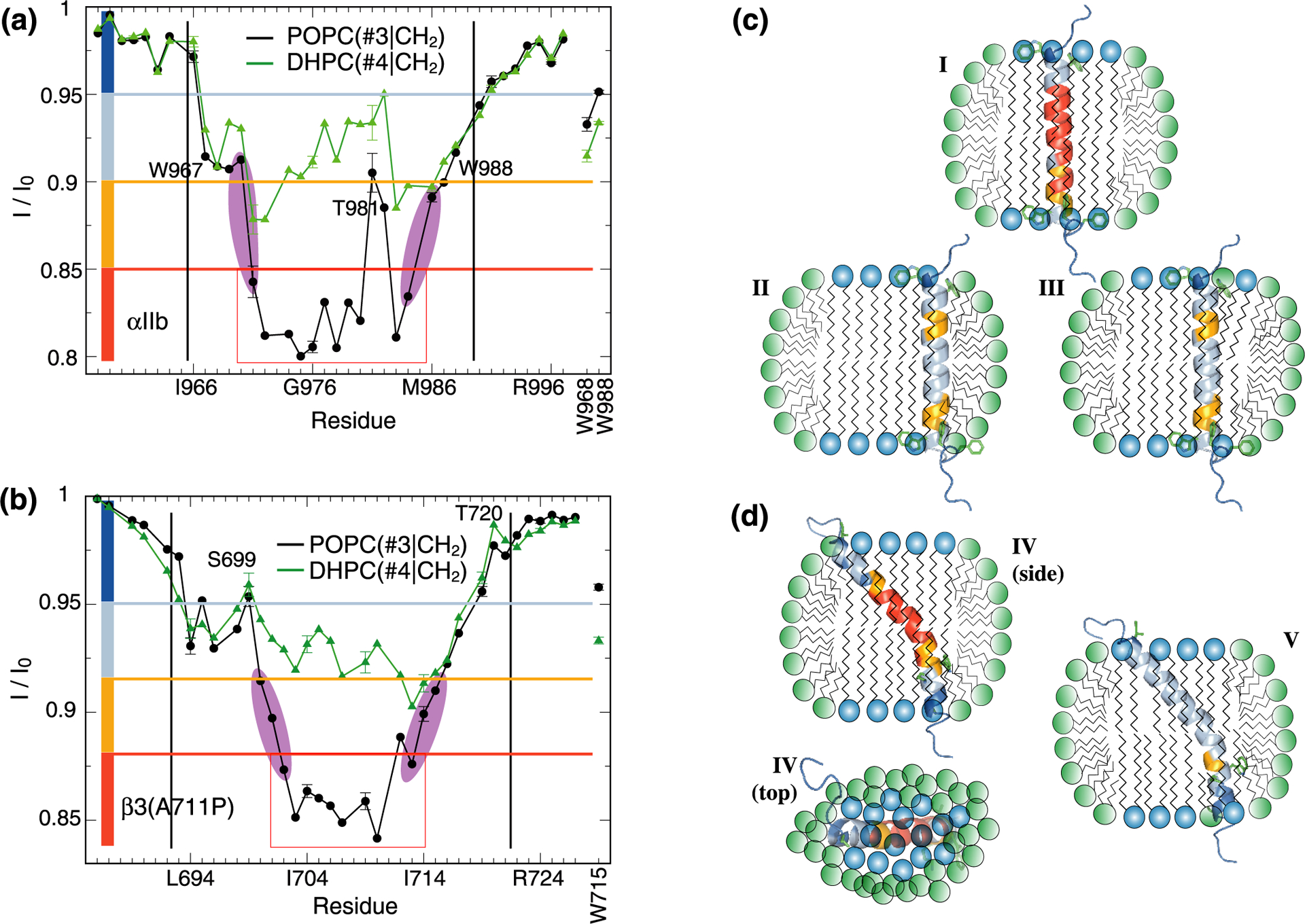
Contacts between protein and lipid hydrocarbon tails. (a-b) Saturation transfers between protein backbone HN nuclei of integrin αIIb or β3(A711P) TM segments and POPC(#3|CH2) or DHPC(#4|CH2) nuclei of lipid hydrocarbon tails (see Figure 1a). Tryptophan sidechain HN nuclei were also evaluated as shown using vertical labels. The helix midpoint was estimated by evaluating the decrease of I/I0 values for POPC(#3|CH2) as highlighted in magenta. Black vertical lines indicate the previously determined membrane borders of αIIb and β3 TM helices.17,18 (c-d) Possible orientations of the αIIb and β3(A711P) TM segments in isotropic bicelles (q=0.5). Bicelles were modeled as outlined in ref19. In model I, the αIIb TM helix, depicted solely in contact with POPC, was color-coded according to the I/I0 values obtained for POPC(#3|CH2). Models II-III emphasize TM helix contacts with DHPC and were color-coded according to the I/I0 values obtained for DHPC(#4|CH2). In model IV, the β3(A711P) TM helix is color-coded according to the I/I0 values obtained for POPC(#3|CH2), while model V depicted the I/I0 values measured for DHPC(#4|CH2). I/I0 values were divided into four groups and color-coded as indicated by the colored rectangles shown in panels a-b.
Figure 3.
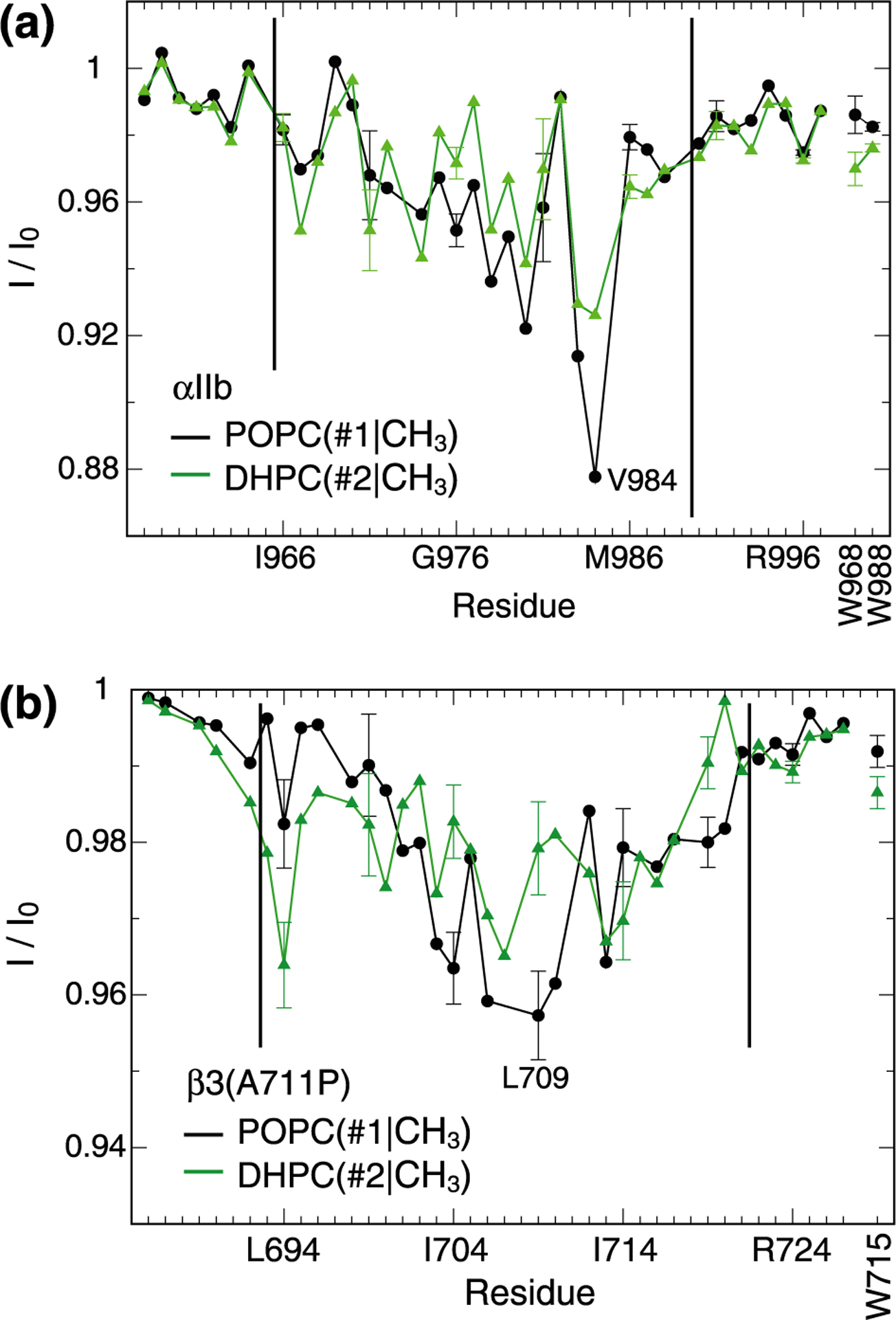
Contacts between protein and the termini of lipid hydrocarbon tails. (a-b) Saturation transfers between protein backbone HN nuclei of integrin αIIb or β3(A711P) TM segments and terminal POPC(#1|CH3) or DHPC(#2|CH3) nuclei of lipid hydrocarbon tails (see Figure 1a). Tryptophan sidechain HN nuclei were also evaluated as shown using vertical labels. Black vertical lines indicate the previously determined membrane borders of αIIb and β3 TM helices.17,18
The tilted and kinked helix of β3(A711P) allows only two principal peptide/DHPC/POPC arrangements (Figure 2d). In model IV, the helix termini contact the DHPC rim constantly while in model V POPC can shield the helix from rim but not interspersed DHPC. In corroboration of model IV, long- and short-chain lipid tail CH2-HN saturation transfers matched for two helical turns at each helix terminus instead of only one for αIIb (Figure 2a–b). Remarkably, DHPC(#4|CH2)-HN transfers were noticeably asymmetric between N- and C-terminal helix segments (Figure 2b), with an I/I0 minimum in the C-terminal half suggesting a helix topology as depicted in all models of Figure 2d. This bicelle-helix orientation would discriminate helix faces with respect to the inside and outside of the bicelle disc (Figure 2d). However, DHPC(#4|CH2)-HN transfers did not favor a particular helix face (Figure 2b), suggesting that, as represented by model V, DHPC interspersed with POPC. As demonstrated, the population of model V is necessary to explain the lipid tail CH2-HN contacts in further support of the mixed bicelle model.
In contrast to the integrin αIIb and β3(A711P) TM helices, NOEs to short-chain lipids were not observed for the β-barrel protein OmpX and the TM helix of TrkA.6,7 Next to differences in bicelle structure, other reasons for this absence are conceivable. First, the cross-relaxation rate scales with the general order parameter S2 that describes fast timescale bond vector dynamics.21 For lipids, S2 is very low at 0.1–0.2,21,22 implying that cross-relaxation is inefficient and strongly modulated by variations in protein-lipid dynamics. Second, the absence or attenuation of expected long-chain lipid-protein NOEs has been implicated in protein structural inhomogeneity.21 NOEs to short-chain lipid have low intensities relative to long-chain lipid (Figure 2a–b) and will be most sensitive to the outlined effects.
The asymmetric DHPC(#4|CH2)-HN transfers with respect to the helix termini of both αIIb and β3(A711P) made these regions unsuitable to determine the helix midpoint in the lipid bilayer (Figure 2a–b). Instead, the plateau that formed for POPC(#3|CH2)-HN transfers at the center of the helices appeared suitable. It is not affected by interfacial amino acids, its midpoint ought to be equidistant from the lipid headgroups, its borders can be estimated to an accuracy of one residue (Figure 2a–b), and it is not significantly smoothened by spin diffusion as illustrated by the sharp changes in I/I0 values observed for αIIb(T981-I982). For αIIb, position 977.5 is the midpoint of the plateau (Figure 2a), which corresponds to the midpoint of the α-helix encompassing Ile966-Lys989 (Figure 1b). For β3(A711P), a plateau midpoint was formed at position 707.5 (Figure 2b). Based on the TM helix border residues Ile693 and Ile721 of the wild-type helix,17 residue 707 is the arithmetic midpoint of the kinked β3(A711P) TM helix (Figure 1b). Thus, despite the structural asymmetry of both helices, they are centered with respect to the lipid bilayer. We further note that POPC(#1|CH3)-HN saturation transfers were not useful in defining the helix centers (Figure 3). For β3(A711P) a broad dip at the helix center was observed that affirmed the bilayer arrangement of POPC in the bicelle. Similarly, an I/I0 dip formed at the αIIb helix center but the minimum was encountered for V984 (Figure 3a). This residue resides on top of re-immersed Phe993 (Figure 1b) and lipids around its aromatic ring may have adjusted in a complex manner.
In addition to the absence of structural inhomogeneity, the plateaus of long-chain lipid tail CH2-HN saturation transfers for both αIIb and β3(A711P) TM helices show that different sidechain sizes along the helix axes (Figure 1b) did not lead to substantial variations in cross-relaxation with lipids. A plateau in POPC(#3|CH2)-HN cross-relaxation rates can therefore be expected for any structurally homogenous, single-pass TM helix. Specifically, the presented approach avoids any complications associated with lipid asymmetry across the lipid bilayer and makes the numerous proteins with single-pass TM helices present in, for example, the human genome23 amenable to the presented technique.
To further examine the orientation of the TM helix relative to the bilayer, the location of the lipid headgroup PO4− moiety is of interest. In pursue of protein-lipid headgroup contacts, we irradiated the PO4− preceding and succeeding methylenes, PC(#9|CH2) and PC(#10|CH2), respectively, which are indistinguishable between DHPC and POPC (Figure 1a). Interestingly, lipid contacts of backbone HN nuclei at TM helix termini were not guaranteed. β3(A711P) displayed few such contacts focusing on L694 and S699 (Figure 4b). For αIIb, more contacts were observed, notably for W967, T981 and V984 (Figure 4a). It appears that protein-lipid dynamics had strong effects on cross-relaxation and only residues with prominent lipid contacts experienced pronounced cross-relaxation. The identified residues showed different depth profiles; β3(L694) showed cross-relaxation only to CH2 preceding PO4−, αIIb(W967) and αIIb(V984) cross-relaxed with CH2 preceding and succeeding PO4−, and αIIb(T981) and β3(S699) only exhibited cross-relaxation with CH2 succeeding PO4−. αIIb(W967) and β3(L694) are one residue from the N-terminal membrane border17,18 fitting with their localization preceding PO4−. The headgroup contacts of αIIb(V984) further highlight a highly asymmetric lipid distribution at this location. The pronounced cross-relaxation experienced by αIIb(T981) and β3(S699) suggest that sidechain HO…PO4− and/or HO…C=O contacts diminished protein-lipid dynamics.
Figure 4.
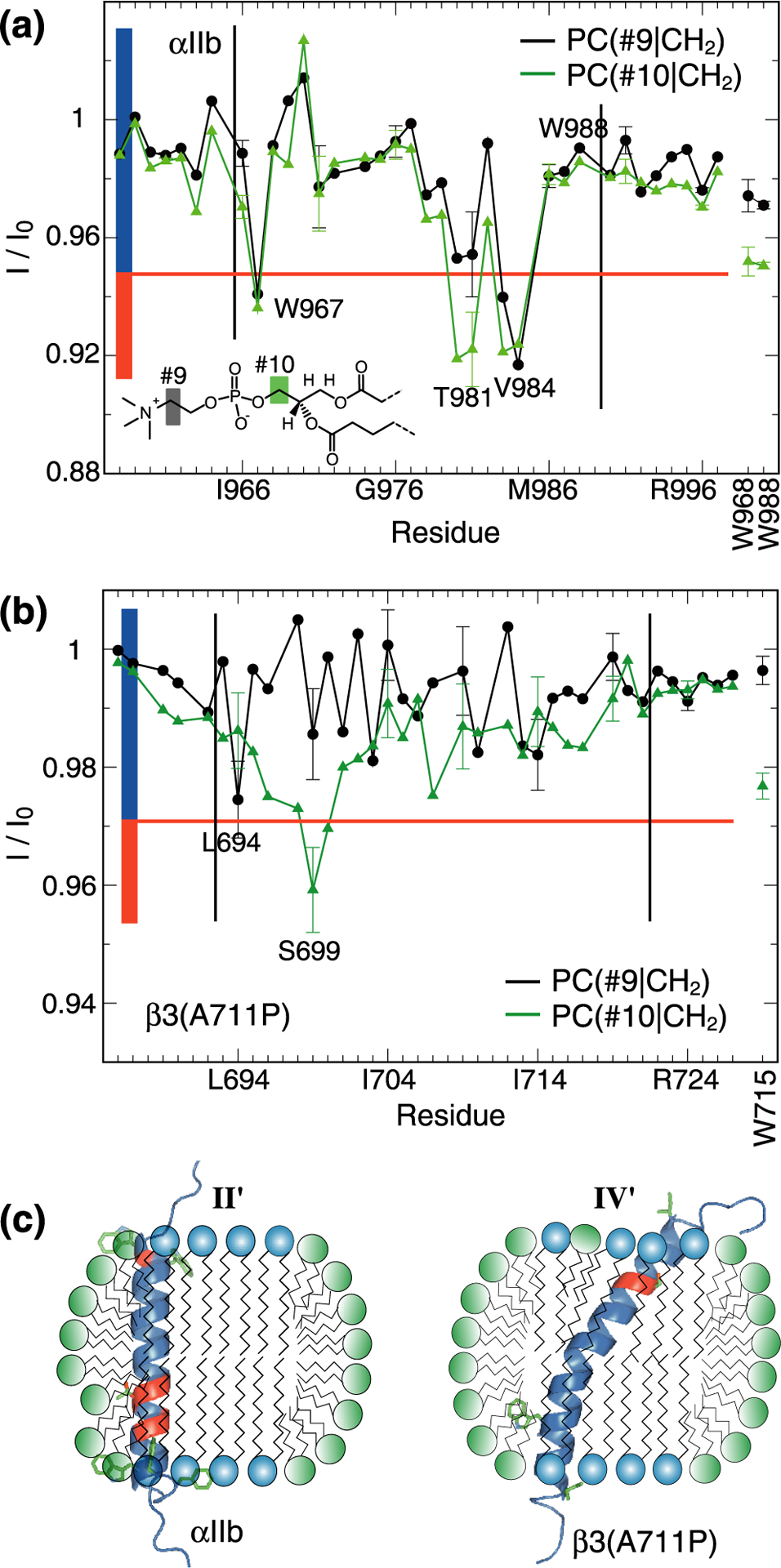
Contacts between protein and lipid headgroups. (a-b) Saturation transfers between protein backbone HN nuclei of integrin αIIb or β3(A711P) TM segments and PC(#9|CH2) or PC(#10|CH2) nuclei of lipid headgroups. Tryptophan sidechain HN nuclei were also evaluated as shown using vertical labels. Black vertical lines indicate the previously determined membrane borders of αIIb and β3 TM helices.17,18 (c) Possible orientations of αIIb and β3(A711P) TM segments in isotropic bicelles (q=0.5) following models II and IV of Figure 2c–d. The TM helices were color-coded according to their I/I0 values. Specifically, I/I0 values were divided into two groups and color-coded as indicated by the colored rectangles shown in panels a-b.
In TM helices, the sidechain HO nuclei of serine and threonine frequently form intrahelical hydrogen bonds with the carbonyl oxygen of residue i-4.25,26 However, in the studied q=0.5 bicelles this interaction appears to be diminished by contacts to lipid PO4− and/or C=O groups. Moreover, in this context we point out the “breaks” in lipid tail POPC(#3|CH2)-HN saturation transfers for αIIb(T981) and β3(S699) relative to neighboring residues (Figure 2a–b). Evidently, their HO nuclei exchanged or cross-relaxed with water molecules within the period of lipid presaturation and backbone HN nuclei then cross-relaxed with these HO nuclei in opposition to CH2. For membrane-immersed HO nuclei, proximity to lipid headgroups and water may have been promoted by the presence of rim short-chain lipids (Figure 2c–d). The tendency of Ser/Thr to engage the rim headgroup region would not be surprising because of their positive free energies of insertion into the membrane core.27,28 On the other hand, certain lipid headgroup contacts can take place in a continuous membrane. We note the proximity of β3(S699) to the membrane border (Figure 1b). To examine whether the relatively small bilayer shell of q=0.5 bicelles had facilitated such interactions, we assessed POPC(#3|CH2)-HN and PC(#10|CH2)-HN saturation transfers in q=0.7 bicelles.
For β3(S699), the larger bicelle disc suppressed water interactions while HO…PO4− and/or HO…C=O contacts persisted (Figure 5b). Interestingly, now L694 exhibited contacts with CH2 preceding PO4−. This pattern indicates reduced contacts with rim lipids and/or a deeper bilayer immersion of the TM helix at the N-terminus in response to the larger q-factor. For αIIb(T981), the situation was reversed; HO…PO4− and/or HO…C=O contacts diminished with increased water interactions (Figure 5a). This situation is incompatible with reduced contacts with rim lipids which likely reflects the deeper membrane immersion of αIIb(T981) than β3(S699) (Figure 1b and 5). An increase in q-factor therefore correlates with diminished contacts of polar sidechains with rim lipids in an immersion depth-dependent manner. For the examined q-factors, we propose dominant contacts between the sidechain of β3(S699) and lipid headgroups in the bilayer region (Figure 4c), which may also occur in a continuous membrane. For the sidechain of αIIb(T981), we postulate contacts to the rim region (Figure 4c). This scenario shows that model II (Figure 2c) was populated, although its proportion must be smaller than the population of model III as discussed above. As such, rim contacts were transient in q=0.5 bicelles. In the bilayer region (models I and III), the intrahelical hydrogen bond can form but, interestingly, the asymmetric lipid distribution around αIIb(V984) (Figure 3a and 4a) may allow T981 to make sidechain contacts to the distorted headgroup region of the lipid bilayer. It is unknown whether αIIb(T981) forms sidechain contacts with a water molecule in a continuous bilayer. Regardless, the effects from perturbing single intrahelical Thr/Ser hydrogen bonds appear small overall.29 More caution is warranted when interhelical hydrogen bonds contribute to membrane protein stability.30
Figure 5.
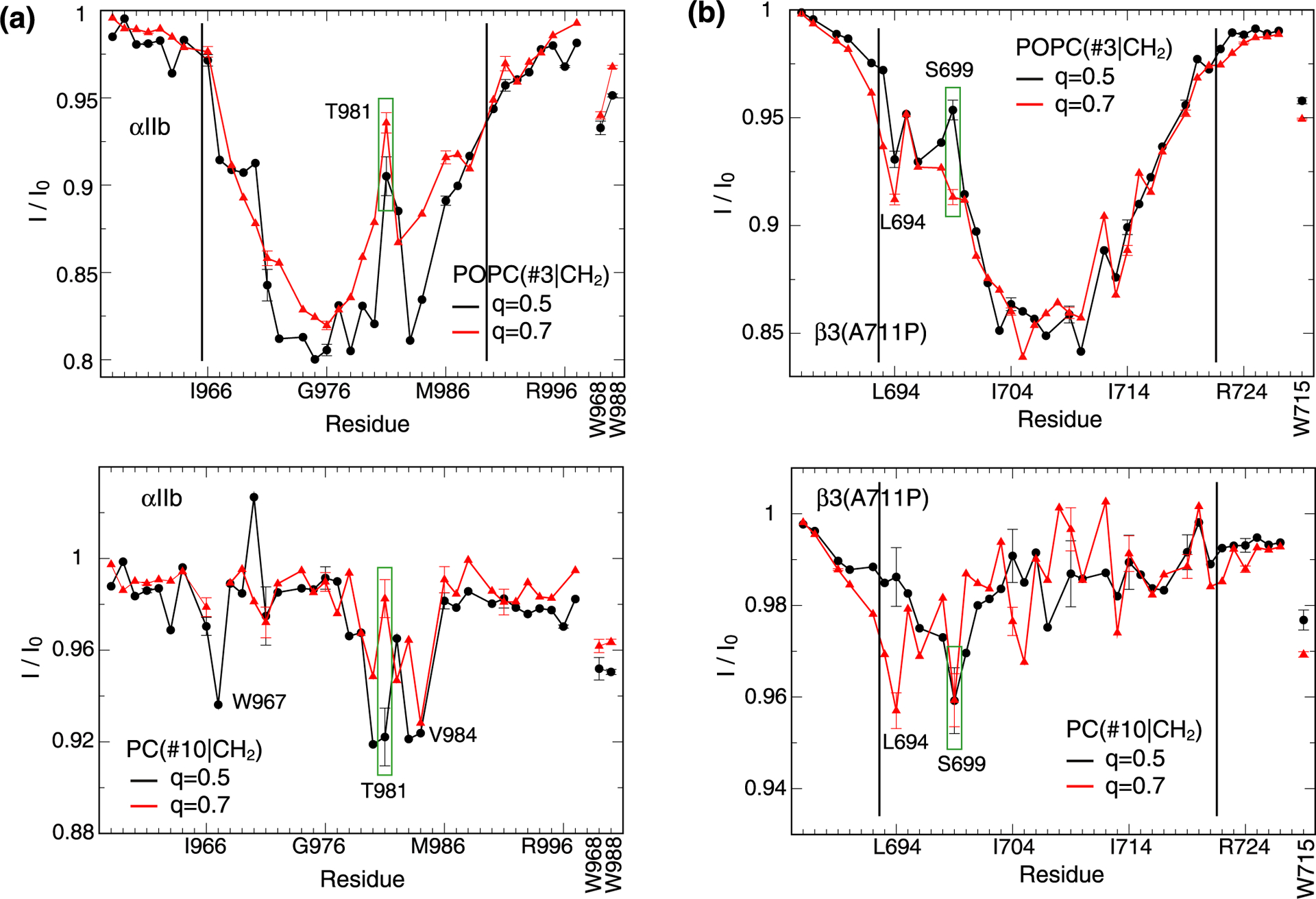
Influence of bicelle size (q-factor) on protein-lipid contacts. Saturation transfers between protein backbone HN nuclei of (a) integrin αIIb and (b) β3(A711P) TM segments and either POPC(#3|CH2) or PC(#10|CH2) nuclei in q=0.5 and 0.7 bicelles. Tryptophan sidechain HN nuclei were also evaluated as shown using vertical labels. Black vertical lines indicate the previously determined membrane borders of αIIb and β3 TM helices.17,18 The POPC(#3|CH2)-HN transfer at the TM helix center of αIIb at q=0.7 is less efficient than at q=0.5 suggesting a heightened proportion of model II (Figure 2c) in q=0.7 bicelles.
In conclusion, the analysis of protein-lipid cross-relaxation provides information on bicelle lipid mixing, the topology of TM helix immersion and associated lipid asymmetry, protein-lipid headgroup contacts, and contacts of polar sidechains with the lipid headgroup region.
Experimental Procedures
Peptides incorporated human integrin sequences αIIb(A958-P998) and β3(P685-F727) with αIIb(A963C) and β3(C687S/G690C/A711P) substitutions were utilized. Perdeuterated peptides were produced by culturing E. coli in minimal medium consisting of 99% d7-glucose, 99% 15ND4Cl and 99% D2O. For backbone assignments, peptides were also produced using 99% 13C-glucose, 99% 15ND4Cl and 99% D2O precursors. Protein purification proceeded as described.18 Freeze-dried peptides were reconstituted at concentrations of 1 mM in 320 μL of 350 mM DHPC, 175 mM POPC, 5 mM tris(2-carboxyethyl)phosphine, 0.02% NaN3, 6% D2O, and 25 mM NaH2PO4/Na2HPO4, pH 7.4, solution. Alternatively, samples with DHPC and POPC concentrations of 400 and 280 mM, respectively, were prepared to obtain q=0.7 bicelles.
NMR data were acquired employing a Bruker Avance 700 spectrometer with cryogenic probehead at 35 °C. Assignments of DHPC and POPC were transferred from the Spectral Database for Organic Compounds entries 16108HSP-45–792 (L-α-dipalmitoylphosphatidylcholine) and 17181HSP-43–236 (oleoyl chloride). Backbone H-N assignments of monomeric αIIb(A963C) and β3(C687S/G690C/A711P) were transferred from the H-N-Cα assignments of the disulfide-linked αIIb(A963C)-β3(C687S/G690C/A711P) TM complex20 applying HNCA experiments.
TROSY-type H-N correlation spectra31 for quantifying lipid saturation transfer were recorded with and without lipid presaturation in an interleaved manner. 15N Boltzmann magnetization was eliminated. The spectra were recorded with 128 × 768 complex points (t1(N)= 71.7 ms, t2(HN)= 85.4 ms) in q=0.5 and with 112 × 512 complex points (t1(N)= 62.7 ms, t2(HN)= 56.9 ms) in q=0.7 bicelles (Figure S1). NMR data were processed and analyzed with the nmrPipe package32 and CARA. Presaturation times of 125, 250 and 500 ms at a field strength of 11.2 Hz were evaluated and 500 ms employed as best conciliation between magnetization transfer, spin diffusion and sensitivity, which is similar to a previous study with perdeuterated protein and protonated detergent.33 Specifically, presaturation of methylene groups did not transfer magnetization to methyl groups and vice versa (Table S1), and had only small effects on spectral neighbors. Peak POPC(#3|CH2) irradiation, for example, attenuated the vicinal signal DHPC(#4|CH2) by 18%. This modest attenuation clearly implied that saturation transfer from the directly irradiated resonance dominated I/I0 profiles.
Supplementary Material
Acknowledgements
This work was supported by American Heart Association grant 15GRNT23200010.
Footnotes
Supporting Information
Figure illustrating the quality of H-N correlation spectra in q=0.5 and 0.7 bicelles. Table quantifying the effect of presaturation on intensity of vicinal signals.
The authors declare no competing financial interest.
References
- (1).Gabriel NE; Roberts MF Interaction of Short-Chain Lecithin with Long-Chain Phospholipids - Characterization of Vesicles That Form Spontaneously. Biochemistry 1986, 25, 2812–2821. [DOI] [PubMed] [Google Scholar]
- (2).Vold RR; Prosser RS; Deese AJ Isotropic Solutions of Phospholipid Bicelles: A New Membrane Mimetic for High-Resolution Nmr Studies of Polypeptides. J. Biomol. NMR 1997, 9, 329–335. [DOI] [PubMed] [Google Scholar]
- (3).Sanders C. R. n.; Landis GC Reconstitution of Membrane Proteins into Lipid-Rich Bilayered Mixed Micelles for Nmr Studies. Biochemistry 1995, 34, 4030–4040. [DOI] [PubMed] [Google Scholar]
- (4).Sanders CR; Schwonek JP Characterization of Magnetically Orientable Bilayers in Mixtures of Dihexanoylphosphatidylcholine and Dimyristoylphosphatidylcholine by Solid-State Nmr. Biochemistry 1992, 31, 8898–8905. [DOI] [PubMed] [Google Scholar]
- (5).Triba MN; Warschawski DE; Devaux PF Reinvestigation by Phosphorus Nmr of Lipid Distribution in Bicelles. Biophys. J 2005, 88, 1887–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Mineev KS; Nadezhdin KD; Goncharuk SA; Arseniev AS Characterization of Small Isotropic Biceiles with Various Compositions. Langmuir 2016, 32, 6624–6637. [DOI] [PubMed] [Google Scholar]
- (7).Lee D; Walter KFA; Bruckner A-K; Hilty C; Becker S; Griesinger C Bilayer in Small Bicelles Revealed by Lipid-Protein Interactions Using Nmr Spectroscopy. J. Am. Chem. Soc 2008, 130, 13822–13823. [DOI] [PubMed] [Google Scholar]
- (8).Vestergaard M; Kraft JF; Vosegaard T; Thogersen L; Schiott B Bicelles and Other Membrane Mimics: Comparison of Structure, Properties, and Dynamics from Md Simulations. J. Phys. Chem. B 2015, 119, 15831–15843. [DOI] [PubMed] [Google Scholar]
- (9).Mineev KS; Bocharov EV; Pustovalova YE; Bocharova OV; Chupin VV; Arseniev AS Spatial Structure of the Transmembrane Domain Heterodimer of Erbb1 and Erbb2 Receptor Tyrosine Kinases. J. Mol. Biol 2010, 400, 231–243. [DOI] [PubMed] [Google Scholar]
- (10).Liu YZ; Kahn RA; Prestegard JH Dynamic Structure of Membrane-Anchored Arf Center Dot Gtp. Nat. Struct. Mol. Biol 2010, 17, 876–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Dev J; Park D; Fu QS; Chen J; Ha HJ; Ghantous F; Herrmann T; Chang WT; Liu ZJ; Frey G, et al. Structural Basis for Membrane Anchoring of Hiv-1 Envelope Spike. Science 2016, 353, 172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Lau T-L; Kim C; Ginsberg MH; Ulmer TS The Structure of the Integrin Alphaiibbeta3 Transmembrane Complex Explains Integrin Transmembrane Signalling. EMBO J. 2009, 28, 1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Nilsson I; Saaf A; Whitley P; Gafvelin G; Waller C; von Heijne G Proline-Induced Disruption of a Transmembrane Alpha-Helix in Its Natural Environment. J. Mol. Biol 1998, 284, 1165–1175. [DOI] [PubMed] [Google Scholar]
- (14).Altenbach C; Greenhalgh DA; Khorana HG; Hubbell WL A Collision Gradient-Method to Determine the Immersion Depth of Nitroxides in Lipid Bilayers - Application to Spin-Labeled Mutants of Bacteriorhodopsin. Proc. Natl. Acad. Sci. U. S. A 1994, 91, 1667–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Hilty C; Wider G; Fernandez C; Wuthrich K Membrane Protein-Lipid Interactions in Mixed Micelles Studied by Nmr Spectroscopy with the Use of Paramagnetic Reagents. Chembiochem 2004, 5, 467–473. [DOI] [PubMed] [Google Scholar]
- (16).Evanics F; Hwang PM; Cheng Y; Kay LE; Prosser RS Topology of an Outer-Membrane Enzyme: Measuring Oxygen and Water Contacts in Solution Nmr Studies of Pagp. J. Am. Chem. Soc 2006, 128, 8256–8264. [DOI] [PubMed] [Google Scholar]
- (17).Lau T-L; Partridge AP; Ginsberg MH; Ulmer TS Structure of the Integrin Beta3 Transmembrane Segment in Phospholipid Bicelles and Detergent Micelles. Biochemistry 2008, 47, 4008–4016. [DOI] [PubMed] [Google Scholar]
- (18).Lau T-L; Dua V; Ulmer TS Structure of the Integrin Alphaiib Transmembrane Segment. J. Biol. Chem 2008, 283, 16162–16168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Situ AJ; Schmidt T; Mazumder P; Ulmer TS Characterization of Membrane Protein Interactions by Isothermal Titration Calorimetry. J. Mol. Biol 2014, 426, 3670–3680. [DOI] [PubMed] [Google Scholar]
- (20).Schmidt T; Situ AJ; Ulmer TS Structural and Thermodynamic Basis of Proline-Induced Transmembrane Complex Stabilization. Sci Rep 2016, 6, 29809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Eichmann C; Orts J; Tzitzilonis C; Vogeli B; Smrt S; Lorieau J; Riek R Intermolecular Detergent-Membrane Protein Noes for the Characterization of the Dynamics of Membrane Protein-Detergent Complexes. J. Phys. Chem. B 2014, 118, 14288–14301. [DOI] [PubMed] [Google Scholar]
- (22).Mors K; Roos C; Scholz F; Wachtveitl J; Dotsch V; Bernhard F; Glaubitz C Modified Lipid and Protein Dynamics in Nanodiscs. Biochim. Biophys. Acta-Biomemb 2013, 1828, 1222–1229. [DOI] [PubMed] [Google Scholar]
- (23).Schmidt T; Ye F; Situ AJ; An W; Ginsberg MH; Ulmer TS A Conserved Ectodomain-Transmembrane Domain Linker Motif Tunes the Allosteric Regulation of Cell Surface Receptors. J. Biol. Chem 2016, 291, 17536–17546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Schmidt T; Suk JE; Ye F; Situ AJ; Mazumder P; Ginsberg MH; Ulmer TS Annular Anionic Lipids Stabilize the Integrin Alpha Iib Beta 3 Transmembrane Complex. J. Biol. Chem 2015, 290, 8283–8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Chamberlain AK; Bowie JU Analysis of Side-Chain Rotamers in Transmembrane Proteins. Biophys. J 2004, 87, 3460–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Gray TM; Matthews BW Intrahelical Hydrogen-Bonding of Serine, Threonine and Cysteine Residues within Alpha-Helices and Its Relevance to Membrane-Bound Proteins. J. Mol. Biol 1984, 175, 75–81. [DOI] [PubMed] [Google Scholar]
- (27).Hessa T; Kim H; Bihlmaier K; Lundin C; Boekel J; Andersson H; Nilsson I; White SH; von Heijne G Recognition of Transmembrane Helices by the Endoplasmic Reticulum Translocon. Nature 2005, 433, 377–381. [DOI] [PubMed] [Google Scholar]
- (28).Moon CP; Fleming KG Side-Chain Hydrophobicity Scale Derived from Transmembrane Protein Folding into Lipid Bilayers. Proc. Natl. Acad. Sci. U. S. A 2011, 108, 10174–10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).del Val C; White SH; Bondar AN Ser/Thr Motifs in Transmembrane Proteins: Conservation Patterns and Effects on Local Protein Structure and Dynamics. J. Membr. Biol 2012, 245, 717–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Dawson JP; Weinger JS; Engelman DM Motifs of Serine and Threonine Can Drive Association of Transmembrane Helices. J. Mol. Biol 2002, 316, 799–805. [DOI] [PubMed] [Google Scholar]
- (31).Pervushin K; Riek R; Wider G; Wuthrich K Attenuated T-2 Relaxation by Mutual Cancellation of Dipole- Dipole Coupling and Chemical Shift Anisotropy Indicates an Avenue to Nmr Structures of Very Large Biological Macromolecules in Solution. Proc. Natl. Acad. Sci. U. S. A 1997, 94, 12366–12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Delaglio F; Grzesiek S; Vuister GW; Zhu G; Pfeifer J; Bax A Nmrpipe - a Multidimensional Spectral Processing System Based on Unix Pipes. J. Biomol. NMR 1995, 6, 277–293. [DOI] [PubMed] [Google Scholar]
- (33).Ulmer TS; Bax A Comparison of Structure and Dynamics of Micelle-Bound Human Alpha-Synuclein and Parkinson’s Disease Variants. J. Biol. Chem 2005, 280, 43179–43187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


