Abstract
Failure of antitumor immunity in cancer was shown to be mediated by myeloid-derived suppressor cells (MDSCs), which are considered to be one of the key factors contributing to the development of malignant diseases. Therefore, the development of pharmacological approaches to effectively eliminate MDSCs in organisms carrying growing tumors is a promising pathway for potential treatment. For this purpose we propose alpha-fetoprotein (AFP) conjugated with a cytotoxic agent as a vector molecule, specifically recognizing MDSCs. The present study was aimed at examination of this suggestion using both in vitro and in vivo approaches. MDSCs, obtained from the spleen of Ehrlich carcinoma bearing mice, selectively bound AFP labeled with fluorescein isothiocyanate. AFP conjugated to daunorubicin (AFP-DR) and DR alone showed similar in vitro cytotoxicity against the granulocytic MDSC subpopulation. The monocytic MDSC subpopulation was resistant to treatment with DR, whereas it was completely depleted in the presence of AFP-DR. Treatment of mice bearing Ehrlich carcinoma with AFP-DR resulted in reduced numbers of splenic MDSCs, normalization of NK cell levels, and inhibition of tumor growth. The obtained results demonstrate that cytotoxic conjugates based on AFP are promising anticancer drugs, which, in addition to the direct effect on tumor cells expressing receptors to AFP, may contribute to elimination of MDSCs.
Keywords: Myeloid-derived suppressor cells, Alpha-fetoprotein, Ehrlich carcinoma, Daunorubicin
Introduction
It is well known that antitumor immunity, including natural killer (NK) cells, macrophages, dendritic cells and cytotoxic T lymphocytes (CTL), ignores tumors at later stages of development, which results in uncontrolled tumor growth [1]. The primary cause of this phenomenon is the immunosuppressive activity of a growing tumor and its microenvironment, the major components of which are T regulatory cells (Tregs) [2, 3] and myeloid-derived suppressor cells (MDSCs) [4]. These cells cooperatively induce functional anergy of cytolytic NK cells, CD8+ CTL and conversion of conventional CD4+ T helper cells into suppressive Tregs. Among suppressive cells that benefit tumor growth, MDSCs surely occupy a special position due to their mandatory role in the progression of malignant tumors, which has been shown in the experiment [5] and the clinic [6].
In mice, MDSCs express integrin CD11b and linear differentiation antigen “granulocytic receptor 1” (Gr-1). Antibodies to Gr-1 recognize both Ly-6G and Ly-6C epitopes. Expression of Ly-6G and Ly-6C discriminates 2 main MDSC subsets: CD11b+Ly6G+Ly6Clow granulocytic (G-MDSCs) and CD11b+Ly6Glow/-Ly6Chigh monocytic (M-MDSCs) [7, 8]. It has also been shown that G-MDSCs have significantly higher expression of Gr-1 compared to M-MDSCs [9].
It is believed that immunosuppressive activity of MDSCs is one of the most important factors limiting the beneficial effects of cancer immunotherapy. Therefore, the development of pharmacological approaches that could effectively eliminate the numbers and/or functions of MDSCs in organisms carrying growing tumors show promise [10–12]. This study proposes the use of alpha-fetoprotein (AFP) conjugated with a cytotoxic agent as a vector molecule that specifically recognizes MDSCs [13]. Although AFP-based cytotoxic conjugates are well known and used for inhibiting growth of experimental tumors expressing receptors to AFP [14–16], the possible use of it for MDSC elimination has not been addressed so far.
AFP is an oncofetal protein with a molecular weight of 68–72 kDa that can be detected at a high concentration in the blood of patients with liver cancer, germ cell cancer, and embryonic carcinomas [17, 18], as well as during pregnancy, where immunosuppressive AFP is involved in maternal immune tolerance [19]. It has been demonstrated that AFP exerts its effects through binding to multiple cellular receptors [20]. Previously, we have shown that AFP is a specific inducer of natural suppressor cells [21], which are identical in its properties to MDSCs [22, 23].
In this study, we substantiated the possibility of MDSC elimination with AFP-daunorubicin (AFP-DR) cytotoxic conjugate. We showed that AFP-FITC binds selectively with MDSCs. Treatment of mice bearing subcutaneous Ehrlich carcinoma with AFP-DR conjugate resulted in reduced numbers of splenic MDSCs, normalization of NK cell levels, and suppression of tumor growth. In vitro, both DR and AFP-DR showed similar cytotoxicity against G-MDSCs. While the M-MDSC subpopulation displayed resistance to treatment with DR, it was completely depleted in the presence of AFP-DR. Furthermore, the mortality rate in the untreated group and the group treated with DR was around 40%, while all the animals in the group treated with AFP-DR remained alive.
Thus, cytotoxic conjugates based on AFP molecule display promise as anticancer drugs, which, in addition to the direct effect on tumor expressing receptors to AFP, may contribute to the elimination of MDSCs, one of the most important tumor-induced immunosuppressive cells.
Materials and methods
AFP obtaining and its evaluation
AFP was obtained from human umbilical cord, blood was collected under standard conditions from healthy women following delivery. For AFP isolation, the previously described method of two-stage affinity chromatography followed by lyophilization was used [24]. The purity of isolated AFP reached 99%, which was assessed by SDS-electrophoresis and HPLC-chromatography.
Preparation of AFP conjugates
To conjugate AFP with FITC, 1 mg of lyophilized AFP was dissolved in 2 ml of borate buffer (pH 9.0). 100 μl of FITC solution (Sigma-Aldrich) in DMSO was then added at a concentration of 1 mg/ml, mixed and incubated for 90 min at 37 °C in the dark with constant rotation. Unreacted FITC was separated from the conjugate by gel filtration on a PD-10 column with Sephadex G-25 equilibrated in 0.005 M PBS, containing 0.15 M NaCl (pH 7.4). The obtained conjugate was stored at − 20 °C.
For conjugation of AFP with DR, 3 mg AFP and 0.5 mg DR (Sigma-Aldrich) were dissolved in 1 ml of PBS, and then 4 μl of 25% glutaraldehyde solution (Sigma-Aldrich) was added at 0.1% final concentration. Solution was incubated at room temperature for 15 min with constant rotation. Unreacted DR was separated from the conjugate by gel filtration on a PD-10 column with Sephadex G-25 equilibrated in PBS. Obtained conjugate was stored at − 20 °C. After flushing PD-10 column, concentration of unreacted DR was determined on a spectrophotometer at 482 nm (maximum absorbance) using a calibration curve (optical density vs native DR concentration). Concentration of AFP in the conjugate was determined by spectrophotometry at 280 nm. The analysis showed that there were 17 molecules of DR per each molecule of AFP in the obtained conjugate.
Murine model of Ehrlich carcinoma
All animals used in the study were males with body weights of 31–38 g. Ehrlich carcinoma cells were collected from ascitic fluid of the peritoneal cavity of mice 10 days after tumor administration (5–10 × 106 cells/ml in sterile PBS). The ascitic fluid was centrifuged (160g, 10 min) and the cells were re-suspended in PBS. Cell viability was determined by trypan blue (Sigma-Aldrich) exclusion method. Experimental and control mice received subcutaneous injection of Ehrlich tumor cells (5 × 105 cells in 0.5 ml sterile PBS) [25] or PBS, respectively. Cervical dislocation of mice and measurements of spleen and tumor weights were performed in 1, 2, 3, 5 weeks after the start of the experiment.
Isolation of spleen and bone marrow mononuclear cells
Suspension of splenocytes was obtained by homogenization of the spleen in PBS with a tissue grinder, filtered through 30 µm pre-separation filters (Miltenyi Biotec) and re-suspended in PBS. Mononuclear cell fraction was obtained by centrifugation of splenocytes on Histopaque-1.083 (Sigma-Aldrich) gradient at 400g for 30 min at 20 °C. Mononuclear cells were washed in RPMI-1640 medium (160g, 10 min) and re-suspended in PBS.
Suspension of bone marrow cells was obtained by flushing femoral bone with PBS, filtered and washed twice in RPMI-1640 medium (300g, 10 min).
Immunomagnetic separation of MDSCs
MDSCs from control and experimental mice were purified by magnetic separation using Myeloid-derived suppressor cell isolation kit (Miltenyi Biotech) according to the manufacturer’s protocol. Briefly, up to 108 mononuclear cells with buoyant density 1.083 g/ml, obtained from the spleen were re-suspended in 350 μl of PBS, containing 0.5% BSA and 2 mM EDTA. FcR blocking reagent (50 μl) was added, mixed well, and incubated for 10 min at 4 °C. After incubation, 100 μl of biotin-conjugated anti-Ly6G antibody was added, and the cells were incubated for further 15 min at 4 °C. Thereafter, cells were washed, re-suspended in 800 μl of buffer; then 200 μl of anti-biotin microbeads was added and incubated for 10 min at 4 °C. Gr-1highLy6Ghigh G-MDSCs were obtained by positive magnetic separation. The negative fraction was re-suspended in 400 μl of buffer with 100 μl of anti-Gr-1-biotin and incubated for 10 min, and then cells were washed and incubated in 900 μl of buffer with 100 μl of Streptavidin MicroBeads for 15 min at 4 °C. Cells were washed and Gr-1dimLy-6G− M-MDSCs were purified by positive selection. Negative fraction contained Ly-6G−Gr-1− cells (non-MDSCs). After separation, cells were washed and re-suspended in PBS for further analysis.
Flow cytometry
Phenotype of cells was evaluated by flow cytometry. Cells were stained with fluorescently labeled monoclonal antibodies [mouse anti-CD3-APC, anti-CD4-PE, anti-CD8a-PerCP, anti-CD11c-PE, anti-CD49b-PE, human anti-CD4-PerCP, anti-CD14-PerCP (BD Biosciences); mouse anti-CD11b-APC, anti-Gr-1-PerCP (Milenyi Biotec); mouse anti-CD45-APC (Biolegend)] according to the manufacturer’s protocol. Samples were analyzed on a FACS Calibur (BD Biosciences).
Tumor tissue dissociation
To obtain single cell suspension, tumors were cut into small pieces and digested with 2 mg/ml collagenase type IV (Gibco) at 37 °C for 1.5 h. The reaction was stopped with RPMI, containing 10% of fetal calf serum. Cells were passed through 30 μm filter and stained with fluorescently labeled antibodies, then subjected to FACS analysis.
Evaluation of binding AFP-FITC conjugate with MDSCs
AFP-FITC binding with different MDSC subpopulations was assessed in tumor-bearing mice in 3 weeks after tumor inoculation using two experimental approaches. First, G-MDSC, M-MDSC and non-MDSC fractions, isolated by immunomagnetic separation were incubated with AFP-FITC at the final concentration 100 μg/ml for 30 min at 4 °C in the dark. Cells were then washed, fixed in Cytofix Fixation Buffer (BD Biosciences) and analyzed by flow cytometry. Second, mononuclear cells were labeled with AFP-FITC and fluorescently labeled antibodies to CD11b and Gr-1, fixed, and analyzed by flow cytometry.
Evaluation of binding AFP-FITC, AFP-DR and DR with Ehrlich carcinoma cells
Ehrlich ascites carcinoma cells were stained with AFP-FITC (100 μg/ml), DR (13 μg/ml), or AFP-DR (13 μg/ml) and mouse anti-CD45-APC for 30 min at 4 °C in the dark, washed, fixed and subjected to FACS analysis. The percentage of FL1-positive (AFP-FITC binding) or FL2-positive (DR binding) events were counted among CD45-negative cells.
Evaluation of cytotoxicity of AFP-DR conjugate
MDSC subsets, as well as non-MDSCs, obtained from the spleen and bone marrow were incubated in 96-well plates (5 × 104 cells/well) in 200 μl RPMI-1640 medium containing 10% fetal calf serum, antibiotics, and different doses of AFP-DR or DR in a CO2 incubator. G-MDSCs were cultured in the presence of recombinant murine GM-CSF (Biolegend) (10 ng/ml final concentration) to support cell viability. In 48 or 24 h, cell viability was determined by trypan blue exclusion method.
Statistical data processing
All experiments were performed in at least triplicates. Variables were analyzed by Student’s t test (two-tailed) and a p < 0.05 was considered statistically significant. Values are expressed as mean ± SD. GraphPad Prism was used to create and design data graphics.
Results
The prevalence of AFP-FITC binding cells is significantly higher in MDSC population compared to non-MDSC population
Taking into account that AFP can exert its effects on target cells only after complementary binding to its cellular receptors, we determined an optimal concentration of AFP-FITC that does not result in non-specific binding. Peripheral blood mononuclear cells obtained from a healthy donor were labeled with different concentrations of AFP-FITC (3.1–150.0 μg/ml), and binding to CD4+ cells and CD14+ cells (cell populations that do not express or express receptors to AFP, respectively [26]) was evaluated. At 100 μg/ml AFP-FITC concentration, we observed a slight background staining of CD4+ cells (6.3 ± 3.4%), whereas 17.1 ± 4.3% of CD14+ cells were positive for AFP-FITC, therefore, this concentration was used for further experiments. Earlier, we have shown that a concentration of 100 μg/ml of AFP-FITC was able to stimulate bone marrow natural suppressor cells [21], which are identical to MDSCs [22, 23].
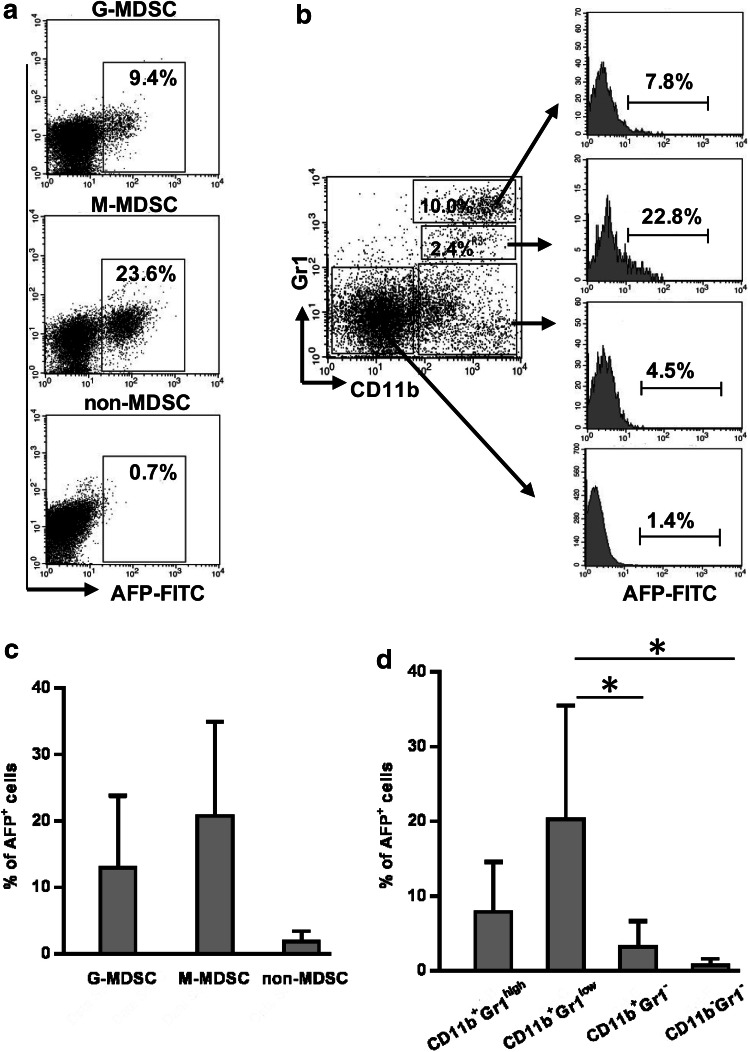
To investigate the ability of MDSC to bind AFP, G-MDSC and M-MDSC subpopulations were magnetically separated from splenocytes of mice 3 weeks after tumor inoculation and analyzed for binding AFP-FITC on a flow cytometer. The analysis demonstrated that both subpopulations were able to bind the protein at significantly higher levels than non-MDSCs (Fig. 1a, c).
Fig. 1.
MDSCs from tumor-bearing mice bind AFP at significantly higher levels than non-MDSCs. Splenocytes were obtained from CBA mice bearing Ehrlich solid carcinoma 3 weeks after tumor inoculation. G-MDSCs (Gr-1highLy-6G+), M-MDSCs (Gr-1dimLy-6G–), and non-MDSCs were isolated by immunomagnetic separation, incubated with 100 µg/ml AFP-FITC and assessed for AFP+ events by flow cytometry (a, c). Mononuclear fraction isolated from tumor-bearing mice was labeled with anti-Gr-1, anti-CD11b, AFP-FITC, and the frequency of AFP+ events was analyzed in G-MDSC (Gr-1highCD11b+), M-MDSC (Gr1dimCD11b+), monocyte (Gr1−CD11b+), and non-MDSC (Gr1−CD11b−) gates using flow cytometry (b, d). Each group included 7 mice. Representative flow cytometry and cumulative results for each group are indicated. Student’s t test showed that there was a significant difference between the groups, as indicated: *p < 0.05
Cytofluorometric analysis of unseparated mononuclears also showed that the percentage of AFP-binding cells was significantly higher in CD11b+Gr-1low M-MDSC and CD11b+Gr-1high G-MDSC subpopulations than in non–MDSC population (Fig. 1b, d). Moreover, the frequency of AFP-binding cells was twofold higher in M-MDSC subpopulation, which comprised 2.6 ± 0.6% of the total pool of mononuclear cells, compared to G-MDSC subpopulation, which comprised 10.1 ± 3.2% of the total pool of mononuclear cells (20.3 ± 15.2 and 7.9 ± 6.7%, respectively). Together, these data show that MDSCs obtained from tumor mice have an increased ability to bind AFP and, therefore, represent a potential target for cytotoxic AFP-conjugate.
AFP-DR exerts a selective cytotoxic effect towards M-MDSCs in vitro
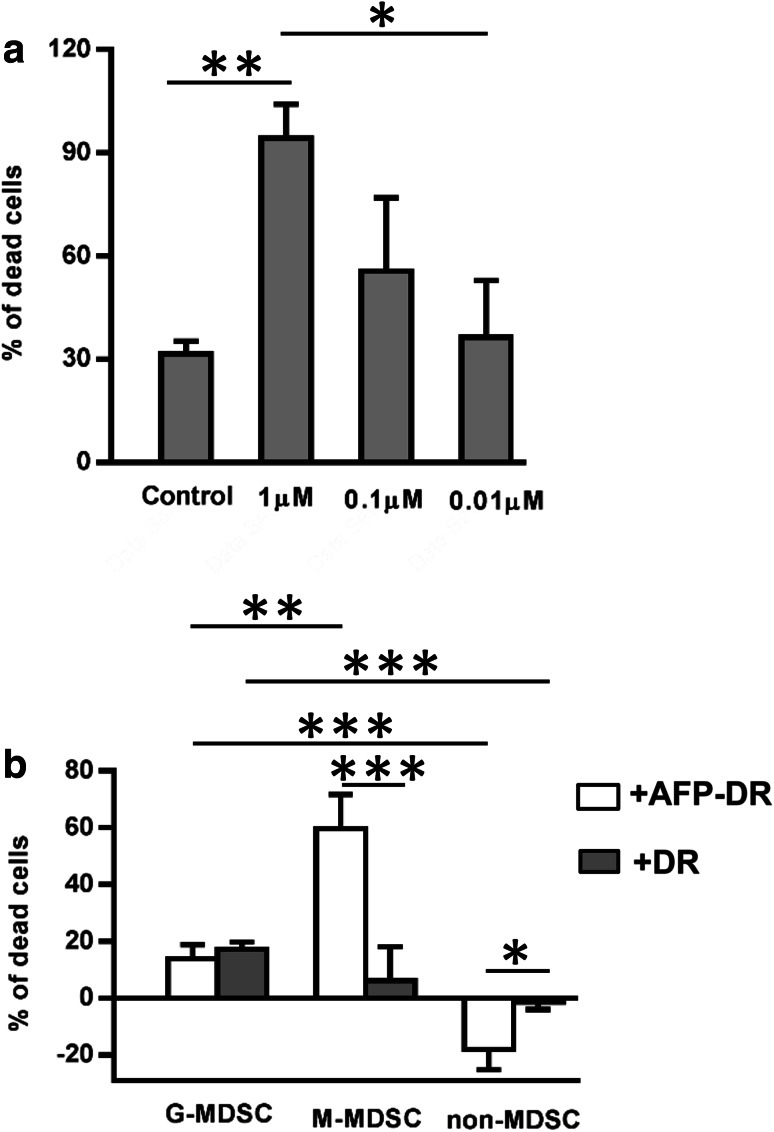
To determine an optimal concentration of cytotoxic conjugate AFP-DR, bone marrow mononuclear cells obtained from intact mice were cultured with different concentrations of conjugate for 48 h. Afterwards, cell viability was examined by the trypan blue exclusion method. AFP-DR concentration of 1 μM was found to display the maximal cell death and used for further experiments (Fig. 2a).
Fig. 2.
M-MDSC subpopulation is more susceptible to the toxic effect of AFP-DR conjugate. To assess the optimal cytotoxic dose of AFP-DR conjugate, bone marrow mononuclear cells obtained from intact mice were cultured with different concentrations of conjugate, then cell viability was examined by trypan blue exclusion method (a). G-MDSCs (Gr-1highLy-6G+), M-MDSCs (Gr-1dimLy-6G–), and non-MDSCs were immunomagnetically isolated from the spleen of mice bearing Ehrlich solid carcinoma 3 weeks after tumor inoculation, incubated with 1 µM of AFP-DR conjugate or DR alone for 24 h. Then cells were labeled with trypan blue and the percentage of dead cells was analyzed (b). Each group included 5 mice. Significant differences between columns assessed by Student’s t test are indicated: *p < 0.05, **p < 0.01, ***p < 0.005
To study the cytotoxic effects of AFP-DR on MDSCs, G-MDSC and M-MDSC subpopulations were obtained from the spleen of tumor mice by magnetic separation and cultured in the presence of 1 μM AFP-DR for 24 h. As shown in Fig. 2b, M-MDSCs were more susceptible to the cytotoxic effect of AFP-DR compared to DR. DR and AFP-DR had similar effects on G-MDSC subpopulation, whereas neither AFP-DR nor DR had effect on non-MDSCs. Moreover, AFP-DR increased viability of non-MDSCs during cultivation in vitro. Together, these data indicate that cytotoxic AFP-conjugate has a selective cytotoxic effect towards MDSCs and displays little to no effect on other cell populations.
AFP-DR treatment prevents an increase in MDSCs and a decrease in NK levels in tumor-bearing mice
Next, we assessed the effect of AFP-DR on Ehrlich carcinoma growth in vivo. For this, three groups of mice (10 mice in each group) received intraperitoneal injections of AFP-DR or DR at a dose of 0.3 μg/g of body mass (DR concentration) or PBS (control group) every day for 5 days starting from the 3rd day after tumor inoculation. The treatment was repeated at 7-day intervals.
In 3 weeks after tumor inoculation, we observed no difference in the tumor weight between all groups (Table 1). In contrast, by the end of the fifth week after tumor inoculation, both groups receiving AFP-DR and DR treatment showed a significantly decreased tumor weight when compared to the control animals. However, in the group treated with DR, one out of five mice died during the first 3 weeks following tumor inoculation and three more mice out of five died during the following 2 weeks, indicating high toxicity of DR treatment. The survival rate in the control group was also considerably lower when compared to the group which received AFP-DR, where all mice survived (Table 1).
Table 1.
Effect of tenfold administration of AFP-DR and DR on tumor mass and survival rate of mice bearing Ehrlich carcinoma
| Weeks after tumor inoculation | Parameter | Untreated mice | Mice treated with DR | Mice treated with AFP-DR |
|---|---|---|---|---|
| 3 weeks | Tumor mass (g) | 1.1 ± 0.4 | 1.8 ± 1.6 | 1.5 ± 0.6 |
| Survival rate | 100% (5/5) | 80% (4/5) | 100% (5/5) | |
| 5 weeks | Tumor mass (g) | 7.2 ± 1.2 | 1.6 ± 0.3* | 1.8 ± 1.1* |
| Survival rate | 40% (2/5) | 40% (2/5) | 100% (5/5) |
Significant differences between untreated mice and mice treated with AFP-DR or DR are indicated: * p ≤ 0.01
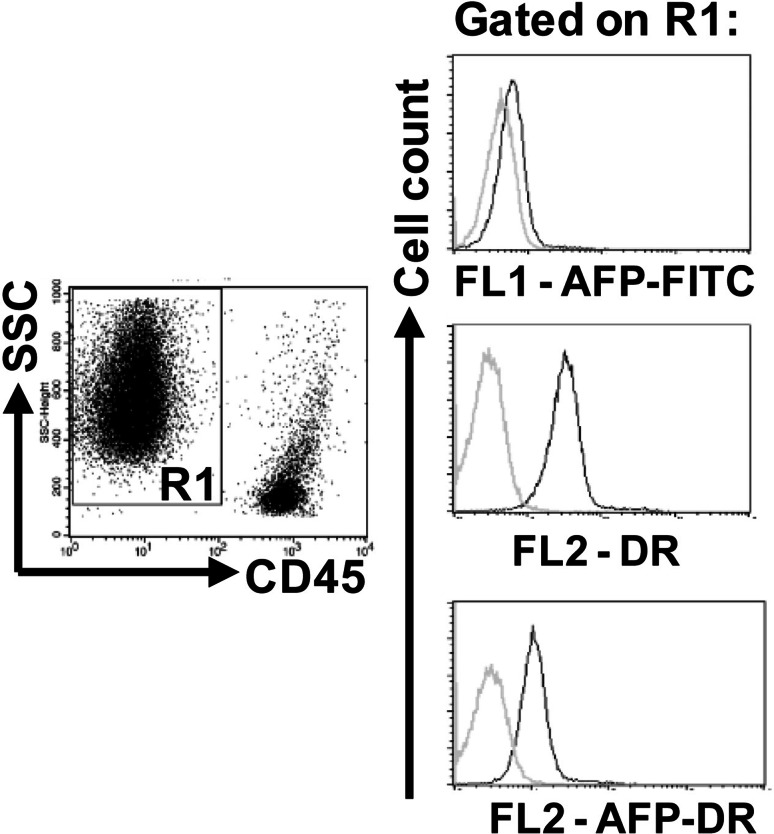
Next, we tried to determine if the observed effect of AFP-DR is mediated by a direct effect on tumor cells. Ehrlich ascites carcinoma cells were stained with AFP-FITC, AFP-DR, and DR and subjected to FACS analysis. As shown in Fig. 3, the tumor cells did not bind AFP-FITC. The ability of DR to emit fluorescence (585 filter) allowed us to assess DR binding to tumor cells. The analysis demonstrated that Ehrlich carcinoma cells bound native DR, whereas DR conjugated with AFP showed notably weaker binding (Fig. 3).
Fig. 3.
Ehrlich carcinoma cells bind both native DR and DR conjugated with AFP but not AFP-FITC. Ehrlich ascites carcinoma cells were stained with anti-CD45-APC and AFP-FITC, AFP-DR, or DR, washed, fixed, and analyzed by flow cytometry. Representative histograms are shown. Grey histograms show cell autofluorescence, black histograms show labeled samples
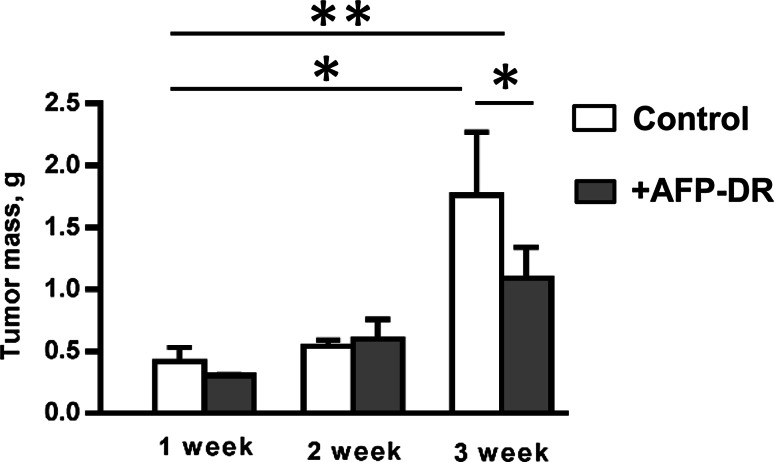
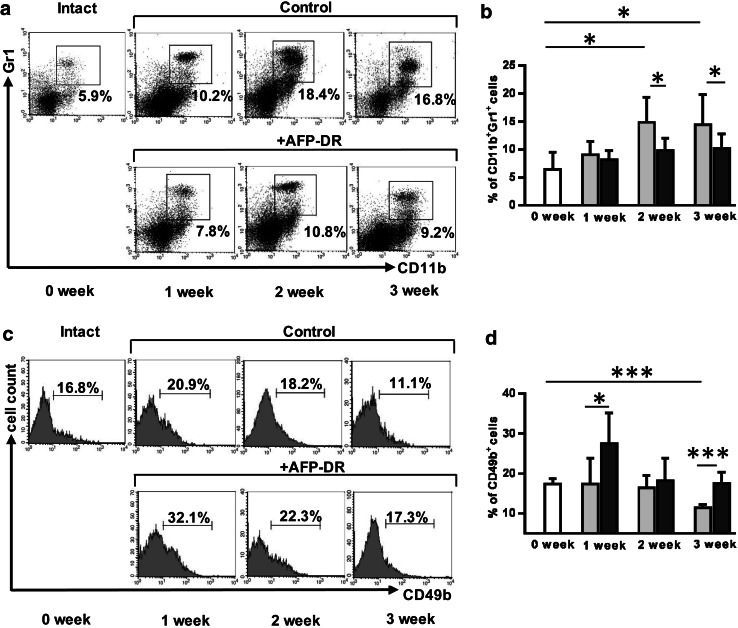
To assess AFP-DR effects on MDSC, NK and T cell content, mice received only five intraperitoneal treatments at the same doze daily for 5 days starting from the 3rd day after tumor inoculation. By the end of the 3rd week after last AFP-DR injection all animals remained alive. Additionally, AFP-DR-treated group had a significantly decreased tumor mass (p = 0.031) compared to the control group (Fig. 4). MDSC content in the spleen of control animals reached a peak at the week 2, while in the experimental group MDSC content did not change compared to intact mice throughout observation periods (Fig. 5a, b). Differences between untreated and treated groups at the 2nd and 3rd weeks were statistically significant (p < 0.05).
Fig. 4.
AFP-DR conjugate delays tumor mass growth in vivo. Mice were treated with PBS or AFP-DR daily for 5 days starting from the 3rd day after tumor inoculation. Each group included 10 mice. Effects of AFP-DR on tumor growth at the 1, 2, and 3 weeks after beginning of the experiment are depicted. Significant differences between columns assessed by Student’s t test are indicated: *p < 0.05, **p < 0.003
Fig. 5.
AFP-DR treatment prevents an increase in splenic MDSCs and a decrease in NK cells in tumor-bearing mice. Tumor mice received five intraperitoneal treatments with AFP-DR (0.3 µg/g mouse mass) or PBS daily for 5 days starting from the 3rd day after tumor inoculation. In 3 weeks splenocytes were obtained, labeled with anti-CD11b and anti-Gr-1, and anti-CD49b, and the frequency of Gr-1+CD11b+ (a, b) and CD49b+ (c, d) cells was analyzed using flow cytometry. Each group included 10 mice (white square box-intact mice, gray square box-tumor-bearing mice, treated with PBS, black square box-tumor-bearing mice, treated with AFP-DR). Representative flow cytometry and cumulative results for each group are depicted. Significant differences between columns assessed by Student’s t test are indicated: *p < 0.05, **p < 0.01, ***p < 0.005
We observed no differences in the levels of splenic T cells (defined as CD3+CD4+ and CD3+CD8+) between AFP-DR-treated and control groups (data not shown). In contrast, the percentage of NK-cells in the control group was significantly decreased by the end of the 3rd week compared to intact mice (p < 0.001), while in the AFP-DR-treated group the percentage of NK cells increased dramatically during the 1st week after tumor inoculation, than reached the control level at the week 2, and remained unchanged at the 3rd week (Fig. 5c, d). The data demonstrate the positive effect of intraperitoneal administration of AFP-DR conjugate on the dynamics of splenic MDSCs and NK cells with simultaneous inhibition of tumor growth.
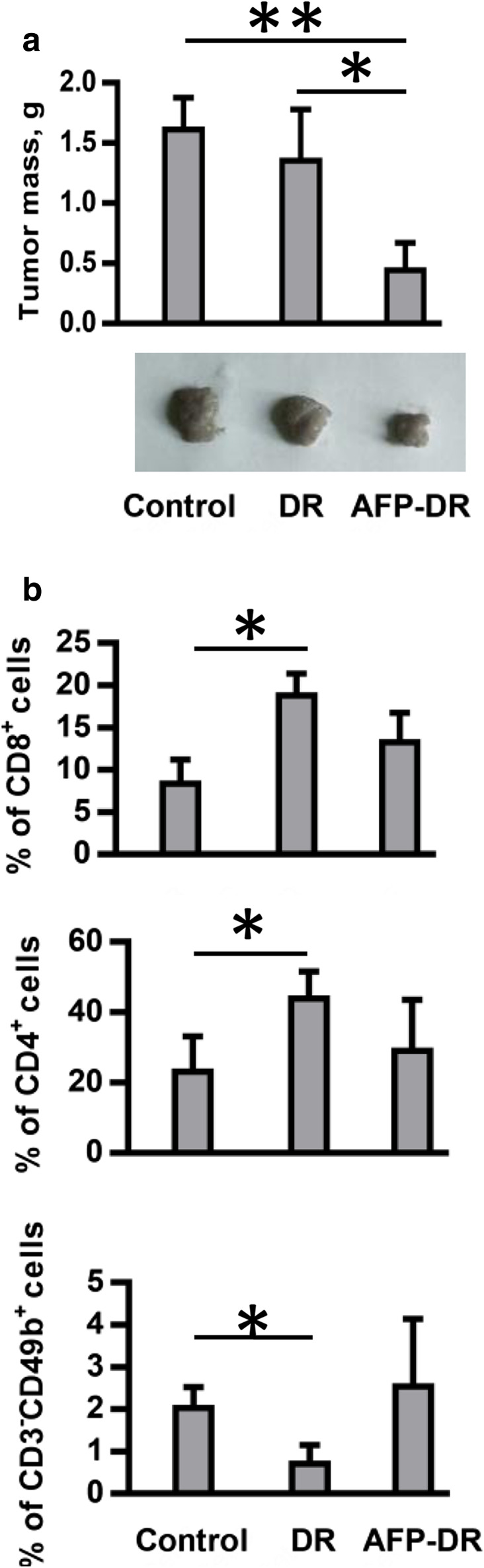
Next, we tried to investigate the effect of AFP-DR on tumor infiltrating lymphocytes. Tumor samples were collected from untreated mice (n = 3) and mice exposed to five intraperitoneal injections of AFP-DR (n = 3) or DR (n = 3) daily for 5 days starting from the 3rd day after tumor inoculation. Animals treated with AFP-DR showed a significantly reduced tumor growth when compared to saline treated or DR-treated mice (Fig. 6a). Surprisingly, we detected no significant differences in the proportions of NK cells, CD4+ and CD8+ T cell populations within the tumors between control and AFP-DR-treated groups, while in the DR-treated group intratumoral CD8+ and CD4+ T cells were significantly up-regulated (Fig. 6b). In addition, the proportion of intratumoral NK cells was decreased in the DR-treated group when compared to the control group. The reasons for these discrepancies are not evident. The majority of works demonstrate that increased tumor-infiltration with lymphocytes is associated with favorable prognosis in various types of cancers [27]. However, other studies have shown that an intense NK or CD8+ infiltration is associated with advanced disease and may even facilitate cancer development [28, 29]. In our study, an increased tumor-infiltration with CD8+ or CD4+ in the DR-treated group did not result in a decreased tumor growth, implying that infiltration of the tumor tissue with these cell populations does not reflect the effective antitumor response. It can be explained by a toxic effect of DR on NK cells that play a critical role in the cytolytic activity towards Ehrlich carcinoma cells which are characterized by the absence of MHC-I expression.
Fig. 6.
AFP-DR treatment decreases tumor weight but does not increase intratumoral CD8+, CD4+ and NK cell infiltration. Tumor mice received five intraperitoneal treatments with DR (0.3 µg/g mouse mass), AFP-DR (0.3 µg/g mouse mass), or PBS daily for 5 days starting from the 3rd day after tumor inoculation. In 3 weeks tumors were surgically removed, weighted, dissociated, labeled with anti-CD3-APC, anti-CD8a-PerCP, anti-CD4-PE, anti-CD49b-PE, and analyzed using flow cytometry. Representative and cumulative data of tumor mass (a) and percentage of CD8+, CD4+, CD3−CD49b+ cells (b) for each group are depicted. Each group included 3 mice. Significant differences between columns assessed by Student’s t test are indicated: *p < 0.05, ** p< 0.01
Discussion
Suppression of the antitumor immune response induced by a growing tumor and its microenvironment is now recognized as a key factor contributing to the development of malignant diseases. The role of MDSCs as a source of immunosuppressive signals in cancer has been demonstrated by numerous studies. Consequently, there has been considerable interest in developing approaches directed at the suppression of MDSC activity, a promising strategy for fighting cancerous diseases, which should be included into traditional regimens of chemo- and immunotherapy of cancer. Some of anti-cancer drugs (docetaxel, doxorubicin, all-transretinoic acid) [30–32], as well as antibodies to MDSC membrane markers [33] have been suggested for pharmacological correction of MDSC activity. However, these anticancer drugs do not target MDSCs with high selectivity and have toxic side effects. Additionally, markers used for identification of animal and human MDSCs are not unique and present on other cells of the immune system; therefore, antibody therapy might result in unpredictable consequences.
It has been shown that receptors to AFP are expressed on tumor and embryonic cells, as well as monocytes/macrophages. AFP functions as an autocrine growth factor for tumor and embryonic cells [19, 34] while for monocytes it is likely an activation factor, whose physiological role is not clear. This may possibly be explained by participation of AFP in the phenomenon of inflammation [35].
Currently, 30 AFP-binding proteins which belong to the family of scavenger receptors have been identified. They include several classes of integral transmembrane proteins with a molecular weight of 18–250 kDa [20], which participate in the transfer of AFP molecules associated with various ligands (bilirubin, fatty acids, retinoids, steroids, heavy metals, flavonoids, phytoestrogens, dioxins, and others) into tumor, fetal, and placental cells through clathrin-mediated endocytosis.
High structural and functional similarities between human and mouse AFP demonstrated previously [36, 37] allowed us to study the effect of human AFP on mouse cells both in vitro and in vivo. We showed that MDSCs isolated from tumor-bearing mice bound AFP. Which membrane receptor is responsible for AFP-binding and whether the process is indeed receptor-mediated remains unclear. Nevertheless, the procedure of labeling MDSC with AFP-FITC at 4 °C excludes the possibility of phagocytosis, and the fact that only a minor part of MDSC population is able to bind AFP assumes involvement of specific receptors on the surface of these cells. The frequency of AFP-binding cells was two times higher in M-MDSC fraction compared to G-MDSC fraction. Consequently, M-MDSC subpopulation was more susceptible to cytotoxic effects of AFP-DR conjugate compared to G-MDSC subpopulation. Native DR exerted similar cytotoxic effects on G-MDSCs while had no effect on M-MDSCs. Previously, it has been reported that some anticancer drugs are able to eliminate G-MDSCs, but not M-MDSCs [12]. Taking into account the higher suppressor activity of M-MDSCs in cancer compared to G-MDSCs [38], we can conclude that the cytotoxic conjugates of AFP appear to be preferable to traditional cytotoxic drugs.
An equal tumor reduction after tenfold treatment with DR or AFP-DR raises the question whether the observed decrease in MDSC expansion in the AFP-DR-treated group is a result of the direct cytolytic effect of the conjugate on tumor cells and the subsequent decrease in tumor mass. The analysis of obtained data allows us to exclude such an assumption suggesting high dissimilarity of pharmacological actions of AFP-DR and DR.
First, it should be noted that Ehrlich carcinoma cells did not bind AFP in vitro, probably due to the absence of specific receptors. At the same time, Ehrlich carcinoma cells were able to bind both native DR and DR conjugated with AFP, but the latter one was significantly weaker.
In addition, the reduction of the total dose of administered DR (native or conjugated) by two times from tenfold to fivefold treatment resulted in a decreased tumor weight only in the AFP-DR-treated group, but not in the DR-treated group. This fact implies that a fivefold administration of DR is insufficient for inhibition of tumor growth in vivo. At the same time, an equal dose of DR conjugated with AFP efficiently inhibited tumor growth in two separate series of experiments, what was accompanied by a decrease in MDSC levels and an increase in NK cell levels.
In the consideration of obtained data, including specific AFP-binding to M-MDSCs and the cytotoxic effect of AFP-DR on the same subpopulation of MDSCs in vitro, we can conclude with reasonable confidence that M-MDSCs are the primary target of AFP-DR. Underlying this conclusion is the assumption that M-MDSC elimination results in a decreased immunosuppressive background within tumor tissue followed by activation of NK cell cytolytic activity.
Thus, AFP-DR conjugate possesses several preferable qualities over native DR. First, the dynamics of mortality among both untreated and treated with DR (tenfold injection) animals were similar in spite of a significant inhibition of tumor growth in both groups at the end of the 5th week. In contrast, all animals treated with AFP-DR remained alive. In addition, a substantial decline in NK cell levels with simultaneous raise in T lymphocytes within tumor tissue was observed only in the animals treated with DR. Ehrlich carcinoma is known to be characterized by the absence of H-2 antigen expression [39], which makes it a specific target for cytolytic activity of NK cells, but it is ignored by cytotoxic T cells. Obviously, the eliminating effect of DR on NK cells in the given tumor model contributes to its overall toxicity which lowers its antitumor value. A separate possibility for the increased level of tumor infiltrating CD4+ T cells in the DR-treated group may lie in the Treg expansion, which negative effect on the antitumor immunity is well known [2]. However, the assumption requires separate examination.
Thus, for the first time we explored the possibility of modulating levels of circulating MDSCs in an experimental tumor model with the aid of AFP cytotoxic conjugate. Resulting data demonstrated that AFP cytotoxic conjugates are a perspective direction in immunotherapy that targets MDSCs, one of the most important factors contributing to the antitumor immunity failure.
Abbreviations
- AFP
Alpha-fetoprotein
- DR
Daunorubicin
- G-MDSCs
Granulocytic myeloid-derived suppressor cells
- Gr-1
Granulocytic receptor 1
- M-MDSCs
Monocytic myeloid-derived suppressor cells
- SD
Standard deviation
- Tregs
T regulatory cells
Compliance with ethical standards
Funding
This study was funded by the Grant number 244/GF3, provided by Ministry of Education and Science of the Republic of Kazakhstan.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving animals were in accordance with the ethical standards of the Kazakh Research Institute of Oncology and Radiology and approved by the Ethical Committee of this Institute.
References
- 1.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 2.Nishikawa H, Sakaguchi Sh. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 3.Byrne WL, Mills KHG, Lederer JA, O’Sullivan GC. Targeting regulatory T cells in cancer. Cancer Res. 2011;71:6915–6920. doi: 10.1158/0008-5472.CAN-11-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Je-In Youn, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;91:167–181. doi: 10.1189/jlb.0311177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11:802–807. doi: 10.1016/j.intimp.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111(8):4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 8.Je-In Youn, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolcetti L, Peranzoni E, Ugel S, Marigo I, Gomez AF, Mesa C, Geilich M, Winkels G, Traggiai E, Casati A, Grassi F, Bronte V. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40:22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 10.Kao J, Ko EC, Eisenstein S, Sikora AG, Fu S, Chen SH. Targeting immune suppressing myeloid-derived suppressor cells in oncology. Crit Rev Oncol Hematol. 2011;77:12–19. doi: 10.1016/j.critrevonc.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin F, Apetoh L, Ghiringhelli F. Role of myeloid-derived suppressor cells in tumor immunotherapy. Immunotherapy. 2012;4:43–57. doi: 10.2217/imt.11.154. [DOI] [PubMed] [Google Scholar]
- 12.Djeu J, Wei Sh. Chemoimmunomodulation of MDSCs as a novel strategy for cancer therapy. Oncoimmunology. 2012;1(1):121–122. doi: 10.4161/onci.1.1.18074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pak VN, Belyaev NN. Alpha-fetoprotein-mediated immune tolerance and its reversal. In: Lakhi N, Moretti M, editors. Alpha-fetoprotein: functions and clinical applications. New York: Nova Science Publishers Inc; 2016. pp. 353–374. [Google Scholar]
- 14.Lutsenko SV, Feldman NB, Finakova GV, Gukasova NV, Petukhov SP, Posypanova GA, Skryabin KG, Severin SE. Antitumor activity of alpha fetoprotein and epidermal growth factor conjugates in vitro and in vivo. Tumor Biol. 2000;21:367–374. doi: 10.1159/000030142. [DOI] [PubMed] [Google Scholar]
- 15.Yabbarov NG, Posypanova GA, Vorontsov EA, Obydenny SI, Severin ES. A new system for targeted delivery of doxorubicin into tumor cells. J Control Release. 2013;168(2):135–141. doi: 10.1016/j.jconrel.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Pak V. The use of a-fetoprotein for the delivery of cytotoxic payloads to cancer cells. Ther Deliv. 2014;5:885–892. doi: 10.4155/tde.14.59. [DOI] [PubMed] [Google Scholar]
- 17.Wu AHB, Sell S. Markers for hepatic carcinoma. In: Herberman RB, Mercer DW, editors. Immunodiagnosis of cancer. New York: Marcel Dekker; 1990. pp. 403–422. [Google Scholar]
- 18.Jacobs I, Bast RC. Immunodiagnosis of germ cell tumors of the gonads. In: Herberman RB, Mercer DW, editors. Immunodiagnosis of cancer. New York: Marcel Dekker; 1990. pp. 323–338. [Google Scholar]
- 19.Mizejewski GJ. Biological roles of alpha-fetoprotein during pregnancy and perinatal development. Exp Biol Med (Maywood) 2004;229:439–463. doi: 10.1177/153537020422900602. [DOI] [PubMed] [Google Scholar]
- 20.Mizejewski GJ. Review of the putative cell-surface receptors for alpha-fetoprotein: identification of a candidate receptor protein family. Tumor Biol. 2011;32:241–258. doi: 10.1007/s13277-010-0134-5. [DOI] [PubMed] [Google Scholar]
- 21.Belyaev NN, Bogdanov AYu, Savvulidi PhG, Krasnoshtanov VK, Tleulieva RT, Alipov GK, Sekine I, Bae JS, Lee JB, Min YK, Yang HM. The influence of alpha-fetoprotein on natural suppressor cell activity and Ehrlich carcinoma growth. Korean J Physiol Pharmacol. 2008;12:193–197. doi: 10.4196/kjpp.2008.12.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forghani P, Khorramizadeh MR, Waller EK. Natural suppressor cells; past, present and future. Front Biosci (Elite Ed) 2012;4:1237–1245. doi: 10.2741/e454. [DOI] [PubMed] [Google Scholar]
- 23.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atemezem A, Mbemba E, Marfaing R, Vaysse J, Pontet M, Saffar L, Charnaux N, Gattegno L. Human α-fetoprotein binds to primary macrophages. Biochem Biophy Res Commun. 2002;296:507–514. doi: 10.1016/S0006-291X(02)00909-9. [DOI] [PubMed] [Google Scholar]
- 25.Subiza JL, Vinuela JE, Rodriguez R, Gil J, Figueredo MA, De la Concha EG. Development of splenic natural suppressor (NS) cells in Ehrlich tumor-bearing mice. Int J Cancer. 1989;44:307–314. doi: 10.1002/ijc.2910440220. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki Y, Zeng CQY, Alpert E. Isolation and partial characterization of a specific alpha-fetoprotein receptor on human monocytes. J Clin Investig. 1992;90:1530–1536. doi: 10.1172/JCI116021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 28.Levi I, Amsalem H, Nissan A, Darash-Yahana M, Peretz T, Mandelboim O, Rachmilewitz J. Characterization of tumor infiltrating natural killer cell subset. Oncotarget. 2015;6(15):13835–13843. doi: 10.18632/oncotarget.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61(13):5132–5316. [PubMed] [Google Scholar]
- 30.Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei Sh, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16(18):4583–4594. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N, Bonnotte B, Katsanis E, Larmonier N. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 2014;74(1):104–118. doi: 10.1158/0008-5472.CAN-13-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iclozan C, Antonia S, Chiappori A, Chen DT, Gabrilovich DI. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother. 2013;62:909–918. doi: 10.1007/s00262-013-1396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srivastava MK, Zhu L, Harris-White M, Kar UK, Huang M, Johnson MF, Lee JM, Elashoff D, Strieter R, Dubinett S, Sharma S. Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS One. 2012;7(7):e40677. doi: 10.1371/journal.pone.0040677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizejewski GJ. Biological role of α-fetoprotein in cancer: prospects for anticancer therapy. Expert Rev Anticancer Ther. 2002;2(6):709–735. doi: 10.1586/14737140.2.6.709. [DOI] [PubMed] [Google Scholar]
- 35.Mizejewski GJ. Alpha-fetoprotein (AFP) and inflammation: is AFP an acute and/or chronic phase reactant? J Hematol Thrombo Dis. 2015;3:191. [Google Scholar]
- 36.Chakraborty M, Mandal C. Immuno-suppressive effect of human alpha-fetoprotein: a cross species study. Immunol Investig. 1993;22(5):329–339. doi: 10.3109/08820139309063412. [DOI] [PubMed] [Google Scholar]
- 37.Baker ME. Evolution of alpha-fetoprotein: sequence comparisons among AFP species and with albumin species. Tumor Biol. 1988;9(2–3):123–136. doi: 10.1159/000217553. [DOI] [PubMed] [Google Scholar]
- 38.Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37(3):208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen L, Watkins GF. Evidence against the presence of H2 histocompatibility antigens in Ehrlich ascites tumour cells. Nature. 1970;225:734–735. doi: 10.1038/225734a0. [DOI] [PubMed] [Google Scholar]