Abstract
Cancer immunotherapy is based on the premise that activated, pro-inflammatory T cell responses to tumor will mostly combat tumor growth. Nowadays accepted as largely valid, this hypothesis has been formed as a result of extensive theoretical and experimental argumentation on the inherent function of the immune system and the nature of the immunological self, dating back to the foundations of immunology. These arguments have also been affected by how current working hypotheses were set by researchers, an issue that has been the focus of study by medical anthropologists. As a result of these processes, cancer immunotherapy has developed into a truly promising anti-cancer strategy, with very substantial benefits in clinical outcomes. However, as immunotherapy still has large margins for improvement, a more thorough examination of both the historical background and evolutionary context of current assumptions for how the immune system responds to cancer can help reveal novel, testable questions. We describe how attempting to answer some of these questions experimentally, such as identifying the contributors of tumor-associated fibrosis, has led to potentially useful insights on how to improve immunotherapy.
Keywords: Tumor, Immunotherapy, Immunological self, T cells, NIBIT 2016
How and why does immunotherapy work?
Immunotherapy has revolutionized the available options for cancer treatment. As a clinical strategy, it is delivering impressive results in hard-to-treat cancers, with lesser collateral side effects compared to the standard therapeutic options. Immunotherapy can be applied as “immune checkpoint blockade”, a collective term standing for inhibition of the natural downregulation of T cell costimulation or inhibition of mechanisms that lead to T cell apoptosis. In this form, the intended effect is to guarantee that any anti-tumor cytotoxic T cells are not restrained by the natural mechanisms that limit the duration and intensity of T cell cytotoxic activity. The second form of immunotherapy is adoptive T cell transfer therapy, where—in its most common form—patient-derived T cells are modified so as to improve their tumor-killing potential; they are then administered to the patient, where hopefully they will attack the tumor or metastasis with improved vigor, in artificially-enhanced numbers, and ideally with improved specificity for tumor antigens [1].
The common assumption underlying both forms of immunotherapy is that the adaptive immune system, and cytotoxic T cells more specifically, have an anti-tumor function. The idea that the immune system affects cancer progression is older than the discovery of adaptive immune cells and as old as immunology itself. Ilya Metchnikoff, the discoverer of macrophages, and the founder of cellular immunology, considered the concept himself more than a 100 years ago [2, 3]. Yet, the current successful surge in using adaptive immunity to fight cancer can partly be traced in key, straightforward, preclinical experiments that demonstrated the existence of immunosurveillance, by showing that tumors grow more easily in mice lacking T cells, even if this anti-tumor activity itself can lead to the subsequent evasion of less immunogenic tumor clones [4]. Importantly, very extensive clinical data demonstrated that exclusion of T cells from tumors such as colorectal, ovarian, and pancreatic ductal carcinoma correlates with worse clinical outcome [5]. Thus, on the basis of both preclinical and clinical observations, it appears safe to state that adaptive immunity reacts against tumors. Exploitation of this feature enables us to perform cancer immunotherapy, and the positive results further confirm the validity of the initial assumption.
And yet, the assumption that the adaptive immune system is inherently anti-tumoral in its function is an oversimplification of a more complex issue. It is easy for questions and doubts to arise: Tumors do express neo-antigens [6], but will undoubtedly also express a wide range of self-antigens. Would not at least some of the latter be tolerated by the antigen-specific cells of the adaptive immune system? After all, a fetus is considered half-self and half-allo with respect to the mother (though, to be precise, the variation at the genomic level due to the allogenic father is much less than 50%) and remains completely tolerated by the maternal adaptive immune system throughout pregnancy [7]. Furthermore, at least until recent advances in therapy, cancer patients do not usually get a chance to pass their genes on to a subsequent generation after the incidence of the tumor. Thus, the way in which the immune system responds to a growing cancer may not have ever been a trait selected for by evolution. As a counter argument, one could hypothesize that immunosurveillance against oncogenesis would confer an evolutionary advantage, by protecting against the incidence of early age-onset tumorigenesis. Yet, many if not most tumors appear later in life, after the end of the reproductively active age of females. From the above questions, which will be discussed in more detail in the next sections, it follows that the assumption that the adaptive immune system is inherently optimized to attack tumors is, in the best of cases, an invalid oversimplification. Given the improvement that immunotherapy has brought about in clinical outcomes, one could argue that the precise reason why immunotherapy works is irrelevant. However, as a large percentage of patients do not, unfortunately, respond to immunotherapy [8], there is still clear space for improvement. Understanding whether and why the adaptive immune system responds to tumors may, hopefully, enable the refinement of current immunotherapy approaches.
The question of how and why the immune system responds to tumors is subordinate to the more fundamental question of what does the immune system do. The answer to this is affected by ideas from several areas of human scholarly thought that can be traced back to the foundations of modern immunology.
What do immunologists perceive as the main function of immunity? Insights from medical anthropology
The conceptualization of immune system function, in the form that we currently take for granted in clinics, universities, and research laboratories world wide, took shape mainly in the twentieth century, with the discovery of the key mechanisms that mediate cellular and humoral immunity. The pioneering theories and experiments of Burnet and Medawar used defense-oriented metaphors to describe an immune system, whose main purpose is to “defend” the organism (the “self”) from invading pathogens, which are recognized due to their being “non-self”, and thus eliminated. Anthropologists such as Emily Martin [9] and David Napier [10–12] have put forward the view that these early immunologists were, as all people, influenced by their surrounding society. Accordingly, the development of modern immunology may have been influenced by the defense-oriented public discourse during the Cold War era. The metaphors thus utilized for the pro-inflammatory cells of the immune system evoked concepts of warfare against an enemy or police surveillance against illegal activity [13]. As we will discuss below, this valid analysis actually can be applied in an equally fitting manner to ideas of the “grandfathers” of immunology, such as Ehrlich, whose pioneering ideas shaped immunology starting from the end of the nineteenth century.
The use of metaphors, whilst very convenient in efficiently introducing and conveying complex ideas, may produce unwanted side effects. In the case of the immunological self, as philosopher and historian of science Alfred Tauber has outlined, the metaphor was borrowed from philosophy and psychology, but it may be limiting our ability to precisely define the true function of the immune system [14, 15]. This may have substantial consequences, discussed below, for our ability to perceive the full range of functions that these cells mediate. It is a problem analogous to the risk of bias from lack of awareness of the imprecision in the nomenclature in immune mediators, which has been recently highlighted [16]. It may also be responsible for the close association of immunity with pathology and its dissociation from physiology [17]. Indeed, the use of metaphors may render us blind to some of the functions that ought to be considered when forming hypotheses about what immune cells do, with consequences for how these cells are then applied in an immunotherapeutic context.
A brief history and philosophy of the conceptualization of immune function
Whilst the defense-oriented bias in the definition of immune function by Burnet is real, it has been argued that both the defense-oriented view of the function of the immune system as well as a more flexible and adaptable view of immune function have both appeared even earlier in human history. Classical medicine, in ancient Greece and Rome, as taught in the tradition of Hippocrates and Galen, viewed the human body as a system in equilibrium, rather than a struggle between a self and a hostile environment. This only changed in the nineteenth century, with the development of modern biology. Metchnikoff identified macrophages and neutrophils (“microphages”), the first cellular component of the immune system to be discovered, as the means via which integrity of the self is preserved. This creates a distinction between the self and non-self (i.e., invading pathogens). Yet, according to Tauber, Metchnikoff’s view was inspired by Darwinian theories of struggle at the developmental level and postulated that the primary immune function was the continuous definition of the self, rather than the defense against pathogens, which was a secondary role. This view was in contrast to the proposed theories humoral-mediated immune responses pioneered by Ehrlich. The immunochemists Robert Koch and Paul Ehrlich were mostly interested in microbial pathogens; as a result, their vision of immune function was more focused on the defense from microbial pathogens [2, 14, 15]. Thus, the first identification of the immune system as a tool for the definition of the self rather than a defender of the self most likely belongs to Metchnikoff, even though the defense function became predominant in subsequent years.
As mentioned above, the self/non-self question was next re-approached by Macfarlane Burnet in 1949, who developed the clonal selection theory to explain how tolerance of self is obtained by elimination of self-reactive cells prior to the birth of a mammal. This was complemented by experiments performed by Medawar and colleagues [18] demonstrating tolerance, leading to their joint Nobel prize with Burnet. The narrative used to describe these seminal contributions crystallized the defense-oriented metaphors that we still use today to explain the function of pro-inflammatory T cells.
At this stage, the identity of the immunological self was considered to be defined by genetically-inherited traits. The fact that mammalian pregnancy requires the acceptance (tolerance) of a fetus that is semi-allogeneic, and hence, “half-non-self” (half-allo, strictly) was not considered to pose a problem. This was justified by Medawar who postulated—erroneously, as it turned out—that no immunological recognition of fetal foreignness occurred [19]. And yet, towards the end of the twentieth century, the strict definition of the self/non-self distinction on the basis of genetically pre-determined traits was put into question, first on a theoretical basis, and subsequently by experimental results.
Melvin Cohn suggested that self/non-self discrimination was a somatic (i.e., not genetically determined) learning process, sufficiently controlled by a two-signal model, proposed by himself and Bretcher [20]. This model envisages an epitope-derived (inactivating) signal 1 that must be licensed by antigen-specific helper T cell populations delivering a second, activating signal [21, 22]. The model accommodates several later findings, such as immunosuppressive regulatory T (Treg) cells, by positioning their effect at the level of modulation of effector functions rather than self/non-self discrimination [23]. It is noteworthy, in the context of our discussion of cancer immunotherapy, that Cohn identifies adaptive immunity as crucial for the rejection of cancer, as such a rejection must occur via the recognition of a non-self epitope. He supports this conclusion by arguing that only in vertebrates, that have both adaptive and innate immunity, is the recognition and consequent rejection of a tumor possible; this is postulated to occur, even though the actual mechanism of rejection involves both adaptive and innate immunity in its effector mechanisms [22]. The two-signal model, in a form closer to that used at present (antigen-derived activating signal 1 and antigen presenting cell-derived co-stimulating signal 2), was actually first proposed by Lafferty and Cunningham, soon after the original Bretcher and Cohn model [24].
A novel theoretical challenge to the postulate of an immune system mediating a binary discrimination of self from non-self came from Niels Jerne, whose network theory [25] postulated an immune system that defines the identity of the self via a dynamic process of complex, self-referential (recursive) feedback interactions, regulated not only at the genetic level but throughout life. By defining the self as a network-based property, and alluding to reactivity to foreign as a secondary by-product, it came closer to the original conceptualization by Metchnikoff of the immune system as a definer of the self [14]. These ideas were further developed by Antonio Coutinho and Irun Cohen, who argued that the self is not simply genetically encoded but dynamically defined [26, 27]. Pradeu, Jaeger, and Vivier have also proposed a more recent equilibrium-based model to explain the initiation or suppression of an immune response [28]. These models, including Cohn’s, were never adopted whole-heartedly by the immunological community, and hence, their predictive power remains substantially untested. Yet, the common idea of a self/non-self definition arising from somatic or network-based interactions is intriguing, and does match well with more recent experimental findings, discussed below. Furthermore, they are all—to different degrees—compatible with Metchnikoff’s idea of an immune system that works to define rather than just defend the self. To an extent, this concept is echoed in recent attempts to study mechanisms of non-aggressive responses to pathogens, where tolerance is used to minimize damage derived from the pathogen or from the response to the pathogen [29–32].
The danger model, put forward by Polly Matzinger, consolidating ideas by Janeway [33, 34], moved further away from an immune system, whose main function is the binary distinction of self/non-self, by replacing it with a system that reacts only in the presence of danger or signs of infection [35, 36]. Thus, what is non-self is decided on the basis of context. This concept was expanded by Janeway, Medzhitov, and others, who, starting from the former’s “infectious non-self model” showed that pattern recognition receptors recognize pathogen-associated molecules, such as Toll-Like Receptor (TLR) 4 that responds to bacterial lipopolysaccaride (LPS). This event is required to drive costimulation (signal 2) of T cells by antigen presenting cells, which may be innate immune cells [37]. Hence, an innate-mediated danger-sensing pathway is required for the “licensing”, or full activation of T cells. Thus, according to this model, the burden of self/non-self discrimination is shifted to both adaptive and innate immune cells [38]. The same line of investigation subsequently showed that TLR-mediated signaling can inhibit the immunosuppressive action of Treg cells [39], opening the way for the initiation of an immune response. According to the danger model, danger signals can be derived from both infectious agents, but also from cells undergoing stress during injury. According to Matzinger, the model predicts that allogenic transplants should be rejected, but fetuses and tumors should not, as they are not as associated with trauma [36]. More recent work has shown that actually both fetuses/placentas and tumors are nonetheless recognized, even if not usually rejected [7, 40]. As a clinical translation of the danger model, the triggering of TLR signals is being explored as an anti-tumor immunotherapy, with promising results [41–43], though not in all contexts [44].
In summary, the sequence of theoretical models described here, in all cases derived from contemporary available experimental data, points towards an immune system that may not just defend but define what is self (and thus tolerated) and what is non-self (and thus rejected). It is clear that this definition is not just germline-encoded, but may be somehow based on functional antigenicity. As Tauber has postulated, indeed, self may be simply what is not rejected by the immune system [15]. This may sound like a truism, yet it is a necessary admission when faced with experimental results of the last 2 decades. Despite the earlier controversial nature of suppressor T cells [45], stemming from noteworthy shortcomings discussed elsewhere [46], Treg cells have been shown to be key mediators of peripheral tolerance. Their importance was formally demonstrated with the description of the dramatic self-reactive responses present in Treg-less Foxp3-deficient mice and the corresponding Immunodysregulation Polyendocrinopathy Enteropathy X-linked disease in humans [47]. It should be noted that the exact dynamics of how immunosuppression prevails over self-reactivity is still not entirely clear in a physiological, non-perturbed state [48]. Yet, what is without doubt is that immune suppression is a crucial mechanism of tolerance. This mechanism, however, does not apply only to germline-encoded self peptides. The immune system acquires tolerance to the commensal gut bacteria, even though they are clearly not germline-encoded self. Indeed, antigen-specific responses to gut flora are affected by the identity of the flora present, leading to changes in the balance of pro-inflammatory versus regulatory T cell subpopulations [49]. Furthermore, we have previously shown that regulatory T cells suppress maternal anti-fetal responses during mammalian pregnancy, as in their absence, it is not possible to obtain successful pregnancies, unless if the father is syngeneic to the mother (i.e., of the same self) [7]. Even in these cases, in the absence of Treg cells, syngeneic fetuses will be predominantly female, as the male-specific antigens will lead to a greater degree of rejection for male fetuses [50]. Importantly for our discourse, the Treg mediating maternal–fetal tolerance can be specific for paternal alloantigens [51]. In other words, during pregnancy, the tolerized self extends to include the half-father identity of the fetus. Hence, it appears that the adaptive immune system will accept (“define as self”) or reject (“define as allo/foreign”) different antigens, according to the physiological or pathological context and not simply according to the germline-encoded self or non-self identity.
What may have T cells evolved to do?
The above suggest that the immune system, most likely via a synergy of its adaptive and innate arms, functions to not just defend, but actively define the self, and hence accept or tolerate it. A functional test for the validity of a hypothesis is to examine whether it could be compatible with evolutionarily selected functions [22]. The role of the immune system as a defender of the self is easy to envisage in all organisms possessing phagocytes that intercept invading pathogens. Yet, the identification of the definition function is not as obvious.
The issue of histocompatibility is usually encountered in the context of artificial transplants, where indeed, the immune system does define whether the graft is sufficiently self or non-self (allo). Yet, surprisingly, similar molecular events can be seen in nature. Colonial protochordates, such as Botryllus schlosseri, form underwater colonies of multicellular individuals that are all clones (different individuals but the same self, the equivalent of twins). These individuals use a polymorphic fusibility/histocompatibility gene product (FuHC), a functional analogue—though unrelated at the sequence level—of vertebrate Major Histocompatibility Complex (MHC) molecules. Via the FuHC, they identify and reject neighboring colonies if they are of the same species but “allo” compared to the original colony, whereas they fuse with them if they are compatible. The molecular mechanism of this complex process is more akin to NK-mediated allorecognition rather than T cell immunity. Nonetheless, it does suggest that, millions of years before the invention of artificial transplants, a primordial immune function may have evolved to assist the definition of the self rather than simply the defense of the self [52, 53].
With the evolution of more complex species, adaptive immunity developed twice—in a compelling example of convergent evolution—in jawless fish and in jawed vertebrates, featuring lymphocytes with variable antigen receptors, able to recognize a wide range of antigens [54, 55]. Yet, as long as growing embryos were kept within the immunologically isolated space of an egg, no issues related to the definition of the self arose. The evolution of eutherian mammals changed this, as now the maternal immune system could—and indeed does—recognize the presence of paternal alloantigens in the fetus. Thus, the mammalian immune system identifies novel, non-self-antigens, and must expand the “effective self” so as to cover these antigens. If this does not occur, for example, when Treg cells are artificially made absent, the fetus is rejected by the adaptive immune system [7]. As reproduction is a key point of evolutionary pressure, it follows that this transient expansion of the definition of the self may have been selected for. It is intriguing that the functional domains of Foxp3, the master regulator gene for Treg cells, appear to be highly conserved in eutherian mammals and in the platypus, an egg-laying mammal [56]. This suggests that the evolution of Treg cells as modifiers of immune function may have occurred prior to the evolution of placental viviparity, as one needs to restrain immune alloreactivity prior to removing the physical barrier of the egg.
The above conclusions are clearly speculative, as they deal with interpretation of past events. Yet, they can add further insight on the question of whether or not the immune system is naturally predisposed to reject or accept a tumor, or indeed whether the response depends on the immunological “context”. As highlighted above, it is unlikely that responses to tumor underwent evolutionary selection. Intriguingly, a pivotal report in 1995 by Tafuri and colleagues demonstrated that a pregnant mouse can tolerate and allow the growth of transplanted tumors that share antigens with the father, whilst rejecting tumors bearing third-party antigens. The tumors, however, could be rejected once the semi-allogeneic pregnancy came to term [40]. These are compatible with subsequent findings on the allo-specificity of the Treg mediating maternal–fetal tolerance [51]. Furthermore, they also match our findings showing that the pregnancy-associated, antigen-specific Treg expansion can temporarily block autoimmune diseases such as arthritis, whilst the pregnancy is ongoing [57]. Yet, what they suggest as far as the anti-tumor response is concerned is that the physiological specific induction of tolerance during pregnancy can completely change the outcome of an anti-tumor response, as long as the antigens involved are matching.
As a distillate of the above considerations, it is sensible to speculate that the adaptive immune system functions as a flexible mediator of the definition of the boundaries of the self. This is a self that includes commensal gut flora. It is also a self that expands to enable fetal implantation, accepting as “piggyback” passengers any other antigenic entity (be it tumor or tissue-specific inflammation) that has a sufficient antigenic overlap and appears simultaneously with pregnancy. Our own work has shown that these changes occur every time the female is fertile [58] and that they are hormonally-driven [59]. Whilst evolutionary pressure would presumably be applied on the females, males may well have inherited any immune functions that are evolutionarily-optimized to suit these key processes. The manner in which the immune system will interact with a tumor will thus be dependent on a series of factors: the physiological or endocrinological context, potential danger signals, as well as the presence of tumor neo-antigens. It cannot, in other words, be taken for granted that it will be pro- or anti-tumoral by default, without examining all these parameters.
Tumor neo-antigens
The study of tumor neo-antigens can be traced back to seminal experiments by Klein et al. [6], who identified that chemically-induced tumors in different mice of the same congenic strain may display different antigenic properties. Extensive studies on neo-antigens have since shed light on the nature of the antigens expressed on tumors. Immune responses against the tumor were shown to be dependent on sufficiently high expression levels of the antigens [60]. A large proportion of these antigens are products of mutated genes, yet fetal antigens not normally expressed in adults are also frequently found. In the case of virally-induced tumors, viral antigens are also often expressed [61, 62]. Nonetheless, many of the tumor-associated antigens will be bona fide germline self-antigens [63]. Responses against such antigens will, thus, be self-reactive or cross-reactive to self-antigens. This cross-reactivity has created substantial, and on occasions lethal, problems during adoptive cell therapy [62]. The cross-reactivity may extend beyond the domain of germline self-antigens. As the effective self includes commensal gut flora, inducing changes in microbiota was recently revealed to have a dramatic impact on the efficacy of anti-CTLA-4 [64] and anti-PD-L1 [65] cancer immunotherapy treatments. These fascinating results, seen in the context of the historical and evolutionary considerations above, are not entirely surprising. However they do suggest that immune responses to tumors are far more complex than the narratives that we commonly use would imply, and thus, updating these narratives would be prudent.
A neglected question
The immune response to the tumor is, therefore, not necessarily anti-tumoral. Immunologists with a focus on innate immunity have produced very extensive data on the tumor-promoting facets of tumor-infiltrating macrophages and other innate cell populations that can help tumors grow [66, 67]. Anti-inflammatory T cells such as Treg clearly can also get in the way of tumor immunosurveillance [68]. But can pro-inflammatory T cells have pro-tumoral roles, if the tumor can be a part of the extended self and the division of pro- and anti-tumoral roles is not as clear-cut as we usually assume it to be? And could some of the obstacles that we consider to be set up by the tumor, be actually part of the physiological T cell response? A tumor that can produce molecules that help evade an anti-tumoral immune response is clearly more likely to grow sufficiently to become a clinical problem. Yet, once we decouple our hypothesis formation from the useful but anthropomorphic assumption that the tumor actively attempts to escape whilst adaptive immunity actively fights this, additional causal relations become possible.
Our journey into this question was accidental. Adoptive T cell therapy is currently burdened by difficulties in ensuring that all transferred T cells reach their intended target site [69, 70] and in enabling the cytotoxic T cells to penetrate solid tumors. Indeed, exclusion of T cells from the tumor correlates with negative clinical outcome, even in immune checkpoint blockade immunotherapy [71]. Part of the problem is that, for a subset of tumors such as lung, breast, and pancreas, a rigid peri-tumoral fibrotic capsule often surrounds the tumor mass, inhibiting T cell access. Such a containment may be good news for the isolation of potentially carcinogenic exogenous substances [72] or for a surgeon in search of a well-defined removable target tumor. However, at surgically inaccessible tumor sites, a fibrotic capsule will keep T cells and chemotherapeutics from accessing the tumor [68, 73–75], whilst transducing pro-growth signals to the tumor itself [76, 77], leading to a boosting of tumor aggressiveness. This could potentially be of most clinical relevance in hard-to-access metastatic sites.
As a potential “tailored” solution to the problem of T cell dispersal and reduced T cell access to the tumor, we considered the idea of sampling a metastatic site so as to analyze the pattern of chemokines (the small chemotactic proteins used by immune cells to guide their migration) expressed by the metastasis itself. After identifying the chemokines differentially and significantly expressed by the metastasis, we retrovirally expressed matching chemokine receptors in the T cells used for adoptive cell therapy, enabling the transferred cells to preferentially home to the required target site. The fact that the cells used for adoptive cell therapy are typically transduced with viruses expressing modified T cell receptors [78] meant that this approach could be readily incorporated in existing therapy protocols. Simultaneously, the tailored identification of the chemokines expressed would allow its theoretical application in different types of tumor or metastases. Applied in a mouse model of spontaneous lymphnode metastasis of prostate tumors, the strategy was indeed able to enhance recruitment of the transferred cytotoxic T cells to the tumor, as intended [79]. Neither prostate tumors nor metastatic lymphnodes belong to the group of sites usually characterized by intense fibrosis. Nonetheless, we were surprised to observe that lymphnodes bearing metastatic Transgenic Adenocarcinoma of Mouse Prostate (TRAMP) prostate tumors displayed significantly higher amounts of collagen deposition compared to tumor-free lymphnodes. This suggested that perhaps, the presence of fibrosis was an inherent, rather than tissue-specific, characteristic of either the tumor, or of the interaction between the tumor and the immune system.
Tumors and fibrosis
Reports in the literature do correlate fibrotic processes with tumor presence [80]. Fibrosis formation itself, in non-oncological contexts, is a process linked to inflammation, requiring the combined contribution of Th2-polarized T cells and macrophages [81]. The presence of the Th2 cells may be required to guarantee the chronic nature of the processes mediated by innate immune cells [82], possibly due to their ability to maintain an ongoing response as long as a “legitimate” target antigen (effective non-self) is present. Indeed, fibrosis-associated tumors are characterized by the presence of Th2 cells [83]. Fibrosis occurs also in wound healing, and, appropriately, the processes taking place in wounds have been paralleled to growing tumors [84]. The surprising finding is that, in the absence of T cells, mice show significantly reduced scar formation [85]. This last observation led us to ask whether indeed the culprit for the formation of the peri-tumoral fibrotic capsule [74] that protects the tumor could be the pro-inflammatory T cells themselves.
A classic experiment revisited
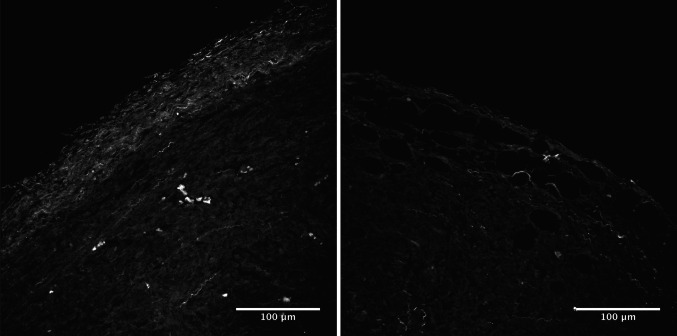
In the paper demonstrating the existence of immunosurveillance [4], mentioned at the beginning of this review, two of the key experiments involve injecting sarcomas in mice that are T cell sufficient or in Recombination Activating Gene (RAG)-deficient mice (lacking B and T cells) or in mice in which T cells have been depleted via antibody treatment. Tumors in the T cell-less mice grew to be significantly larger. We performed a variation of this experiment, injecting a (fibrosis-prone) pancreatic tumor cell line into wild-type or RAG-deficient mice. As expected, the tumors growing in T cell-sufficient mice were indeed significantly smaller. However, surprisingly, the tumors grown in the absence of T cells were larger, but had significantly less collagen deposition in their peri-tumoral fibrotic capsule. Furthermore, to demonstrate that this difference was relevant for the accessibility of the tumor to T cells, we analyzed the ability of reintroduced T cells to penetrate the larger, but less fibrotic tumors grown in the absence of T cells. The results suggested that these larger tumors were significantly more permeable to T cells [79]. Thus, the presence of the pro-tumoral fibrotic capsule that inhibits T cells access appears to be partially dependent on T cells for its formation. Importantly, this finding would have been impossible to detect in a xenotransplantation setting, where the interactions of the tumor with the host environment (including the immune response) are artificially kept in check. Results from similar experiments in a model of breast cancer, shown in Fig. 1, confirm that this phenomenon is not limited to the conditions of the specific experiment.
Fig. 1.
T cell depletion reduces the collagen density of T cell-excluding peri-tumoral capsules. Two-photon microscopy images displaying second harmonic generation signals derived from the collagen-rich peri-tumoral capsule in mice orthotopically injected with 4T1 breast cancer cells. Image on the left: wild-type mouse. Image on the right: mouse depleted of T cells prior to tumor injection. Image courtesy of Debora Vignali and Diego Morone
Consequences and conclusions
The immediate deduction from the experiments described above is that pro-inflammatory T cells appear to pro-actively assist tumor evasion from T cell attack. This may sound like a recursive riddle; however, it is not difficult to envisage a situation, where such an effect would be of potential clinical relevance. Adoptive T cell therapy requires that the transferred T cells have maximal in vivo proliferation capacity. As a consequence, they need to be as undifferentiated as possible [86]. Whilst cell therapy is mainly utilizing CD8+ cytotoxic T cells, the presence of CD4+ T cells is beneficial, as it increases the overall cytotoxic potential of the transferred cells [87]. It is reasonable to assume that the fibrosis-promoting T cells are Th2-polarized CD4+ cells, as type 2 cytokine IL-13 is a known driver of fibrosis [88]. An ideal transfer of a mixed, undifferentiated pro-inflammatory T cell population could indeed enable the clearance of a target tumor. Yet, an extrapolation of our findings would predict that a surviving metastasis could become more fibrotic, and thus more resistant to T cell attack, due to the action of—a subset of—the transferred “therapeutic” T cells. As a remedy, it would, possibly, suffice to combine adoptive cell therapy with a treatment targeting type-2 polarized responses, to minimize such a risk.
The above conclusion is not entirely novel. There is ample evidence that in pancreatic and breast cancers, Th2-polarized cells correlate with decreased survival and enhanced tumor growth [83, 89–91]. Thus, Th2-polarized cells at least correlate with pro-tumoral function. Yet, before more generalized conclusions can be drawn on the nature of this pro-inflammatory T cell subpopulation, further experiments demonstrating formal proof of their pro- versus anti-tumoral functions in different settings will be required. Currently, many of the characteristics of different types of tumors are attributed to the unique features of the tissue involved. For example, as mentioned above, breast, lung, and pancreatic cancers are considered tumors of “fibrotic-prone” tissues. However, we have seen that fibrosis formation is a result of an immune response to the tumor. The decision of whether the immune system will respond to a tumor with a “ridding” type 1/type 17 response, a “repairing”/fibrosis-associated type 2 response or a tolerizing immunoregulatory response will depend on how the immune system will define the antigenicity (or effective non-selfness) of the tumor. This will, in turn, depend on the antigens presented, the context and danger signals. By decoupling this assessment from the uniqueness of each tissue and approaching it, in an unbiased manner, as a systemic immune function problem, we may be able to come up with better, testable hypotheses, leading us closer to improved clinically-relevant immunotherapies. This does not necessarily have to be limited to cancer immunotherapy; by following a very similar rationale, we have very recently identified a noxious role for pro-inflammatory T cells in the progression of cardiac fibrosis and heart failure. Inhibition of their action using an immune checkpoint activator led to very significant therapeutic effects [92]. One would hope that the rational identification of untested hypotheses regarding causal relationships, in cancer and other pathologies, may help pave the way for innovative experimental efforts and an eventual improvement of therapy strategies.
Acknowledgements
The author is indebted to Dr. A.G. Betz, Dr. D. Kallikourdis, and Prof. M. Holbraad for initiating discussions on the definition of immunological self and pregnancy, postulate identification in scientific theory, and medical anthropology, respectively. The author is also grateful to Prof. A. Mantovani, Prof. S. Meri, Dr. G.C. Ramos, and the reviewers for helpful suggestions and critical reading of the manuscript.
Abbreviations
- FuHC
Fusibility/histocompatibility gene product
- RAG
Recombination activating gene
- Treg
Regulatory T
Funding
Aspects of the work described in this review received support from Associazione Italiana per la Ricerca su Cancro (AIRC) (MFAG10752), the Italian Ministry of Health (GR-2009-1558698 and GR-2013-02355011), Fondazione Veronesi as well as Fondazione Cariplo (2014-1184).
Compliance with ethical standards
Conflict of interest
The author declares to have no conflicts of interest.
Ethical approval and ethical standards
The data shown in this review have been authorized by the institutional animal welfare committee of the Humanitas Clinical and Research Center, as well as by the Italian Ministry of Health (authorization code 39/2014-PR).
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Fourteenth Meeting of the Network Italiano per la Bioterapia dei Tumori (NIBIT) on Cancer Bio-Immunotherapy, held in Siena, Italy, 13th–15th October 2016. It is part of a series of Focussed Research Reviews and meeting report in Cancer Immunology, Immunotherapy.
References
- 1.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 2.Tauber AI. The immunological self: a centenary perspective. Perspect Biol Med. 1991;35:74–86. doi: 10.1353/pbm.1991.0050. [DOI] [PubMed] [Google Scholar]
- 3.Vikhanski L. Immunity. Chicago: Chicago Review Press; 2016. [Google Scholar]
- 4.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 5.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 6.Klein G, Sjogren HO, Klein E, Hellstrom KE. Demonstration of resistance against methylcholanthrene-induced sarcomas in the primary autochthonous host. Cancer Res. 1960;20:1561–1572. [PubMed] [Google Scholar]
- 7.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 8.Barbee MS, Ogunniyi A, Horvat TZ, Dang TO. Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology. Ann Pharmacother. 2015;49:907–937. doi: 10.1177/1060028015586218. [DOI] [PubMed] [Google Scholar]
- 9.Martin E. Toward an anthropology of immunology: the body as nation state. Med Anthropol Q. 1990;4:410–426. doi: 10.1525/maq.1990.4.4.02a00030. [DOI] [Google Scholar]
- 10.Napier AD. The age of immunology: conceiving a future in an alienating world. Chicago: University of Chicago Press; 2003. [Google Scholar]
- 11.Napier AD. Introduction. Cult Anthropol. 2012;27:118–121. doi: 10.1111/j.1548-1360.2012.01129.x. [DOI] [PubMed] [Google Scholar]
- 12.Napier AD. A new sociobiology: immunity, alterity, and the social repertoire. Camb J Anthropol. 2013;35:20–43. doi: 10.3167/ca.2013.310202. [DOI] [Google Scholar]
- 13.Anderson W. Getting ahead of one’s self? The common culture of immunology and philosophy. Isis. 2014;105:606–616. doi: 10.1086/678176. [DOI] [PubMed] [Google Scholar]
- 14.Tauber AI. The immune self: theory or metaphor. Immunol Today. 1994;15:134–136. doi: 10.1016/0167-5699(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 15.Tauber AI. The elusive immune self: a case of category errors. Perspect Biol Med. 1999;42:459–474. doi: 10.1353/pbm.1999.0008. [DOI] [PubMed] [Google Scholar]
- 16.Mantovani A. Reflections on immunological nomenclature: in praise of imperfection. Nat Immunol. 2016;17:215–216. doi: 10.1038/ni.3354. [DOI] [PubMed] [Google Scholar]
- 17.Ramos GC. Inflammation as an animal development phenomenon. Clin Dev Immunol. 2012;2012:983203. doi: 10.1155/2012/983203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol Med. 1953;7:320–338. [Google Scholar]
- 19.Billington WD. The immunological problem of pregnancy: 50 years with the hope of progress. A tribute to Peter Medawar. J Reprod Immunol. 2003;60:1–11. doi: 10.1016/S0165-0378(03)00083-4. [DOI] [PubMed] [Google Scholar]
- 20.Bretscher P, Cohn M. A theory of self-nonself discrimination. Science. 1970;169:1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 21.Cohn M. The wisdom of hindsight. Annu Rev Immunol. 1994;12:1–62. doi: 10.1146/annurev.iy.12.040194.000245. [DOI] [PubMed] [Google Scholar]
- 22.Cohn M. The evolutionary context for a self-nonself discrimination. Cell Mol Life Sci. 2010;67:2851–2862. doi: 10.1007/s00018-010-0438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohn M. Analysis of Paris meeting redefining the “Self” of the immune system. Immunol Res. 2015;62:106–124. doi: 10.1007/s12026-015-8641-5. [DOI] [PubMed] [Google Scholar]
- 24.Lafferty KJ, Cunningham AJ. A new analysis of allogeneic interactions. Aust J Exp Biol Med Sci. 1975;53:27–42. doi: 10.1038/icb.1975.3. [DOI] [PubMed] [Google Scholar]
- 25.Jerne NK. Towards a network theory of the immune system. Ann Immunol (Paris) 1974;125C:373–389. [PubMed] [Google Scholar]
- 26.Varela FJ, Coutinho A, Dupire B, Vaz N. Cognitive networks: Immune, neural, and otherwise. In: Perelson A, editor. Theoretical immunology, Part II. New Jersey: Addison-Wesley; 1988. pp. 359–375. [Google Scholar]
- 27.Cohen IR, Young DB. Autoimmunity, microbial immunity and the immunological homunculus. Immunol Today. 1991;12:105–110. doi: 10.1016/0167-5699(91)90093-9. [DOI] [PubMed] [Google Scholar]
- 28.Pradeu T, Jaeger S, Vivier E. The speed of change: towards a discontinuity theory of immunity. Nat Rev Immunol. 2013;13:764–769. doi: 10.1038/nri3521. [DOI] [PubMed] [Google Scholar]
- 29.Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol. 2008;8:889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Råberg L, Graham AL, Read AF. Decomposing health: tolerance and resistance to parasites in animals. Philos Trans R Soc Lond B Biol Sci. 2009;364:37–49. doi: 10.1098/rstb.2008.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sears BF, Rohr JR, Allen JE, Martin LB. The economy of inflammation: when is less more. Trends Parasitol. 2011;27:382–387. doi: 10.1016/j.pt.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janeway CA. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/SQB.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Janeway CA. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 35.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 36.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 37.Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 38.Medzhitov R, Janeway CAJ. How does the immune system distinguish self from nonself. Sem Immunol. 2000;12:185–188. doi: 10.1006/smim.2000.0230. [DOI] [PubMed] [Google Scholar]
- 39.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+ CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 40.Tafuri A, Alferink J, Moller P, Hammerling GJ, Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science. 1995;270:630–633. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- 41.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 42.Salaun B, Lebecque S, Matikainen S, Rimoldi D, Romero P. Toll-like receptor 3 expressed by melanoma cells as a target for therapy. Clin Cancer Res. 2007;13:4565–4574. doi: 10.1158/1078-0432.CCR-07-0274. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez-Gugel E, Saxena M, Bhardwaj N. Modulation of innate immunity in the tumor microenvironment. Cancer Immunol Immunother. 2016;65:1261–1268. doi: 10.1007/s00262-016-1859-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chatterjee S, Crozet L, Damotte D, Iribarren K, Schramm C, Alifano M, et al. TLR7 promotes tumor progression, chemotherapy resistance, and poor clinical outcomes in non-small cell lung cancer. Cancer Res. 2014;74:5008–5018. doi: 10.1158/0008-5472.CAN-13-2698. [DOI] [PubMed] [Google Scholar]
- 45.Moller G. Do suppressor T cells exist? Scand J Immunol. 1988;27:247–250. doi: 10.1111/j.1365-3083.1988.tb02344.x. [DOI] [PubMed] [Google Scholar]
- 46.Aluvihare VR, Kallikourdis M, Betz AG. Tolerance, suppression and the fetal allograft. J Mol Med. 2005;83:88–96. doi: 10.1007/s00109-004-0608-2. [DOI] [PubMed] [Google Scholar]
- 47.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 48.Garetto S, Trovato AE, Lleo A, Sala F, Martini E, Betz AG, et al. Peak inflammation in atherosclerosis, primary biliary cirrhosis and autoimmune arthritis is counter-intuitively associated with regulatory T cell enrichment. Immunobiology. 2015;220:1025–1029. doi: 10.1016/j.imbio.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 50.Kahn DA, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci USA. 2010;107:9299–9304. doi: 10.1073/pnas.1003909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Magor BG, De Tomaso A, Rinkevich B, Weissman IL. Allorecognition in colonial tunicates: protection against predatory cell lineages? Immunol Rev. 1999;167:69–79. doi: 10.1111/j.1600-065X.1999.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 53.De Tomaso AW, Nyholm SV, Palmeri KJ, Ishizuka KJ, Ludington WB, Mitchel K, et al. Isolation and characterization of a protochordate histocompatibility locus. Nature. 2005;438:454–459. doi: 10.1038/nature04150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alder MN, Rogozin IB, Iyer LM, Glazko GV, Cooper MD, Pancer Z. Diversity and function of adaptive immune receptors in a jawless vertebrate. Science. 2005;310:1970–1973. doi: 10.1126/science.1119420. [DOI] [PubMed] [Google Scholar]
- 55.Dzik JM. The ancestry and cumulative evolution of immune reactions. Acta Biochim Pol. 2010;57:443–466. [PubMed] [Google Scholar]
- 56.Andersen KG, Nissen JK, Betz AG. Comparative genomics reveals key gain-of-function events in Foxp3 during regulatory T Cell evolution. Front Immunol. 2012;3:113. doi: 10.3389/fimmu.2012.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Munoz-Suano A, Kallikourdis M, Sarris M, Betz AG. Regulatory T cells protect from autoimmune arthritis during pregnancy. J Autoimmun. 2012;38:J103–J108. doi: 10.1016/j.jaut.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kallikourdis M, Betz AG. Periodic accumulation of regulatory T cells in the uterus: preparation for the implantation of a semi-allogeneic fetus? PLoS One. 2007;2:e382. doi: 10.1371/journal.pone.0000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benedusi V, Martini E, Kallikourdis M, Villa A, Meda C, Maggi A. Ovariectomy shortens the life span of female mice. Oncotarget. 2015;6:10801–10811. doi: 10.18632/oncotarget.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weinhold M, Sommermeyer D, Uckert W, Blankenstein T. Dual T cell receptor expressing CD8+ T cells with tumor- and self-specificity can inhibit tumor growth without causing severe autoimmunity. J Immunol. 2007;179:5534–5542. doi: 10.4049/jimmunol.179.8.5534. [DOI] [PubMed] [Google Scholar]
- 61.McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14:135–146. doi: 10.1038/nrc3670. [DOI] [PubMed] [Google Scholar]
- 63.Stewart TJ, Smyth MJ. Improving cancer immunotherapy by targeting tumor-induced immune suppression. Cancer Metastasis Rev. 2011;30:125–140. doi: 10.1007/s10555-011-9280-5. [DOI] [PubMed] [Google Scholar]
- 64.Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow. Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 67.Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33–40. doi: 10.1016/j.semcancer.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 68.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–1962. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20:607–615. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qin Z, Kim HJ, Hemme J, Blankenstein T. Inhibition of methylcholanthrene-induced carcinogenesis by an interferon gamma receptor-dependent foreign body reaction. J Exp Med. 2002;195:1479–1490. doi: 10.1084/jem.20011887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peranzoni E, Rivas-Caicedo A, Bougherara H, Salmon H, Donnadieu E. Positive and negative influence of the matrix architecture on antitumor immune surveillance. Cell Mol Life Sci. 2013;70:4431–4448. doi: 10.1007/s00018-013-1339-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. 2012;122:899–910. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salmon H, Donnadieu E. Within tumors, interactions between T cells and tumor cells are impeded by the extracellular matrix. Oncoimmunology. 2012;1:992–994. doi: 10.4161/onci.20239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garetto S, Sardi C, Martini E, Roselli G, Morone D, Angioni R, et al. Tailored chemokine receptor modification improves homing of adoptive therapy T cells in a spontaneous tumor model. Oncotarget. 2016;7:43010–43026. doi: 10.18632/oncotarget.9280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang H, Maric I, Diprima MJ, Khan J, Orentas RJ, Kaplan RN, et al. Fibrocytes represent a novel MDSC subset circulating in patients with metastatic cancer. Blood. 2013;122:1105–1113. doi: 10.1182/blood-2012-08-449413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marra F, Aleffi S, Galastri S, Provenzano A. Mononuclear cells in liver fibrosis. Semin Immunopathol. 2009;31:345–358. doi: 10.1007/s00281-009-0169-0. [DOI] [PubMed] [Google Scholar]
- 82.Loke P, Gallagher I, Nair MG, Zang X, Brombacher F, Mohrs M, et al. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol. 2007;179:3926–3936. doi: 10.4049/jimmunol.179.6.3926. [DOI] [PubMed] [Google Scholar]
- 83.De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469–478. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 85.Gawronska-Kozak B, Bogacki M, Rim JS, Monroe WT, Manuel JA. Scarless skin repair in immunodeficient mice. Wound Repair Regen. 2006;14:265–276. doi: 10.1111/j.1743-6109.2006.00121.x. [DOI] [PubMed] [Google Scholar]
- 86.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Caserta S, Kleczkowska J, Mondino A, Zamoyska R. Reduced functional avidity promotes central and effector memory CD4 T cell responses to tumor-associated antigens. J Immunol. 2010;185:6545–6554. doi: 10.4049/jimmunol.1001867. [DOI] [PubMed] [Google Scholar]
- 88.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 89.Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, et al. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–1047. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, Marches F, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med. 2011;208:479–490. doi: 10.1084/jem.20102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shiao SL, Ruffell B, DeNardo DG, Faddegon BA, Park CC, Coussens LM. TH2-Polarized CD4(+) T Cells and Macrophages Limit Efficacy of Radiotherapy. Cancer Immunol Res. 2015;3:518–525. doi: 10.1158/2326-6066.CIR-14-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kallikourdis M, Martini E, Carullo P, Sardi C, Roselli G, Greco CM, et al. T cell costimulation blockade blunts pressure overload-induced heart failure. Nat Commun. 2017;8:14680. doi: 10.1038/ncomms14680. [DOI] [PMC free article] [PubMed] [Google Scholar]