Abstract
Rectal cancer, which comprises 30% of all colorectal cancer cases, is one of the most common forms of cancer in the world. Patients with locally advanced rectal cancer (LARC) are often treated with neoadjuvant chemoradiotherapy (neoCRT) followed by surgery. However, after neoCRT treatment, approximately one-third of the patients progress to local recurrence or distant metastasis. In these studies, we found that patients with tumors that exhibited cytosolic HMGB1(Cyto-HMGB1) translocation and/or the presence of PD-1+ tumor-infiltrating lymphocytes (TILs) before treatment had a better clinical outcome. The better outcome is likely due to the release of HMGB1, which triggers the maturation of dendritic cells (DCs) via TLR4 activation, and the subsequent recruitment of PD-1+ tumor-infiltrating lymphocytes to the tumor site, where they participate in immune-scavenging. In conclusion, our results provide evidence that cyto-HMGB1 and/or PD-1+TIL are not only predictive biomarkers before treatment, but they can also potentially designate patients for personalized oncological management including immunotherapy.
Electronic supplementary material
The online version of this article (10.1007/s00262-017-2109-5) contains supplementary material, which is available to authorized users.
Keywords: HMGB1, PD-1, NeoCRT, LARC, TLR4
Introduction
Colorectal cancer (CRC) is one of the leading causes of death worldwide [1]. The proportion of rectal cancer cases among all CRC cases ranges from 27 to 58% [2]. For patients with locally advanced rectal cancer (LARC, stage T3–T4, or lymph node-positive disease), preoperative (neoadjuvant) chemoradiotherapy (neoCRT) has been shown to improve local control [3]. For most patients, preoperative chemoradiotherapy results in clinical tumor regression, but the degree of response varies among patients. Approximately 40–60% of LARC patients treated with neoadjuvant CRT achieve some degree of pathologic response [4]. However, no effective method has been developed that can be used before the commencement of neoCRT that can predict how patients will respond to neoCRT and that can subsequently lead to better survival [5]. The identification of patients who will benefit most from neoCRT is crucial not only for the avoidance of CRT-related morbidity but also for the improvement in the survival rate of patients with LARC.
Tumor-infiltrating lymphocytes (TILs) are frequently found in tumor tissue, which suggest a tumor-associated immune response within the tumor microenvironment [6–8]. Moreover, many studies have reported that a high abundance of TILs is associated with a favorable clinical outcome [9, 10]. High infiltration of tumors with effector and memory T cells also correlates with improved relapse-free and overall survival in patients with CRC [11, 12]. Based on these pioneering studies and many subsequent investigations, the presence of CD8+ TILs has been established as an independent prognostic factor in CRC and has an even stronger prognostic significance than conventional TNM staging [13, 14].
However, the recruitment of TILs requires several preparatory processes. First is the release of damage-associated molecular pattern molecules (DAMPs) by dying cancer cells and stressed cancer cells, such as high-mobility group box 1 (HMGB1), heat shock protein 70 (Hsp70), ATP and calreticulin (CRT), and by tumors. Among these DAMPs, HMGB1 normally localizes in the nucleus under physiologic conditions. When cells are under stress or affected by chemotherapy and radiotherapy, HMGB1 is translocated and released into the extracellular matrix, where it promotes immune responses via the activation of dendritic cell (DC) maturation [15, 16]. Second, HMGB1 binds to surface receptors (Toll-like receptor 4, TLR4) of immature DCs, which leads to the maturation of DCs; this is followed by tumor neoantigen processing and presentation to CD4+ and CD8+ T cells. Furthermore, germ-line single-nucleotide polymorphisms (SNPs) in TLR4 such as Asp299Gly (rs4986790) and Thr399Ile (rs4986791) are known to reduce the interaction between TLR4 and the danger signal HMGB1 [17]. Defects in the binding of HMGB1 to the mutated TLR4 receptor impair the maturation of DCs and negatively affect the capacity of DCs to cross-present antigens to cytotoxic T cells. It has also been reported that patients who carry those loss-of-function allele of TLR4 present with accelerated tumor progression after chemotherapy and have a shortened disease-free survival [17–19], which suggests that HMGB1 release and TLR4 polymorphisms may be able to predict clinical outcome after chemoradiotherapy.
The programmed death 1 receptor (PD-1, also known as CD279) is one of the receptors that is similar to cytotoxic T-lymphocyte antigen 4 (CTLA4) and was reported to be involved in dampening anti-tumor T-cell responses [20, 21]. The blockade of CTLA-4 and PD-1 with their ligands using blocking antibodies alone or in combination has been shown to unleash an immune response against melanoma [22, 23], renal cell carcinoma [22], and non–small cell lung cancer [22]. The anti-tumor responses observed in these clinical trials support the presence of naturally occurring tumor-reactive CD8+ T cells and indicate their immunotherapeutic potential. Despite the well-accepted negative regulatory role of PD-1 in T cells, few studies have established the idea that expression of PD-1 on CD8+ TILs represents the repertoire of clonally expanded tumor-reactive, mutation-specific lymphocytes, which reveals a dual role of PD-1 expression in the tumor microenvironment [24, 25]. Moreover, a high number of PD-1+ lymphocytes predict a favorable outcome for follicular lymphoma [21].
In the current study, we found that patients with high cyto-HMGB1 translocation and/or PD1+ TILs in the tumor microenvironment, demonstrate better local control before neoCRT, a low distant metastasis (DM) rate, a better disease-free survival (DFS) rate and a better overall survival (OS) rate in patients with LARC.
Materials and methods
Patient characteristics
One hundred twenty-one patients with locally advanced rectal cancer were treated at our hospital from 2006 to 2013. Among these patients, 89 received neoCRT followed by surgery. Patients with biopsy-proven locally advanced rectal cancer (cT3-4 or cN+ by endorectal ultrasonography, computed tomography, or magnetic resonance imaging) and treated with preoperative chemoradiotherapy followed by radical resection at China Medical University Hospital were the study cohort. This study was reviewed and approved by the Internal Review Board (IRB) of China Medical University Hospital [Protocol number: CMUH105-REC2-072]. Patients with concurrent distant metastasis or concurrent inflammatory bowel disease, hereditary colorectal cancer syndromes, concurrent malignancy, emergent surgery, prior history of radiotherapy to the pelvis, or prior history of malignancy were excluded from this study. Tumors were staged based on the American Joint Committee on Cancer (AJCC) staging system. Survival time was defined as the time from surgery until death.
Table 1 presents the clinical pathological characteristics of these patients. The mean age at diagnosis was 59.2 ± 12.9 years (31–90 years of age). The majority of patients were men (70.0%). The median distance from the tumor to the anal verge was 7 cm (53.9%). Most tumors were cT3 at preoperative evaluation (82.0%). The median radiation dose was 50.4 Gy given in 28 fractions (minimal dose: 44.8 Gy; and maximal dose: 50.4 Gy). Concurrent chemotherapy was fluorouracil-based in 39.3% and capecitabine in 60.7% of patients. All patients underwent total or tumor-specific mesorectal excision depending on the extent and location of the tumor after neoCRT.
Table 1.
Patient and tumor characteristics (Pre-neoCRT, n = 89)
| Clinicopathological parameters | Total cases | Cyto-HMGB1 translocation | p value | PD-1+ TILs | p value | Cyto-HMGB1 and/or PD-1+ TILs | p value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| High | Low | High | Low | High | Low | |||||
| 89 (100%) | 44 (49%) | 45 (51%) | 29 (33%) | 60 (67%) | 56 (63%) | 33 (37%) | ||||
| cN stage | 0.24 | 0.17 | 0.68 | |||||||
| Negative | 46 (52%) | 20 (45%) | 26 (58%) | 18 (62%) | 28 (47%) | 28 (50%) | 18 (55%) | |||
| Positive | 43 (48%) | 24 (56%) | 19 (42%) | 11 (38%) | 32 (53%) | 28 (50%) | 15 (45%) | |||
| pN stage | 0.64 | 0.03* | 0.2 | |||||||
| Negative | 71 (80%) | 36 (82%) | 35 (78%) | 27 (93%) | 44 (73%) | 47 (84%) | 24 (73%) | |||
| Positive | 18 (20%) | 8 (18%) | 10 (22%) | 2 (7%) | 16 (27%) | 9 (16%) | 9 (27%) | |||
| Primary response | 1.00 | 0.36 | 0.82 | |||||||
| CR | 11 (12%) | 5 (11%) | 6 (13%) | 3 (10%) | 8 (13%) | 6 (11%) | 5 (15%) | |||
| PR | 40 (45%) | 20 (44%) | 20 (44%) | 15 (52%) | 25 (42%) | 26 (47%) | 14 (42%) | |||
| SD | 32 (36%) | 16 (36%) | 16 (36%) | 11 (38%) | 21 (35%) | 20 (36%) | 11 (33%) | |||
| PD | 6 (7%) | 3 (7%) | 3 (7%) | 0 (0%) | 6 (10%) | 3 (5%) | 3 (9%) | |||
| Tumor regression grade (TRG) | 0.02* | 0.35 | 0.05 | |||||||
| 4 | 12 (14%) | 5 (11%) | 7 (16%) | 3 (10%) | 9 (15%) | 6 (11%) | 6 (18%) | |||
| 3 | 52 (58%) | 32 (73%) | 20 (44%) | 21 (72%) | 31 (52%) | 38 (68%) | 14 (42%) | |||
| 2 | 16 (18%) | 3 (7%) | 13 (29%) | 3 (10%) | 13 (22%) | 6 (11%) | 10 (30%) | |||
| 1 | 9 (10%) | 4 (9%) | 5 (11%) | 2 (7%) | 7 (12%) | 6 (11%) | 3 (9%) | |||
| Chemotherapy | 0.01* | 0.97 | 0.04* | |||||||
| Xeloda | 53 (60%) | 32 (73%) | 21 (47%) | 17 (59%) | 36 (60%) | 37 (66%) | 16 (48%) | |||
| UFT | 30 (34%) | 9 (20%) | 21 (47%) | 11 (38%) | 19 (32%) | 15 (27%) | 15 (45%) | |||
| FOLFOX | 1 (1%) | 0 (0%) | 1 (2%) | 0 (0%) | 1 (2%) | 0 (0%) | 1 (3%) | |||
| 5-FU | 4 (4%) | 3 (7%) | 1 (2%) | 1 (3%) | 3 (5%) | 4 (7%) | 0 (0%) | |||
| Xeloda+UFT | 1 (1%) | 0 (0%) | 1 (2%) | 0 (0%) | 1 (2%) | 0 (0%) | 1 (3%) | |||
Fisher’s exact test was used when > 25% of the cells have expected counts < 5
cN stage negative (Stage 0) vs positive (Stage 1+ 2), pN stage negative (Stage 0 + 1a + X) vs positive (Stage 1b + 2), CR complete response, PR partial response, SD stable disease, PD progression disease, TRG tumor regression grade
Clinical staging, treatment, and pathological evaluation
EUS, MRI or CT was used to assess the pretreatment clinical stage. All pretreatment biopsies were reviewed by pathologists, as previously described [26]. All patients underwent colonoscopic evaluation to exclude synchronous tumors, and digital rectal examination and proctoscopy to identify the tumor distance from the anal verge. Patients were treated with chemoradiotherapy with a median radiotherapy dose of 50.4 Gy and concurrent fluoropyrimidine-based chemotherapy (mainly single-agent infusional capecitabine). After chemoradiotherapy regime was completed, surgery was performed 6 to 8 weeks later. Low anterior resection, proctectomy with coloanal reconstruction, abdominoperineal resection, or multivisceral rectal resection was included according to total mesorectal excision principles. Neoadjuvant chemotherapy was recommended for patients with metastatic lymph node(s) in surgical specimens and consisted of infusional fluorouracil or capecitabine for a period of 4–6 months. Oxaliplatin-containing regimens were introduced later at the discretion of the treating physician.
Resected specimen pathologic staging was performed after resection in accordance with the guidelines of the College of American Pathologists. Expert pathologists in gastrointestinal cancer rendered the histopathology diagnosis. Pathologic complete response (pCR) is the absence of viable adenocarcinoma cells in the surgical specimen (ypT0N0).
Construction of the tissue microarray (TMA)
Tissue microarrays were constructed from pre-neoCRT biopsies and post-neoCRT surgical tissue from rectal cancer patients (n = 89) (Table 1). Areas of tumor are marked on the hematoxylin & eosin (H & E)-stained slides. The corresponding area on the matching paraffin block (donor block) was then identified and marked. We used the AutoTiss 10C system (EverBio Technology Inc., Taiwan) to remove the tissue core from these areas of the donor blocks into the recipient block in a precise, arrayed fashion. The punches were 2 mm in diameter, and a maximum of 60 punches were placed on a single block. Sample sections (cut on a microtome) were then mounted on capillary-gap slides (Dako, Hamburg, Germany) and baked overnight.
Antibodies and reagents
These antibodies used in this study are as follows: anti-β-actin (sc-8432, Santa Cruz, CA, USA), anti-HMGB1 (ab18256, Abcam, Cambridge, UK), anti-PD1 (ab137132, Abcam, Cambridge, UK) and anti-p-NFκB (#3033, Cell Signaling Technology, MA, USA). All secondary antibodies (HRP-conjugated anti-rabbit, anti-mouse and anti-goat) were purchased from Santa Cruz Biotechnology. Recombinant HMGB1 proteins were purchased from Sigma (MO, USA).
Cell culture and treatment
Two colorectal cancer cell lines (SW480 and SW620) were obtained from the American Type Culture Collection (ATCC). Cells were cultured and maintained in RPMI1640 supplemented with 10% fetal bovine serum (Life Technologies, Grand Island, New York, USA), 2 mM glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin, and 1 mM pyruvate at 37 °C in a humidified atmosphere of 5% CO2.
SW480-HMGB1-GFP cells, which stably expressed HMGB1-GFP in SW480 cells, were seeded onto a six-well plate at ~ 80% confluence in RPMI1640 supplemented with 10% FBS on the day before radiation treatment. After exposure to radiation, cells were fixed at the indicated time using 4% paraformaldehyde for 15 min. and then observed using immunofluorescence microscopy.
The radiation dose distributions were measured using EBT3 films (Gafchromic EBT self-developing film for radiotherapy dosimetry, International Specialty Products, ISP, Wayne, NJ) for a small circular 6 MV photon beam 10 mm diameter produced using a Varian Linac.
Western blot analysis
For chemotherapeutic drug treatment, cells were incubated in serum-free media containing different chemotherapeutic drugs for 1 day prior to harvest [27]. The conditioned medium was collected by ultrafiltration using a Microcon filter (10,000-Da cutoff; Millipore, MA) to purify secreted proteins. Total lysates (30 μg) or subcellular fractions (10 μg) were separated using 6–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE), and transferred to a PVDF membrane (GE, Amersham, UK). Membranes were blocked with 5% non-fat milk and probed with specific antibodies overnight at 4 °C. Horseradish peroxidase (HRP)-conjugated secondary antibodies (1:10,000, GE Healthcare, Amersham, UK) and the Immobilon western chemiluminescent HRP Substrate (Millipore, MA, USA) were used. Western blot densitometry analysis was performed using the AlphaImager2200 digital imaging system (Digital Imaging System, CA, USA). Digital images were processed with Adobe Photoshop 7.0. Each blot was stripped in Restore Western Blot Stripping Buffer (Pierce, IO, USA) and incubated with the other antibodies. The results were assessed via Image J software (NIH, MD, USA).
Immunohistochemistry
TMA slides were stained individually with horseradish peroxidase-conjugated avidin biotin complex (ABC) using a Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA, USA) and NovaRed chromogen (Vector Laboratories) and were counterstained in hematoxylin. Staining for PD-1 was positive when detected in the cytoplasm or in the cell membrane of tumor-infiltrating lymphocytes (TILs). HMGB1 was positive when detected in the nucleus and cytoplasm of tumor cells. The sections were evaluated for PD-1 or HMGB1 staining patterns using microscopy (OLYMPUS BX53, Tokyo, Japan) according to the proportion of positively stained tumor cells and TILs. Two pathologists, blind to all information about the samples, evaluated the infiltration of PD-1+ TILs and cytoplasmic HMGB1 staining. With respect to the detection of PD-1+ TILs, the tissue was reviewed at 40× magnification and the area with the highest density of PD-1+ TILs adjacent to malignant cells was counted at ×400 (No. of PD-1+ TILs/high-power field). The average number of PD-1+ TILs in five high-power fields was included in the evaluation. For PD-1, a count of zero PD-1+ TILs in a high-power field was given a score of 0, 1–3 PD-1+ TILs was given a score of 1, 4–10 PD-1+ TILs was given a score of 2, and > 10 PD-1+ TILs was given a score of 3. For cyto-HMGB1, the cytosolic expression was evaluated on a semi-quantitative scale, as follows: 0 for absent, 1+ for weak, 2+ for moderate, and 3+ for strong cytoplasmic staining intensity. The percentage of cyto-HMGB1+ cells was recorded as follows: a score of 0 was given when the positive tumor cell proportion ≤ 10%; a score of 1 was given when the positive cell proportion ranged from 11 to 25%; a score of 2 was given when the positive cell proportion ranged from 26 to 50%; a score of 3 was given when the positive cell proportion ranged from 51% to 100. The final immunoreactivity scoring system was determined by multiplying the staining intensity (SI) and the percentage of positivity scores (PP) [28], with a minimum score of 0 and a maximum score of 9. The basis on cyto-HMGB1 staining is > 25% cells stained with moderate cyto-HMGB1 were considered as “High”. Using score of 3 as a cutoff, the immunostains were defined as ‘low’ for scores from 0 to 3 and as ‘high’ for scores from 4 to 9 (Supplementary Table 1).
Statistical analysis
SAS statistical software, version PC 9.4 (SAS Institute, Cary, NC, USA), performed the statistical analysis. All tests reported two-sided p value with the significance level set at 0.05. Student’s t test, Pearson Chi-square and Fisher’s exact test performed group comparisons. Cox regression analysis was used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for univariate and multivariate models. Influential factors that affect the rectal cancer patient survival rate were adjusted in the Cox models, including sex (female vs male), age (< 65 versus ≥ 65), TRG (4 vs 1 & 2 & 3), primary response (CR vs PR, stable disease and progression), and ypN (positive vs negative). The Kaplan–Meier estimation method assessed the three-year overall survival, disease-free survival, non-recurrent survival, and the survival after distant metastasis. The univariate comparison was performed using the log-rank test.
Results
Cytosolic HMGB1 translocation and PD-1+ TILs in biopsy specimens before neoCRT
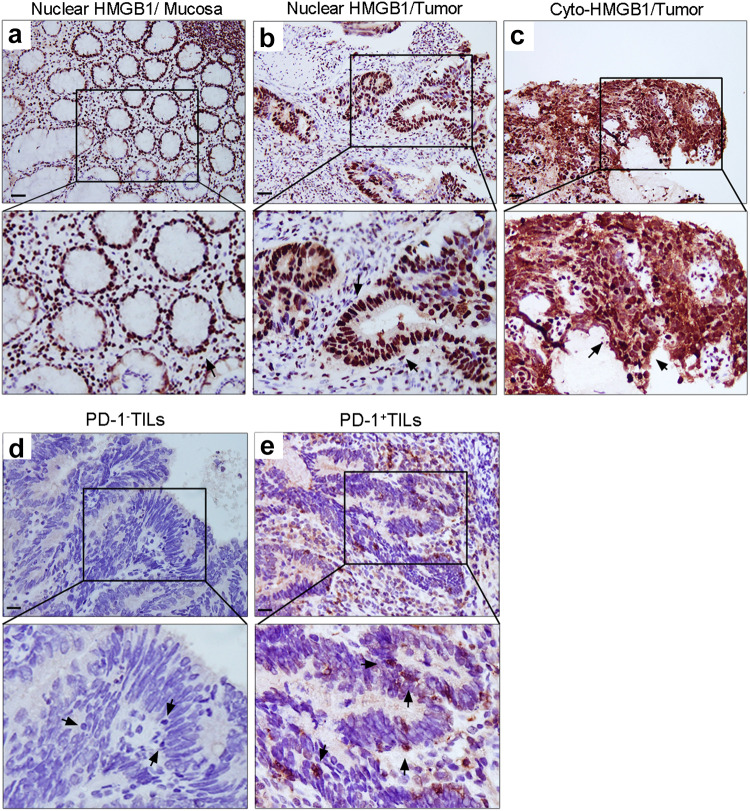
Surgical specimens were reviewed and scored based on the Tumor Regression Grade (TRG) system [29]. In all, 13.5% (12/89) of patients exhibited a pathologic complete response (pCR, TRG 4), while 86.5% (77/89) of patients exhibited a pathologic partial response (TRG 1–3). We first examined the expression pattern of the danger signal HMGB1 in pretreatment biopsy specimens using an immunohistochemical approach in a rectal cancer tissue microarray (TMA) [30]. All of the cases were scored based on their cytoplasmic staining and intensity. HMGB1 staining was present not only in the nuclei of adjacent normal mucosa (Fig. 1a), but the co-expression of nuclear and cytoplasmic HMGB1 (co-expression pattern) was also observed in a subset of cancer tissues [44/89 (49.4%); Fig. 1c, arrow]. Patients whose biopsy specimens demonstrated high cytosolic translocation of HMGB1 had better outcomes according to the tumor regression grade (TRG, p = 0.02) system (Table 1).
Fig. 1.
Expression patterns of HMGB1+ and PD-1+ tumor-infiltrating lymphocytes (TILs) within tumor microenvironment. a Nuclear staining of HMGB1 (200×) in pretreatment normal mucosa obtained by biopsy. b Nuclear staining of HMGB1 (200×) in pretreatment biopsy tumor tissue. c Nuclear/cytoplasmic staining of HMGB1 (200×) in pretreatment biopsy tumor tissue. d PD-1-negative TILs (200×) in pretreatment biopsy tumor tissue. e PD-1-positive TILs (200×) in pretreatment biopsy tumor tissue. Scale bar = 20 µm
Recent studies have found that PD-1+ TILs accurately identified the repertoire of clonally expanded tumor-reactive cells in the tumor microenvironment [24, 25]. We, therefore, examined the pattern of PD-1 expression on tumor-infiltrating lymphocytes and found that a substantial proportion of TILs-expressed PD-1 (29/89 [32.5%]; Fig. 1d, e, arrow). The expression of PD-1 on TILs was correlated with the pathologic lymph node stage (pN stage = 0.03) (Table 1).
Since HMGB1 translocation triggered the maturation of DCs as well as antigen presentation, it is reasonable to classify tumors that show cytosolic HMGB1 translocation and PD-1+ TILs in the tumor microenvironment as derived from patients with more favorable adaptive immunity. Patients with high cyto-HMGB1 and/or PD-1+ TILs were found in a substantial proportion of LARC cases (56/ 89 [62.9%]), while the remaining 37.1% (33/89) of patients were deemed to have inactive adaptive immunity, low levels of cyto-HMGB1 translocation/PD-1+ TILs in their biopsy (Table 1).
Prognostic impact of cyto-HMGB1 and/or PD-1+ TILs on treatment outcome
Moreover, patients with a high level of cytosolic HMGB1 translocation evident in their pretreatment biopsy specimen demonstrated no local recurrence (LR, 0/44 = 0%) compared with those with a low level of HMGB1 translocation (10/45 = 22%, p = 0.001, Supplementary Table 2). Patients with a high level of cytosolic HMGB1 translocation also demonstrated a lower distant metastasis (DM) rate (5 vs 22%, p = 0.01). However, patients with high numbers of PD-1+ TILs within the tumor microenvironment alone did not demonstrate significant differences with respect to the local recurrence rate and the distant metastasis rate compared with those with low numbers of PD-1+ TILs.
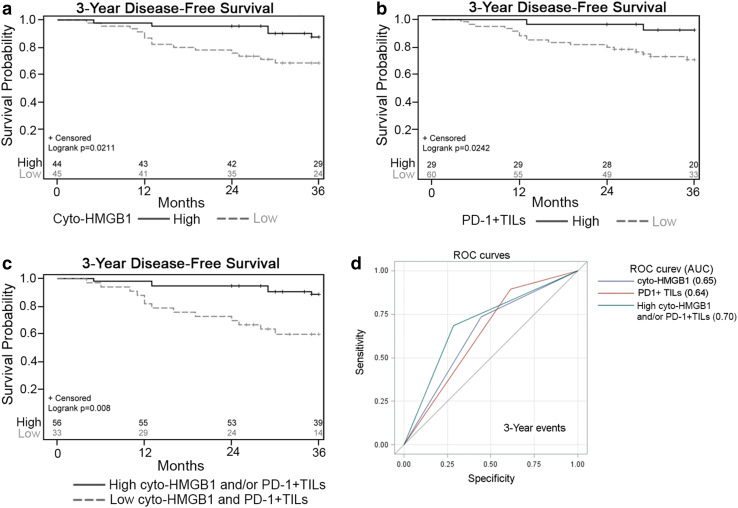
Since it is necessary that immune-surveillance and immune-scavenging cooperate to achieve tumor control, we found that patients with high cyto-HMGB1 and/or PD-1+ TILs were significantly likely to experience a low local recurrence rate (LR, p = 0.001) and distant metastasis rate (DM, p = 0.006, Supplementary Table 2). We then assessed survival outcomes, specifically overall survival (OS) and disease-free survival (DFS), among LARC patients. Within the 3-year follow-up period, 19 patients (21.3%) died. The estimated DFS was 78% (Table 2), and the estimated OS was 92%. For patients with high cyto-HMGB1 release and PD-1+ TILs, the estimated DFS was 87 and 93%, respectively (Table 2). Kaplan–Meier survival analysis indicated that high cyto-HMGB1 release and PD-1+ TILs were associated with better DFS, respectively (p = 0.0211 and p = 0.0242; Fig. 2a, b).
Table 2.
3-year survival outcome in LARC patients by clinicopathologic parameters, cyto-HMGB1 and/or PD-1+ TILs (Pre-neoCRT, n = 89)
| Variable | Total cases | 3-Year DFS | |
|---|---|---|---|
| % | p value* | ||
| 89 | 78 | ||
| cN stage | 0.7 | ||
| Negative | 46 | 79 | |
| Positive | 43 | 76 | |
| pN stage | 0.003* | ||
| Negative | 71 | 83 | |
| Positive | 18 | 56 | |
| Primary response | 0.72 | ||
| Good response | 11 | 82 | |
| Poor response | 78 | 77 | |
| TRG | 0.58 | ||
| pCR | 12 | 75 | |
| pPR | 77 | 78 | |
| Cyto-HMGB1 translocation | 0.02* | ||
| High | 44 | 87 | |
| Low | 45 | 68 | |
| PD-1+ TILs | 0.02* | ||
| High | 29 | 93 | |
| Low | 60 | 71 | |
| Cyto-HMGB1 and/or PD-1+ TILs | 0.008* | ||
| High | 56 | 88 | |
| Low | 33 | 60 | |
Kaplan–Meier method was used for survival analysis
cN stage positive (Stage 1+ 2) vs negative (Stage 0), pN stage positive (Stage 1b + 2) vs negative (Stage 0 + 1a + X), primary response good response (complete response, CR) vs poor response (PR + SD + PD), TRG pCR (pathological complete response, Grade 4) vs pPR (pathological partial response, grade 1+ 2 + 3), SE standard error
*p value was obtained from log-rank test
Fig. 2.
The association between disease-free survival (DFS) and cyto-HMGB1 and/or PD-1+ TILs in LARC. a Kaplan–Meier curves show that cyto-HMGB1 within the tumor microenvironment is associated with 3-year DFS (p = 0.0211). b Kaplan–Meier curves show that PD-1+ TILs within the tumor microenvironment is associated with 3-year DFS (p = 0.0242). c Kaplan–Meier curves show that the level of cyto-HMGB1 and/or PD-1+ TILs within the tumor microenvironment is associated with 3-year DFS (p = 0.008). d ROC curves show sensitivity/specificity for predicting clinical outcome in LARC patients receiving neoCRT using the level of cyto-HMGB1 and/or PD-1+ TILs
Next, we assessed the survival differences between groups classified by these two factors. For patients with high cyto-HMGB1 and/or PD-1+ TILs, the estimated DFS was 88%, and the estimated OS was 94%. In comparison, patients with less cyto-HMGB1 and/or PD-1+ TILs had a DFS of 60% and an OS of 87%. The differences in DFS between the two groups were statistically significant (p = 0.008, Table 2). Kaplan–Meier survival analysis indicated that high cyto-HMGB1 and/or PD-1+ TILs were associated with a better disease-free status (p = 0.008; Fig. 2c), lower local recurrence rate (p = 0.01, Supplementary Fig. 1a), and a lower distant metastasis rate (p = 0.006, Supplementary Fig. 1b).
In the univariate analysis, patients with low cyto-HMGB1 (HR = 3.12, 95% CI = 1.12–8.65, p = 0.03) or low PD-1+ TILs (HR = 4.61, 95% CI = 1.06–19.94, p = 0.04) had an increased risk of poor DFS. Moreover, patient with low cyto-HMGB1 and PD-1+ TILs within tumor microenvironment of pretreatment biopsy also had a significant risk of DFS (HR = 4.54, 95% CI = 1.72–11.97, p = 0.002, Table 3). The overall area under curves (AUCs) was 0.65 for cyto-HMGB1 and 0.64 for PD-1+ TILs. However, compared to the 2-factor combination model (cyto-HMGB1/PD-1+ TILs, p = 0.008, Fig. 2c), the results of the ROC curve showed that high cyto-HMGB1 and/or PD-1+ TILs is a more powerful predictive model (Fig. 2d, AUC = 0.70). These results indicate that the cyto-HMGB1 and/or PD-1+ TILs significantly elevated the diagnostic accuracy for LARC patient.
Table 3.
Univariate and multivariate analysis of cyto-HMGB1 and/or PD-1+ TILs on survival outcome (Pre-neoCRT, n = 89)
| 3-Year DFS | ||||||
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Age (≥ 65 vs < 65) | 1 (0.00–3.00) | 1.00 | 0.96 (0.34–2.72) | 0.95 | 0.97 (0.34–2.81) | 0.96 |
| Sex (female vs male) | 0.87 (0.34–2.23) | 0.08 | 0.73 (0.24–2.19) | 0.57 | 0.68 (0.22–2.06) | 0.50 |
| pN stage (positive vs negative) | 2.76 (1.17–6.52) | 0.02* | 3.51 (1.23–9.97) | 0.02* | 3.70 (1.33–10.31) | 0.01* |
| Primary response (CR vs others) | 0.99 (0.29–3.33) | 0.98 | 3.54 (0.27–47.11) | 0.34 | 3.49 (0.26–47.10) | 0.35 |
| TRG (pCR vs pPR) | 1.35 (0.46–3.98) | 0.59 | 3.85 (0.43–34.10) | 0.23 | 3.75 (0.42–33.15) | 0.23 |
| Cyto-HMGB1 translocation (low vs high) | 3.12 (1.12–8.65) | 0.03* | 3.15 (1.10-9.00) | 0.03* | – | |
| PD-1+ TILs (low vs high) | 4.61 (1.06–19.94) | 0.04* | 2.99 (0.65–13.68) | 0.16 | – | |
| cyto-HMGB1 and/or PD-1+ TILs (low vs high) | 4.54 (1.72–11.97) | 0.002* | – | 4.09 (1.51–11.10) | 0.006* | |
Age: ≥65 vs < 65; sex: female vs male; pN stage: positive (Stage 1b + 2) vs negative (Stage 0 + 1a + X); primary response: CR (complete response) vs others (PR + SD + PD); TRG: pCR (pathological complete response, Grade 4) vs pPR (pathological partial response, grade 1+ 2 + 3)
Moreover, in the multivariate analysis, patients with low cyto-HMGB1 and PD-1+ TILs in the tumor microenvironment presented increased risk for poor DFS (HR = 4.09, 95% CI = 1.51–11.10, p = 0.006, Table 3), local recurrence (HR = 5.17, 95% CI = 1.59–16.85, p = 0.006, Supplementary Table 3), and distant metastasis (HR = 3.28, 95% CI = 1.19–9.05, p = 0.02, Supplementary Table 3). These results showed that combinational cyto-HMGB1 and/or PD-1+ TILs is an independent prognostic factor (Table 3).
Radiotherapy and chemotherapy trigger cytosolic translocation of HMGB1 in CRC
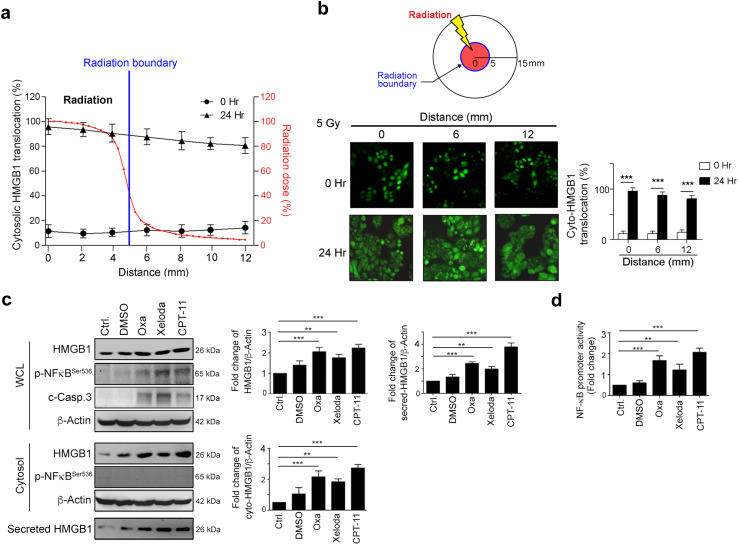
HMGB1 release is associated with environmental stresses, such as inflammation, hypoxia, and chemoradiotherapy [31]. It has been reported that CRC patients have an increased serum level of HMGB1, which is caused by a systemic inflammatory response or a local reaction within the tumor microenvironment [32]. To examine whether the phenomenon of bystander effect (out-of-field) contributes to the HMGB1 translocation/release in the tumor microenvironment, we used SW480-HMGB1-GFP cells, which stably expressed HMGB1-GFP, and exposed them to 5 Gy using a customized circular radiation field 10 mm in diameter. First, we measured the relative radiation dose from the central zone to the boundary (Fig. 3a). At the boundary zone (5 mm away from the isocenter), the relative radiation dose was 41.2% that of the central zone. Moreover, as seen in Fig. 3a, b, we found that cytosolic HMGB1 translocation still occurred at the marginal region beyond the area of direct radiation exposure (12 mm from the isocenter with only 4.5% of the isocenter dose). These in vitro results indicated that local radiation could trigger cytosolic HMGB1 translocation beyond the radiation field.
Fig. 3.
Radiotherapy and chemotherapeutic agents fostered cytosolic HMGB1 translocation in CRC. a The relative radiation dose and cyto-HMGB1 distribution in terms of distance. The radiation dose distributions were measured using EBT3 films for a small circular 6 MV photon beam of 10 mm diameter produced by a Varian Linac. b SW480-HMGB1-GFP cells were exposed to 5 Gy radiations and fixed at the indicated time points by 4% paraformaldehyde for 15 min. After fixation, the location of GFP-HMGB1 was observed using immunofluorescent microscopy. In all, 100 cells were scored and the quantification of these results is shown, ***p < 0.001. c SW620 cells were treated with oxaliplatin (10 μM), Xeloda (capecitabine, 10 μM) and CPT-11 (5 μM) for 24 h. Cell lysates and medium were then analyzed using immunoblotting, and the quantification of these results is shown (n = 3). **p < 0.01 and ***p < 0.001. d SW620 cells were treated with DMSO (DMSO, 0.05%), oxaliplatin (10 μM), Xeloda (capecitabine, 10 μM) and CPT-11 (5 μM) for 24 h. The NF-κB promoter activities were analyzed using luciferase assays. These results were quantified (n = 3). **p < 0.01 and ***p < 0.001. These data were obtained from at least three independent experiments. The values represent the means ± S.D
Furthermore, the common chemotherapy drugs used in CRCs, such as Capecitabine (Xeloda), Irinotecan (CPT-11), and oxaliplatin, all up-regulated HMGB1, triggered cytosolic translocation and released HMGB1 to the extracellular space. The upregulation of HMGB1 is associated with the NF-κB signal transduction pathway (Fig. 3c, d). These results provided evidence that chemoradiotherapy drugs exert their tumor killing function not only by direct cytotoxic events but also potentially via triggering an adaptive immune response by DAMP signaling.
Discussion
Here, we showed that the cytosolic translocation and extracellular release of HMGB1 and the presence of PD-1+ TILs in the tumor microenvironment were significant predictive factors in LARC. Such a combination implies active immune-surveillance by the host and the presence of tumor scavenging events in tumors. Our study is the first to show that the active adaptive immunity in LARC is associated with significantly better local control, less distant metastasis and prolonged DFS.
One of the mechanisms that enhances the tumoricidal effect of radiotherapy or chemotherapy is the induction of immunogenic cell death (ICD) [33]. ICD is characterized by the release of DAMP from stressed or dying tumor cells after chemotherapy and/or radiotherapy [34]. Among various DAMPs, HMGB1 is pivotal to tumor antigen-specific T-cell immunity [35, 36]. HMGB1 release enhances the engulfment of antigenic components by DCs through TLR4 and mediates cross-presentation of tumor antigens onto CD4 and CD8 T cells, which effectively leads to tumor antigen-specific T-cell responses for tumor immune-surveillance [35, 36]. In the current study, we showed that cytosolic HMGB1 translocation served as a surrogate marker, likely via its function in adaptive immune response, to determine the therapeutic outcome of patients with LARC. The magnitude of cytosolic translocation of HMGB1 in the tumor microenvironment before neoCRT positively correlated with patient survival. Attractively, previous studies have shown that several post-translational modifications, such as acetylation, are involved in the translocation of HMGB1 to the cytoplasm and its subsequent secretion [37, 38]. Hyperacetylation of HMGB1 via JAK/STAT1 signaling redirected this protein to the cytoplasm in preparation for HMGB1 secretion during inflammation [37, 38]. Moreover, inflammation and TILs were also found in the tumor microenvironment before neoCRT. These observations strongly support the idea that the state of tumor immunogenicity might predetermine the outcome of patients who receive neoCRT. Most importantly, this pivotal predictive information can be obtained from pretreatment biopsy specimens and from a patient’s blood, as HMGB1 is released and may be detected in the peripheral blood. It has been reported that an increase in plasma HMGB1 occurs within 3 days after patients undergo neoadjuvant chemotherapy for breast and esophageal cancers [39, 40]. In addition, HMGB1 was significantly upregulated within the tumor microenvironment in patients who underwent preoperative chemoradiotherapy for esophageal cancer. The level of HMGB1 in the serum after chemoradiotherapy positively correlated with tumor antigen-specific T-cell responses and patient survival [41]. It is reasonable to assume that HMGB1 translocation triggers the cascade of tumor scavenging through the activation of the host immune-surveillance system, which enhances therapeutic efficacy.
Moreover, the neutralization or knockdown of HMGB1 or its receptor TLR4 on DCs has been reported to be associated with reduced anticancer immune response and has been shown to be correlated with poor treatment outcomes [42, 43]. Patients who carry a loss-of-function allele of TLR4, exhibit accelerated tumor relapses after chemotherapy and shortened disease-free survival in several cancer types. Thus, these observations strongly indicated that the HMGB1-related immune response after chemoradiotherapy may play a critical role in cancer treatments and that cytosolic HMGB1 translocation and TLR4 polymorphisms could serve as biomarkers to direct patients toward the most effective therapy.
Tumor-infiltrating lymphocytes have been reported to have clinical significance in rectal cancer [7, 44] and colorectal cancer [8, 11, 12]. Teng et al., indicated that high densities of TILs before treatment are associated with a good response to neoadjuvant chemoradiotherapy and a favorable prognosis in patients with LARC [7]. On the contrary, PD-1 plays an important role to attenuate T-cell activation and promote T-cell tolerance [45]. PD-1 also participates in the regulation of the immune response against cancer cells and facilitates the evasion of the immune system by tumor cells [46]. Moreover, recent reports have indicated that PD-1+ TILs represent a subpopulation of CD8+ immune effector cells. Here, we found that the density of PD-1+ TILs in tumor tissues predicts better therapeutic results. In line with our observation, it has been reported that the presence of CD8+/PD-1+ lymphocytes can serve as a biomarker for the identification of the tumor-reactive repertoire of immune cells [24, 25] and act as a predictor of a better overall survival rate in follicular lymphoma [21]. Moreover, recent studies supported our finding that high HMGB1 expression in cytoplasm was associated with abundant TILs [47]. Lee et al., indicated that high cyto-HMGB1 expression was associated with high numbers of CD8+ cells in the breast cancer, suggesting that cyto-HMGB1 expression is involved in DC maturation for antigen presentation and TILs recruitment within tumor microenvironment.
In summary, our results showed that patients with locally advanced rectal cancer and with high cyto-HMGB1 and PD-1+ TILs before neoCRT had better outcomes. Cytosolic HMGB1 translocation, which activates the maturation of DCs via TLR4 and recruits PD-1+ TILs within the tumor microenvironment, can serve as a surrogate of immune-surveillance (sending out danger signals) and immune-scavenging (sending in effector cells). Hence, HMGB1 can not only serve as predictive biomarkers before treatment but may also help to verify the status of patients during neoCRT. We are conducting a prospective study to further uncover how HMGB1 and PD-1+ TILs evolve during a course of chemoradiotherapy. This study involves the longitudinal collection of tissues and blood from patients who are undergoing neoCRT for LARC. A better understanding of adaptive immune activity will pave the way for future precision immunotherapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful for the tissue microarray (TMA) support from the Translation Research Core, China Medical University Hospital. Ms. Vicky Lin assisted in the preparation of the manuscript.
Abbreviations
- AUC
Area under curve
- CRC
Colorectal cancer
- DAMPs
Damage-associated molecular pattern molecules
- DC
Dendritic cell
- DFS
Disease-free survival
- DM
Distant metastasis
- HMGB1
High-mobility group box 1
- ICD
Immunogenic cell death
- LARC
Locally advanced rectal cancer
- LR
Local recurrence
- neoCRT
Neoadjuvant chemoradiotherapy
- OS
Overall survival
- pCR
Pathologic complete response
- PD-1
Programmed death 1 receptor
- pN stage
Pathologic lymph node stage
- pPR
Pathologic partial response
- ROC
Receiver operating characteristic
- TILs
Tumor-infiltrating lymphocytes
- TLR4
Toll-like receptor 4
- TMA
Tissue microarray
- TRG
Tumor regression grade
Author contributions
Chih-Yang Huang and Shu-Fen Chiang conducted and performed the experiments; William Tzu-Liang Chen, Tao-Wei Ke and Tsung-Wei Chen participated in the collection and IHC evaluation of the LARC patients; Yu-Ching Lan and Ying-Shu You performed the statistical analysis; An-Cheng Shiau performed radical experiments; Shu-Fen Chiang, William Tzu-Liang Chen and K. S. Clifford Chao supervised this study; Chih-Yang Huang, Shu-Fen Chiang and K. S. Clifford Chao analyzed the data and wrote the manuscript.
Compliance with ethical standards
Funding resource
This study is supported in part by China Medical University Hospital (DMR-106-107 and DMR-CELL-17,018), Ministry of Science and Technology (MOST106-2314-B-039-005) and Ministry of Health, and Welfare (MOHW106-TDU-B-212-113004).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
This study was reviewed and approved by the Internal Review Board (IRB) of China Medical University Hospital [Protocol number: CMUH105-REC2-072].
Informed consent
Informed consents were obtained from all participants in the study.
Footnotes
William Tzu-Liang Chen and K. S. Clifford Chao contributed equally to this work.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Conde-Muino R, Cuadros M, Zambudio N, Segura-Jimenez I, Cano C, Palma P (2015) Predictive biomarkers to chemoradiation in locally advanced rectal cancer. Biomed Res Int 2015:921435. 10.1155/2015/921435 [DOI] [PMC free article] [PubMed]
- 3.Yoon WH, Kim HJ, Kim CH, Joo JK, Kim YJ, Kim HR. Oncologic impact of pathologic response on clinical outcome after preoperative chemoradiotherapy in locally advanced rectal cancer. Ann Surg Treat Res. 2015;88(1):15–20. doi: 10.4174/astr.2015.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balko JM, Black EP. A gene expression predictor of response to EGFR-targeted therapy stratifies progression-free survival to cetuximab in KRAS wild-type metastatic colorectal cancer. BMC Cancer. 2009;9:145. doi: 10.1186/1471-2407-9-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palma P, Conde-Muino R, Rodriguez-Fernandez A, Segura-Jimenez I, Sanchez-Sanchez R, Martin-Cano J, Gomez-Rio M, Ferron JA, Llamas-Elvira JM. The value of metabolic imaging to predict tumour response after chemoradiation in locally advanced rectal cancer. Radiat Oncol. 2010;5:119. doi: 10.1186/1748-717X-5-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105(1):93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teng F, Mu D, Meng X, Kong L, Zhu H, Liu S, Zhang J, Yu J. Tumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemoradiotherapy and its clinical utility for rectal cancer. Am J Cancer Res. 2015;5(6):2064–2074. [PMC free article] [PubMed] [Google Scholar]
- 8.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 9.Fridman WH, Galon J, Pages F, Tartour E, Sautes-Fridman C, Kroemer G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 2011;71(17):5601–5605. doi: 10.1158/0008-5472.CAN-11-1316. [DOI] [PubMed] [Google Scholar]
- 10.Dieci MV, Criscitiello C, Goubar A, Viale G, Conte P, Guarneri V, Ficarra G, Mathieu MC, Delaloge S, Curigliano G, Andre F. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol. 2014;25(3):611–618. doi: 10.1093/annonc/mdt556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 12.Ohtani H. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human colorectal cancer. Cancer Immun. 2007;7:4. [PMC free article] [PubMed] [Google Scholar]
- 13.Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, Zatloukal K, Trajanoski Z, Berger A, Fridman WH, Galon J. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27(35):5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 14.Halama N, Michel S, Kloor M, Zoernig I, Benner A, Spille A, Pommerencke T, von Knebel DM, Folprecht G, Luber B, Feyen N, Martens UM, Beckhove P, Gnjatic S, Schirmacher P, Herpel E, Weitz J, Grabe N, Jaeger D. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011;71(17):5670–5677. doi: 10.1158/0008-5472.CAN-11-0268. [DOI] [PubMed] [Google Scholar]
- 15.Tesniere A, Apetoh L, Ghiringhelli F, Joza N, Panaretakis T, Kepp O, Schlemmer F, Zitvogel L, Kroemer G. Immunogenic cancer cell death: a key-lock paradigm. Curr Opin Immunol. 2008;20(5):504–511. doi: 10.1016/j.coi.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Kusume A, Sasahira T, Luo Y, Isobe M, Nakagawa N, Tatsumoto N, Fujii K, Ohmori H, Kuniyasu H. Suppression of dendritic cells by HMGB1 is associated with lymph node metastasis of human colon cancer. Pathobiology. 2009;76(4):155–162. doi: 10.1159/000218331. [DOI] [PubMed] [Google Scholar]
- 17.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 18.Bergmann C, Bachmann HS, Bankfalvi A, Lotfi R, Putter C, Wild CA, Schuler PJ, Greve J, Hoffmann TK, Lang S, Scherag A, Lehnerdt GF. Toll-like receptor 4 single-nucleotide polymorphisms Asp299Gly and Thr399Ile in head and neck squamous cell carcinomas. J Transl Med. 2011;9:139. doi: 10.1186/1479-5876-9-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, Mendiboure J, Pignon JP, Jooste V, van Endert P, Ducreux M, Zitvogel L, Piard F, Kroemer G. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- 20.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carreras J, Lopez-Guillermo A, Roncador G, Villamor N, Colomo L, Martinez A, Hamoudi R, Howat WJ, Montserrat E, Campo E. High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J Clin Oncol. 2009;27(9):1470–1476. doi: 10.1200/JCO.2008.18.0513. [DOI] [PubMed] [Google Scholar]
- 22.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inozume T, Hanada K, Wang QJ, Ahmadzadeh M, Wunderlich JR, Rosenberg SA, Yang JC. Selection of CD8 + PD-1 + lymphocytes in fresh human melanomas enriches for tumor-reactive T cells. J Immunother. 2010;33(9):956–964. doi: 10.1097/CJI.0b013e3181fad2b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME, Hanada K, Almeida JR, Darko S, Douek DC, Yang JC, Rosenberg SA. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J Clin Invest. 2014;124(5):2246–2259. doi: 10.1172/JCI73639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park IJ, You YN, Agarwal A, Skibber JM, Rodriguez-Bigas MA, Eng C, Feig BW, Das P, Krishnan S, Crane CH, Hu C-Y, Chang GJ. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30(15):1770–1776. doi: 10.1200/JCO.2011.39.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee H, Song M, Shin N, Shin CH, Min BS, Kim HS, Yoo JS, Kim H. Diagnostic significance of serum HMGB1 in colorectal carcinomas. PLoS One. 2012;7(4):e34318. doi: 10.1371/journal.pone.0034318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fedchenko N, Reifenrath J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue—a review. Diagn Pathol. 2014;9:221. doi: 10.1186/s13000-014-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodel C, Martus P, Papadoupolos T, Fuzesi L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W, Raab R, Sauer R, Wittekind C. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23(34):8688–8696. doi: 10.1200/JCO.2005.02.1329. [DOI] [PubMed] [Google Scholar]
- 30.Clifford Chao KS, Ke WT-LC,Tao-Wei, Chiang S-F, Chen T-W, Huang C-Y, You Y-S, Yuh-Cherng Kuo. Acquired immunity trumps ypN + and TRG as the sole prognostic biomarker for locally advanced rectal cancer (LARC) treated with neoadjuvant chemoradiation therapy (NeoCRT) Int J Radiat Oncol Biol Phys. 2016;96(2S):S108. doi: 10.1016/j.ijrobp.2016.06.265. [DOI] [Google Scholar]
- 31.Kono K, Mimura K. Immunogenic tumor cell death induced by chemoradiotherapy in a clinical setting. Oncoimmunology. 2013;2(1):e22197. doi: 10.4161/onci.22197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ueda M, Takahashi Y, Shinden Y, Sakimura S, Hirata H, Uchi R, Takano Y, Kurashige J, Iguchi T, Eguchi H, Sugimachi K, Yamamoto H, Doki Y, Mori M, Mimori K. Prognostic significance of high mobility group box 1 (HMGB1) expression in patients with colorectal cancer. Anticancer Res. 2014;34(10):5357–5362. [PubMed] [Google Scholar]
- 33.Kroemer G, Senovilla L, Galluzzi L, Andre F, Zitvogel L. Natural and therapy-induced immunosurveillance in breast cancer. Nat Med. 2015;21(10):1128–1138. doi: 10.1038/nm.3944. [DOI] [PubMed] [Google Scholar]
- 34.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 35.Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, Dumitriu IE, Muller S, Iannacone M, Traversari C, Bianchi ME, Manfredi AA. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5(8):825–830. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B, Tracey KJ, Chiorazzi N. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol. 2004;173(1):307–313. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- 37.Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22(20):5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu B, Antoine DJ, Kwan K, Lundback P, Wahamaa H, Schierbeck H, Robinson M, Van Zoelen MA, Yang H, Li J, Erlandsson-Harris H, Chavan SS, Wang H, Andersson U, Tracey KJ. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc Natl Acad Sci USA. 2014;111(8):3068–3073. doi: 10.1073/pnas.1316925111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arnold T, Michlmayr A, Baumann S, Burghuber C, Pluschnig U, Bartsch R, Steger G, Gnant M, Bergmann M, Bachleitner-Hofmann T, Oehler R. Plasma HMGB-1 after the initial dose of epirubicin/docetaxel in cancer. Eur J Clin Invest. 2013;43(3):286–291. doi: 10.1111/eci.12043. [DOI] [PubMed] [Google Scholar]
- 40.Exner R, Sachet M, Arnold T, Zinn-Zinnenburg M, Michlmayr A, Dubsky P, Bartsch R, Steger G, Gnant M, Bergmann M, Bachleitner-Hofmann T, Oehler R. Prognostic value of HMGB1 in early breast cancer patients under neoadjuvant chemotherapy. Cancer Med. 2016;5(9):2350–2358. doi: 10.1002/cam4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki Y, Mimura K, Yoshimoto Y, Watanabe M, Ohkubo Y, Izawa S, Murata K, Fujii H, Nakano T, Kono K. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res. 2012;72(16):3967–3976. doi: 10.1158/0008-5472.CAN-12-0851. [DOI] [PubMed] [Google Scholar]
- 42.Apetoh L, Tesniere A, Ghiringhelli F, Kroemer G, Zitvogel L. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res. 2008;68(11):4026–4030. doi: 10.1158/0008-5472.CAN-08-0427. [DOI] [PubMed] [Google Scholar]
- 43.Kepp O, Tesniere A, Schlemmer F, Michaud M, Senovilla L, Zitvogel L, Kroemer G. Immunogenic cell death modalities and their impact on cancer treatment. Apoptosis. 2009;14(4):364–375. doi: 10.1007/s10495-008-0303-9. [DOI] [PubMed] [Google Scholar]
- 44.Choi CH, Kim WD, Lee SJ, Park WY. Clinical predictive factors of pathologic tumor response after preoperative chemoradiotherapy in rectal cancer. Radiat Oncol J. 2012;30(3):99–107. doi: 10.3857/roj.2012.30.3.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 46.Blank C, Kuball J, Voelkl S, Wiendl H, Becker B, Walter B, Majdic O, Gajewski TF, Theobald M, Andreesen R, Mackensen A. Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell responses in vitro. Int J Cancer. 2006;119(2):317–327. doi: 10.1002/ijc.21775. [DOI] [PubMed] [Google Scholar]
- 47.Lee HJ, Kim A, Song IH, Park IA, Yu JH, Ahn JH, Gong G. Cytoplasmic expression of high mobility group B1 (HMGB1) is associated with tumor-infiltrating lymphocytes (TILs) in breast cancer. Pathol Int. 2016;66(4):202–209. doi: 10.1111/pin.12393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.