Abstract
Background:
More than 30,000 mpox cases have been confirmed in the United States since May 2022. Mpox cases have disproportionally occurred among adult gay, bisexual, and other men who have sex with men; transgender persons; and Black and Hispanic/Latino persons. We examined knowledge, attitudes, and practices regarding mpox vaccination among adults presenting for vaccination to inform prevention efforts.
Methods:
We collected mixed-methods data from a convenience sample of adults presenting for JYNNEOS vaccination at 3 DC Health mpox vaccine clinics during August–October 2022. Survey and interview topics included knowledge about mpox symptoms and vaccine protection, beliefs about vaccine access, and trusted sources of information.
Results:
In total, 352 participants completed self-administered surveys and 62 participants completed an in-depth interview. Three main themes emerged from survey and interview data. First, most participants had a general understanding about mpox, but gaps remained in comprehensive understanding about mpox symptoms, modes of transmission, vaccine protection, personal risk, and vaccine dosing strategies. Second, participants had high trust in public health agencies. Third, participants wanted more equitable and less stigmatizing access to mpox vaccine services.
Conclusions:
Nonstigmatizing, inclusive, and clear communication from trusted sources, including public health agencies, is needed to address mpox knowledge gaps and increase vaccine access and uptake in affected communities. Mpox outreach efforts should continue innovative approaches, including person-level risk assessment tools, to address community needs.
As of June 2023, more than 30,000 confirmed mpox cases have been reported in the United States since the global outbreak began in May 2022.1 Cases have dispioportionally occurred among adult gay, bisexual, and other men who have sex with men (MSM); transgender persons; and Black and Hispanic/Latino persons.2 During this outbreak, transmission has mostly occurred from contact with mpox lesions during sexual activity; transmission from contact with surfaces is thought to be low.3 Mpox prevention strategies include behavioral changes to reduce sustained skin-to-skin contact and expanding access to mpox vaccination.2
The Food and Drug Administration licensed JYNNEOS (Modified Vaccinia Ankara vaccine, Bavarian Nordic) vaccine in 2019 as a subcutaneous 0.5-mL 2-dose series administered 28 days apart to prevent smallpox and mpox disease in adults 18 years or older.4 Peak protection is expected 2 weeks after the second dose. During the current outbreak, JYNNEOS vaccine was initially available to jurisdictions by request for persons with known mpox exposure. In June 2022, the national vaccination strategy broadened recommendations to include persons with known or presumed mpox exposure.5 In August 2022, the Food and Drug Administration issued an Emergency Use Authorization for administration of 0.1-mL doses by intradermal injection to increase vaccine supply.6 Vaccine administration continues to ensure sustained control of mpox.
Despite more than 700,000 administered vaccine doses between May and December 2022, inequities in vaccine uptake were observed. By the end of 2022, vaccination rates were higher among male individuals in racial and ethnic minority groups than among White male individuals but not proportionate to higher infection rates among these groups, indicating that disparities remain despite improvements in vaccine uptake.7 To support equitable vaccine distribution among mpox priority populations, it is critical to understand mpox perceptions, and barriers and facilitators to vaccine uptake. Among other policy and programmatic efforts, this information would aid in development and dissemination of tailored public health messages.
To inform mpox prevention strategies, during August–October 2022, we collected information on knowledge, attitudes, and practices related to mpox and mpox vaccine as part of a larger investigation to assess mpox prevalence and immune response in the District of Columbia (DC), which had seen more than 300 mpox cases by August 2022.8,9 This analysis incorporated quantitative survey data with qualitative interview data to provide social context to findings and a person-centered approach to data collection and analysis.10 We focused on perceptions of mpox and experiences with vaccine access to inform ongoing mpox prevention strategies and future outbreaks of similar infectious diseases.
MATERIALS AND METHODS
Eligibility
We enrolled individuals who presented for JYNNEOS vaccine at 3 DC Health clinics during August 11–31 and September 7–11, 2022. Before August 13, 2022, persons were eligible if they were a DC resident, 18 years or older, and were (1) MSM who had multiple or anonymous sexual partners in the prior 14 days, (2) transgender women or nonbinary persons assigned male sex at birth who had sex with men, or (3) sex workers or staff at establishments where sexual activity occurs (e.g., bathhouses, saunas, sex clubs). Starting August 13, 2022, vaccine eligibility expanded to people of any sexual orientation or gender who reported multiple sexual partners in the prior 14 days.9 Individuals were ineligible to participate in the investigation if they self-reported a characteristic mpox rash or history of mpox infection.
Recruitment
During August 11–31, 2022, we recruited individuals to participate during their first dose after vaccination waiting period. We described the larger investigation,8 determined eligibility, and obtained informed consent. Participants were asked to attend 3 clinic visits across 2 months (days 0 [first dose], 28 [second dose], and 42 [2 weeks after second dose]) for the larger study.8 Participants completed a self-administered survey at each visit. Those who completed a self-administered survey at their first and second dose visits were included in the quantitative analysis of this study.
We also approached individuals during August 12–21, 2022, for in-depth interviews (IDIs). Participants were eligible for IDIs regardless of whether they were presenting for their first or second vaccine dose at the time of enrollment. During September 7–11, 2022, we recruited additional participants for IDIs, and participants were eligible if they were presenting for their second vaccine dose. All IDI participants had to be able to complete the interview in English.
Participants received $51 for completing a survey at their second visit and $51 for their participation in an IDI.
Data Collection
Survey participants completed self-administered paper surveys that captured information on demographics, knowledge of mpox signs/symptoms, beliefs about infection risk, vaccine protection and availability, and their trusted sources of mpox information. We developed our survey based on other previously developed cross-sectional surveys on mpox and topics of interest for the mpox response at the time of the study. These data were transcribed into a REDCap11 database hosted at the CDC and exported to RStudio for analysis.
For IDIs, coauthors with extensive experience in rapid qualitative methods developed a semistructured interview guide based on guides used in past rapid outbreak responses and topics of interest for the mpox response, including mpox knowledge, perceptions of mpox risk and the vaccine, experience with vaccine access, preferences for sources of information about mpox, perceptions of health departments, and behavior change. In-depth interviews were conducted in a private room in the DC Health clinic or outside at a private table by CDC and DC Health staff trained on IDIs. Two staff members conducted each interview: one to conduct the interview and one to take detailed notes. In-depth interviews lasted approximately 30 to 45 minutes and were audio recorded.
Analysis
We calculated frequencies and proportions for survey results collected at first and second dose visits. We used multivariable Poisson regression with robust estimation of standard errors12 to determine an adjusted prevalence ratio (aPR) of reported trust in mpox information from health care providers, CDC, and local or state public health officials at the second visit, adjusting for demographic variables selected a priori (age, race/ethnicity, and gender/gender of sex partners). Gender/gender of sex partners incorporates gender identity, sexual orientation, and reported genders of recent sex partners to create categories of MSM (including men who have sex with men and women), men who have sex with women only (MSW), women, transgender persons, and persons with other gender identities. We defined significance at 2-tailed α = 0.05. We performed all statistical analyses in RStudio (v2022.07.1).
We analyzed IDI data rapidly in the field.13,14 After each interview, the interviewer and notetaker discussed and documented emerging themes and recommendations for future interviews. At the end of each day, at least one member of each interview team participated in a group debrief lasting 45 to 60 minutes to discuss themes and recommendations across all interviews from the day and assess whether data collection was nearing saturation. Based on daily debriefs, minor edits to the interview guide were made as needed for clarity.
Within 24 to 48 hours of each interview, interviewers entered notes into a matrix to organize findings, summarizing participant responses and including illustrative quotes. The matrix columns aligned with the interview guide and were designed to facilitate rapid review of themes. Interviewers reviewed audio files to confirm notes, capture missed information, or verify quotes. At the conclusion of each round of data collection, we reviewed the matrix entries and summarized findings. After all data were collected, key themes were compiled by reviewing the matrix entries, interview and debrief notes, and summaries, and organizing findings and participant quotes by main themes. Findings were summarized and discussed with team members to refine and reach consensus on key themes.
Quantitative and qualitative data were analyzed independently with findings integrated at the interpretation stage.
This activity was reviewed by the CDC and conducted in accordance with applicable federal law and CDC policy.15
RESULTS
Participant Characteristics
A total of 352 participants completed a self-administered survey at their first and second vaccine dose visits (458 participants who completed only a first dose visit and survey were excluded from analysis), and 62 participants completed an IDI.
Survey participants ranged in age from 18 to 82 years (median, 34 years; Table 1). Most participants identified as men (68%), were lesbian or gay (56%), and identified as non-Hispanic White (53%) or non-Hispanic Black/African American (20%).
TABLE 1.
Characteristics of Survey and In-Depth Interview Participants Presenting for JYNNEOS Vaccination—District of Columbia, August–October 2022
| Characteristic | Survey Participants, n(%) | In-Depth Interview Participants, n (%) |
|---|---|---|
| Total | 352 (100) | 62 (100) |
| Age group, y | ||
| 18–24 | 60 (17) | 8 (13) |
| 25–34 | 118 (34) | 27 (44) |
| 35–44 | 86 (24) | 13 (21) |
| 45–54 | 40 (11) | 6 (10) |
| >55 | 48 (14) | 8 (13) |
| Sex at birth | ||
| Male | 248 (70) | 52 (84) |
| Female | 104 (30) | 10 (16) |
| Gender identity* | ||
| Man | 238 (68) | 46 (74) |
| Woman | 93 (26) | 6 (10) |
| Nonbinary | 11 (3) | 7 (11) |
| Transgender woman | 1 (0.3) | 1 (2) |
| Transgender man | 3 (1) | 0 (0) |
| Genderqueer | 5 (1) | 1 (2) |
| Other | 1 (0.3) | 1 (2) |
| Sexual orientation | ||
| Lesbian or gay | 196 (56) | 43 (69) |
| Straight | 51 (14) | 7 (11) |
| Bisexual | 86 (24) | 5 (8) |
| Another sexual orientation | 15 (4) | 6 (10) |
| Prefer not to answer | 4 (1) | 0 (0) |
| Race/Hispanic ethnicity† | ||
| American Indian or Alaska Native, non-Hispanic | 2 (1) | 1 (2) |
| Asian, non-Hispanic | 28 (8) | 4 (6) |
| Black or African American, non-Hispanic | 72 (20) | 11 (18) |
| Hispanic or Latino | 42 (12) | 12 (19) |
| Native Hawaiian or Other Pacific Islander, non-Hispanic | 0 (0) | 0 (0) |
| Multiple races, non-Hispanic | 9 (3) | 3 (5) |
| White, non-Hispanic | 188 (53) | 28 (45) |
| Missing or declined to answer | 11 (3) | 3 (5) |
| Self-reported HTV positive status | 37 (11) | 5 (8) |
No participants reported transmasculime or transferminine gender identities.
Persons with Hispanic or Latino (Hispanic) ethnicity were categorized as Hispanic and might be of any race; persons with non-Hispanic ethnicity were categorized into single race groups or as multiracial (more than 1 race category selected). Persons with missing data for either race or ethnicity were categorized as missing race and ethnicity.
In-depth interview participants ranged in age from 19 to 69 years (median, 33 years; Table 1). Most participants identified as men (74%), were lesbian or gay (69%), and identified as non-Hispanic White (45%) or Hispanic or Latino (19%). About half (53%) of the IDI participants received their first vaccine dose at the time of the IDI, and the remainder (47%) received their second dose at the time of the IDI.
Key Findings
Three key themes emerged from survey and IDI data. First, although most participants had a general understanding about mpox, gaps remained in comprehensive understanding about mpox symptoms, modes of transmission, vaccine protection, and personal risk. Second, participants visiting mpox vaccine clinics had high trust in public health agencies. Third, participants wanted more equitable and less stigmatizing access to mpox vaccine services.
Gaps Remain in Understanding Mpox Symptoms, Modes of Transmission, Timing of Protection From Vaccination, and Personal Risk
The top 3 recognized mpox signs or symptoms among survey participants at first dose of vaccination were rash, fever, and swollen lymph nodes, reported by 57% to 70% of participants (Table 2). At their second dose visit, a greater proportion recognized any mpox sign or symptom, with 72% to 81% recognizing rash, fever, and swollen lymph nodes as associated with mpox infection. The 3 least recognized symptoms were cough (14%–25%), mild pain (23%–33%), and sore throat (23%–30%).
TABLE 2.
Summary of Survey Results on Mpox Knowledge, Attitudes, and Practices Among Adults Presenting for JYNNEOS Vaccination (N = 352)—District of Columbia. Auqust–October 2022
| First-Dose Visit, n (%) | Second-Dose Visit, n (%) | |
|---|---|---|
| Recognized signs and symptoms of mpox* | ||
| Rash | 246 (70) | 285 (81) |
| Fever | 226 (64) | 268 (76) |
| Swollen lymph nodes | 201 (57) | 255 (72) |
| Fatigue | 160 (45) | 209 (59) |
| Chills | 154 (44) | 209 (59) |
| Scarring | 138 (39) | 165 (47) |
| Muscle aches | 128 (36) | 177 (50) |
| Headache | 120 (34) | 164 (47) |
| Severe pain | 119 (34) | 150 (43) |
| Sore throat | 82 (23) | 107 (30) |
| Mild pain | 80 (23) | 117 (33) |
| Cough | 50 (14) | 89 (25) |
| I don’t know | 44 (12) | 22 (6) |
| Only certain groups of people can get mpox | ||
| True | 4 (1) | 5 (1) |
| False | 344 (98) | 345 (98) |
| Missing | 4 (1) | 2 (1) |
| Protection against mpox occurs immediately after the first or second dose of vaccine | ||
| True | 19 (5) | 40 (11) |
| False | 326 (93) | 300 (85) |
| Missing | 7 (2) | 12 (3) |
| Vaccines for mpox should be available to anyone who wants one | ||
| Agree or strongly agree | 293 (83) | 306 (87) |
| Neutral | 10 (3) | 8 (2) |
| Disagree or strongly disagree | 44 (12) | 32 (9) |
| Missing | 5 (1) | 6 (2) |
| Trusted sources for accurate mpox information* | ||
| Doctor/health care provider | 330 (94) | 327 (93) |
| CDC | 290 (82) | 311 (88) |
| State/local public health | 256 (73) | 255 (72) |
| WHO | 235 (67) | 244 (69) |
| Health information websites | 147 (42) | 131 (37) |
| News media | 87 (25) | 95 (27) |
| Friends/family | 41 (12) | 38 (11) |
| Social media | 17 (5) | 22 (6) |
| Dating apps | 16 (5) | 19 (5) |
| Religious organizations | Not asked | 5 (1) |
| Prefer not to answer | 3 (1) | 2 (1) |
| I don’t trust any of these | 1 (0.3) | 1 (0.3) |
| Other | Not asked | 4 (1)† |
Participants could select more than one answer.
Other answers included National Institutes of Health, academic/institutional, and DC government. WHO indicates World Health Organization.
In-depth interview participants were well informed, and many did their own research on mpox. However, knowledge gaps were identified. Participants were not fully aware of modes of transmission and were confused about the role of droplets, body fluids, and fomites in spreading disease. In addition, few participants recalled seeing clear messages on mpox prevention strategies.
“I’m not sure I fully understand how it’s transmitted.”
(Joint interview with 30- and 32-year-old White, non-Hispanic/Latino men)
In general, IDI participants wanted to see more messaging about mpox epidemiology, disease trends, geographical spread, and vaccine coverage. Some compared the information they would like to see to information they were used to seeing for COVID-19.
Most survey and IDI participants understood that the mpox vaccine is not immediately protective. Ninety-five percent (317 of 335) of survey participants believed they would not be immediately protected after their first vaccine dose (Fig. 1). Among these participants, a majority (91% [289 of 317]) continued to understand that they would not be immediately protected after the second dose. However, 9% (28 of 317) believed they would be immediately protected after the second dose.
Figure 1.
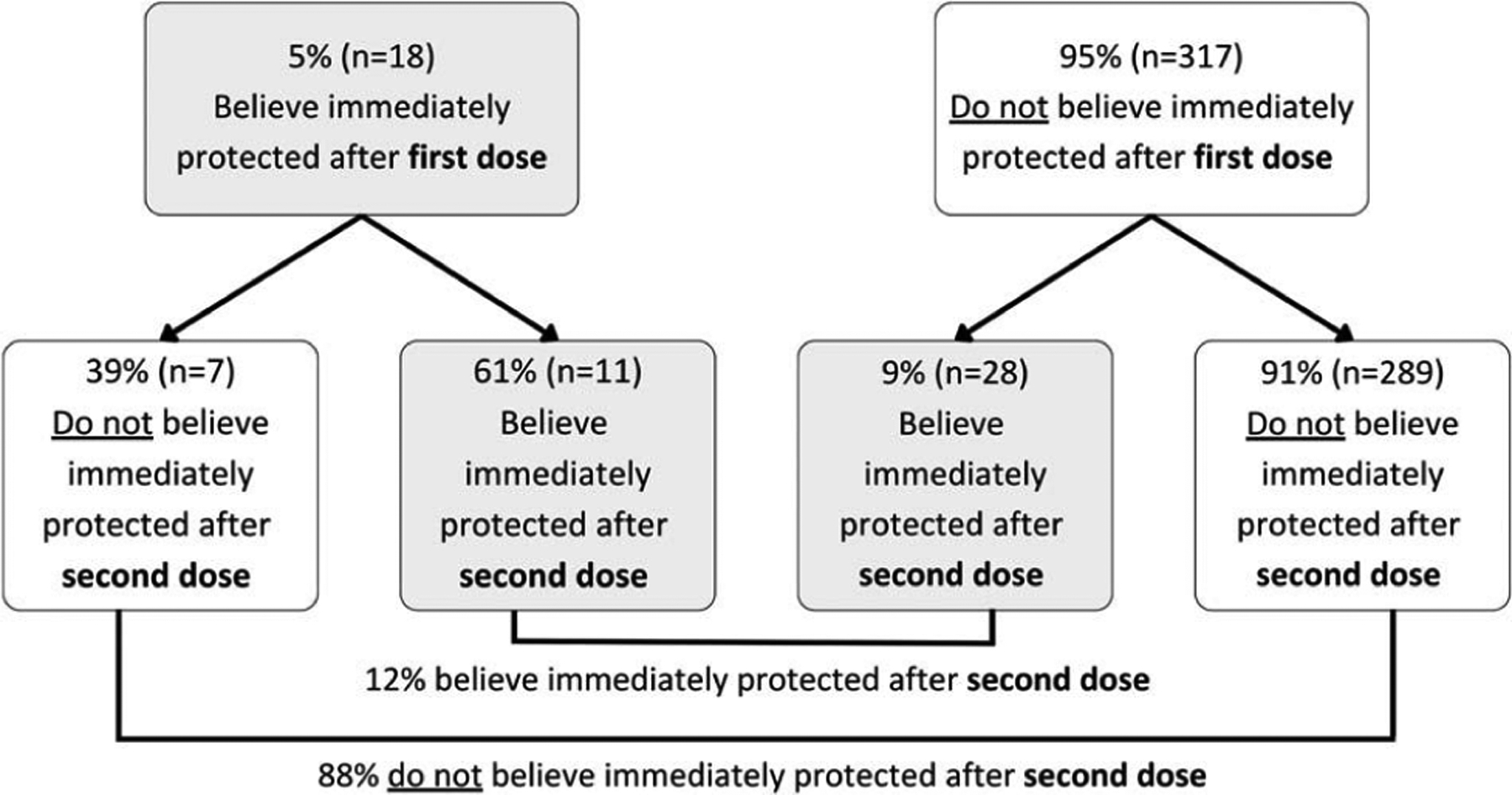
Change in beliefs about timing of protection from mpox vaccine among adults presenting for first- and second-dose JYNNEOS vaccination—District of Columbia, August–October 2022 (N = 335). Note: Figure excludes 17 participants with a missing response in their first- or second-dose visit surveys.
All IDI participants understood that the vaccine is not fully protective and thought they would be most protected anywhere from 1 to 4 weeks after their second dose.
“Vaccines aren’t invincibility shields.”
(32-year-old White, non-Hispanic/Latino man)
In-depth interview participants had varied levels of personal concern about mpox and determined their personal risk based on their behaviors, knowledge of the virus and transmission, and feelings about vaccine protectiveness. Many participants were more concerned about the risk of infection from nonsexual contact and contact with surfaces, particularly because these were often situations more outside of their control.
“At gay clubs it is common for people to rub up against you and you don’t have control of who might touch you”
(37-year-old, Multiracial, non-Hispanic/Latino man)
Overall, participants desired clearer, succinct information on personal risk and steps they could take for mpox protection.
Vaccine Clinics Reached Populations That Trust Public Health Agencies
Doctors and other health care providers were most frequently reported as trusted sources of mpox information as indicated by 93% (327 of 352) of survey participants at their second visit (Table 2). There were no significant differences in trust in health care providers by age, race/ethnicity, or gender/gender of sex partner(s) (Table 3).
TABLE 3.
Reported Trust in Health Care Providers, CDC, and Local/State Health Officials for Accurate Mpox Information Among Adults Presenting for Second Dose of JYNNEOS Vaccination by Age, Race/Ethnicity, and Gender Identity (N = 352)—District of Columbia, August to October 2022
| Trust in Health Care Providers | Trust in CDC | Trust in Local/State Health Officials | ||||
|---|---|---|---|---|---|---|
| Characteristic (No.) | n (%) | aPR (95% CI) | n (%) | aPR (95% CI) | n (%) | aPR (95% CI) |
| Age group, y | ||||||
| 18–24 (60) | 55 (92) | Reference | 53 (88) | Reference | 47 (78) | Reference |
| 25–34 (118) | 111 (94) | 1.02 (0.94–1.12) | 102 (86) | 0.98 (0.87–1.10) | 84 (71) | 0.93 (0.79–1.11) |
| 35–44 (86) | 76 (88) | 0.97 (0.87–1.08) | 75 (87) | 1.02 (0.90–1.16) | 60 (70) | 0.97 (0.81–1.16) |
| 45–54 (40) | 37 (92) | 0.99 (0.88–1.12) | 36 (90) | 1.00 (0.87–1.16) | 28 (70) | 0.89 (0.69–1.14) |
| >55 (48) | 48 (100) | 1.07 (0.99–1.16) | 45 (94) | 1.06 (0.92–1.23) | 36 (75) | 1.04 (0.84–1.29) |
| Race/ethnicity* | ||||||
| American Indian/Alaska Native, non-Hispanic (2) | 178 (95) | Reference | 171 (91) | Reference | 153 (81) | Reference |
| Asian, non-Hispanic (28) | 65 (90) | 0.97 (0.90–1.05) | 58 (81) | 0.89 (0.80–1.01) | 35 (49) | 0.59 (0.46–0.76)† |
| Black, non-Hispanic (72) | 24 (86) | 0.90 (0.78–1.04) | 26 (93) | 1.04 (0.93–1.17) | 21 (75) | 0.93 (0.75–1.17) |
| Hispanic/Latino (42) | 2 (100) | 1.08 (0.97–1.19) | 1 (50) | 0.58 (0.13–2.58) | 1 (50) | 0.58 (0.16–2.15) |
| Multiple races, non-Hispanic (9) | 8 (89) | 0.96 (0.77–1.20) | 8 (89) | 1.01 (0.82–1.25) | 8 (89) | 1.05 (0.80–1.39) |
| White, non-Hispanic (188) | 39 (93) | 0.99 (0.91–1.08) | 37 (88) | 0.97 (0.86–1.10) | 29 (69) | 0.86 (0.70–1.07) |
| Gender/gender of sex partner(s)‡ | ||||||
| MSM (220) | 208 (95) | Reference | 198 (90) | Reference | 156 (71) | Reference |
| MSW (16) | 14 (88) | 0.92 (0.75–1.14) | 12 (75) | 0.82 (0.59–1.15) | 12 (75) | 1.07 (0.79–1.47) |
| Woman (93) | 86 (92) | 0.98 (0.91–1.05) | 84 (90) | 1.01 (0.93–1.10) | 71 (76) | 1.09 (0.94–1.26) |
| Transgender Persons (4) | 3 (75) | 0.80 (0.46–1.39) | 3 (75) | 0.85 (0.47–1.52) | 3 (75) | 1.10 (0.60–2.03) |
| Another identity (17) | 15 (88) | 0.95 (0.79–1.12) | 13 (76) | 0.85 (0.64–1.11) | 13 (76) | 1.01 (0.76–1.33) |
Persons with Hispanic or Latino (Hispanic) ethnicity were categorized as Hispanic and might be of any race; persons with non-Hispanic ethnicity were categorized into single-race groups or as multiracial (more than 1 race category selected).
p < 0.001.
MSM includes gay, bisexual, and other men who reported having sex with men, as well as men who reported sex with both men and women. MSW includes men who reported having sex with women only.
Survey and IDI participants reported strong levels of trust in government public health agencies. The CDC and state or local public health officials were selected as trusted sources of information by 88% and 72% of survey participants, respectively, at their second visit Although there were no significant differences in reported trust of CDC, the proportion of non-Hispanic Black participants reporting trust in local or state public health officials (aPR, 0.59; 95% confidence interval [CI], 0.46–0.76) was significantly lower than among non-Hispanic White participants, controlling for age and gender/gender of sex partners (Table 3). Although social media was only reported by 6% of survey participants, IDI participants reported trusting information posted on social media by public health agencies, such as the CDC.
Some IDI participants reported that their trust in CDC had decreased because of perceived mishandling of messaging during the COVID-19 pandemic. However, participants still generally trusted information from CDC because they still felt that CDC was the most trustworthy source available, even if they now consume public health messages with renewed skepticism.
“[Government sources] better be trustworthy or who do we trust?”
(22-year-old, Asian, non-Hispanic/Latino nonbinary participant)
“Even though a lot of people doesn’t trust the government, the government has the resources to do data, research and get the message out”
(37-year-old, White, non-Hispanic/Latino man)
In-depth interview participants described an underlying trust in science itself, and some participants did less research on mpox and the mpox vaccine before coming to the clinic, because their trust in science and vaccines was generally high.
“I fully trust science and medicine, if a doctor says this works, I believe them.”
(54-year-old, Black, Hispanic/Latino man)
In-depth interview participants described doing their own research on mpox and referring to multiple sources to verify information. Although most participants trusted government agencies for mpox information, some expressed concern that messaging and recommendations were not completely trustworthy and may be swayed by competing interests. One participant mentioned seeing a public health message that described riding public transportation and trying on clothes at a store as low-risk activities for mpox but dancing in a crowd as a high-risk activity. According to the participant, these messages seemed driven by a desire to protect capitalism and gave the impression that public health agencies “don’t want [us] to participate in things that are associated with joy, sexual pleasure, and enjoyment.” (30-year-old, White, non-Hispanic/Latino nonbinary participant).
Participants Wanted Equitable Access to Mpox Vaccine Services and Are Concerned About the Potential Stigmatizing Impact of Mpox Messaging
Most survey participants believed that mpox vaccines should be available to anyone who wants one, with 87% agreeing or strongly agreeing with this statement at their second visit (Table 2). In-depth interview participants expressed concerns that vaccine access was inequitable, particularly for communities of color and those without access to technology and transportation.
“In this part of this epidemic we still need more outreach…the people who need it the most are left out.”
(47-year-old, Black, Hispanic/Latino man)
In-depth interview participants expressed frustration and confusion about eligibility requirements. Many felt that early vaccine criteria were not inclusive of all populations potentially at risk, such as people in the queer community who do not identify as MSM but who do have close contact with this population. One participant described frustrations with early criteria being limited to those assigned male sex at birth, because their partner was assigned female sex at birth but shared the same risks as the participant; they described this disparity in access as due to a “way of thinking that was maybe a bit old fashioned” by public health agencies. A few participants disclosed inflating the number of recent sexual partners when registering for a vaccine appointment to ensure they would be deemed eligible.
“It affects everyone and should be targeted to everyone”
(41-year-old, Multiracial, non-Hispanic/Latino woman)
“I liked getting early access, it feels rare to be prioritized that way, but I don’t want to be selfish and exclude people for the same reason…Intimacy is intimacy, it doesn’t matter whether you’re gay or not”
(29-year-old, White, Hispanic/Latino woman)
In-depth interview participants were grateful that lesbian, gay, bisexual, transgender, queer, and other sexual and gender minority communities were given priority vaccine access. However, many saw the eligibility criteria as stigmatizing and upholding stereotypes or misconceptions about this community and perceived promiscuity. Participants felt that this messaging also reinforced misinformation associating mpox as a gay disease and could prevent some people from seeking out the vaccine and taking other preventative measures.
DISCUSSION
Although participants demonstrated a general understanding about mpox, we found gaps in knowledge about sign/symptoms, modes of transmission, and timing of vaccine protection, which influence perceptions of personal risk Perceived risk is a determinant of engagement in preventive health behaviors16,17; higher perceived risk of mpox infection was associated with uptake of mpox vaccination in studies among transgender persons and MSM.18,19 As of January 31, 2023, an estimated 23% of persons at risk for mpox infection were fully vaccinated nationally, emphasizing continued gaps in vaccine uptake.20 We recommend clear, concise communication about mpox to address knowledge gaps and better inform comprehensive person-level risk assessments and decision making regarding vaccination. Because understanding of mpox risk has advanced since the time of this study, this could now include lists of certain activities (such as dancing at a club, trying on clothes in a store, sharing kitchen items, or intimate contact), the level of potential mpox risk, and specific actions individuals can take to protect themselves in these situations.
Many participants drew parallels between receiving information about COVID-19 and mpox public health messaging. For some, frequent and detailed updates from CDC and other public health agencies about COVID-19 established a data standard, and the level of information provided about mpox, by comparison, was insufficient. Many also felt their perceptions of and trust in public health agencies were shaped by how agencies messaged about and responded to the COVID-19 pandemic. Previous research on trusted information sources for COVID-19 aligns with our findings that physicians and health care workers were most trusted; trust in public health agencies was lower overall and more polarized by political beliefs.21,22 A 2022 study found that those who trusted federal public health agencies most commonly did so because of their perceived scientific expertise,22 similar to our IDI findings. The COVID-19 pandemic has had profound and lasting impacts on public perceptions of public health information and messaging, and future communication strategies need to consider this context in formulating campaigns that enhance trust and communicate clear and effective information.
A predominate concern raised by participants, all of whom were able to obtain mpox vaccine for themselves, was the need for more equitable access to mpox vaccines for others. In the 2022 outbreak, mpox infections disproportionately occurred among sexual and gender minorities and Black and Hispanic/Latino persons.2,7,23 Unfortunately, similar racial and ethnic disparities were observed among those who received the vaccine.24,25 Structural barriers, inequitable vaccine allocation and distribution, vaccine confidence, and medical mistrust are all known barriers to equitable vaccine access and uptake from previous public health responses26–28 Lessons learned from prior responses can inform strategies to boost vaccine equity, and participants in our investigation frequently cited vaccine efforts for COVID-19 as examples of best practices. Some potential strategies include the following: stigma-reducing language and messaging, partnering with trusted community organizations and leaders, low barrier community-based vaccine efforts to meet people where they are, and the creation and dissemination of culturally and linguistically appropriate education materials.26–29 Our findings were limited to those who were English speaking, willing to seek vaccination, willing to participate in a study conducted by CDC and DC Health staff, and already had access to vaccine clinics in DC, which, as of January 2023, was the only jurisdiction in the United States that achieved >50% 2-dose vaccine coverage among persons at increased risk for mpox.20 To better understand barriers for other populations in accessing vaccines, it is important to speak with persons unable or unwilling to seek out vaccination in diverse geographic locations and languages to inform development of tailored, barrier-reducing strategies to increase vaccine uptake and reduce disparities.
This mixed-methods analysis allowed for a person-first approach to public health research. Although quantitative methods play a fundamental role in providing epidemiological descriptions during an outbreak, qualitative data allow for additional insight into social context, including social implications of disease, social factors that contribute to differential access and lived experience during an outbreak, and the psychological, cultural, and social factors that inform health behaviors and acceptance of public health response strategies.30 Infectious disease outbreaks demand real-time data collection, analysis, and sharing of actionable research findings to inform practice. Rapid qualitative methods address challenges with timeliness of traditional qualitative methods and have been used in infectious epidemics and natural disaster response efforts since the early 2000s.13,14 Our use of rapid thematic analytic techniques to facilitate collection and analysis of qualitative data in parallel allowed for early and continuous sharing of both qualitative and quantitative findings to inform real-time recommendations for the mpox response.
Knowledge gaps and concerns about equity remain among adults presenting for mpox vaccination, highlighting the need for further nonstigmatizing, inclusive, clear, and consistent messaging from trusted sources. Perceived gaps in mpox vaccine access suggest that there may be missed opportunities to reach prioritized populations. Mpox outreach efforts should continue innovative approaches to information sharing to address community needs. Our findings can be used to improve outreach and vaccination strategies in current and future public health responses.
Acknowledgments:
DC PEP++ Project Team: Ausaf Ahmad, Eric Anthony, Marie Argyriou, Jaia Armstrong, Leslie Ayuk-Takor, Marie Brake, Paige Bunkley, Perri Callaway, Ayan K. Chakrabarti, Azam Elnour, Jennifer Folster, Catalina Forero, Bruce (Bryce) Furness, L. Claire Godino, Lauren Greenberg, Sarah Anne J. Guagliardo, Dionnie Israel, Samadhan Jadhao, Tekisha Jordan, Emiko Kamitani, Barbara Keino, Erik Kopping, Joo Lee, Leah Lopez, David Lowe, Ie Meh, John Metz, Shanna Miko, Victoria Mrotz, Adi Noiman, Tiffany Notigan, James Partin, Melissa Brykailo Pearce, Kevin Pettus, Saumya Rajamohan, Sergio Rodriguez, Julia Rowse, Susanna Sabin, Loredana Santo, Caroline A. Schrodt, Brandon Shapiro, Pankaj Sharma, Victoria Shelus, Dallas Shi, Olutomi Sodeke, Nora Springstubb, Jennifer Steppe, Christan Halverson Stager, Roxana Rodriguez Stewart, Xiaoling Tang, Moukaram Tertuliano, Logan Timm, Casey Vantucci, Saria Widatalla, Yong Yang.
The authors thank Susan Clark, Karl Fehrenbach, Otto Ike, Christiana Ogbuokiri, Carolyn M. Tunstall, and Tanya Williams for their assistance and support of project staff.
Footnotes
Conflict of Interest and Sources of Funding: None declared.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2022–2023. U.S. Map & Case Count. Available at: https://www.cdc.gov/poxvirus/mpox/response/2022/us-map.html. Accessed March 2, 2023.
- 2.Kava CM, RohrafFDM Wallace B, et al. Epidemiologic features of the monkeypox outbreak and the public health response—United States, May 17–October 6, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1449–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Preventioa Mpox Science Brief: Detection and Transmission of Mpox (Formerly Monkeypox) Virus During the 2022 Clade Hb Outbreak. Available at: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/poxvirus/mpox/about/science-behind-transmission.html. Accessed May 12, 2023.
- 4.U.S. Food and Drug Administration. JYNNEOS [package insert, revised April 2022]. US Department of Health and Human Services, Food and Drug Administration. Available at: https://www.fda.gov/media/131078/download. Accessed Match 6, 2023. [Google Scholar]
- 5.U.S. Department of Health and Human Services. HHS Announces Enhanced Strategy to Vaccinate and Protect At-Risk Individuals From the Current Monkeypox Outbreak. Available at: https://www.hhs.gov/about/news/2022/06/28/hhs-announces-enhanced-strategy-vaccinate-protect-at-risk-individuals-from-current-monkeypox-outbreak.html. Accessed March 6, 2023.
- 6.U.S. Food and Drug Administration. Fact sheet for healthcare providers administering vaccine: emergency use authorization of JYNNEOS (smallpox and monkeypox vaccine, live, non-replicating) for prevention of monkeypox disease in individuals determined to be at high risk for monkeypox infection. Available at: https://www.fda.gov/media/160774/download. Accessed March 6, 2023.
- 7.Kota KK, Hong J, Zelaya C, et al. Racial and ethnic disparities in mpox cases and vaccination among adult males—United States, May–December 2022. MMWR Morb Mortal Wkly Rep 2023; 72:398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogale YP, Baird N, Townsend MB, et al. Evidence of mpox virus infection among persons without characteristic lesions or rash presenting for first dose of JYNNEOS vaccine—District of Columbia, August 2022. Clin Infect Dis 2023; 77:298–302. [DOI] [PubMed] [Google Scholar]
- 9.DC Health. DC Health Expands Eligibility Criteria for Monkeypox Vaccinations. Available at: https://dchealth.dc.gov/release/dc-health-expands-eligibility-criteria-monkeypox-vaccinations. Accessed May 12, 2023.
- 10.Regnault A, Willgoss T, Barbie S, et al. Towards the use of mixed methods inquiry as best practice in health outcomes research. J Patient Rep Outcomes 2017; 2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159:702–706. [DOI] [PubMed] [Google Scholar]
- 13.Johnson GA, Vindrola-Padros C. Rapid qualitative research methods during complex health emergencies: A systematic review of the literature. Soc Sci Med 2017; 189:63–75. [DOI] [PubMed] [Google Scholar]
- 14.Vindrola-Padros C, Chisnall G, Cooper S, et al. Carrying out rapid qualitative research during a pandemic: Emeiging lessons from COVID-19. Qual Health Res 2020; 30:2192–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.45 C.F.R. part 46,21 C.F.R. part 56; 42 U.S.C. Sect 241(d); 5 U.S.C. Sect 552a; 44 U.S.C. Sect. 3501 et seq.
- 16.Ferrer R, Klein WM. Risk perceptions and health behavior. Curr Opin Psychol 2015; 5:85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brewer NT, Chapman GB, Gibbons FX, et al. Meta-analysis of the relationship between risk perception and health behavior: The example of vaccination. Health Psychol 2007; 26:136–145. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert M, Ablona A, Chang HJ, et al. Uptake of Mpox vaccination among transgender people and gay, bisexual and other men who have sex with men among sexually-transmitted infection clinic clients in Vancouver, British Columbia. Vaccine 2023; 41:2485–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dukers-Muijrers N, Evers Y, Widdershoven Y et al. Mpox vaccination willingness, determinants, and communication needs in gay, bisexual, and other men who have sex with men, in the context of limited vaccine availability in the Netherlands (Dutch Mpox-survey). Front Public Health 2023; 10:1058807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owens LE, Currie DW, Kramarow EA, et al. JYNNEOS vaccination coverage among persons at risk for Mpox—United States, May 22, 2022-January 31, 2023. MMWR Morb Mortal Wkly Rep 2023; 72:342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Findling MG, Blendon RJ, Benson JM. Polarized public opinion about public health during the COVID-19 pandemic: Political divides and future implications. JAMA Health Forum 2022; 3:e220016. [DOI] [PubMed] [Google Scholar]
- 22.SteelFisher GK, Findling MG, Caporello HL, et al. Trust in US federal, state, and local public Health agencies during COVID-19: Responses and policy implications. Health Aff (Millwood) 2023; 42:328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Mpox Cases by Age and Gender and Race and Ethnicity. Available at: https://www.cdc.gov/poxvirus/mpox/response/2022/demographics.html. Accessed March 22, 2023.
- 24.Kriss JL, Boersma PM Martin E, et al. Receipt of first and second doses of JYNNEOS vaccine for prevention of monkeypox—United States, May 22–October 10, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1374–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Mpox Vaccine Administration in the U.S Available at: https://www.cdc.gov/poxvirus/mpox/response/2022/vaccines_data.html. Accessed March 22, 2023.
- 26.Baack BN, Abad N, Yankey D, et al. COVID-19 vaccination coverage and intent among adults aged 18–39 years—United Stales, March–May 2021. MMWR Morb Mortal Wkly Rep 2021; 70:928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khubchandani J, Macias Y. COVID-19 vaccination hesitancy in Hispanics and African-Americans: A review and recommendations for practice. Brain Behav Immun Health 2021; 15:100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kricorian K, Turner K. COVID-19 vaccine acceptance and beliefs among Black and Hispanic Americans. PloS One 2021; 16:e0256122. Available at: https://joumals.plos.org/plosone/. Accessed March 22, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Mpox Equity and Anti-Stigma Toolkit. Available at: https://archive.cdc.gOv/#/details7urMittps://www.cdc.gov/poxvirus/rnpox/resources/toolkite/equity.html. Accessed March 22, 2023.
- 30.Teti M, Schatz E, Liebenbeig L. Methods in the time of COVID-19: The vital role of qualitative inquiries. Int J Qual Methods 2020; 19:1–5. [Google Scholar]


