Abstract
Introduction
The utility of immunotherapy in elderly melanoma patients is debated. We aimed in this study to evaluate the efficacy and tolerability of immunotherapy among elderly patients.
Method
This is a retrospective single-institution cohort study. Patients aged 75 years and above who had been treated with nivolumab, pembrolizumab or ipilimumab for advanced or metastatic melanoma, were included. Patients and disease characteristics were collected using electronic medical records. Objective response was determined according to the immune-related response criteria. Drug-related toxicities (DRT) were graded according to the CTCAE v4.03.
Results
99 patients were included with a mean age of 80 years (SD = 4). One patient received nivolumab and ipilimumab combination, but died because of drug-related diverticulitis. Median PFS on pembrolizumab, nivolumab or ipilimumab were equal to 11.9 (95% CI 5.4–18.4), 1.4 (95% CI 0.01–2.8), and 2.8 months (95% CI 2.6-3), respectively, while objective response rates were equal to 51.6, 12.5, and 17.3%, respectively. Median OS was not reached in patients who received only pembrolizumab, 8.7 months in the ipilimumab only group, and 23 months in patients receiving several immune therapies sequentially. Pembrolizumab, nivolumab, and ipilimumab grade 3–4 DRT rates were equal to 24.2, 62.5, and 32.7% respectively, while discontinuation rates were equal to 43.5, 62.5, and 28.8%, respectively.
Conclusions
Our study suggests that immunotherapy is effective and well tolerated in the elderly. The PFS on pembrolizumab was greater than expected, a finding that needs to be investigated further.
Keywords: Melanoma, Immunotherapy, Elderly, Anti-PD1, Anti-CTLA4
Introduction
The incidence of melanoma is increasing, especially among the elderly [1]. Immunotherapy is a major breakthrough in modern cancer therapy which has led to the approval of anti-CTLA-4 and anti-PD-(L)1 monoclonal antibodies (mAb) for the treatment of patients with advanced or metastatic melanoma [2]. Although there was no age limit among the criteria for inclusion of clinical trials that led to the approval of these drugs, the proportion of elderly people was small [3]. This is partly due to physicians and patients worries about adverse events in a more vulnerable population. Furthermore, some physicians believe that immunotherapy is less effective in the elderly because of immune senescence. This is supported by in vitro and animal studies which showed that immune function is weakened by age [4, 5]. A meta-analysis was published by Nishijima et al. and concluded that immunotherapy could be of less or no benefit in patients aged more than 75 years [6]. However, this meta-analysis included different types of cancer and different age cut-offs. A few studies suggested the opposite, but included a limited number of patients [7–9]. Furthermore, tolerability of anti-PD-(L)1 and anti-CTLA-4 mAb has not been specifically evaluated in the elderly [10, 11], except in the study reported by Betof et al. [9] which found no significant difference in immune-related toxicity across age groups.
The aim of the present study was to evaluate the efficacy and tolerability of immunotherapy among elderly patients diagnosed with advanced or metastatic melanoma.
Method
This retrospective cohort study included all patients aged 75 years and above and who were treated with nivolumab, pembrolizumab or ipilimumab for advanced or metastatic melanoma between January 2013 and December 2016 in the dermatology department of the Gustave Roussy Institut (Villejuif–France). Patients who lacked essential information about the efficacy and tolerability of treatment were excluded. Data were collected using electronic medical records. The following characteristics were registered prior to immunotherapy: age, sex, known or unknown primary, histologic sub-type of melanoma [12], presence or absence of ulceration, BRAF and NRAS mutational status, cancer stage according to the American Joint Committee on Cancer (AJCC) seventh version [13], performance status (PS) according to the Eastern Cooperative Oncology Group (ECOG) score [14], previous cancer therapy, presence or absence of lymph node invasion, and the presence or absence of visceral or central nervous system (CNS) metastasis. Progression-free survival (PFS) was defined as the time from initiation of immunotherapy to objective clinical or radiological tumor progression. Overall survival (OS) was defined as the time from initiation of immunotherapy till death from any reason. Overall response rate (ORR) was defined as the sum of complete response (CR) and partial response (PR) according to the immune-related response criteria (irRC) [15], while disease control rate (DCR) was defined as the sum of ORR and stable disease (StD). Drug-related toxicities (DRT) were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 [16]. July 2017 was the last date of follow up. Statistical analysis was performed using the Predictive Analytics SoftWare (PASW) version 22. Kaplan–Meier estimates were used to calculate the probability of survival and multivariate Cox regression model to explore the association between survival and patients/disease characteristics. The first-degree error alpha was fixed to 0.05 bilaterally.
Results
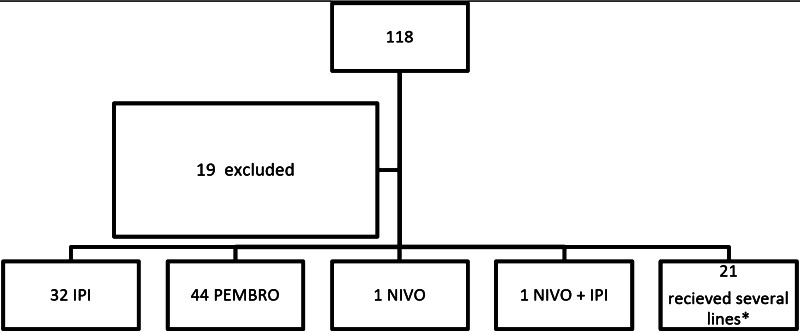
One hundred eighteen patients were included among whom 19 patients had to be excluded (Fig. 1). Among the excluded patients, 7 received only one cycle of treatment in our institution but were lost to follow up, 7 other patients took part in randomized double-blind trials evaluating immunotherapy versus placebo, and 5 patients received immunotherapy combined with other drugs. Of the remaining 99 eligible patients, 53 were males and 46 females. BRAF was mutated in 18.9% of cases, NRAS in 37%, and cKIT in 6.2%. Mean age at first immunotherapy was 80 years [standard deviation (SD) = 4 and a range between 75 and 92 years]. Most patients had metastatic melanoma before first immunotherapy (77.8%). As for the PS, 68.7, 27.3, and 4% had ECOG PS 0, 1, and 2, respectively. Tumor characteristics at diagnosis as well as previous therapies received before immunotherapy are summarized in Table 1.
Fig. 1.
Consort diagram. *Eighteen patients received 2 lines of immunotherapy as follows: 8 received ipilimumab then pembrolizumab, 6 pembrolizumab then ipilimumab, 3 ipilimumab then nivolumab, and 1 pembrolizumab then nivolumab. Three patients received 3 lines of immunotherapy as follows: 2 received ipilimumab then pembrolizumab then nivolumab and 1 pembrolizumab then ipilimumab then nivolumab. ipi ipilimumab, nivo nivolumab, pembro pembrolizumab
Table 1.
Tumor characteristics at diagnosis
| % | |
|---|---|
| Histologic subtype n = 73 | |
| Superficial spreading melanoma of the skin | 39.8 |
| Nodular melanoma | 19.2 |
| Acral lentiginous melanoma | 17.8 |
| Mucosal melanoma | 13.7 |
| Lentigo maligna melanoma | 5.5 |
| Not otherwise specified | 3.8 |
| Uveal melanoma | 1.4 |
| Ulceration n = 49 | |
| No | 40.8 |
| Yes | 59.2 |
| Primary tumor location n = 99 | |
| Known | 91.8 |
| Unknown | 8.1 |
| Stage at diagnosis n = 99 | |
| Stage I–II | 58.6 |
| Stage III | 28.3 |
| Stage IV | 13.1 |
| Type of systemic therapies prior to first immunotherapy n = 99 | |
| Interferon | 3 |
| Chemotherapy | 22.2 |
| BRAF inhibitors | 8.1 |
| BRAF + MEK inhibitors | 5.1 |
| No prior therapy | 61.6 |
| The site of metastatic involvement prior to immunotherapy n = 99 | |
| Lymph nodes | 80.8 |
| Visceral metastasis | 62.6 |
| Central nervous system metastasis | 20.2 |
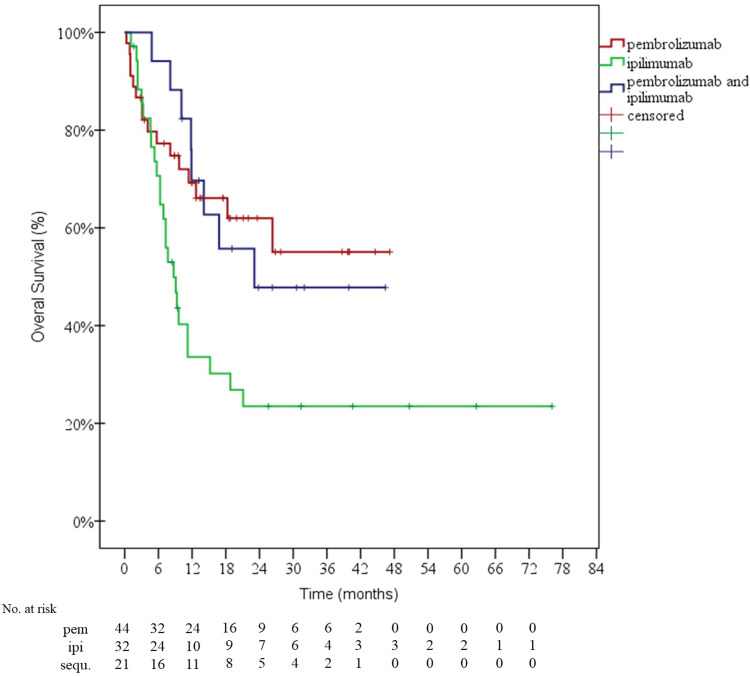
Only one patient received the combination of nivolumab and ipilimumab for one cycle but died because of severe drug-related diverticulitis. Sixty-two patients (62.2%) received pembrolizumab, 8 (8.1%) nivolumab, and 52 (52.5%) ipilimumab. Response rates are summarized in Table 2, while PFS and OS survival curves are illustrated in Figs. 2 and 3, respectively. After a median follow-up of 24 months, 41 patients were still alive (among whom 5 patients were still receiving immunotherapy), 51 were reported to be dead and 7 lost to follow up (4 patients in the pembrolizumab group after a median follow up of 8 months, and 3 patients in the ipilimumab group after a median follow up of 10 months). Median follow up of the whole sample was equal to 18.3 months [95% confidence interval (CI) 8.6–28]. Median OS in the group of 44 patients who received only pembrolizumab as immunotherapy was not reached, while it was equal to 8.7 months for the 32 patients who received only ipilimumab, and 23 months for the 21 patients who received several types of immunotherapy sequentially (Fig. 3).
Table 2.
Best response according to each immunotherapy
| ORR (%) | DCR (%) | |
|---|---|---|
| Pembrolizumab | 51.6 | 67.7 |
| Nivolumab | 12.5 | 37.5 |
| Ipilimumab | 17.3 | 23.1 |
DCR disease control rate, ORR overall response rate
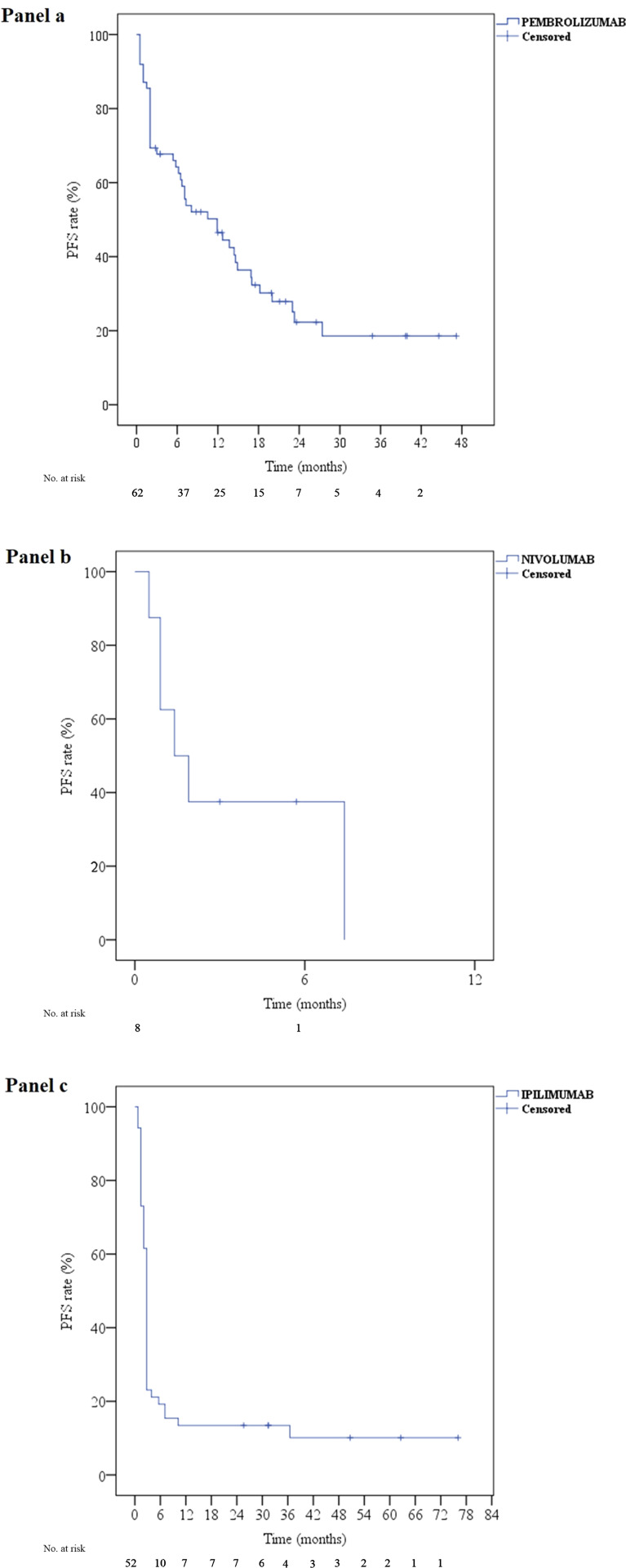
Fig. 2.
progression free survival curves for pembrolizumab (a), nivolumab (b) and ipilimumab (c). PFS progression free survival
Fig. 3.
overall survival curves of the three groups of patients: group A who received only pembrolizumab, group B who received only ipilimumab and group C who received several lines of immunotherapy sequentially. ipi ipilimumab, pem pembrolizumab, sequ sequential therapy
Among the 62 patients who received pembrolizumab, the median number of cycles was equal to 9 (SD 8, range 1–31). After a median follow up of 26.5 months, median PFS was equal to 11.9 months (95% CI 5.4–18.4) (Fig. 2a), and 1 and 2-year PFS rates were equal to 46 and 23%, respectively. A Cox regression model was used to explore the association between PFS and the following factors: age, sex, BRAF and NRAS status, ulceration, ECOG PS, and cancer stage (metastatic or locally advanced). Age was found to be independently associated with a better survival (regression coefficient B = 0.2, p value 0.045).
Among the 8 patients who received nivolumab, the median number of cycles was equal to 5 (SD 7, range 2–24). After a median follow up of 5.7 months, median PFS was equal to 1.4 months (95% CI 0.01–2.8) (Fig. 2b). Among the 4 patients who received nivolumab as a re-challenge after pembrolizumab (Fig. 1), 2 patients had StD (PFS 14.1 and 34.8 months) but discontinued treatment because of DRT, while 2 other patients progressed at 7.1 and 13.7 months.
Among the 52 patients who received ipilimumab, the full course (4 cycles) was administered to 30 patients (57.7%) among whom one patient received 2 additional cycles as a re-challenge. As for the others, 3 (5.8%), 12 (23%), and 7 patients (13.5%) received 1, 2 and 3 cycles, respectively. After a median follow up of 50 months, median PFS was equal to 2.8 months (95% CI 2.6–3), while 1 and 2-year PFS rates were both equal to 13% (Fig. 2c). No factor was independently associated with survival on the Cox regression model (data not shown).
Tolerability
In total, 80.6, 100, and 86.5% of patients had at least grade 1 DRT during the treatment with pembrolizumab, nivolumab, and ipilimumab respectively. Because of drug toxicity, 43.5, 62.5, and 28.8% discontinued pembrolizumab, nivolumab, and ipilimumab, respectively. Serious DRT occurred in 15 patients (24.2%) receiving pembrolizumab, in 5 patients (62.5%) receiving nivolumab, and in 17 patients (32.7%) receiving ipilimumab. During treatment with pembrolizumab, 4 patients suffered from acute kidney injury (interstitial nephritis), 3 developed hypersensitivity pneumonitis, 3 severe colitis, 3 severe fatigue limiting self-care activities of daily living (ADL), 1 liver failure, 1 severe deep vein thrombosis (DVT), and 1 peripheral auditory neuropathy. During nivolumab treatment, 1 patient developed acute hepatitis, 1 fatigue limiting ADL, 1 severe colitis, 1 peripheral motor neuropathy, and 1 pneumonitis. During ipilimumab treatment, 9 patients developed colitis, 1 confusion, 3 fatigue limiting ADL, 2 severe maculopapular rash, 1 acute kidney injury, and 1 acute hepatitis. All immune-related toxicities were successfully treated with steroids, except 2 patients who suffered severe colitis refractory to steroids and who had required infliximab therapy (one patient receiving pembrolizumab and another patient receiving ipilimumab).
Grade 1–2 DRT were mainly cutaneous rash and diarrhea. Vitiligo occurred in 11 patients (17.7%) during pembrolizumab treatment. Endocrine disorders occurred in 5 patients among whom 4 had hypothyroidism and 1 adrenal insufficiency treated with hormone replacement therapies. Immune-related grade 2 adverse events occurred in 3 patients: 1 bullous pemphigoid, 1 anterior uveitis, and 1 Sjogren’s syndrome, and were all successfully treated with steroids.
Discussion
This study showed that nearly half of elderly patients treated with pembrolizumab did not experience melanoma progression after 1 year of follow up, and one-fourth after 2 years. Median PFS was equal to nearly 1 year, and the survival curve reached a plateau of 18% after 28 months of follow up. If we compare these results with those reported in randomized controlled trials (RCT) we may assume that pembrolizumab could perform better in elderly patients than in younger ones. For instance, median PFS was equal to 4.1 months in naïve melanoma patients [17], and 2.9 months in ipilimumab refractory patients [18]. The higher the age of the patient the better the efficacy of pembrolizumab was, as shown by the Cox regression model. As for nivolumab, the results reported in this study may not adequately assess its efficacy neither its tolerability. In fact, 7 out of 8 patients had received nivolumab as a re-challenge after pembrolizumab (Fig. 1). In a recent systematic review, Daste et al. showed that the hazard ratio for death among the 67 patients included in the checkmate 006 and who were aged more than 75 years, was equal to 0.25 (95% CI 0.10–0.61) [2, 19]. Regarding the treatment with ipilimumab, 57.7% of patients received 4 cycles of induction while 11.2% progressed during treatment. The results shown in this study are similar to those reported by clinical trials, with a median PFS of nearly 3 months, an ORR between 10 and 15%, and a 1-year PFS rate between 10 and 15% [20–23]. Chiarion Sileni et al. reported the efficacy and safety of ipilimumab in 188 pretreated patients aged 70 years and above who were enrolled in an expanded access program in Italian centers [24]. The results were similar to those reported in this study, with a DCR of 38%, a median PFS of 4 months, and a 1-year PFS rate of 21%.
The combination of nivolumab and ipilimumab could not be evaluated in our study because only one patient had received the combination, but died because of digestive toxicity. In the clinical trials which evaluated nivolumab and ipilimumab combination in patients with advanced or metastatic melanoma, there was no age limit among the inclusion criteria [15, 25, 26], but the proportion of elderly patients was small. For example, only 11.1% of the patients who received the nivolumab and ipilimumab in the checkmate 067 were aged 75 years and above [27]. The results regarding the efficacy and tolerability of the combination in this age group were not available.
Even though the results of this descriptive real-life study could not be directly compared with registration trials, the fact that the response to anti-CTLA-4 mAb was similar to those reported in clinical trials while the response to pembrolizumab was higher than expected, is intriguing. This could be related to the particular molecular profile of melanoma in this age group. The BRAF gene was less frequently mutated in this study compared to the literature (18 versus 40–60% in patients with cutaneous melanoma regardless of age). In contrast, NRAS mutation rate was higher in this study (37 versus 10–20% in large studies) [28, 29]. These findings are consistent with previous studies which have shown that melanoma in the elderly has a different molecular profile than that in young people [30]. It has been suggested that UV-radiations have an active role in inducing RAS mutations [31]. In addition, advanced age was associated with a higher incidence of TP53 mutations in cutaneous melanoma [30]. Additionally, studies have shown that the mutational load of cancer cells increased with age [32], which might explain why the efficacy of anti-PD(L)-1 is more pronounced in the elderly. Another potential explanation could be related to the cytotoxic T cells. The percentage of highly differentiated CD 28− and CD 27− T cells within the CD8+ T cell pool increases significantly with age [33, 34]. This subgroup is less efficient and contributes to immunosenescence. Henson et al. showed that this subgroup of T cells expresses significantly higher levels of PD1 compared to the undifferentiated more efficient type. Anti-PD-(L)1 mAb were able to enhance the proliferation and activation of differentiated T cells taken from older individuals [34]. More studies highlighted the importance of PD-(L)1 in the age-dependent decline of T cells function, which could be at least partially restored by antibodies targeting PD-(L)1 [35]. Little is known concerning the role of CTLA-4 in the immunosenescence. One study showed that anti-CTLA-4 mAb alone were able to deplete regulatory T cells and induce tumor rejection in young but not in old BL6 mice (melanoma model) [36]. However, this hypothesis is not conclusive, and the difference in efficacy between anti-PD-(1) and anti-CTLA-4 in elderly melanoma patients warrants further investigation.
Except for the only patient who had received the combination of nivolumab and ipilimumab, there was no treatment-related death. The rate of grade 1–2 toxicity related to pembrolizumab was similar to those reported in the literature (between 65 and 85%), with a predominance of skin rash, fatigue, and diarrhea [17, 18, 37]. However, it seemed that vitiligo appears more frequently in the elderly during the treatment with pembrolizumab. The frequency of vitiligo in this study was equal to 17.7% compared to 8.3% (95% CI 4.4–15.2%) in the meta-analysis conducted by Bellum et al. [38].
The frequency of patients who discontinued pembrolizumab due to adverse events was higher than what have been reported in clinical trials (43.5% in our study versus 4–8% in RCTs). In the same way, the frequency of grade 3–4 DRT was also higher in this study (24.2% compared to 10–15% in clinical trials) [17, 18, 39]. It should be noted, however, that the median follow-up and the median number of cycles (or the duration of exposure to treatment) were higher in our study. The types of toxicity were similar to those reported in RCTs apart from immune nephropathy which was described more frequently in our study. Even if we have found higher toxicity rate in patients who received nivolumab, no conclusion could be withdrawn from this study for the same reasons mentioned above. As for ipilimumab, the frequency of DRT as well as treatment discontinuation rate were also higher than what have been reported in clinical trials [20–23], but did not differ from the results published in real-life studies [40–42]. This could be related to patients selection.
Limitation:
This study was mainly limited by its retrospective design. We tried to limit this bias by collecting all the information from electronic medical records. On the other hand, not all patients had geriatric evaluation before treatment initiation, and as it has been already shown in different studies ECOG PS or Karnofsky performance status scale are not good estimators of fitness in the elderly. The use of validated tools like the G8 is highly recommended before starting immunotherapy.
Conclusions
This study suggested that checkpoint inhibitor monotherapy is effective and well tolerated in elderly melanoma patients. The PFS of patients treated with pembrolizumab in this study was greater than expected which could be due to a higher mutational load. However, this finding should be interpreted with caution taking into account the retrospective design of this study and needs to be investigated further.
Abbreviations
- ADL
Activities of daily living
- AJCC
American Joint Committee on Cancer
- CI
Confidence interval
- CNS
Central nervous system
- CR
Complete response
- CTCAE
Common Terminology Criteria for Adverse Events
- DCR
Disease control rate
- DRT
Drug-related toxicity
- DVT
Deep vein thrombosis
- ECOG
Eastern Cooperative Oncology Group
- irRC
Immune-related response criteria
- mAb
Monoclonal antibodies
- ORR
Overall response rate
- PASW
Predictive Analytics Software
- PR
Partial response
- PS
Performance status
- RCT
Randomized controlled trial
- StD
Stable disease
Author contributions
Study design: TI, CR. Data acquisition: TI, CM, MB. Statistical analysis: TI, MB. Manuscript writing: TI, CR. Manuscript editing: CM. Final manuscript approval: TI, CM, MB, CR.
Funding
No relevant funding.
Compliance with ethical standards
Conflict of interest
the authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Ethical Committee of the Gustave Roussy Institut.
Informed consent
all patients signed a written informed consent allowing authors to exploit data anonymously.
References
- 1.Arnold M, Holterhues C, Hollestein L, et al. Trends in incidence and predictions of cutaneous melanoma across Europe up to 2015. J Eur Acad Dermatol Venereol. 2014;28:1170–1178. doi: 10.1111/jdv.12236. [DOI] [PubMed] [Google Scholar]
- 2.Daste A, Domblides C, Gross-Goupil M, et al. Immune checkpoint inhibitors and elderly people: a review. Eur J Cancer. 2017;82:155–166. doi: 10.1016/j.ejca.2017.05.044. [DOI] [PubMed] [Google Scholar]
- 3.Shenoy P, Harugeri A. Elderly patients’ participation in clinical trials. Perspect Clin Res. 2015;6:184–189. doi: 10.4103/2229-3485.167099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pawelec G, Lustgarten J, Ruby C, Gravekamp C. Impact of aging on cancer immunity and immunotherapy. Cancer Immunol Immunother CII. 2009;58:1907–1908. doi: 10.1007/s00262-009-0743-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lustgarten J. Cancer, aging and immunotherapy: lessons learned from animal models. Cancer Immunol Immunother CII. 2009;58:1979. doi: 10.1007/s00262-009-0677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishijima TF, Muss HB, Shachar SS, Moschos SJ. Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: a systematic review and meta-analysis. Cancer Treat Rev. 2016;45:30–37. doi: 10.1016/j.ctrv.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Hegde Upendra P., Kerr Phil, Masternak Sophie, Brenner Bruce Mark, grant-Kels Jane. Immune checkpoint inhibitor treatment of advanced age metastatic melanoma (MM) patients. Journal of Clinical Oncology. 2017;35(15_suppl):e14603–e14603. doi: 10.1200/JCO.2017.35.15_suppl.e14603. [DOI] [Google Scholar]
- 8.Perier-Muzet M, Gatt E, Péron J, et al. Association of immunotherapy with overall survival in elderly patients with melanoma. JAMA Dermatol. 2018;154:82–87. doi: 10.1001/jamadermatol.2017.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betof AS, Nipp RD, Giobbie-Hurder A, et al. Impact of age on outcomes with immunotherapy for patients with melanoma. Oncologist. 2017;22:963–971. doi: 10.1634/theoncologist.2016-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785–792. doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Bao Z, Zhang X, et al. Effectiveness and safety of PD-1/PD-L1 inhibitors in the treatment of solid tumors: a systematic review and meta-analysis. Oncotarget. 2017;8:59901–59914. doi: 10.18632/oncotarget.18316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahendraraj K, Sidhu K, Lau CSM, et al. Malignant melanoma in African-Americans: a population-based clinical outcomes study involving 1106 African-American patients from the surveillance, epidemiology, and end result (SEER) database (1988–2011) Medicine (Baltim) 2017;96:e6258. doi: 10.1097/MD.0000000000006258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:472–492. doi: 10.3322/caac.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manig L, Käsmann L, Janssen S, et al. Simplified comorbidity score and Eastern Cooperative Oncology Group performance score predicts survival in patients receiving organ-preserving treatment for bladder cancer. Anticancer Res. 2017;37:2693–2696. doi: 10.21873/anticanres.11618. [DOI] [PubMed] [Google Scholar]
- 15.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Department of Health and Health and Human Services, National Institutes of Health, National Cancer Institute (2010) Common terminology criteria for adverse events (CTCAE) version 4.03. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed 12 Dec 2017
- 17.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 18.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomized, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 20.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 22.Maio M, Grob J-J, Aamdal S, et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J Clin Oncol. 2015;33:1191–1196. doi: 10.1200/JCO.2014.56.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiarion Sileni V, Pigozzo J, Ascierto PA, et al. Efficacy and safety of ipilimumab in elderly patients with pretreated advanced melanoma treated at Italian centers through the expanded access programme. J Exp Clin Cancer Res. 2014;33:30. doi: 10.1186/1756-9966-33-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomized, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558–1568. doi: 10.1016/S1470-2045(16)30366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas NE, Edmiston SN, Alexander A, et al. Association between NRAS and BRAF mutational status and melanoma-specific survival among patients with higher-risk primary melanoma. JAMA Oncol. 2015;1:359–368. doi: 10.1001/jamaoncol.2015.0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adler NR, Wolfe R, Kelly JW, et al. Tumour mutation status and sites of metastasis in patients with cutaneous melanoma. Br J Cancer. 2017;117:1026–1035. doi: 10.1038/bjc.2017.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim DW, Haydu LE, Joon AY, et al. Clinicopathological features and clinical outcomes associated with TP53 and BRAF(N)(on-)(V)(600) mutations in cutaneous melanoma patients. Cancer. 2017;123:1372–1381. doi: 10.1002/cncr.30463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van ‘t Veer LJ, Burgering BM, Versteeg R, et al. N-ras mutations in human cutaneous melanoma from sun-exposed body sites. Mol Cell Biol. 1989;9:3114–3116. doi: 10.1128/MCB.9.7.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aunan JR, Cho WC, Søreide K. The Biology of aging and cancer: a brief overview of shared and divergent molecular hallmarks. Aging Dis. 2017;8:628–642. doi: 10.14336/AD.2017.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henson SM, Franzese O, Macaulay R, et al. KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood. 2009;113:6619–6628. doi: 10.1182/blood-2009-01-199588. [DOI] [PubMed] [Google Scholar]
- 34.Henson SM, Macaulay R, Franzese O, Akbar AN. Reversal of functional defects in highly differentiated young and old CD8 T cells by PDL blockade. Immunology. 2012;135:355–363. doi: 10.1111/j.1365-2567.2011.03550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurez V, Padrón ÁS, Svatek RS, Curiel TJ. Considerations for successful cancer immunotherapy in aged hosts. Clin Exp Immunol. 2017;187:53–63. doi: 10.1111/cei.12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Figueiredo ASP, Hurez V, Liu A, Curiel TJ (2016) Age and sex affect αCTLA-4 efficacy alone and combined with αB7-H1 or regulatory T cell depletion in a melanoma model. In: The AAI annual meeting 2016. J Immunol 196 (suppl; Abstract 213.4)
- 37.Deeks ED. Pembrolizumab: a review in advanced melanoma. Drugs. 2016;76:375–386. doi: 10.1007/s40265-016-0543-x. [DOI] [PubMed] [Google Scholar]
- 38.Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12–25. doi: 10.1016/j.ejca.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600–1609. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- 40.Ahmad S, Qian S, Ellis W, et al. Ipilimumab in the real world: the UK expanded access programme experience in previously treated advanced melanoma patients. Melanoma Res. 2015;25:432–442. doi: 10.1097/CMR.0000000000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung M, Lee J, Kim TM, et al. Ipilimumab real-world efficacy and safety in Korean melanoma patients from the korean named-patient program cohort. Cancer Res Treat. 2017;49:44–53. doi: 10.4143/crt.2016.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berrocal A, Arance A, Lopez Martin JA, et al. Ipilimumab for advanced melanoma: experience from the Spanish Expanded Access Program. Melanoma Res. 2014;24:577–583. doi: 10.1097/CMR.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]