Abstract
Dendritic cell (DC)-based vaccination is a promising approach for active-specific immunotherapy, but is currently of limited efficacy. The safety and effectiveness of a DC vaccine (DCV) loaded with glioblastoma stem cell-like (GSC) antigens was assessed in glioblastoma multiforme (GBM) patients. In this double-blind, placebo-controlled phase II clinical trial, 43 GBM patients were randomized after surgery at a 1:1 ratio to receive either DCV (n = 22) or normal saline placebo (n = 21). Overall survival (OS) and progression-free survival (PFS) were analysed. Participants were stratified into different molecular subgroups based on the mutation (MT) status of isocitrate dehydrogenase (IDH1/2) and telomerase reverse transcriptase (TERT). Plasma cytokine levels, tumor-infiltrating lymphocyte numbers and immune co-inhibitory molecules PD-L1 and B7-H4 were also assessed. Multivariate Cox regression analysis revealed that DCV treatment significantly prolonged OS (p = 0.02) after adjusting for IDH1 and TERT promoter MT and B7-H4 expression, primary vs recurrent GBM. Among IDH1wild type (WT) TERTMT patients, DCV treatment significantly prolonged OS (p < 0.01) and PFS (p = 0.03) and increased plasma levels of cytokines CCL22 and IFN-γ compared with placebo. Patients with low B7-H4 expression showed significantly prolonged OS (p = 0.02) after DCV treatment. Therefore, IDH1WTTERTMT and low B7-H4 expression identified subgroups of GBM patients more responsive to GSC DCV-based specific active-immunotherapy.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2232-y) contains supplementary material, which is available to authorized users.
Keywords: Dendritic cell vaccine, Glioblastoma multiforme, B7-H4, IDH, TERT, Active-specific immunotherapy
Background
Glioblastoma multiforme (GBM) is the most common malignant brain tumor, and 5-year survival rate remains at 9.8% with standard of care treatment [2, 3]. Therefore, alternative treatment options are urgently needed for this devastating primary brain tumor.
Dendritic cells (DCs) are antigen-presenting cells that initiate immune responses. Several early-stage clinical trials showed that DC vaccination (DCV) prolonged the survival of GBM patients [4]. Our previous study revealed that the glioblastoma stem-like cell (GSC) antigens could elicit intense immune responses against gliomas [5, 6]. We previously performed a phase I clinical trial to confirm the safety of DCVs pulsed with GSC antigens.
GBM can elicit immune suppression to escape immune surveillance. In our previous studies B7-H4, a member of the costimulatory B7 family, was highly expressed in high-grade gliomas [7, 8]. T cell-mediated antitumor immunity could be suppressed by GSC antigen-induced B7-H4 expression in macrophages/microglia, while suppression of B7-H4 leads to T-cell activation and tumor regression in glioma xenografts [9]; thus B7-H4 may serve as a biomarker predicting the efficacy of DCV immunotherapy.
Molecularly defined GBM subtypes are associated with the expression of certain tumor antigens [10]. Studies from our group and others have demonstrated that molecular classification on the basis of the mutation status of isocitrate dehydrogenase (IDH) 1 and /2 and telomerase reverse transcriptase (TERT) has significant predictive and prognostic value in gliomas [11, 12]. However, the relationship between tumor immunity and the new molecular glioma subtype classification has not been well understood to date.
In this study [1], a randomized phase II clinical trial, we sought to assess the efficacy of GSC antigen-primed DCV in terms of patient survival and tumor progression, and to identify the molecular characteristics of GBM associated with a higher likelihood of responding to DCV.
Methods
Patient eligibility criteria
Inclusion criteria included the following: new or recurrent GBM; resection of the tumor (≥ 95%); age range of 17–70 years; Karnofsky performance scale (KPS) score of ≥ 60; adequate organ function; within the normal ranges for absolute neutrophil (segmented and bands) count, platelets, hemoglobin, bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and normal serum creatinine.
Exclusion criteria included the following: ongoing or active infection; symptomatic congestive heart failure; unstable angina pectoris; psychiatric illness; immune system abnormalities; or currently receiving cortisol medication or investigational agents.
Surgery, radiotherapy and chemotherapy
Maximum resection of the tumor (≥ 95%) with conventional or intraoperative magnetic resonance imaging (MRI) neuronavigation was performed. Patients with newly diagnosed GBM received a standard therapy regimen [2]. Patients with recurrent GBM received additional chemotherapy or radiotherapy after a new maximal, safe resection during vaccination.
Preparation of GSC-pulsed dendritic cell vaccine (GSC-DCV)
Peripheral blood mononuclear cells from each GBM patient were isolated using lymphocyte separation medium (Sinopharm Chemical Reagent Co., Ltd, China). Monocytes were enriched by plate adherence and subsequently cultured for 6 days in 6-well plates at 2–4 × 106 cells per well in GT-T551 medium (TAKARA, China) supplemented with 3% autologous blood plasma, 50 ng/mL rhu GM-CSF and 100 ng/mL rhu IL-4 (PeproTech, USA). Glioma stem cell (GSC) antigens were prepared as previously described [5, 6]. Briefly, primary glioma tumor cells were harvested from each GBM patient and cultured in DMEM/F12 medium (Thermo Fisher, USA) supplemented with 20 μg/mL bFGF, 20 μg/mL EGF (PeproTech), and 20 μg/mL B27 neuronal cell culture supplement (Thermo Fisher).
1 day or 2 days later, A2B5 + glioma tumor cells were sorted by MoFlo XDP cell sorter to enrich GSCs. Then, these purified GSCs were digested with highly purified, recombinant cell-dissociation enzyme, TrypLE Express (Thermo Fisher), and resuspended in fresh culture medium every 3 days or 4 days. After 15–20 days, GSCs were irradiated with 6 cGy. GSC lysates were generated by five freezing/thawing cycles, followed by centrifugation at 800×g to remove intact cells.
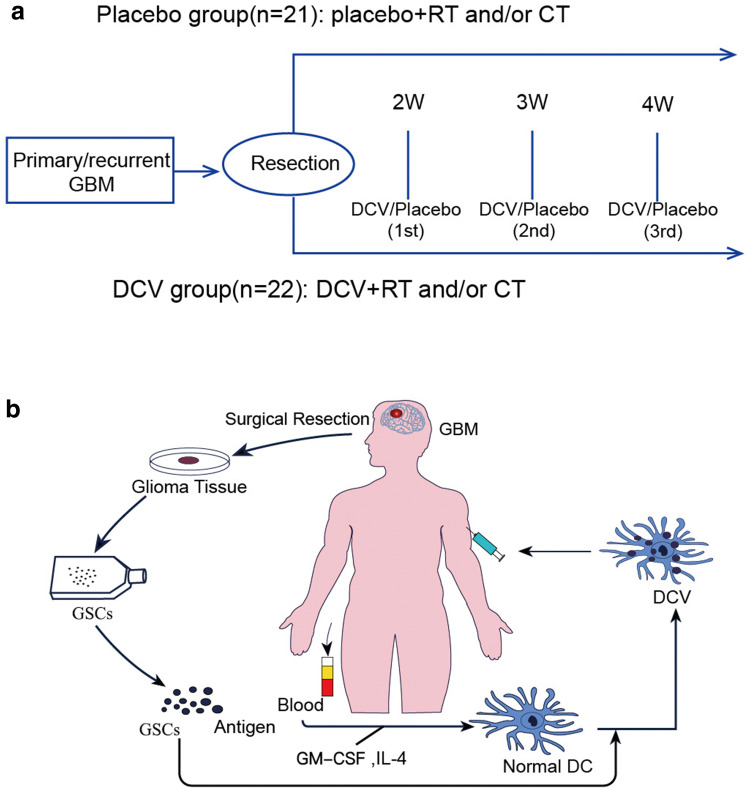
After 6 days culture, the immature DCs were stimulated with GSC lysates (100 μg GSC lysates per 4 × 106 immature DCs). 24 h later, mature DCs were harvested and analysed. Approximately 4 weeks were required for the first GSC-DCV production and administration. Ten ml blood samples were collected three times from each patient to prepare GSC-DCV. Each patient received an injection of 2–4 million autologous GSC-DCV once a week from the 2nd to 4th week for a total of three vaccinations (Fig. 1).
Fig. 1.
Overview of the phase II clinical trial of DCV therapy for GBM patients. a Flow chart of the phase II clinical trial of DCV therapy for GBM patients. Post-operatively, eligible patients were randomly assigned to either the placebo group or the DCV group on a 1:1 basis. Both the placebo and DCV patients with recurrent GBM received additional chemotherapy or radiotherapy after surgical of recurrent disease. Patients with newly diagnosed GBM received vaccination according to a standard therapy regimen. b DCV based on the immunotherapeutic protocol for GBM: harvest peripheral blood mononuclear cells, generate immature DC with GM-CSF and rhu IL-4 stimulation, load with A2B5 + GSC antigen, followed by transfer activated antigen-presenting DC back to respective GBM patients
The GSC-DCV assessment criteria are summarized in the Supplementary Table 1, in which HLA and expression of costimulatory molecules (HLA-DR, CD86, CD83, CD80) reflecting the maturation of DCV were assessed. IL-12 secretion was assessed to determine the capacity of induction of antitumor immunity by GSC-DCV, and CD14 expression was assessed to assess monocyte contamination. Once the percentage of CD14+ monocyte was more than 5%, FACS sorting was used to remove the monocytes.
Treatment and GSC-DCV administration protocol
After elective tumor surgery, eligible GBM patients were randomly assigned to either placebo or to a DCV group on a 1:1 basis; the randomization code was prepared by the clinical trial statistician using a computer-based random number table. Patients were consented by IRB protocol for this treatment randomization.
GSC-DCV was administered via intradermal injections in 0.5 mL of physiological saline in the shoulder near the back of the neck to facilitate trafficking of the DCs to the cervical lymph nodes. For the placebo group, patients were given physiological saline injections.
Clinical parameters of outcomes
Adverse effects were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. The end points of this study were evaluation of toxicity, immunological cytokine response and clinical response after DC therapy. The extent of resection was measured through the comparative analysis of preoperative and early postoperative MRI within 72 h [13].
The primary efficacy end point was PFS, and the secondary efficacy end point was OS. OS and PFS were calculated from the random assignment arms for the first month and every 3–4 months until disease progression or treatment discontinuation (whichever occurred later). Response was measured using Response Assessment in Neuro-Oncology (RANO) [13].
Cytokine analysis
Plasma samples were obtained from peripheral blood collected from each patient before and after the first DCV administration in both the vaccine and placebo groups. Aliquots of plasma were stored at − 80 °C in sterile NUNC-Cryovial tubes. The Luminex 200 System (Merck Millipore, Billerica, Massachusetts, USA) was used to evaluate the sera. Levels of 12 cytokines consisting of DC-associated cytokines (EGF, IL-12, FGF, GM-CSF, CCL22, and MCP-1), effector T cell–associated cytokines (IFN-γ, TNF-α, IL-17, and IP-10), and tumor-associated inhibitory cytokines (IL-10 and VEGF) were assessed as described by the manufacturer The percentage differences of the plasma cytokines before and after the first DCV treatment in each group were assessed.
Pathology and immunohistochemical analysis
Formaldehyde-fixed paraffin-embedded (FFPE) sections of the surgical tumor specimens were prepared for immunohistochemical analysis as previously described [8] and subsequently stained with anti-human antigen antibodies (Abcam, Cambridge, UK). Low or high expression was interpreted via immunoreactivity using the 0–6 semi-quantitative scoring systems for both the intensity of staining and the percentage of positive cells according to our previous study [9]. The staining pattern of the biopsies was defined as follows: 0–2, low expression; 3–6, high expression. The semi-quantitative evaluation of CD4+ and CD8+ TILs was as previously reported [14].
Analysis of molecular markers
MGMT methylation of GBM tissues was detected as previously described [15]. IDH1/2 and TERT promoter mutations were detected by direct sequencing as we previously described [12].
Statistical analyses
Statistical analyses were performed using SPSS software version 19.0 (IBM Corp., Armonk, NY, USA). The differences in data distribution among the groups were evaluated by the Chi-square (× 2) test. Differential molecular expression was analysed using one-way ANOVA and Holm-Sidak’s multiple comparisons test between each group. A multivariate Cox proportional hazards model was used to study the relationships among a set of predictors: DCV treatment, molecular characteristics, B7-H4 expression and primary vs recurrent tumor, based on the variables selected by the univariate analysis. Proportional assumption was checked by the log-minus-log plot. The DCV treatment effect on the OS and PFS was also analysed by the log-rank test in subgroups based on molecular characteristics and B7-H4 expression. p < 0.05 was considered statistically significant.
Results
Clinical characteristics
From December 1, 2012, to January 20, 2016, 43 patients with newly diagnosed or recurrent GBM were enrolled in the study. All patients had undergone resection surgery Demographic and clinical characteristics (Table 1) did not differ significantly with regard to baseline characteristics between the DCV and placebo group (p > 0.05). Of note, these characteristics include age, the proportion of primary and recurrent patients, treatment regimens, the extent of tumor resection, and MGMT promoter methylation, which might have prognostic implications and could contribute to different outcomes in the two arms (Table 1). As shown in Table 1, in the DCV treatment group, 17 had been treated with temozolomide and 16 with radiotherapy 60 Gy. In the placebo group, 19 had been treated with temozolomide and 17 with radiotherapy 60 Gy.
Table 1.
Demographic and baseline characteristics in placebo and DCV group GBM patients
| Placebo group (n = 21) | DCV group (n = 22) | p value | |
|---|---|---|---|
| Age (years) | NS | ||
| Mean | 50 | 48 | |
| Range | 22–71 | 25–71 | |
| Gender | NS | ||
| Male | 11 | 13 | |
| Female | 10 | 9 | |
| KPS | 79 | 83 | NS |
| Recurrence | NS | ||
| Yes | 10 | 9 | |
| No | 11 | 13 | |
| Radiotherapy | NS | ||
| Yes | 17 | 16 | |
| No | 4 | 6 | |
| Chemotherapy | NS | ||
| Yes | 19 | 17 | |
| No | 2 | 5 | |
| Treatment after progression | NS | ||
| Surgery | 6 | 4 | |
| Radiotherapy | 8 | 10 | |
| Chemotherapy | 19 | 17 | |
| Molecular profiles | NS | ||
| IDH1MTTERTWT | 1 | 3 | |
| IDH1WTTERTWT | 10 | 10 | |
| IDH1WTTERTMT | 10 | 9 | |
| MGMTp methylation | 12 | 9 | NS |
DCV Dendritic cell vaccine, GBM glioblastoma multiforme, IDH isocitrate dehydrogenase, KPS Karnofsky performance status, MGMTp O6-methylguanine-DNA methyltransferase promotor, MT mutation, TERT telomerase reverse transcriptase, WT wild type
Safety and incidence of adverse events
DCV was well tolerated in patients with no major adverse clinical events. One patient (DCV group, No. 5) had a mild fever, which lasted for 4 h after vaccination. Another patient (DCV group, No. 8) had mild erythema at the vaccine injection site.
Clinical outcomes
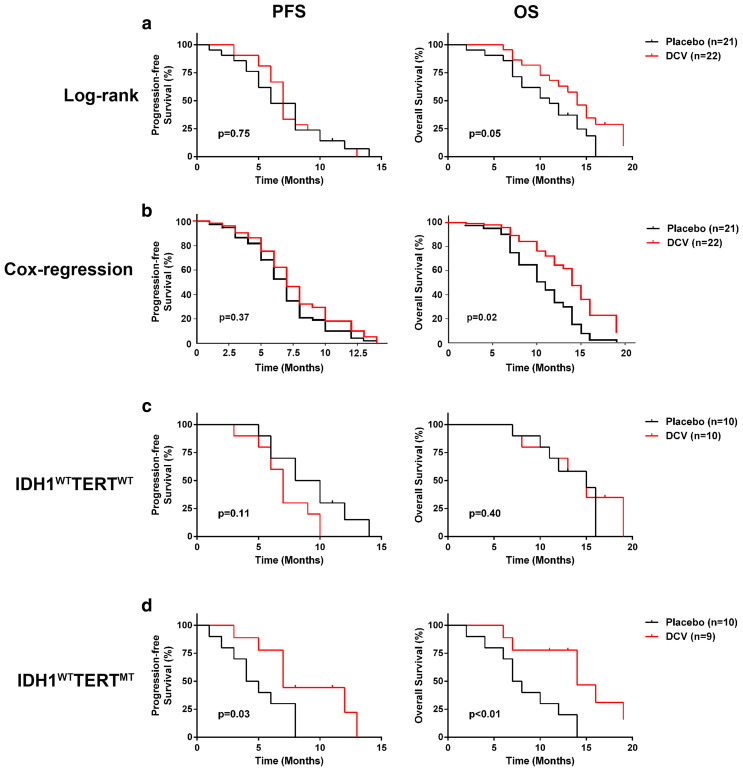
The median follow-up time of the patients was 14 months (range 9–23 months). In 24 of the DCV group, local or distant tumor recurrence developed in 22 patients, while 2 patients are alive without progression (follow-up to 11 months). In the placebo group, 23 recurrences developed; there was no significant difference between two groups. Without any stratification, Kaplan–Meier and log-rank test analyses of the 43 GBM patients indicated that the group with the DCV treatment showed prolonged overall survival (OS) (median OS 13.7 months vs 10.7 months, p = 0.05), but not progression-free survival (PFS) (median PFS 7.7 months vs 6.9 months, p = 0.75) when compared with the placebo group (Fig. 2a). However, when the analysis was conducted using a multivariate Cox proportional hazards model adjusted for IDH1 and TERT promoter MT, primary vs recurrent GBM and B7-H4 expression (Table 2), the DCV treatment group showed more significantly prolonged OS (p = 0.02; HR 2.5; 95% confidence interval [CI] 1.15–5.45) and a non-significant trend of improvement of PFS (p = 0.37; HR 1.37; 95% CI 0.68–2.74) compared with the placebo group (Fig. 2b).
Fig. 2.
DCV treatment prolonged OS and PFS of IDH1WTTERTMT GBM patients. a In assessing all 43 GBM patients, Kaplan–Meier and log-rank test analyses indicated that the DCV group showed no significant difference in OS (p = 0.05) with a PFS (p = 0.75) compared with the placebo group. b Using a Cox regression analysis to adjust for IDH1 and TERT promoter MT and B7-H4 expression, the group with DCV treatment showed significantly longer OS (p = 0.03) and better PFS (p = 0.48). c Of IDH1WTTERTWT patients, the group with DCV treatment showed no significant difference OS (p = 0.40) and PFS (p = 0.11) compared with the placebo group. d Of IDH1WTTERTMT patients, Kaplan–Meier and log-rank test analyses indicated that DCV group showed significant response outcome; OS (p < 0.01) and PFS (p = 0.03) compared with the placebo group
Table 2.
Overall survival and progression-free survival: multivariate Cox proportional hazards model was used to study the relationship among a set of predictors: DCV treatment, molecular characteristics, and B7-H4 expression, primary vs recurrent tumor
| Factor | OS | PFS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CL | p | HR | 95% CL | p | |
| DCV treatment | 0.40 | 0.18–0.87 | 0.02 | 0.73 | 0.37–1.46 | 0.37 |
| IDH1 and TERT promoter MT | 0.27 | 0.26–1.46 | 0.27 | 0.76 | 0.37–1.57 | 0.45 |
| primary vs recurrent GBM | 0.24 | 0.09–0.66 | 0.01 | 0.55 | 0.25–1.23 | 0.15 |
| B7-H4 expression | 3.69 | 1.33–10.20 | 0.01 | 1.52 | 0.69–3.35 | 0.29 |
CI Confidence interval, DCV dendritic cell vaccine, GBM glioblastoma multiforme, HR hazard ratio, IDH isocitrate dehydrogenase, MT mutation, OS overall survival, PFS progression-free survival, TERT telomerase reverse transcriptase
We sought to determine the in-depth impact of the mutation status of TERT and IDH1/2 on patients’ responses to the DCV treatment. For the IDH1WTTERTWT GBM patients, Kaplan–Meier and log-rank test analyses indicated that the group with the DCV treatment and the placebo group showed no significant difference in OS (p = 0.40) or PFS (p = 0.11) (Fig. 2c). In contrast, for the IDH1WTTERTMT GBM patients, Kaplan–Meier and log-rank test analyses indicated that the group with the DCV treatment showed significantly prolonged OS (p < 0.01) and PFS (p = 0.03) (Fig. 2d). Due to the limited number (n = 4), statistical comparative analyses were not used in IDH1MTTERTWT patients.
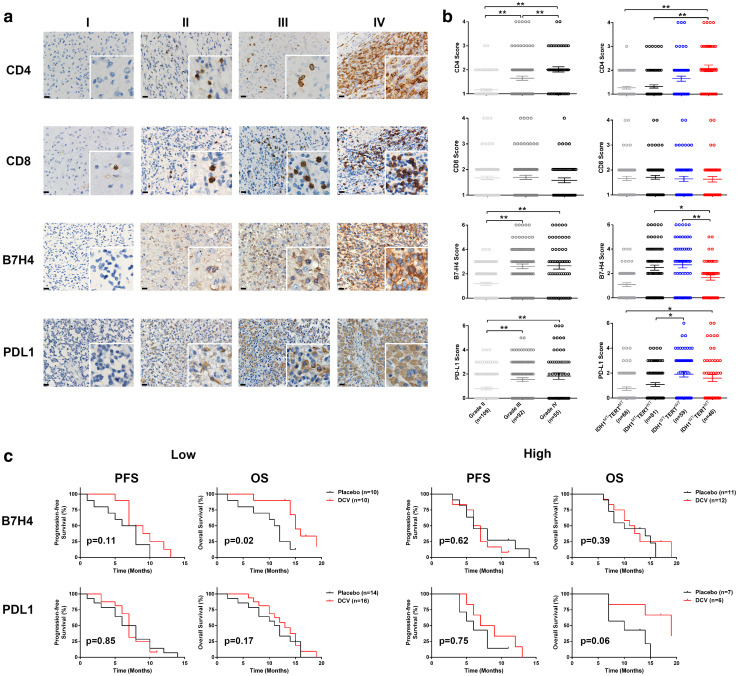
To identify the factors that could potentially affect patients’ responses to the DCV treatment with different molecular characteristics, we retrospectively, examined the level of CD4+ and CD8+ tumor-infiltrating lymphocytes (TILs) as well as the levels of PD-L1 and B7-H4 in samples with different molecular characteristics in a separate cohort of glioma patients from Huashan Hospital. For this analysis 109 low-grade glioma and 147 high-grade glioma samples were analysed retrospectively. To create a more uniform study population, these patients were randomly selected within the same period as the clinical trial enrolled patients and underwent routine clinical care from December 1, 2012, to February 10, 2016. As shown in Fig. 3b, expression B7-H4 in IDHWTTERTMT patients was lower than IDHWTTERTWT patients (p = 0.01, p < 0.05); expression of B7-H4 in IDHWTTERTMT patients was lower than IDHMTTERTWT patients (p = 0.02, p < 0.05), whereas no significant differences in the numbers of CD4+, CD8+ TILs and in PD-L1 protein levels were observed between IDH1WTTERTWTand IDH1WTTERTMT samples. In addition, we also assessed the expression levels of other immune checkpoint genes including B7-H3, CTLA4, PD-1 and TIM-3, and found that none of them showed significant differential expression among the aforementioned subgroups.
Fig. 3.
B7H4, but not PDL1, predicts patients’ survival after DCV treatment. a Representative demonstration of CD4, CD8, B7-H4, and PD-L1 expression. No or sporadic CD4 + or CD8 + TILs infiltration corresponding to score ‘1 point’ (I); moderate CD4 + or CD8 + TILs infiltration corresponding to score ‘2 points’ (II). Dense CD4 + or CD8 + TILs infiltration corresponding to score ‘3 points’ (III); very dense CD4 + TILs or CD8 + infiltration corresponding to score ‘4 points’ (IV). B7-H4 or PD-L1 low expression (I, II: corresponding to score ‘0’ and ‘2’ respectively) and high expression (III, IV: corresponding to score ‘4’ and ‘6’ respectively). Scale bar = 20 µm; b the levels of CD4 + TIL, CD8 + TIL, B7-H4, and PD-L1 were analysed in grade II, III or IV-glioma, and also in IDH1MTTERTMT, IDH1MTTERTWT, IDH1WTTERTWT or IDH1WTTERTMT patients Expression B7-H4 in IDHWTTERTMT is significantly lower than IDHWTTERTWT patients (p = 0.01); expression of B7-H4 in IDHWTTERTMT is significantly lower than IDHMTTERTWT patients (p = 0.02), whereas no significant differences in the numbers of CD4+, CD8 + TILs and in PD-L1 protein levels were observed between IDH1WTTERTWTand IDH1WTTERTMT samples. c Of low (score 0–2) or high (score 3–6) B7H4 or PD-L1 expression patients, PFS and OS were analysed using Kaplan–Meier and log-rank test. Of the low B7-H4 expression patients, Kaplan–Meier and log-rank test analyses indicated that the DCV group showed significantly better OS (p = 0.02) and not significant PFS (p = 0.11) compared with the placebo group. Among patients with high expression of B7-H4, the group with DCV treatment showed no significant difference in OS (p = 0.39) or PFS (p = 0.62) compared with the placebo group
It has been shown that the expression levels of immune checkpoint genes could predict patient responses to the DCV treatment in GBM [16]; therefore, we sought to determine whether the protein levels of B7-H4 and PD-L1 could be related to GBM patient survival in response to DCV treatment. In GBM patients with low protein expression of B7-H4, the DCV treatment group showed significantly prolonged OS (p = 0.02) and a trend towards better PFS (p = 0.11) compared with the placebo group, whereas in GBM patients with high expression of B7-H4, the DCV treatment made no significant impact on either OS (p = 0.39) or PFS (p = 0.62) (Fig. 3c). In contrast to the predictive function of B7-H4 protein, PD-L1 protein levels were not related to patient survival in response to DCV treatment (Fig. 3c).
Cytokine analysis
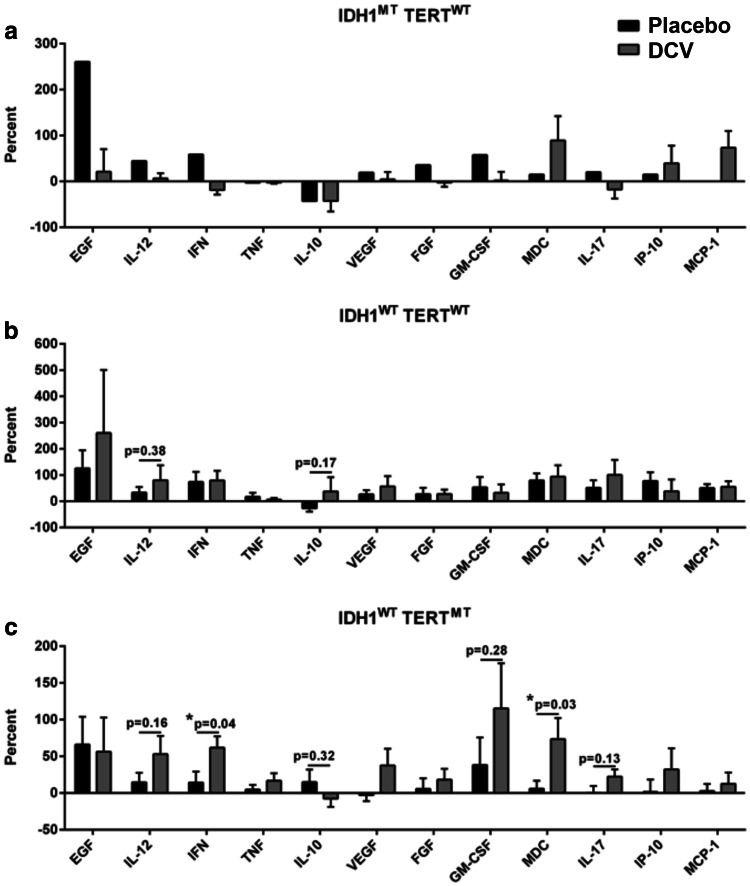
To determine how the immune system of the patients responded to DCV treatment, plasma cytokines were measured with the Luminex cytokine array before and after the first treatment. In IDH1WTTERTMT patients, CCL22 (p < 0.05) and IFN-γ (p < 0.05) levels were significantly increased compared to placebo patients with IDH1WTTERTMT genotype, whereas other cytokines such as EGF, IL-12, TNF-α, IL-10, VEGF, FGF, GM-CSF, IL-17, IP-10, MCP-1 did not change significantly. In contrast, in IDH1WTTERTWT patients, there were no significant differences in cytokine levels compared to placebo patients with IDH1WTTERTWT genotype. For IDH1MTTERTWT patients, the statistical significance of differences could not be calculated due to the limited number of patients (n = 4) (Fig. 4). These data indicate that only the IDH1WTTERTMT patients, but not the other groups, showed positively elevated responses of cytokine activity after the DCV treatment.
Fig. 4.
DCV treatment induces cytokine responses in IDH1WTTERTMT patients. Plasma samples were collected from peripheral blood collected from each patient before and after the first vaccination. A Luminex cytokine assay was used to evaluate the levels of specific cytokines, which included EGF, IL-12, IFN-γ, TNF-α, IL-10, VEGF, FGF, GM-CSF, CCL22, IL-17, IP-10, and MCP-1 in IDH1MTTERTWT (a), IDH1WTTERTWT (b) or IDH1WTTERTMT (c) glioma patients. DCV had significantly enhanced IFN-γ and CCL22 levels compared with the placebo group in the IDH1WTTERTMT group (p < 0.05) after DCV treatment. Other cytokines such as EGF, IL-12, TNF-α, IL-10, VEGF, FGF, GM-CSF, IL-17, IP-10, MCP-1 did not change significantly in the IDH1WTTERTWT groups
Individual patient response
To validate the role of T-cell infiltration and the influence of immune checkpoints in response to the DCV treatment, the expression levels of CD4, CD8, B7-H4, and PD-L1 before and after DCV treatment for two patients were then analysed (Supplementary Fig. 1). During the DCV treatment, these two patients received standard-of-care treatment with external beam radiation to a dose of 60 Gy and with concurrent temozolomide at a targeted daily dose of 75 mg/m2/day, followed by temozolomide (150–200 mg/m2/day) on days 1–5 of every 28-day cycle. For the IDH1WTTERTMT patient, the numbers of CD4+ and CD8+ TILs increased after combination therapy, whereas the B7-H4 protein level was low both before and after the combination therapy, which was consistent with the better prognosis of this patient. In the second patient with IDH1MTTERTWT tumor, both CD4+ and CD8+ TILs were low, whereas B7-H4 protein levels were high both before and after the combination therapy, which might be associated with the poor response of this patient. In contrast, PD-L1 levels were below detection in both patients before and after combination therapy, which was consistent with our previous finding that the PD-L1 level does not predict the response of patients to DCV treatment.
As noted, in these patients’ analysis, two patients were primary gliomas and recurrent GBM respectively, in which the immunological response could be different and might influence the results. Further studies are needed on patients with different tumor status.
Discussion
DCV-based active immunotherapy against GBM has shown promising results in clinical trials [4]. However, as with other treatments, only subsets of patients responded to this therapy [17]. This may be due to the molecular heterogeneity of GBM which is clinically and therapeutically challenging. In the present study, our clinical trial suggests that GBM patients with low tumor B7-H4 protein levels are more responsive to DCV loaded with GSC antigen.
The results of our phase II clinical trial showed that DCV was safe. Maximal resection of the tumor was the major inclusion criteria for DC vaccination strategies since, from an immunological point of view, tumor-induced immune suppression may be reduced by the maximal resection of the tumor. In addition, steroids can generally be weaned faster in case of maximal resection, so the patients who were currently receiving cortisol medication were excluded in our study.
After correction for recurrence, molecular characteristics, and B7-H4 expression, DCV was significantly associated with improved OS of GBM patients, indicating that a fraction of, but not all of, GBM patients benefited from the DCV treatment. With regard to PFS, detection of radiographic progression according to RANO criteria with follow-up imaging was considered to be the development of new lesions during immunotherapy. However, these early new progressive radiographic lesions could be a sign of a beneficial immune response in some patients after immunotherapy, directed against invading brain tumor cells. For instance, increased CD8 + TIL, rather than viable tumor cells, could be found in the tumor on examination [18], which could explain the observation that OS improved more significantly than radiographic evidence of PFS in our study. Supporting this notion, other studies have found early progressive radiographic changes in patients undergoing active immunotherapy [19, 20] and early progressive radiographic changes do not always preclude therapeutic benefit [21].
Recently, the status of TERT promoter MT and IDH1/2 MT has been used to define molecular subtypes of gliomas that could correlate with different prognoses [11]. Our previous work also demonstrated that TERT promoter MT combined with IDH1/2MT had prognostic value and enhanced sensitivity to adjuvant radiotherapy or chemotherapy in gliomas [12, 22]. Accordingly, we investigated whether these molecular groups were associated with different clinical outcomes in DC-based immunotherapy. Typically, the GBM subgroup with only TERTMT has a poorer prognosis than other subgroups [11]. In fact, the IDH1WTTERTMT subtype has similar genetic alterations to the mesenchymal subtype of GBM according to the classification based on the TCGA dataset [11]. Consistently, it has been reported that mesenchymal GBM may be more responsive to immune-based therapies [23]. Our results also showed that the survival of IDH1WTTERTMT patients was improved significantly after DCV therapy, which might be associated with up-regulated levels of cytokines (CCL22 and IFN-γ) in the patients’ sera. The increased production of IFN-γ indicated that DCV might induce a Th1-type protective immunity against GBM in IDH1WTTERTMT patients.
A variable that hypothetically may predict prognosis of patients in response to DCV treatment is the presence of activated TILs [24]. We examined the TILs in GBM patients who had undergone DCV treatment and discovered that the majority of TILs in solid tumors are of the CD3 + T-cell phenotype, which includes CD4 + helper cells, CD4 + regulatory T cells and CD8 + T lymphocytes. Increased numbers of CD4+ and CD8+ TILs were found in a patient with the molecular classified IDH1WTTERTMT subset of GBM. However, the up-regulation of TIL infiltration was not observed in IDH1MTTERTWT patients.
B7 molecules are important mediators of immune evasion in the tumor microenvironment. Among these molecules, PD-1 and its ligand, programmed cell death ligand 1 (PD-L1, also called B7-H1), mediate the immunosuppression of tumors by promoting T-cell apoptosis and inducing regulatory T cells (Tregs) in many cancers [25, 26]. However, the incidence of PD-L1 expression in GBM patients is confined to only a small subpopulation [27], and the antitumor effects of antibody therapy targeting PD-L1 and PD-1 have not been confirmed [28]. In the present study, the level of PD-L1 expression did not appear to affect patient survival in response to DCV treatment. B7-H4 is a member of the T-cell costimulatory and coinhibitory B7 family, which seemed to have some predictive value in GBM patients’ response to DCV treatment. Our previous work suggested that B7-H4 activation on macrophages/microglia in the glioma microenvironment could block effective T-cell immune responses [29] and that B7-H4 is an immunity-associated biomarker for progression of GBM [7, 8]. In the present study, the IDH1WTTERTMT GBM subtype exhibited lower expression of B7-H4 compared with the other two groups, which could explain why IDH1WTTERTMT GBM patients showed more clinical benefit from DCV treatment. It is noted that B7-H4 expression was analysed in a cohort including both low-grade glioma and high-grade glioma samples. Therefore, the results of the present study should be confirmed in a larger cohort of GBM patients to develop immunotherapy guidelines. The present study population is too small to evaluate conclusively demographic criteria for entry and patient recruitment. A high level of B7-H4 expression in GBM patients might block DC vaccine efficiency, By contrast, DCV treatment showed significantly prolonged OS and a trend towards better PFS for patients with low expression of B7-H4. Taken together, these might suggest that the B7-H4 pathway plays an important role in the adaptive immune resistance of GBM and may provide rationale for the combination therapy of anti-B7-H4 antibody and DCV in GBM patients.
Conclusion
In summary, our study provides evidence that stratification of GMB patients based on molecular biomarkers may enable identification of those more susceptible to DCV treatment. However, subgroup analysis was limited by the study size. Stratification according to these molecular biomarkers should be further explored in the future design of a larger randomizes trial with predefined subgroups.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Ian V Hutchinson for editing the final manuscript.
Abbreviations
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- CT
Chemotherapy
- CTCAE
Common terminology criteria for adverse events
- DC
Dendritic cell
- DCV
Dendritic cells vaccine
- FFPE
Formaldehyde-fixed paraffin-embedded
- GBM
Glioblastoma multiforme
- GSC
Glioblastoma stem-like cells
- IDH
Isocitrate dehydrogenase
- KPS
Karnofsky performance scale
- MGMT
O6-Methylguanine-DNA methyltransferase
- MRI
Magnetic resonance imaging
- MT
Mutation
- OS
Overall survival
- PD-L1
Programmed cell death ligand 1
- PFS
Progression-free survival
- RANO
Response assessment in neuro-oncology criteria
- RT
Radiotherapy
- TERT
Telomerase reverse transcriptase
- TIL
Tumor-infiltrating lymphocytes
- WT
Wild type
Author contributions
Authors LfZ, YwC, YY, JH analysed the data, designed the research studies, and wrote the manuscript. Authors FfL, CT and DkC conducted experiments, acquired and analysed data, and wrote the manuscript. Authors ZyQ, WH, MX, PZ, SqY, DC, XjD, YZ, XjZ, JY, JwQ and YtD conducted experiments, and acquired data. Author DSBH consulted on the study, analysed data, and wrote the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China Grants (81472347 to Liangfu Zhou, 81572478, 81372708 to Yu Yao, 81772672 to Chao Tang, 31400772 to Feifei Luo, 31570892 to Yiwei Chu); and the Science and Technology Commission of Shanghai Municipality Grants (13JC1408000 to Liangfu Zhou, 13JC1407700 to Yiwei Chu).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
Patients with GBM were enrolled after signing informed consent forms. The Institutional Review Board of Huashan Hospital, Fudan University, Shanghai approved the protocol (KY2011-006). The trial was registered with ClinicalTrials.gov(USA) as NCT 01567202. This study does not contain any studies with animals performed by any of the authors.
Footnotes
An Abstract of this paper was submitted to WFNS XVI, World Congress of Neurosurgery held in Istanbul, Turkey, August 20–25, 2017, and published as an abstract with the title of "TERT mutations and B7-H4 expression predict responses to DC vaccines in GBM" [1].
Yu Yao, Feifei Luo, Chao Tang and Dikang Chen contributed equally.
Contributor Information
Yiwei Chu, Email: ywchu@shmu.edu.cn.
Liangfu Zhou, Email: lfzhouc@126.com.
References
- 1.Yao Y, Tang C, Luo F, Chen D, Qin Z, Wu J, Hua W, Hoon DS, Hu J, Chu Y, Zhou L. TERT mutations and B7-H4 expression predict responses to DC vaccines. Abstact. Turkey: World Congress of Neurosurgery; 2017. [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/s1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 4.Bregy A, Wong TM, Shah AH, Goldberg JM, Komotar RJ. Active immunotherapy using dendritic cells in the treatment of glioblastoma multiforme. Cancer Treat Rev. 2013;39(8):891–907. doi: 10.1016/j.ctrv.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Hua W, Yao Y, Chu Y, Zhong P, Sheng X, Xiao B, Wu J, Yang B, Mao Y, Zhou L. The CD133+ tumor stem-like cell-associated antigen may elicit highly intense immune responses against human malignant glioma. J Neurooncol. 2011;105(2):149–157. doi: 10.1007/s11060-011-0572-y. [DOI] [PubMed] [Google Scholar]
- 6.Xu M, Yao Y, Hua W, Wu Z, Zhong P, Mao Y, Zhou L, Luo F, Chu Y. Mouse glioma immunotherapy mediated by A2B5+GL261 cell lysate-pulsed dendritic cells. J Neurooncol. 2014;116(3):497–504. doi: 10.1007/s11060-013-1334-9. [DOI] [PubMed] [Google Scholar]
- 7.Yao Y, Wang X, Jin K, Zhu J, Wang Y, Xiong S, Mao Y, Zhou L. B7-H4 is preferentially expressed in non-dividing brain tumor cells and in a subset of brain tumor stem-like cells. J Neurooncol. 2008;89(2):121–129. doi: 10.1007/s11060-008-9601-x. [DOI] [PubMed] [Google Scholar]
- 8.Mo LJ, Ye HX, Mao Y, Yao Y, Zhang JM. B7-H4 expression is elevated in human U251 glioma stem-like cells and is inducible in monocytes cultured with U251 stem-like cell conditioned medium. Chin J Cancer. 2013;32(12):653–660. doi: 10.5732/cjc.012.10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao Y, Ye H, Qi Z, Mo L, Yue Q, Baral A, Hoon DS, Vera JC, Heiss JD, Chen CC, Hua W, Zhang J, Jin K, Wang Y, Zang X, Mao Y, Zhou L. B7-H4(B7x)-mediated cross-talk between glioma-initiating cells and macrophages via the IL6/JAK/STAT3 pathway lead to poor prognosis in glioma patients. Clin Cancer Res. 2016;22(11):2778–2790. doi: 10.1158/1078-0432.ccr-15-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doucette T, Rao G, Rao A, Shen L, Aldape K, Wei J, Dziurzynski K, Gilbert M, Heimberger AB. Immune heterogeneity of glioblastoma subtypes: extrapolation from the cancer genome atlas. Cancer Immunol Res. 2013;1(2):112–122. doi: 10.1158/2326-6066.CIR-13-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, Pekmezci M, Rice T, Kosel ML, Smirnov IV, Sarkar G, Caron AA, Kollmeyer TM, Praska CE, Chada AR, Halder C, Hansen HM, McCoy LS, Bracci PM, Marshall R, Zheng S, Reis GF, Pico AR, O’Neill BP, Buckner JC, Giannini C, Huse JT, Perry A, Tihan T, Berger MS, Chang SM, Prados MD, Wiemels J, Wiencke JK, Wrensch MR, Jenkins RB. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–2508. doi: 10.1056/NEJMoa1407279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan AK, Yao Y, Zhang Z, Chung NY, Liu JS, Li KK, Shi Z, Chan DT, Poon WS, Zhou L, Ng HK. TERT promoter mutations contribute to subset prognostication of lower-grade gliomas. Mod Pathol. 2015;28(2):177–186. doi: 10.1038/modpathol.2014.94. [DOI] [PubMed] [Google Scholar]
- 13.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 14.Dahlin AM, Henriksson ML, Van Guelpen B, Stenling R, Oberg A, Rutegard J, Palmqvist R. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod Pathol. 2011;24(5):671–682. doi: 10.1038/modpathol.2010.234. [DOI] [PubMed] [Google Scholar]
- 15.Cankovic M, Mikkelsen T, Rosenblum ML, Zarbo RJ. A simplified laboratory validated assay for MGMT promoter hypermethylation analysis of glioma specimens from formalin-fixed paraffin-embedded tissue. Lab Investig. 2007;87(4):392–397. doi: 10.1038/labinvest.3700520. [DOI] [PubMed] [Google Scholar]
- 16.Fong B, Jin R, Wang X, Safaee M, Lisiero DN, Yang I, Li G, Liau LM, Prins RM. Monitoring of regulatory T cell frequencies and expression of CTLA-4 on T cells, before and after DC vaccination, can predict survival in GBM patients. PloS one. 2012;7(4):e32614. doi: 10.1371/journal.pone.0032614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12(4):265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, Mintz AH, Engh JA, Bartlett DL, Brown CK, Zeh H, Holtzman MP, Reinhart TA, Whiteside TL, Butterfield LH, Hamilton RL, Potter DM, Pollack IF, Salazar AM, Lieberman FS. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29(3):330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, Gilbert MR, Herndon JE, 2nd, McLendon RE, Mitchell DA, Reardon DA, Sawaya R, Schmittling RJ, Shi W, Vredenburgh JJ, Bigner DD. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(31):4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, Ellingson BM, Hashimoto N, Pollack IF, Brandes AA, Franceschi E, Herold-Mende C, Nayak L, Panigrahy A, Pope WB, Prins R, Sampson JH, Wen PY, Reardon DA. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–e542. doi: 10.1016/s1470-2045(15)00088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang ZY, Chan AK, Ding XJ, Qin ZY, Hong CS, Chen LC, Zhang X, Zhao FP, Wang Y, Wang Y, Zhou LF, Zhuang Z, Ng HK, Yan H, Yao Y, Mao Y. TERT promoter mutations contribute to IDH mutations in predicting differential responses to adjuvant therapies in WHO grade II and III diffuse gliomas. Oncotarget. 2015;6(28):24871–24883. doi: 10.18632/oncotarget.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, Nelson SF, Liau LM. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17(6):1603–1615. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han S, Zhang C, Li Q, Dong J, Liu Y, Huang Y, Jiang T, Wu A. Tumour-infiltrating CD4+ and CD8+ lymphocytes as predictors of clinical outcome in glioma. Br J Cancer. 2014;110(10):2560–2568. doi: 10.1038/bjc.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 26.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 27.Nduom EK, Wei J, Yaghi NK, Huang N, Kong LY, Gabrusiewicz K, Ling X, Zhou S, Ivan C, Chen JQ, Burks JK, Fuller GN, Calin GA, Conrad CA, Creasy C, Ritthipichai K, Radvanyi L, Heimberger AB. PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol. 2016;18(2):195–205. doi: 10.1093/neuonc/nov172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brower V. Hyperprogressive disease with anti-PD-1 and anti-PD-L1. Lancet Oncol. 2016;17(12):e527. doi: 10.1016/s1470-2045(16)30590-3. [DOI] [PubMed] [Google Scholar]
- 29.Jeon H, Vigdorovich V, Garrett-Thomson SC, Janakiram M, Ramagopal UA, Abadi YM, Lee JS, Scandiuzzi L, Ohaegbulam KC, Chinai JM, Zhao R, Yao Y, Mao Y, Sparano JA, Almo SC, Zang X. Structure and cancer immunotherapy of the B7 family member B7x. Cell Rep. 2014;9(3):1089–1098. doi: 10.1016/j.celrep.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.