Abstract
Renal cell carcinoma (RCC) is one of the most lethal urologic malignancies. Its incidence continues to rise worldwide with a rate of 2% per year. Approximately, one-third of the RCC patients are diagnosed at advanced stages due to the asymptomatic nature of its early stages. This represents a great hurdle, since RCC is largely chemoresistant/radioresistant, and targeted therapy of mRCC still has limited efficacy. The 5-year survival rate of metastatic RCC (mRCC) is only around 10%. Adoptive cell transfer (ACT), a particular form of cell-based anticancer immunotherapy, is a promising approach in the treatment of mRCC. The vaccination principle, however, faces unique challenges that preclude the efficacy of ACT. In this article, we review the main challenges of ACT in the treatment of mRCC and describe multiple methods that can be used to overcome these challenges. In this respect, the ultimate purpose of this review is to provide a descriptive tool by which to improve the development of novel protocols for ACT of mRCC.
Keywords: Renal cell carcinoma, ACT, Tumor-infiltrating lymphocytes, PECAM, Peritumoral lymphocytes, PIVAC 18
Renal cell carcinoma (RCC)
One of the leading malignancies of central Europe, renal cell carcinoma (RCC), is considered to be the most lethal malignant tumor of the urologic system. It is broadly divided into clear cell renal carcinoma (ccRCC, approx. 80% of cases) and non-clear cell renal carcinoma (non-ccRCC, approx. 20% of cases). The overall incidence is still on the rise with a rate of 2% per year [1, 3]. If identified at an early stage, radical surgical intervention offers a favorable prognosis, with the survival rate of 60% within 5 years [1]. However, approximately one-third of RCC patients are diagnosed at advanced stages due to the asymptomatic nature of the early ones. The advanced stages are characterized by invasive growth or distant metastases. Unfortunately, there are currently no effective imaging or cytology-based screening methods for the early detection of RCC necessitating the need for appropriate treatment selection [2].
RCC is a highly chemoresistant/radioresistant malignancy. The landscape of metastatic RCC (mRCC) treatment has changed during the last decade with the introduction of targeted therapies, and the first-line treatment options are currently mTOR and tyrosine kinase inhibitors together with biological therapy, such as anti-VEGF drugs [3]. Immunotherapeutic approaches, based on the inhibition of the immune checkpoint molecules PD-1 and/or CTLA-4, have also shown favorable results and have been approved by the FDA and EMA for the treatment of mRCC [3]. Nevertheless, targeted therapies still share limited efficacy in the treatment of mRCC. The 5-year survival rate of mRCC is around 10% [1]. Since the wide tumor-immune system interplay in RCC is evident, immunotherapies aimed at increasing intratumoral T cell numbers or increasing their cytotoxicity may have a unique potential to improve the survival of patients with mRCC [3, 4].
Adoptive transfer of expanded tumor-infiltrating lymphocytes (TILs) in RCC
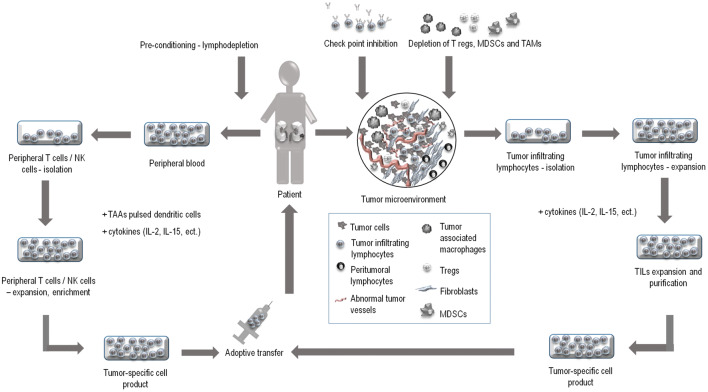
Adoptive cell transfer (ACT), a particular form of cell-based anticancer immunotherapy, may be one potential treatment modality in metastatic diseases, where conventional therapy tends to fail. As shown in Fig. 1, ACT is a cell therapy based on immune cells extracted from the patient, processed in vitro, extensively expanded, then transferred back to the patient [5, 6]. For ACT, both peripheral and tumor-infiltrating lymphocytes (TILs) can be used as the source of cells [5]. The in vitro processing of these source cells may include either purification or the enrichment of the cell culture. Alternatively, the cells can be genetically modified [4]. The following in vitro expansion of the cells allows the tumor-specific cells to be grown outside the immunosuppressive tumor microenvironment these cells encounter in vivo. Moreover, the patient can undergo lymphodepletion to reduce the number of suppressor immune cells prior to the transfer [5, 6].
Fig. 1.
In renal cell carcinoma (RCC), the tumor-immune system interplay is evident; therefore, immunotherapeutic approaches may be the most promising treatment modalities. Adoptive cell transfer (ACT) is a cell-based vaccination approach, where tumor-specific lymphocytes can be selected from the patient, stimulated, expanded to large numbers in vitro and injected back to the patient. For the purpose of ACT, both peripheral and tumor-infiltrating lymphocytes (TILs) can be used. TILs are obtained from freshly resected tumors, expanded with IL-2, IL-15 and/or other cytokines and purified. TILs are a very heterogenous cell population. The population, however, contains cells capable of recognizing tumor-associated antigens (TAAs).This represents a great advantage in using TILs as a source material. On the other hand, peripheral blood mononuclear cells (PBMCs) can be readily extracted from the whole blood of patients and their isolation does not require surgical resection of the tumor. PBMCs can be stimulated in vitro with cytokines and to achieve the specific anti-tumor response, priming with TAA-pulsed dendritic cells is often required. The efficacy of ACT can be amplified by patient’s pre-conditioning. The therapy itself can be combined with other immunotherapeutic approaches, such as check point inhibition and/or the biological therapy
ACT has been shown in clinical trials to cause objective clinical responses in 40–72% of patients with metastatic melanoma. Moreover, up to 40% of these patients experienced complete regressions lasting up to 7 years ongoing [6]. These results caused an overall optimism and led to the exploration of the efficacy of ACT in a number of non-melanoma solid tumors. Unfortunately, ACT in the treatment of mRCC has shown only limited efficacy [7]. Therefore, novel approaches and novel combinations of therapeutic strategies in this field are needed. Successful ACT requires the isolation of immune cells, their expansion and, once transferred back to the patient, efficient trafficking of the tumor-specific immune cells to the tumor. TILs isolated from excised tumors and non-specifically expanded in vitro are the traditional source for ACT [6, 8]. However, TILs are a very heterogeneous cell population, and the subpopulations that could provide the most convenient source of TILs for ACT have not yet been defined [8].
Main challenges of ACT
TIL-based ACT, in combination with high-dose IL-2, has shown up to 50% objective and durable responses in metastatic melanoma patients with proper lymphodepleting pre-conditioning [9]. Out of twenty patients, two patients had complete remissions, eight had partial responses with significantly prolonged progression-free survival and four patients showed disease stabilization [9]. However, in the treatment of other solid tumors, TIL-based ACT still faces unique challenges. Partially, it is because of the biological barrier that prevents immune cells from penetrating the tumor tissues. Recently, Torcellan et al. provided in vivo imaging of immune cell migration towards multiple solid tumors and showed that the collagen fibers in the tumor ECM affect intratumoral T cell distribution and migration [11]. The biological barrier that accompanies the growth of the tumor is characterized by a high density of extracellular matrix (ECM) and production of a broad amount of pro-fibrotic molecules, such as transforming growth factor beta (TGF-β), fibroblast growth factor (FGF), and others [10]. The barrier, together with the abnormal tumor vessel barrier and interstitium, causes insufficient trafficking of the immune cells towards the tumor. Imaging techniques allowed a deep insight into the intratumoral T cell dynamics and in lung cancer specifically, the ECM fibers surrounding the tumor were shown to significantly influence T cell migration and to cause limited T cell trafficking into the tumor [12]. Moreover, in human ovarian carcinomas, the high density of ECM resulted in impaired CD8+ T cell migration and caused alternative forward and backward movements of the cells in the peritumoral area [13]. Taken together, the pathologic nature of the tumor microenvironment may restrain a direct contact of adoptively transferred immune cells with tumor antigens and limit the efficacy of ACT [10, 13].
As shown in Table 1, other obstacles that preclude the efficacy of ACT in mRCC are immunosuppressive and hostile tumor microenvironments, poor immune cell recruitment into tumors [14] and the loss of cytotoxic functions of the immune cells [14, 15]. How to improve the unpredictable lifespan of transferred immune cells in the hostile tumor microenvironment has been a matter of intensive debate [14]. Survival of both NK and CD8+ T cells was shown to be prolonged in the presence of IL-2, IL-7, and IL-15 and, together with IL-21, these cytokines stimulated the telomerase activity and inhibited apoptosis of the transferred cells [15]. Nevertheless, successful augmentation of the in vivo survival and effector functions of transferred cells along with facilitating the tumor infiltration with these cells still remains to be determined.
Table 1.
Adoptive cell transfer (ACT) faces unique challenges
| Constant challenges |
| Patient’s non-compliance |
| Non-resectable tumor |
| Low immunogenicity of the tumor |
| Pre-treatment intolerance |
| Complete/partial resistance to therapy |
| Processing difficulties |
| External factors |
| Variable challenges |
| Lack of chemoattraction to the tumor site |
| Poor trafficking to the tumor site |
| Lack of cytotoxicity of the transferred cells |
| Exhaustion of the transferred cells |
| Short in vivo persistence of transferred cells |
Constant challenges include factors that are difficult to predict and modify, such as immunogenicity of the tumor, resistance to the therapy, and also the factors that individually vary among the patients, such as compliance of the patient and the individual tolerance of lymphodepleting regimens. Variable challenges are factors that can be modified by changing the tumor microenvironment or changing the mechanisms of isolation, expansion and transferring the immune cells
Isolating TILs from different RCC compartments for the purpose of phenotypic analyses
The lymphocyte infiltration in the RCC tissue may serve not only as a valuable prognostic biomarker, but also as a unique source material for ACT [16]. As TILs are very heterogeneous, many studies attempted to identify and evaluate those populations of TILs which have the highest cytotoxic and migratory potential [16]. However, this approach is limited not only by the tumor sample size, but also by the types of analyses that can be performed with the limited number of cells available.
The tumor size, the surgical resectability of the tumor, and the intensity of intratumoral lymphocyte infiltration might be the major aspects that favor malignant melanoma in TIL preparation while causing difficulties in other solid tumors. Several strategies have been devised to find the optimal lymphocyte populations for ACT [8]. Since malignant melanoma is the most immunogenic cancer, successful generation and phenotyping of TILs are widely reported by a number of studies [17].
In RCC patients, the phenotypic signature of intratumoral NK cells and T cells patients has also been described [18, 19]. However, only a few studies considered the importance of peritumoral lymphocytes [16, 20]. Recently, it has been shown that the presence of mature dendritic cells in the peritumoral immune aggregates, in combination with CD8+ TILs, in ccRCC patients is associated with a good prognosis [18]. Nevertheless, a comparative analysis that would evaluate the phenotypic signatures of infiltrating immune cell in the pertinent tissue compartments, i.e. the tumor, peritumoral tissue, and the adjacent healthy renal tissue, has not been conducted until recently [20].
The strategy of TIL preparation and analysis in RCC patients
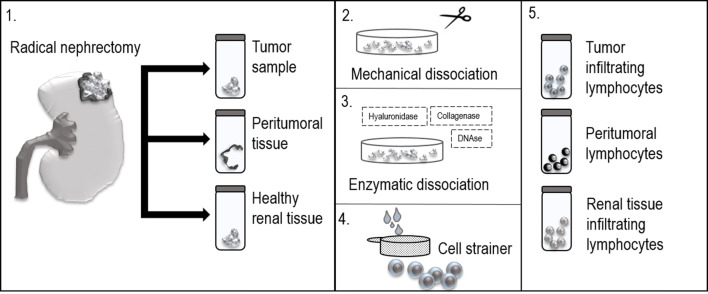
The preparation and administration of TILs for clinical purposes have been limited to highly specialized research centers where a tight interdisciplinary relationship is secured. Isolation of TILs from RCC patients is a complex multi-step process. In the first step, tumor samples are aseptically resected from the kidney after radical nephrectomy. The optimal mean size of the sample is at least 0.5 cm in diameter [21, 22]. To analyze lymphocyte migration within different microenvironmental compartments, peritumoral tissue can be also obtained and its infiltrating lymphocytes evaluated. Peritumoral tissue is characterized as the boundary between the normal tissue and the tumor. However, to reach comparable data among different studies, peritumoral tissue needs to be established as the tissue which is localized approximately 0.5–1.0 cm from the macroscopic tumor edge [23]. After the resection, it is essential to transport the tissue samples in an appropriate liquid solution, such as a saline solution or, preferably, a cell culture medium. The samples are transported to and processed in a specialized laboratory that is compliant with Good Manufacturing Practice (GMP). Each sample is then sliced into small pieces (1 mm3) and transferred into the cell culture medium. The medium is then supplemented with a mixture of collagenase, hyaluronidase, and DNAse to enzymatically dissociate the sliced tissue. The dissociated tissue is passed through a sterile 70 µm or 100 µm nylon cell strainer to separate the cells (Fig. 2). The separated cells are rinsed with phosphate-buffered saline (PBS) and the excess of red blood cells is removed by centrifugation or by specific lysis. To perform phenotypic analysis, the cell surface markers are stained with fluorophore-conjugated monoclonal antibodies. To decrease the formation of cell aggregates and antibody internalization, 2 mM EDTA is often added. The stained cells are then rinsed to remove unbound antibodies and analyzed with a flow cytometer [20–22]. For the purpose of ACT, non-homogenized cut pieces of the tumor can be also used, once the cultures are supplemented with high doses of IL-2. This cytokine leads to the rapid expansion of lymphocytes in the culture, which is further followed by the elimination of tumor cells by the expanded lymphocytes [5].
Fig. 2.
Isolation of tissue-infiltrating lymphocytes from RCC patients is a complex multi-step process. 1. In the first step, tumor samples, peritumoral tissue, and healthy renal tissue are aseptically resected from the kidney after radical nephrectomy. 2. Each sample is then sliced into small pieces (1 mm3) and transferred into the cell culture medium. 3. The medium is then supplemented with a mixture of collagenase, hyaluronidase, and DNAse to enzymatically dissociate the sliced tissue. 4. The dissociated tissue is passed through a sterile 70 µm or 100 µm nylon cell strainer to singularize the cells. 5. To perform phenotypic analysis, the cell surface markers can be stained with fluorophore-conjugated monoclonal antibodies
Overcoming insufficient trafficking to the tumor site
ACT often utilizes extensively in vitro-expanded TILs. However, the therapeutic efficacy of the expanded TILs is largely compromised by poor trafficking to the tumor site. To improve the therapeutic efficacy of ACT, it is crucial to enable the tumor-specific lymphocytes to achieve direct contact with their target cancer cells. To this aim, the lymphocytes need to cross the aberrant fibrotic and vessel barrier of the tumors [10, 24]. Intratumoral fibrosis (ITF), together with abnormal tumor vasculature, is the main factor protecting the tumor against the immune cells [10, 24]. In most tumors, the ITF results in a worse prognosis. This fibroprotection is present in many solid tumor malignancies. RCC is a highly vascularized cancer. The most common type, ccRCC, is often associated with a significant peritumoral or intratumoral fibrosis. In ccRCC, the ITF not only defines worse prognosis but also relates to other poor prognostic factors, such as increased Fuhrman nuclear grade, intratumoral necrosis and lymphovascular invasion [25].
A number of new strategies have been tested in mouse models to enhance the trafficking of adoptively transferred lymphocytes. One approach is to control the remodeling of tumor vasculature, either with angiogenesis inhibitors, such as anginex, endostatin and angiostatin [26], or with anti-VEGF or anti-VEGFR antibodies [26]. Another approach that has been extensively studied is focused on increasing the permeability of small vessels with NGR-containing ligands [27]. This approach is being currently tested in a randomized phase III clinical trial and does, to date, not cause serious adverse reactions (NCT01098266) [28]. Recently, chimeric antigen receptor T (CAR-T) cells engineered to express extracellular matrix-degrading enzymes or chemokine receptors have also been reported to assist the engineered T cells in infiltrating the tumor microenvironment [29].
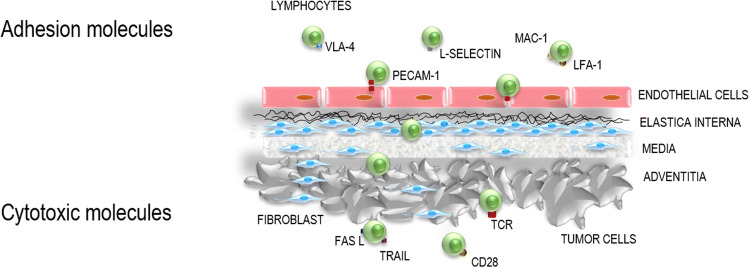
When searching for optimal TIL subsets for ACT, the cell subsets which express the transendothelial migration (TEM)-enhancing molecules should be taken into consideration [10, 30]. There are a number of surface molecules regulating extravasation, paracellular migration and endothelial transmigration of leukocytes to the site of inflammation [31] (Fig. 3). Among these, platelet/endothelial cell adhesion molecule 1 (PECAM-1, also known as CD31), strongly defines the TEM capacity of leukocytes and was found to be required for TEM [31, 32]. The PECAM-1-mediated leukocyte migration can be effectively prevented by PECAM-1-specific blocking antibodies or by inhibition of PECAM-1 expression [31, 32]. PECAM-1 is expressed diffusely on leukocytes and platelets. However, some cells may lack the expression of PECAM-1, resulting in their arrest along the tight junctions and the apical membrane of the endothelium [31]. Moreover, it has been demonstrated that PECAM-1, as opposed to other adhesion molecules, when transfected into cells that normally lack it, supports TEM and allows cells to transmigrate efficiently [31, 32]. From that vantage point, it is surprising that PECAM-1-expressing tumor-infiltrating T cells or NK cells have not been studied yet for their potential use in ACT. PECAM-1 shows a great deal of promise in enhancing the trafficking of the immune cells towards and potentially into the tumors. However, to improve the therapeutic efficacy of ACT, the search for the tumor-specific infiltrating lymphocytes that express this molecule that could be then used for ex vivo expansion for ACT will certainly also necessitate the analysis of the phenotypes of the infiltrating lymphocytes beyond the tumor compartments and also in addition to beyond the tumors themselves [20]. We hypothesize that adoptive transfer of cells with enhanced migratory capacity could be the optimal phenotype for improving the therapeutic efficacy of ACT of RCC.
Fig. 3.
There are a number of surface molecules regulating extravasation, paracellular migration and transendothelial migration (TEM) of leukocytes to the site of inflammation. Among these, platelet/endothelial cell adhesion molecule 1 (PECAM-1, also known as CD31) strongly defines TEM capacity of leukocytes and was found to be required for TEM. The PECAM-1 molecule shows a great deal of promise in enhancing the trafficking of the immune cells towards the tumor. TEM allows T cells to get in direct contact with tumor-associated antigens (TAAs) and to use cytotoxic tools, such as FasL and TRAIL
Cytotoxicity of adoptively transferred TILs
Our ability to define and expand therapeutically potent migratory and cytotoxic lymphocytes may be critical in developing effective TIL-based ACT for mRCC. Andersen et al. searched in RCC patients for cytotoxic TILs that could be efficiently exploited for ACT. The study showed that TILs, isolated from primary RCC specimens, can recognize tumors. Nevertheless, the immune responses of TILs against RCC tumor cells remained weak [33].
In TIL-based ACT, to achieve a desired therapeutic outcome, only a small number of TILs with appropriate effector functions may be necessary. Once identified, these TILs can be specifically isolated, ex vivo expanded to large numbers and used for cancer treatment [5]. Among these TIL populations, there are lymphocytes that express the cytotoxic markers TRAIL (CD253) [34, 35] and FasL (CD178) [36]. It has been reported that sub-lethal ionizing radiation enhanced FasL- and TRAIL-mediated apoptosis in cancer cells. This event was further shown to increase Fas/TRAIL-mediated apoptosis, even in chemoresistant ovarian cancer cells [37]. Clinical trials testing the TRAIL agonists, however, did not show similar therapeutic effects [38]. However, none of these TRAIL-focused trials aimed to specifically isolate and expand TRAIL+ TILs. Due to these disappointing results, it may be prudent to terminate the use of indiscriminate pan-specific expansion of TILs for ACT and to direct more attention to the use of specific TIL populations with promising phenotype for ex vivo expansion and ACT. The rationale for this approach stems not only from the current disappointing results, but also from pre-clinical observations which suggested that adoptive transfer of one particular subset of cells showed better therapeutic efficacy than transferring immune cells non-selectively [39, 40]. No clinical comparison has, however, been done to favor one selected subset over the other [39, 40]. Whether the use of specifically expanded TRAIL+ or Fas+ or TRAIL+/Fas+ TILs can really improve the efficacy of ACT in the treatment of mRCC still remains to be determined.
Increasing the potency of TIL-based ACT
The approaches to increase the potency of adoptively transferred TILs are being largely debated and investigated in most of the solid tumors. Different pre-conditioning approaches have been utilized. In malignant melanoma patients, lymphodepletion with cyclophosphamide (CTX) and fludarabine (FLU) increases the response to ACT with TILs [5]. Total body irradiation (TBI) appears to augment the efficacy of adoptively transferred cells in malignant melanoma patients [5].
In addition to pre-conditioning regimens, approaches utilizing ACT with phenotypically diverse T cells and NK cells have been tested [15]. It is still unclear whether improved cytotoxicity of transferred cells can be achieved by transferring a particular subset of αβ T cells, γδ T cells, by co-transferring different cell subsets, or by transferring CAR T cells [15]. Different cell-based clinical trials in RCC have been initiated (NCT00328861, NCT02926053), including CAR T cell therapy for RCC which is currently being tested in phase I/II clinical trial (NCT02830724). Particularly relevant to this issue may also be the boosting effect of dendritic cell-based immunotherapy when given in combination with TIL ACT [41].
Immunosuppressive tumor microenvironment in RCC
One of the aspects that limits the effector functions of the transferred TILs is the inhibitory tumor microenvironment in RCC. The causes of tumor-induced immunosuppression are primarily due to the production of checkpoint receptor ligands and to the production and release of anti-inflammatory cytokines by the tumor cells. The strategy to block immune checkpoints has shown encouraging results in clinical trials and has already been approved for the treatment of mRCC [3]. However, in some patients, primary resistance to checkpoint inhibitors has been reported [3]. Thus, other immunotherapies, such as the ACT, should also be investigated for the treatment of this condition.
One factor that suppresses the effector functions of transferred cells is the pathological generation of inhibitory immune cells, such as myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs) and regulatory T cells (Tregs). Increased levels of circulating Tregs have been reported in patients with RCC [42, 43]. The immunosuppressive properties of MDSCs, TAMs, and Tregs may be, however, reversed by decreasing their numbers [44], by blocking their suppressive activity [45] or by antagonizing chemokine receptors, and thus causing a failure of their migration towards the inflammatory environment [46]. Depletion of inhibitory host cells, without impairing effector function of adoptively transferred cells, is currently an area of intense investigation for the treatment of cancer patients, including those with RCC.
Discussion
It remains a challenge for current therapeutic strategies to effectively treat late stages of RCC. Immunotherapy has led to major breakthroughs in the treatment of a number of cancers and, thus, represents a promising approach for the treatment of mRCC.
Immunotherapy, with immune checkpoint blockade, has become an approved treatment for RCC patients and has proved to be clinically effective [3]. However, resistance to immune checkpoint blockade has been reported in a subgroup of individuals [3]. TIL-based ACT is a promising approach in the treatment of diverse human malignancies. However, TIL-based ACT also faces unique challenges that preclude its efficacy and wide use in the treatment of mRCC. To overcome the challenges, the current approaches based on selecting non-terminally differentiated cells that recognize tumor cells appear to be inadequate [47]. These cells need to additionally have an enhanced capacity to reach their targets in the challenging environment of RCC tumors. Furthermore, they need to have an enhanced capacity to eradicate cancer cells and need to expand to sufficient numbers to face the presence of the large population of immunosuppressive cells in RCC tumors. To achieve these properties, the method of the preparation of cells for ACT needs to be substantially changed. Firstly, the methods for the expansion of these populations need to be developed. This cannot be restricted to only the tumor site but should also expand to adjacent tissues [20]. Secondly, the populations need to have a strong capacity to effectively penetrate the enhanced fibroprotection of RCC tumors. Immune cells which express markers of transendothelial migratory capacity, such as PECAM-1, should be then considered. Thirdly, the populations must be able to effectively eradicate the cancer cells. The cells that express TRAIL and Fas ligand receptors may represent the lead candidates to consider in this way. Finally, the populations with the intended migratory and cytotoxic phenotypes need to outnumber the immunosuppressive cells in the tumors. Therefore, cells with these phenotypes need to be specifically expanded for ACT and used in sufficient numbers for RCC treatment.
To develop protocols for preparation of cells with such features for ACT of RCC will certainly require intensive and challenging research. However, as preclinical data are already promising, these approaches may indeed prove to be worthwhile and bring new hope in the form of novel ACT-based treatment of such challenging cancer as mRCC.
Acknowledgement
We would like to thank Prof. Ilja Striz and Dr. Alasdair M. Gilfillan for a critical review of the manuscript.
Abbreviations
- ACT
Adoptive cell transfer
- ccRCC
Clear cell RCC
- IL
Interleukin
- ITF
Intratumoral fibrosis
- mRCC
Metastatic RCC
- NK cell
Natural killer cell
- non-ccRCC
Non-clear cell RCC
- PBMC
Peripheral blood mononuclear cell
- PECAM
Platelet endothelial cell adhesion molecule
- RCC
Renal cell carcinoma
- TIL
Tumor-infiltrating lymphocyte
- TRAIL
TNF-related apoptosis-inducing ligand
Author contributions
Zuzana Strizova drafted and wrote the manuscript. Jirina Bartunkova contributed to the intellectual content and writing of the manuscript. Daniel Smrz contributed to the intellectual content and with Zuzana Strizova wrote the manuscript. All authors read and approved the final version of the manuscript.
Funding
Charles University—project GA UK No. 364218 and PRIMUS/MED/12 and funding by the Ministry of Health, Czech Republic—project AZV 16-28135A.
Compliance with ethical standards
Conflict of interest
The authors Zuzana Strizova and Daniel Smrz declare that there is no conflict of interest regarding this article. Jirina Bartunkova is a part-time employee and a minority shareholder of SOTIO, a.s., a biotech company developing cell-based immunotherapy.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kabaria R, Klaassen Z, Terris MK. Renal cell carcinoma: links and risks. Int J Nephrol Renovasc Dis. 2016;9:45–52. doi: 10.2147/IJNRD.S75916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shea MW. A proposal for a targeted screening program for renal cancer. Front Oncol. 2013;3:207. doi: 10.3389/fonc.2013.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santoni M, Massari F, Di Nunno V, Conti A, Cimadamore A, Scarpelli M, et al. Immunotherapy in renal cell carcinoma: latest evidence and clinical implications. Drugs Context. 2018;7:212528. doi: 10.7573/dic.212528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen JE, Merims S, Frank S, Engelstein R, Peretz T, Lotem M. Adoptive cell therapy: past, present and future. Immunotherapy. 2017;9(2):183–196. doi: 10.2217/imt-2016-0112. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348(6230):62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phan GQ, Rosenberg SA. Adoptive cell transfer for patients with metastatic melanoma: the potential and promise of cancer immunotherapy. Cancer Control. 2013;20(4):289–297. doi: 10.1177/107327481302000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang X, Liu T, Zang X, Liu H, Wang D, Chen H, et al. Adoptive cellular immunotherapy in metastatic renal cell carcinoma: a systematic review and meta-analysis. PLoS ONE. 2013;8(5):e62847. doi: 10.1371/journal.pone.0062847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013;39(1):49–60. doi: 10.1016/j.immuni.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16(9):2646–2655. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 10.Bellone M, Calcinotto A, Corti A. Won’t you come on in? How to favor lymphocyte infiltration in tumors. Oncoimmunology. 2012;1(6):986–988. doi: 10.4161/onci.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torcellan T, Stolp J, Chtanova T. In vivo imaging sheds light on immune cell migration and function in cancer. Front Immunol. 2017;8:309. doi: 10.3389/fimmu.2017.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. 2012;122(3):899–910. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bougherara H, Mansuet-Lupo A, Alifano M, Ngo C, Damotte D, Le Frere-Belda MA, et al. Real-time imaging of resident T cells in human lung and ovarian carcinomas reveals how different tumor microenvironments control T lymphocyte migration. Front Immunol. 2015;6:500. doi: 10.3389/fimmu.2015.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Idorn M, Thor Straten P. Chemokine receptors and exercise to tackle the inadequacy of T cell homing to the tumor site. Cells. 2018 doi: 10.3390/cells7080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nayar S, Dasgupta P, Galustian C. Extending the lifespan and efficacies of immune cells used in adoptive transfer for cancer immunotherapies—a review. Oncoimmunology. 2015;4(4):e1002720. doi: 10.1080/2162402X.2014.1002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fridman WH, Galon J, Pages F, Tartour E, Sautes-Fridman C, Kroemer G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 2011;71(17):5601–5605. doi: 10.1158/0008-5472.CAN-11-1316. [DOI] [PubMed] [Google Scholar]
- 17.Lee S, Margolin K. Tumor-infiltrating lymphocytes in melanoma. Curr Oncol Rep. 2012;14(5):468–474. doi: 10.1007/s11912-012-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giraldo NA, Becht E, Pages F, Skliris G, Verkarre V, Vano Y, Mejean A, Saint-Aubert N, Lacroix L, Natario I, Lupo A, Alifano M, Damotte D, Cazes A, Triebel F, Freeman GJ, Dieu-Nosjean MC, Oudard S, Fridman WH, Sautes-Fridman C. Orchestration and prognostic significance of immune checkpoints in the microenvironment of primary and metastatic renal cell cancer. Clin Cancer Res. 2015;21(13):3031–3040. doi: 10.1158/1078-0432.CCR-14-2926. [DOI] [PubMed] [Google Scholar]
- 19.Geissler K, Fornara P, Lautenschlager C, Holzhausen HJ, Seliger B, Riemann D. Immune signature of tumor infiltrating immune cells in renal cancer. Oncoimmunology. 2015;4(1):e985082. doi: 10.4161/2162402X.2014.985082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strizova Z, Taborska P, Stakheev D, Partlova S, Havlova K, Vesely S, Bartunkova J, Smrz D. NK and T cells with a cytotoxic/migratory phenotype accumulate in peritumoral tissue of patients with clear cell renal carcinoma. Urol Oncol. 2019;37(7):503–509. doi: 10.1016/j.urolonc.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Crossey F, Marx S, Holters S, Schmitt K, Bohle RM, Schmidt T, et al. Robust method for isolation of tumor infiltrating lymphocytes with a high vital cell yield from small samples of renal cell carcinomas by a new collagenase-free mechanical procedure. Urol Oncol. 2018;36(9):402e1–402e10. doi: 10.1016/j.urolonc.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Mayor P, Starbuck K, Zsiros E. Adoptive cell transfer using autologous tumor infiltrating lymphocytes in gynecologic malignancies. Gynecol Oncol. 2018;150(2):361–369. doi: 10.1016/j.ygyno.2018.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voigt H, Kleeberg UR. Malignes melanom. Berlin: Springer; 1986. p. 185. [Google Scholar]
- 24.Cox TR, Erler JT. Fibrosis and cancer: Partners in crime or opposing forces? Trends Cancer. 2016;2(6):279–282. doi: 10.1016/j.trecan.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Joung JW, Oh HK, Lee SJ, Kim YA, Jung HJ. Significance of intratumoral fibrosis in clear cell renal cell carcinoma. J Pathol Transl Med. 2018;52(5):323–330. doi: 10.4132/jptm.2018.07.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171–6180. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corti A, Pastorino F, Curnis F, Arap W, Ponzoni M, Pasqualini R. Targeted drug delivery and penetration into solid tumors. Med Res Rev. 2012;32(5):1078–1091. doi: 10.1002/med.20238. [DOI] [PubMed] [Google Scholar]
- 28.Gregorc V, Gaafar RM, Favaretto A, Grossi F, Jassem J, Polychronis A, et al. NGR-hTNF in combination with best investigator choice in previously treated malignant pleural mesothelioma (NGR015): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2018;19(6):799–811. doi: 10.1016/S1470-2045(18)30193-1. [DOI] [PubMed] [Google Scholar]
- 29.Zhang E, Xu H. A new insight in chimeric antigen receptor-engineered T cells for cancer immunotherapy. J Hematol Oncol. 2017;10(1):1. doi: 10.1186/s13045-016-0379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berman ME, Xie Y, Muller WA. Roles of platelet/endothelial cell adhesion molecule-1 (PECAM-1, CD31) in natural killer cell transendothelial migration and beta 2 integrin activation. J Immunol. 1996;156(4):1515–1524. [PubMed] [Google Scholar]
- 32.Dasgupta B, Dufour E, Mamdouh Z, Muller WA. A novel and critical role for tyrosine 663 in platelet endothelial cell adhesion molecule-1 trafficking and transendothelial migration. J Immunol. 2009;182(8):5041–5051. doi: 10.4049/jimmunol.0803192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen R, Westergaard MCW, Kjeldsen JW, Muller A, Pedersen NW, Hadrup SR, et al. T-cell responses in the microenvironment of primary renal cell carcinoma-implications for adoptive cell therapy. Cancer Immunol Res. 2018;6(2):222–235. doi: 10.1158/2326-6066.CIR-17-0467. [DOI] [PubMed] [Google Scholar]
- 34.Tian JQ, Wang ZP, Rodriguez R, Fu JS, Lu JZ, Ma BL. In vitro enhanced cytotoxicity of tumor-infiltrating lymphocytes transfected with tumor necrosis factor-related apoptosis-inducing ligand and/or interleukin-2 gene in human renal cell carcinoma. Urology. 2006;67(5):1093–1098. doi: 10.1016/j.urology.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 35.de Bruyn M, Wei Y, Wiersma VR, Samplonius DF, Klip HG, van der Zee AG, Yang B, Helfrich W, Bremer E. Cell surface delivery of TRAIL strongly augments the tumoricidal activity of T cells. Clin Cancer Res. 2011;17(17):5626–5637. doi: 10.1158/1078-0432.CCR-11-0303. [DOI] [PubMed] [Google Scholar]
- 36.Strater J, Hinz U, Hasel C, Bhanot U, Mechtersheimer G, Lehnert T, Moller P. Impaired CD95 expression predisposes for recurrence in curatively resected colon carcinoma: clinical evidence for immunoselection and CD95L mediated control of minimal residual disease. Gut. 2005;54(5):661–665. doi: 10.1136/gut.2004.052696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cacan E. Enhancing sensitivity of chemoresistant ovarian cancer cells to TRAIL and FAS mediated apoptosis by radiation. Turk Hij Den Biyol Derg. 2017;74(3):185–192. doi: 10.5505/TurkHijyen.2017.12499. [DOI] [Google Scholar]
- 38.de Miguel D, Lemke J, Anel A, Walczak H, Martinez-Lostao L. Onto better TRAILs for cancer treatment. Cell Death Differ. 2016;23(5):733–747. doi: 10.1038/cdd.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinrichs C, Borman Z, Cassard L, Gattinoni L, Spolski R, Yu Z, Sanchez-Perez L, Muranski P, Kern S, Logun C, et al. Adoptively transferred effector cells derived from naïve rather than central memory CD8 T cells mediate superior antitumor immunity. PNAS. 2009;106:17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen MC, Popplewell L, Cooper LJ, DiGiusto D, Kalos M, Ostberg JR, Forman SJ. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 2010;16:1245–1256. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poschke I, Lovgren T, Adamson L, Nystrom M, Andersson E, Hansson J, et al. A phase I clinical trial combining dendritic cell vaccination with adoptive T cell transfer in patients with stage IV melanoma. Cancer Immunol Immunother. 2014;63(10):1061–1071. doi: 10.1007/s00262-014-1575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cesana GC, DeRaffele G, Cohen S, et al. Characterization of CD4*CD25* regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol. 2006;24:1169–1177. doi: 10.1200/JCO.2005.03.6830. [DOI] [PubMed] [Google Scholar]
- 43.Siddiqui SA, Frigola X, Bonne-Annee S, et al. Tumor-infiltrating Foxp3-CD4+CD25+ T cells predict poor survival in renal cell carcinoma. Clin Cancer Res. 2007;13:2075–2081. doi: 10.1158/1078-0432.CCR-06-2139. [DOI] [PubMed] [Google Scholar]
- 44.Finke JH, Rini B, Ireland J, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14(20):6674–6682. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
- 45.Nagaraj S, Youn JI, Weber H, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 2010;16(6):1812–1823. doi: 10.1158/1078-0432.CCR-09-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicholson IC, Mavrangelos C, Bird DR, et al. PI16 is expressed by a subset of human memory Treg with enhanced migration to CCL17 and CCL20. Cell Immunol. 2012;275(1–2):12–18. doi: 10.1016/j.cellimm.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Knutson KL, Wagner W, Disis ML. Adoptive T cell therapy of solid cancers. Cancer Immunol Immunother. 2006;55(1):96–103. doi: 10.1007/s00262-005-0706-1. [DOI] [PMC free article] [PubMed] [Google Scholar]