Abstract
Lung cancer is the most common malignancy worldwide. Despite significant advances in diagnosis and treatment, mortality rates remain extremely high, close to incidence rates. Several targeted therapies have been recently introduced for the treatment of non-small cell lung cancer (NSCLC), the most common type of lung cancer. Nivolumab, a monoclonal antibody that targets programmed death-1 (PD-1), was the first immune checkpoint inhibitor approved for the treatment of patients with advanced/metastatic NSCLC not responding to platinum-based chemotherapy. Biomarkers predicting response to these therapies would allow early identification of non-responders and timely implementation of appropriate combination strategies, avoiding inadequate and expensive therapies. The role of the neutrophil to lymphocyte ratio and other blood cell count indexes as possible biomarkers of response has been recently investigated. We discuss the encouraging results reported on the topic, provide new data from our personal experience, and discuss opportunities for further research.
Keywords: Lung cancer, Nivolumab, Immunotherapy, Blood cell counts, NLR
Lung cancer is the most common malignancy worldwide [1]. Despite significant advances in diagnosis and treatment, mortality rates remain extremely high, close to incidence rates. This is due to several factors, including the insidious onset, with consequent delay in diagnosis and clinical assessment, the gap in the knowledge of the pathophysiological mechanisms of the disease and the lack of effective treatment strategies, particularly in advanced-stage patients [2].
In the recent years, several targeted therapies have been introduced for the treatment of non-small cell lung cancer (NSCLC), the most common type of lung cancer. Several drugs against specific molecular targets in cancers harboring particular genetic alterations, such as mutations of the epidermal growth factor receptor (EGFR) or the anaplastic lymphoma kinase (ALK) genes, are currently in use. Targeted immunotherapies have been also developed. Nivolumab, a monoclonal antibody that targets programmed death 1 protein (PD-1), was the first immune checkpoint inhibitor approved for the treatment of patients with advanced NSCLC not responding to platinum-based chemotherapy [3, 4]. Used as single, second-line agent in NSCLC, nivolumab showed durable responses in 10 (37%) of 27 confirmed responders with squamous NSCLC and 19 (34%) of 56 with non-squamous NSCLC that had ongoing response after a minimum follow-up of 2 years [5]. This medication, as well as other PD-1 and programmed death-ligand 1 (PDL1) inhibitors such as pembrolizumab and atezolizumab, opened a new era in the treatment of advanced NSCLC.
Biomarkers that are able to differentiate between potential durable responders and non-responders might be particularly useful in guiding clinical decisions, because early identification of non-responders allows timely implementation of appropriate treatment strategies [6]. PDL1 expression on tumor tissue seems to correlate with the activity of immune checkpoint inhibitors (ICI); however, 10% of responders lack PDL1 expression [5]. Therefore, PDL1 positivity alone cannot be considered a biomarker of response, rather a bioselector useful to identify the patient population more likely to benefit from ICI. Recently, the prognostic role of some blood cell count indexes, particularly the neutrophil to lymphocyte ratio (NLR), has also been investigated.
The NLR, a reliable index of systemic inflammation, predicts the severity and prognosis of numerous pathological conditions, including chronic inflammatory diseases [7, 8] and cancer [9–12]. In lung cancer, high pretreatment NLR values have been associated with poor prognosis [12, 13]. Other blood cell count-related indexes, such as the platelet to lymphocyte ratio (PLR), the monocyte to lymphocyte ratio (LMR), and the red cell distribution width (RDW), might also predict outcomes in patients with cancer.
Bagley et al. reviewed the medical records of 175 patients with advanced NSCLC treated with nivolumab at the University of Pennsylvania [14]. The median age at the time of treatment was 68 years (range 33–88; IQR 60–74), 46% of the patients were men, and 75% were Caucasian. Nearly all patients had received prior platinum-based therapy (174 patients, 99%), 12 patients (7%) had received prior EGFR tyrosine kinase inhibitor therapy, and 3 patients (2%) had received prior ALK inhibitor therapy. Seventeen patients (10%) underwent PD-L1 testing on tumor samples. Median baseline NLR was 5.5 (range 0.9–117; IQR 3.1–9.4), with NLR < 5 in 73 patients (42%) and ≥ 5 in 102 patients (58%). Median overall survival (OS) was 6.5 months (95% CI 5.2–8.0 months). In univariate analysis, a NLR ≥ 5 was associated with decreased OS (median 5.5 versus 8.4 months; HR 1.83, 95% CI 1.2–2.8; p = 0.006). In multivariate analysis, a NLR ≥ 5 (HR 2.07, 95% CI 1.3–3.3, p = 0.002), an ECOG PS ≥ 2 (HR 2.49, 95% CI 1.6–3.9, p < 0.001), and liver metastases (HR 2.13, 95% CI 1.3–3.4, p = 0.002) were associated with decreased OS. Furthermore, only a NLR ≥ 5 (HR 1.43, 95% CI 1.02–2.0, p = 0.04) and an ECOG PS ≥ 2 (HR 1.89, 95% CI 1.3–2.8, p = 0.001) remained independently associated with decreased progression-free survival (PFS) in multivariate analysis. Nevertheless, the NLR was not associated with response to nivolumab in this study. The authors concluded that it is unclear whether NLR is predictive or prognostic in the context of PD-1 therapy [14].
Another retrospective study showed that a simple index, the lung immune prognostic index (LIPI), calculated by adding the differential NLR (dNLR, neutrophils/leukocytes minus neutrophils) to the lactate dehydrogenase (LDH), might stratify NSCLC patients treated with ICIs on the basis of their prognostic outcomes. A dNLR greater than 3 and LDH greater than the upper limit of its normal reference range identifies a population with a poor prognosis when treated with ICIs, with a median OS of 3 months compared to the 34 months of those with lower dNLR and LDH values. These results were not confirmed in the cohort treated with chemotherapy [15]. Similarly, another predictive algorithm based on several variables including sex, Eastern Cooperative Oncology Group performance status (ECOG PS), NLR and dNLR (iSEND model) was retrospectively tested in 159 advanced NSCLC patients. The model revealed significant differences in patients with advanced NSCLC treated with nivolumab, with good, intermediate and poor iSEND associated with a mean PFS at 13 months of 52.2%, 25.9% and 17.8%, respectively [6].
Shiroyama et al. studied a total of 201 patients with NSCLC undergoing therapy with nivolumab; median age was 68 years (range 27–87 years), 67% were men, and 24% had an ECOG PS of 2 or higher [16]. Associations between several parameters, including the pretreatment NLR and the advanced lung cancer inflammation index (ALI, body mass index × albumin/NLR) with PFS and early progression of the disease were studied. Pretreatment NLR greater than 4 was significantly associated with poor PFS and early progression on univariate analysis. Multivariate analyses revealed that only pretreatment ALI < 18 was independently associated with reduced PFS and higher likelihood of early progression. Furthermore, Kiriu et al. reported that pretreatment NLR values higher than 5 were associated with poor OS in 19 NSCL patients treated with nivolumab; in addition, the authors showed that a post-treatment NLR greater than 5 was also significantly associated with poor OS [17]. In this study, the NLR increased from its pretreatment values in five out of seven patients with disease progression and in all the four patients that discontinued the treatment due to toxicity. This suggests that the NLR temporal changes might reflecting disease progression in patients undergoing nivolumab therapy for advanced NSCLC.
Recently, Suh et al. published a retrospective study investigating the prognostic role of NLR, PLR and SII (Systemic Inflammation Index), a composite index calculated as the platelet count multiplied by the NLR, at week 6 in patients undergoing therapy with anti-PD 1 antibodies [18]. In this study, 54 consecutive patients (77.8% men, 27.8% non-smokers) treated at the Seoul National University Hospital (SNUH) and the Seoul National University Bundang Hospital (SNUBH) were included. Among them, 31 patients received nivolumab and 23 patients received pembrolizumab as single agent regimen. Only seven out of 36 patients (19.4%) evaluated were positive for PD-L1. Eighteen (33.3%) patients had clinical objective partial response, but none of them had an NLR value ≥ 5. The baseline values of NLR, PLR and SII were not associated with response to the therapy or to PFS in univariate and multivariate analysis. In multivariate analysis, a high post-treatment NLR at 6 weeks (HR 15.09, 95% CI 4.55–50.06, p < 0.001) independently predicted a decreased PFS and OS. The authors advocated that the NLR value at 6 weeks after initiation of treatment is a prognostic marker for patients with advanced NSCLC treated with anti-PD-1 antibody, and a potential predictive marker of response.
We performed a similar retrospective analysis in 78 consecutive NSCLC patients treated with nivolumab at the Units of Oncology of the University of Sassari and at the San Gerardo Hospital of Monza (Italy) to investigate correlations between blood cell count indexes and PFS or OS. Table 1 reports the clinical characteristics of the patients included in the study. Nivolumab was initially administered at 3 mg/kg intravenously (I.V.) over 60 min every 2 weeks and later at 240 mg I.V. Patients underwent serial clinical evaluations and radiographic imaging every 8–12 weeks and were evaluated for response either using computed tomography (CT) or positron emission tomography (PET) scans. PFS and OS were determined with a mean follow-up time of approximately 11 months (range 3–23); no patients were lost to follow-up.
Table 1.
Main demographic and clinical features of the patients enrolled
| Feature | |
|---|---|
| Total cases, n | 78 |
| Males, n (%) | 66 (84.6) |
| Age, mean (SD) years | 67 ± 7 |
| Smoking history | |
| Never smokers, n (%) | 4 (5.1) |
| Current smokers, n (%) | 28 (35.9) |
| Ex-smokers, n (%) | 46 (59) |
| Histology | |
| Adenocarcinoma, n (%) | 29 (37.2) |
| Squamous carcinoma, n (%) | 49 (62.8) |
| Stage | |
| III, n (%) | 10 (12.8) |
| IV, n (%) | 68 (87.2) |
| PDL-1 positive, n (%) | 3/11 (27.3) |
| EGFR mutations, n (%) | 1/23 (4.3) |
| ALK, n (%) | 1/24 (4.2) |
| Number of previous therapies | |
| 1, n (%) | 37 (47.4) |
| 2, n (%) | 26 (33.3) |
| 3, n (%) | 15 (19.2) |
| Time from diagnosis to nivolumab, median (IQR) months | 17.4 (10.4–32.0) |
| Therapy duration, median (IQR) days | 126 (52–216) |
| Number of cycles, median (IQR) | 10 (5–22) |
| Side effects | |
| Yes, n (%) | 38 (48.7) |
| No, n (%) | 40 (51.3) |
| Grade 1–2, n (% side effects) | 32 (84.2) |
| Grade 3–4, n (% side effects) | 6 (15.8) |
| Progression free survival, median (IQR) months | 4.8 (2.3–10.8) |
| Overall survival, median (IQR) months | 6.1 (3.7–13.6) |
| Pre-treatment | |
| NLR, median (IQR) | 3.0 (2.2–4.9) |
| PLR, median (IQR) | 164 (128–250) |
| MLR, median (IQR) | 0.5 (0.4–0.6) |
| SII, median (IQR) | 2.25 (1.50–3.40) |
| AISI, median (IQR) | 498 (293–1130) |
| At 6 weeks | |
| NLR, median (IQR) | 3.2 (2.4–4.9) |
| PLR, median (IQR) | 156 (129–215) |
| MLR, median (IQR) | 0.5 (0.4–0.6) |
| SII, median (IQR) | 2.45 (1.45–3.40) |
| AISI, median (IQR) | 637 (294–1092) |
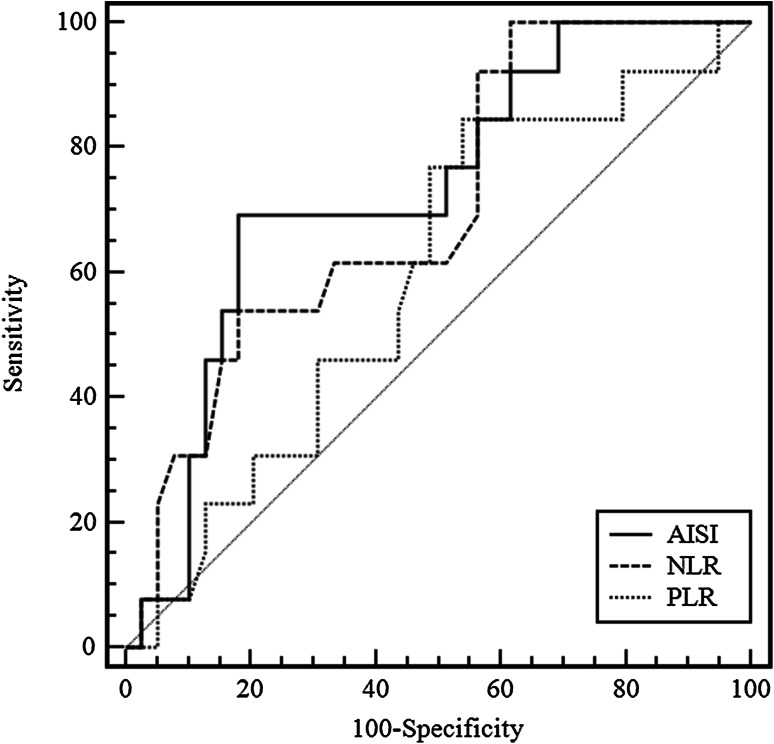
We evaluated the associations of PFS and OS with the following blood cell count indexes before starting treatment with nivolumab and at 6 weeks thereafter: NLR, PLR, MLR, SII and the Aggregate Index of Systemic Inflammation (AISI), calculated as the platelet count multiplied by the monocyte count and by the NLR, in relation to PFS and OS. None of the indexes evaluated at baseline showed any association with the outcomes under evaluation, while the NLR, PLR and AISI values at 6 weeks were significantly associated with both PFS and OS (Table 2). Furthermore, the SII at 6 weeks was significantly correlated only with PFS. Moreover, we performed ROC analysis to determine cut-offs and evaluate the sensitivity and specificity of the indexes examined (Table 3). Figure 1 shows the ROC curves of the NLR, PLR and AISI obtained.
Table 2.
Correlations between blood cell count indexes and prognosis at baseline and 6 weeks from nivolumab starting
| NLR | PLR | MLR | SII | AISI | |
|---|---|---|---|---|---|
| Baseline | |||||
| PFS | |||||
| Correlation coefficient | − 0.177 | − 0.208 | − 0.137 | − 0.181 | − 0.234 |
| p value | 0.1794 | 0.1143 | 0.3041 | 0.1734 | 0.0775 |
| OS | |||||
| Correlation coefficient | − 0.061 | − 0.208 | − 0.051 | − 0.052 | − 0.118 |
| p value | 0.5930 | 0.0672 | 0.6579 | 0.6515 | 0.3068 |
| 6th week | |||||
| PFS | |||||
| Correlation coefficient | − 0.408 | − 0.290 | − 0.253 | − 0.293 | − 0.374 |
| p value | 0.0029 | 0.0389 | 0.0757 | 0.0368 | 0.0068 |
| OS | |||||
| Correlation coefficient | − 0.367 | − 0.292 | − 0.215 | − 0.233 | − 0.372 |
| p value | 0.0075 | 0.0360 | 0.1292 | 0.0960 | 0.0066 |
PFS progression-free survival, OS overall survival, NLR neutrophil to lymphocyte ratio, PLR platelet to lymphocyte ratio, MLR monocyte to lymphocyte ratio, SII systemic inflammation index, AISI aggregate index of systemic inflammation
Statistically significant values are in bold (p < 0.05)
Table 3.
ROC analysis of NLR, PLR and AISI as predictors of survival in NSCLC patients treated with nivolumab
| Index | Cut-off | AUC | 95% CI | p value | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| NLR | > 4.4 | 0.706 | 0.564–0.824 | 0.011 | 100 | 38 |
| PLR | – | 0.596 | 0.451–0.730 | 0.284 | – | – |
| AISI | > 351 | 0.732 | 0.591–0.845 | 0.004 | 69 | 82 |
NLR neutrophil to lymphocyte ratio, PLR platelet to lymphocyte ratio, AISI aggregate index of systemic inflammation
Fig. 1.
ROC curves of NLR, PLR and AISI
Our results are in contrast with those published by Bagley et al. and other authors as we did not find any significant correlation between the pretreatment NLR and prognosis. On the other hand, our findings are in accordance with those of Suh et al., despite we did not include patients treated with pembrolizumab. The discrepancy between pretreatment and post-treatment correlations of the indices under evaluation with prognosis is difficult to interpret; it may depend on a higher inflammatory response involving specific white blood cell populations during treatment in patients with worst prognosis. In both our study and that of Suh et al. the NLR at 6 weeks from treatment initiation was predictive of PFS and OS. The authors did not find such a role for the SII. By contrast, the SII was significantly correlated with PFS and our AISI was a better predictor of outcomes than the other indices. The optimal cut-off value identified for the NLR in our study was 4.4, similar to the cut-off value (5) tested in other studies [14, 16, 17]. Nevertheless, the contrasting results, the lack of a correlation with the response to ICI therapy, and the relatively weak areas under the curve (AUC) in our study, suggest that the exact clinical significance of the blood cell count indexes in predicting outcomes of anti-PD 1 therapies in NSCLC patients remains far from clear. The studies have several limitations mainly due to the retrospective design, the different drug used (nivolumab and pembrolizumab) and the sample size. Therefore, further well-designed studies with a greater number of patients are warranted to establish the clinical utility of the blood cell count indexes in monitoring the outcomes of anti-PD 1 therapies and the prognosis of NSCLC patients.
Acknowledgements
The authors wish to thank professor Arduino Aleksander Mangoni, Department of Clinical Pharmacology, College of Medicine and Public Health, Flinders University, Adelaide, Australia, for his contribution.
Abbreviations
- (d)NLR
(Differential) neutrophil to lymphocyte ratio
- AISI
Aggregate index of systemic inflammation
- ALI
Advanced lung cancer inflammation index
- ECOG
Eastern Cooperative Oncology Group
- ICI
Immune checkpoint inhibitors
- iSEND
Sex, ECOG PS, NLR and dNLR index
- MLR
Monocyte to lymphocyte ratio
- PLR
Platelet to lymphocyte ratio
- PS
Performance status
- SII
Systemic inflammation index
Author contributions
CP conceived and designed the study, supervised data collection and contributed in writing and editing the manuscript. DLC conceived and designed the study, co-supervised data collection and contributed in writing and editing the manuscript. FC contributed in collecting, analysing and interpreting data. SC contributed in collecting, analysing and interpreting data. CC contributed in analysing and interpreting data and wrote part of the manuscript. AZ contributed in analysing and interpreting data and wrote part of the manuscript. PP conceived and designed the study, supervised all phases of the study, contributed in drafting the manuscript and approved its final version. All the authors read and approved the final version.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
The study was performed in accordance with the Declaration of Helsinki.
Informed consent
All patients signed an informed consent for the treatments performed and for the anonymous use of their demographic and clinical data for research purposes.
Footnotes
This Commentary discusses 10.1007/s00262-017-2092-x, published in Cancer Immunology, Immunotherapy, and other publications.
References
- 1.Paliogiannis P, Attene F, Cossu A, Budroni M, Cesaraccio R, Tanda F, Trignano M, Palmieri G. Lung cancer epidemiology in North Sardinia, Italy. Multidiscip Respir Med. 2013;8:45. doi: 10.1186/2049-6958-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paliogiannis P, Attene F, Cossu A, et al. Impact of tissue type and content of neoplastic cells of samples on the quality of epidermal growth factor receptor mutation analysis among patients with lung adenocarcinoma. Mol Med Rep. 2015;12:187–191. doi: 10.3892/mmr.2015.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortinovis DL, Canova S, Abbate M, Colonese F, Bidoli P. Focus on nivolumab in NSCLC. Front Med (Lausanne) 2016;3:67. doi: 10.3389/fmed.2016.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057) J Clin Oncol. 2017;35:3924–3933. doi: 10.1200/JCO.2017.74.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park W, Kwon D, Saravia D, Desai A, Vargas F, El Dinali M, Warsch J, Elias R, Chae YK, Kim DW, Warsch S, Ishkanian A, Ikpeazu C, Mudad R, Lopes G, Jahanzeb M. Developing a predictive model for clinical outcomes of advanced non-small cell lung cancer patients treated with nivolumab. Clin Lung Cancer. 2017 doi: 10.1016/j.cllc.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Yao C, Liu X, Tang Z. Prognostic role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio for hospital mortality in patients with AECOPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2285–2290. doi: 10.2147/COPD.S141760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Z, Zhang Z, Lin F, Ren Y, Liu D, Zhong R, Liang Y. Comparisons of neutrophil-, monocyte-, eosinophil-, and basophil- lymphocyte ratios among various systemic autoimmune rheumatic diseases. APMIS. 2017;125:863–871. doi: 10.1111/apm.12722. [DOI] [PubMed] [Google Scholar]
- 9.Paliogiannis P, Scognamillo F, Bellomo M, Pittalis ML, Pisano IP, Karligkiotis A, Bozzo C, Sotgiu G, Attene F. Neutrophil to lymphocyte ratio as a predictor of thyroid papillary carcinoma. Acta Med Mediterr. 2015;31:371–375. [Google Scholar]
- 10.Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, Dong J, Cheng JW, Liu ZW, Ma L, Lv Y. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer. 2014;134:2403–2413. doi: 10.1002/ijc.28536. [DOI] [PubMed] [Google Scholar]
- 11.Kara M, Uysal S, Altinişik U, Cevizci S, Güçlü O, Dereköy FS. The pre-treatment neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and red cell distribution width predict prognosis in patients with laryngeal carcinoma. Eur Arch Otorhinolaryngol. 2017;274:535–542. doi: 10.1007/s00405-016-4250-8. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Liang D, Xu X, Jin J, Li S, Tian G, Gao Z, Liu C, He Y. The prognostic value of neutrophil to lymphocyte and platelet to lymphocyte ratios for patients with lung cancer. Oncol Lett. 2017;14:6449–6456. doi: 10.3892/ol.2017.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Y, Qian L, Cui J. Value of neutrophil-to-lymphocyte ratio for predicting lung cancer prognosis: a meta-analysis of 7,219 patients. Mol Clin Oncol. 2017;7:498–506. doi: 10.3892/mco.2017.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagley SJ, Kothari S, Aggarwal C, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1–7. doi: 10.1016/j.lungcan.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Mezquita L, Auclin E, Ferrara R, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4:351–357. doi: 10.1001/jamaoncol.2017.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiroyama T, Suzuki H, Tamiya M, et al. Pretreatment advanced lung cancer inflammation index (ALI) for predicting early progression in nivolumab-treated patients with advanced non-small cell lung cancer. Cancer Med. 2018;7:13–20. doi: 10.1002/cam4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiriu T, Yamamoto M, Nagano T, et al. The time-series behavior of neutrophil-to-lymphocyte ratio is useful as a predictive marker in non-small cell lung cancer. PLoS One. 2018;13:e0193018. doi: 10.1371/journal.pone.0193018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suh KJ, Kim SH, Kim YJ, Kim M, Keam B, Kim TM, Kim DW, Heo DS, Lee JS. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother. 2017;67:459–470. doi: 10.1007/s00262-017-2092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]