Abstract
Cholangiocarcinoma (CCA) is a cancer of the bile ducts that is associated with poor prognosis and poor treatment outcome. Approximately one-third of CCA patients can undergo surgery, but the recurrence rate is high and chemotherapy often cannot satisfactorily prolong survival. Cellular immunotherapy based on adoptive T-cell transfer is a potential treatment for CCA; however, the development of this technology and the search for an appropriate tumor-associated antigen are still ongoing. To enhance the cytotoxic activity of effector T cells against CCA, we developed self-differentiated monocyte-derived dendritic cells (SD-DC) presenting cAMP-dependent protein kinase type I-alpha regulatory subunit (PRKAR1A), which is an overexpressed protein that plays a role in the regulation of tumor growth to activate T cells for CCA cell killing. Dendritic cells (DCs) transduced with lentivirus harboring tri-cistronic cDNA sequences (SD-DC-PR) could produce granulocyte–macrophage colony-stimulating factor, interleukin-4, and PRKAR1A. SD-DC showed similar phenotypes to those of DCs derived by conventional method. Autologous effector T cells (CD3+, CD8+) activated by SD-DC-PR exhibited greater cytotoxic activity against CCA than those activated by conventionally-derived DCs. Effector T cells activated by SD-DC-PR killed 60% of CCA cells at an effector-to-target ratio of 15:1, which is approximately twofold greater than the cell killing performance of those stimulated with control DC. The cytotoxic activities of effector T cells activated by SD-DC-PR against CCA cells were significantly associated with the expression levels of PRKR1A in CCA cells. This finding that SD-DC-PR effectively stimulated autologous effector T cells to kill CCA cells may help to accelerate the development of novel therapies for treating CCA.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2212-2) contains supplementary material, which is available to authorized users.
Keywords: Cholangiocarcinoma, Cellular immunotherapy, Dendritic cells, Self-differentiated monocyte-derived dendritic cells, Cytotoxic T cells
Introduction
Cholangiocarcinoma (CCA) [1] is a fatal bile duct cancer that has poor prognosis and treatment outcome, with a 5-year overall survival rate of only 5–10%. In patients who underwent surgery for CCA, the 5-year recurrence rate was approximately 70–80% [2]. In patients with advanced and metastatic CCA, systemic chemotherapy using a combination of gemcitabine and cisplatin was reported to prolong median overall survival by about 1 year [3]. Given the poor survival rates associated with the current treatments used to manage this disease, a novel approach for treating CCA is urgently needed.
Adoptive T-cell transfer is a type of cellular immunotherapy that is performed by transferring ex vivo-activated autologous effector T cells to the patient for cancer treatment. This approach is considered to be a promising treatment alternative in patients with CCA. Since adoptive T-cell transfer requires T-cell activation, a new method was developed to improve ex vivo activation of autologous effector T cells. Specifically, self-differentiated monocyte-derived dendritic cells (SD-DC) for ex vivo activation of effector T cells were recently developed to overcome the obstacle of large-scale DC production [4, 5]. SD-DC are promoted by transduction with lentivirus construct encoding granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4), both of which are required for DC differentiation and activation. In addition to promoting DC differentiation, lentivirus transduction to express GM-CSF and IL-4 in combination with a tumor antigen can also influence tumor antigen processing and presentation. Recently, transduction of C57BL/6 bone marrow precursor cells or CD14+ monocytes with lentivirus encoding GM-CSF, IL-4, and melanoma antigen (tyrosinase-related protein 2, TRP2) was reported to promote DC differentiation without toxicity. These SD-DC markedly enhanced T-cell expansion and cytotoxic activity against melanoma [4, 5]. Thus, SD-DC demonstrated an ability to present tumor-associated antigen (TAA) that activated effector T cells to kill cancer cells.
The ideal TAA is one that is specifically expressed at high level in tumor cells, but that is not expressed in normal cells. cAMP-dependent protein kinase type I-alpha regulatory subunit (PRKAR1A) was previously reported to overexpress and contribute to the induction of biliary cell transformation and cell growth in cholangiocarcinogenesis [6]. Interestingly, a high expression of PRKAR1A is restricted to CCA tissue and not to normal bile duct epithelia, which suggests the use of PRKAR1A as the TAA to be presented by SD-DC [7]. Herein, we report the generation of SD-DC-PR by lentivirus transduction to activate effector T cells ex vivo. SD-DC-PR demonstrated an ability to stimulate effector T cells to kill CCA cells at a rate approximately twofold greater than the cell killing performance of those stimulated with control DCs.
Materials and methods
Immunohistochemistry
Paraffin-embedded liver tissues from primary tumor of CCA patients were obtained from the specimen bank of the Cholangiocarcinoma Research Institute, Faculty of Medicine, Khon Kaen University, Thailand. PRKAR1A protein was stained according to standard immunohistochemistry protocol. Briefly, tissue sections were deparaffinized and rehydrated by stepwise ethanol treatments. Mouse anti-PRKAR1A antibody at a dilution of 1:400 (Abcam, Cambridge, UK) was added and incubated at 4 °C overnight. After washing, sections were incubated with HRP-conjugated EnVision™ secondary antibody (Agilent Technologies, Inc., Santa Clara, CA, USA), and peroxidase activity was detected by diaminobenzidine (DAB) solution. Tissues were stained with hematoxylin and then dehydrated. Stained sections were mounted and evaluated under a light microscope (400× magnification, Eclipse Ti2, Nikon, Nikon Instrument Inc. Melville, NY, USA).
Construction of lentivirus harboring tri-cistronic cDNA sequences and lentivirus production
Lentivirus harboring tri-cistronic complementary DNA sequences encoding granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin-4 (IL-4), and PRKAR1A protein (pCDH-GM/IL4/PR) was constructed as previously described [5]. Control vector expressing only PRKAR1A (pCDH-PR) or vector expressing unrelated protein, phytochrome-based near-infrared fluorescent protein (iRFP), GM-CSF, and IL-4 (pCDH-GM/IL4/iRFP) was also generated. HEK293T cells were transformed with pCDH-GM/IL4/PR, pCDH-PR, or pCDH-GM/IL4/iRFP for viral packing to produce lentiviruses, namely LV-GM/IL4/PR, LV-PR, and LV-GM/IL4/iRFP, respectively. Lentiviral titers were quantitated by measuring p24 concentration (Clontech Laboratories, Inc., Mountain View, CA, USA).
Western blot analysis and enzyme-linked immunosorbent assay (ELISA)
CCA cells [KKU-100 (JCRB1568), KKU-213 (JCRB1557), and KKU-055 (JCRB1551)] were obtained from the Japanese Collection of Research Bioresources Cell Bank (JCRB), National Institute of Biomedical Innovation, Osaka, Japan. CCA cells were treated with 20 µM cycloheximide (Sigma-Aldrich Corporation, St. Louis, MO, USA) at various time points to investigate PRKAR1A half-life, or were treated with 10 µM MG132 (Sigma-Aldrich) and/or 20 µM cycloheximide for 12 h to determine proteasome degradation of PRKAR1A, which were analyzed by Western blot method. Cell lysates were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted to detect PRKAR1A or actin using mouse anti-human PRKAR1A (Abcam, Cambridge) or mouse anti-actin antibody (Santa Cruz Biotechnology, Dallas, TX, USA), respectively. For ELISA, culture supernatant was harvested on the indicated day and subjected to measurement of cytokines using Human GM-CSF Quantikine ELISA Kit (R&D Systems, Inc., Minneapolis, MN, USA), Human IL-4 ELISA (ImmunoTool, Friesoythe, Germany), and Human IL-12 ELISA (ImmunoTool).
Preparation of conventional DC and SD-DC-PR
Monocytes that were isolated from human peripheral blood mononuclear cells (PBMC) were used to prepare dendritic cells (DCs) by culturing in AIM-V media (Invitrogen, Carlsbad, CA, USA) containing GM-CSF and IL-4 (ImmunoTool). Whole cell lysate from CCA as a source of TAA was added to pulse DCs to achieve a broad antitumor response at day 5, after which the DC culture was continued in AIM-V media containing tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ) (ImmunoTool). For preparation of DC-PRKAR1A, monocytes were transduced with LV-PR and cultured in AIM-V media containing GM-CSF and IL-4. For preparation of SD-DC-PR, monocytes were transduced with LV-GM/IL4/PR and cultured in fresh AIM-V media without cytokine supplementation.
Activation of effector T cells with DC
Isolated effector T cells were subjected to DC stimulation by seeding the effector cells with conventionally prepared DCs, DC-PR, or SD-DC-PR at an effector cell to DC ratio of 10:1 in RPMI1640 Medium (Invitrogen) containing 5% human serum for 3 days. Medium was then replaced with AIM-V containing IL-2, IL-7, and IL-15 (ImmunoTool), after which the culture was continued for other 7 days.
Cytotoxicity assay
Target KKU-213 or KKU-100 cells were seeded into a 24-well plate. Stimulated effector cells were incubated with target cells at effector cell (E) to target cell (T) ratios of 3:1, 7.5:1, and 15:1. After 24 h of co-culturing, target cells were harvested, and dead cells were measured by Annexin V/PI staining. Cells were analyzed using a BD FACSCalibur™ flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Immunophenotype assay
The following monoclonal antibodies reactive to DC markers were used in DC immunophenotype analysis: APC-conjugated CD14 or CD11c; PE-conjugated CD83, CD86, or CD11c; FITC-conjugated HLA-DR, CD40, or CD14 (all eBioscience, San Diego, CA, USA), as well as their respective isotype controls (eBioscience). DCs were analyzed using a BD FACSCalibur™ flow cytometer (BD Biosciences).
Statistical analysis
All data analyses were performed using GraphPad Prism software version 7 (GraphPad Software, La Jolla, CA, USA). At least three independent assays were performed for all experiments. Data sets of multiple groups were compared using one-way analysis of variance (ANOVA) and Tukey’s HSD (honest significant difference) post hoc test. Data sets of two groups were compared using Student’s t test. A p value less than 0.05 was considered to be statistically significant (* indicates p < 0.05; ** indicates p < 0.01; and, *** indicates p < 0.001).
Results
PRKAR1A expression in human CCA tissue and cell lines
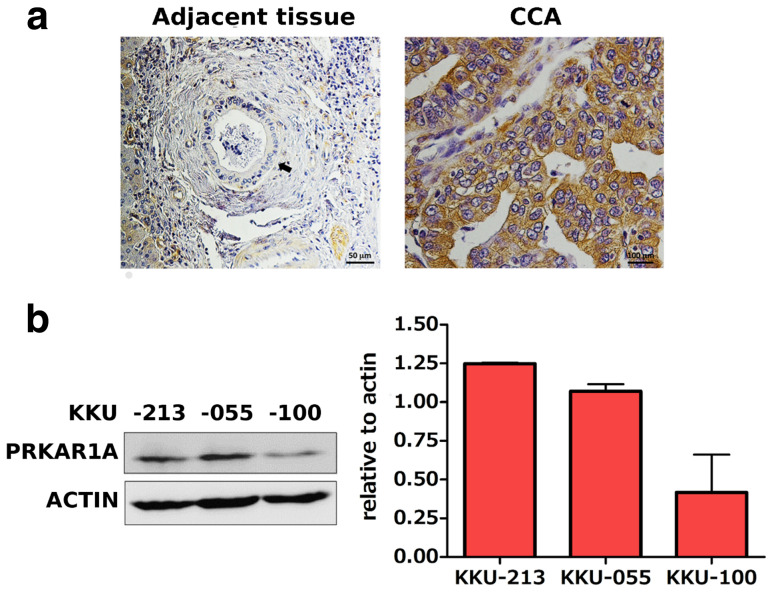
When examined by immunohistochemical staining, PRKAR1A was highly expressed in human CCA tissues, but poorly expressed in normal bile duct tissue (Fig. 1a). PRKAR1A was also expressed in CCA cell lines, including KKU-213, KKU-055, and KKU-100, as demonstrated by Western blot analysis. PRKAR1A expression was greater in KKU-213 and KKU-055 cells than in KKU-100 cells (Fig. 1b).
Fig. 1.
PRKAR1A expression in normal bile duct and CCA tissues. a Immunohistochemistry showing PRKAR1A expression in normal bile duct (black arrow) and in CCA tissues. b Western blot analysis showing PRKAR1A expression in CCA cell lines KKU-213, KKU-055, and KKU-100
PRKAR1A degradation and inhibition
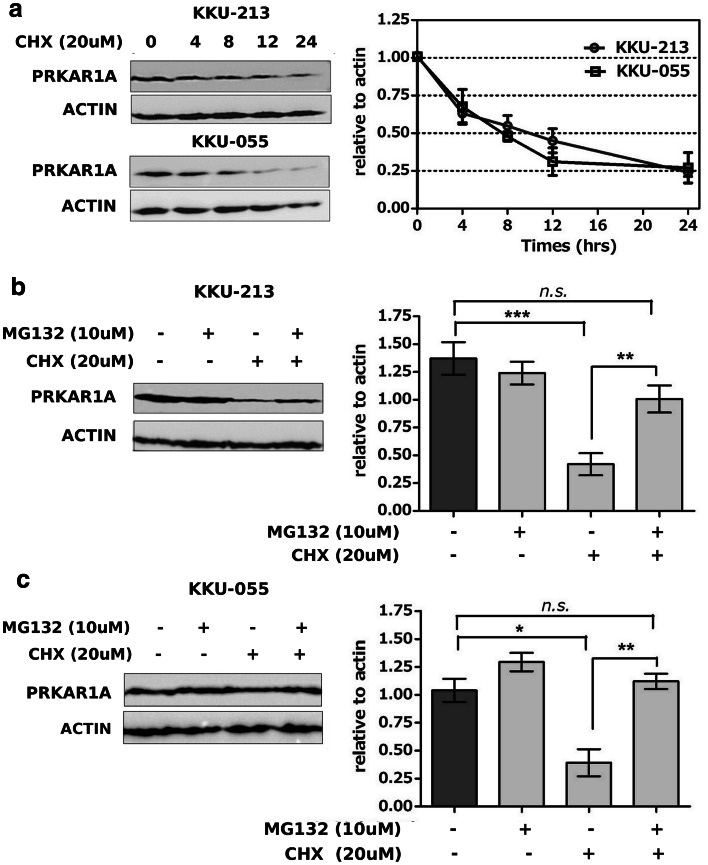
PRKAR1A degradation was examined in two CCA cell lines (KKU-213 and KKU-055) by inhibition of newly synthesized proteins with 20 µM cycloheximide. Relative PRKAR1A quantities were measured at 4, 8, 12, and 24 h after treatment by Western blot analysis. PRKAR1A half-life was approximately 6 h, and the turn-over rate was about 24 h. There was no statistically significant difference between the proteins prepared from the two different cell lines (Fig. 2a). Treatment of KKU-213 or KKU-055 cells with cycloheximide combined with proteasome inhibitor MG132 could significantly restore PRKAR1A in both cell lines when compared with cycloheximide treatment alone (Fig. 2b, c). These results indicated that PRKAR1A that was overexpressed in CCA cell lines had a short half-life. Furthermore, PRKAR1A was mainly processed via proteasome degradation. This finding strongly suggested the use of PRKAR1A as a novel tumor antigen target for DC presentation and activation of cytotoxic effector T cells.
Fig. 2.
PRKAR1A degradation. a PRKAR1A analyzed by Western blot method in KKU-213 and KKU-055 cell lines after inhibition of protein synthesis by cycloheximide and examination at 0, 4, 8, 12, and 24 h. b, c Inhibition of PRKAR1A protein synthesis in KKU-213 and KKU-055 cell lines by cycloheximide and protein degradation by proteasome inhibitor MG132
Generation of SD-DC-PR by LV-GM/IL4/PR transduction into human monocytes
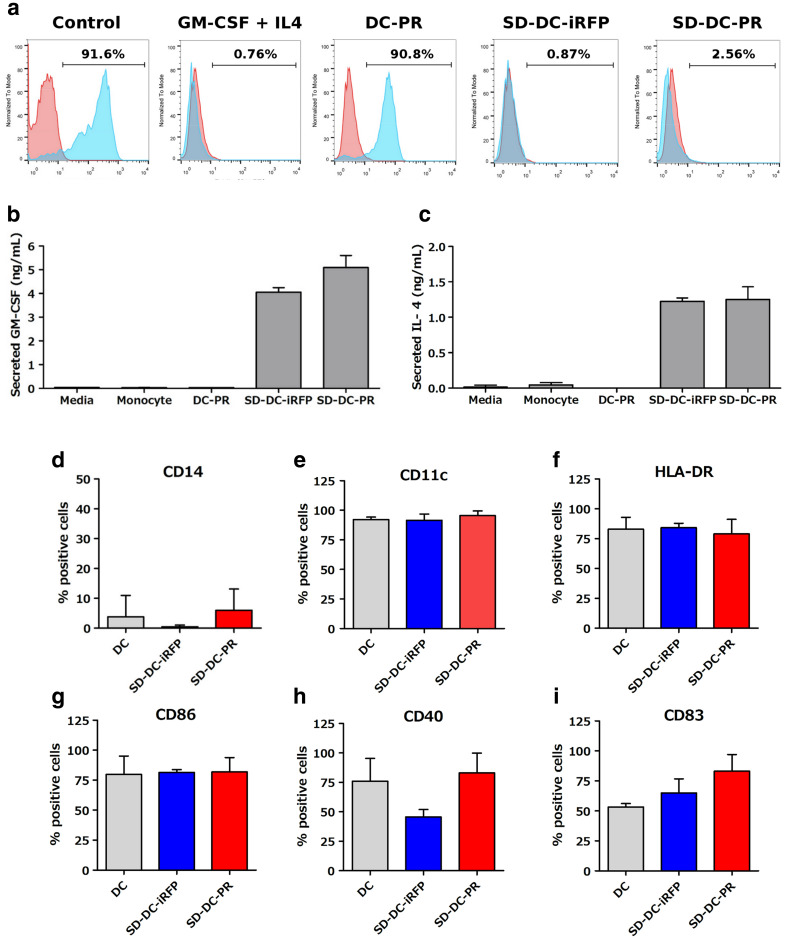
Tri-cistronic lentivirus vector containing GM-CSF, IL4, and PRKAR1A (pCDH-GM/IL4/PR) was constructed to produce lentivirus (LV-GM/IL4/PR) in HEK293T cells. The viruses were transduced into human monocytes to generate self-differentiated DCs expressing PRKAR1A (namely SD-DC-PR). The construct containing only PRKAR1A gene (pCDH-PR) or containing GM-CSF, IL4, and unrelated gene iRFP (pCDH-GM/IL4/iRFP) to produce lentivirus in HEK293T cells (LV-PR) or LV-GM/IL4/iRFP was used to generate DC-PR or SD-DC-iRFP as negative controls. To test the feasibility of LV-GM/IL4/PR to drive DC differentiation, we transduced human monocytes isolated from healthy donors with LV-GM/IL4/PR. Immunophenotypes of LV-GM/IL4/PR- and LV-GM/IL4/iRFP-transduced cells at day 5 after transduction were assayed. The results showed remarkable reduction in CD14-positive cells (> 80%) compared to the reduction in CD14-positive cells in control parental cells. This result was similar to that observed in the monocytes derived by the addition of GM-CSF and IL-4 (Fig. 3a). In contrast, transduction of cells with LV-PR could only slightly decrease CD14-positive cells (< 10%). We also examined GM-CSF and IL-4 expression in culture supernatant. The results revealed these two cytokines to be at high levels in the culture supernatant of LV-GM/IL4/PR- and LV-GM/IL4/iRFP-transduced cells (Fig. 3b, c). DC marker and costimulatory molecules, including CD14, CD11c, HLA-DR, CD86, CD40, and CD83, were determined and compared with those of the conventional DCs, SD-DC-iRFP, and SD-DC-PR from three donors. The results showed significant reduction in CD14-positive cells in all three donors (Fig. 3d). The cells positive for costimulatory molecules varied among donors, and the cells positive for CD83 (a maturation marker) were greatly varied when they were transduced with SD-DC-PR, as compared to cells derived by conventional method in all three donors (Fig. 3e–i).
Fig. 3.
Analysis of dendritic cells derived from human monocytes. a Results of flow cytometry analysis showing representative mean fluorescence intensity profiles and percentages of viable CD14+ cells after GM-CSF and IL-4 treatment or LV-PR transduction or LV-GM/IL4/iRFP transduction or LV-GM/IL4/PR transduction. b, c Levels of secreted GM-CSF and IL-4 in cell-culture supernatants. d–i Immunophenotypes and expression of costimulatory molecules in DC generated by the conventional method (gray column) or by LV-GM/IL4/iRFP transduction (blue column) or by LV-GM/IL4/PR transduction (red column) from three healthy donors
Effector T cells stimulated with SD-DC-PR
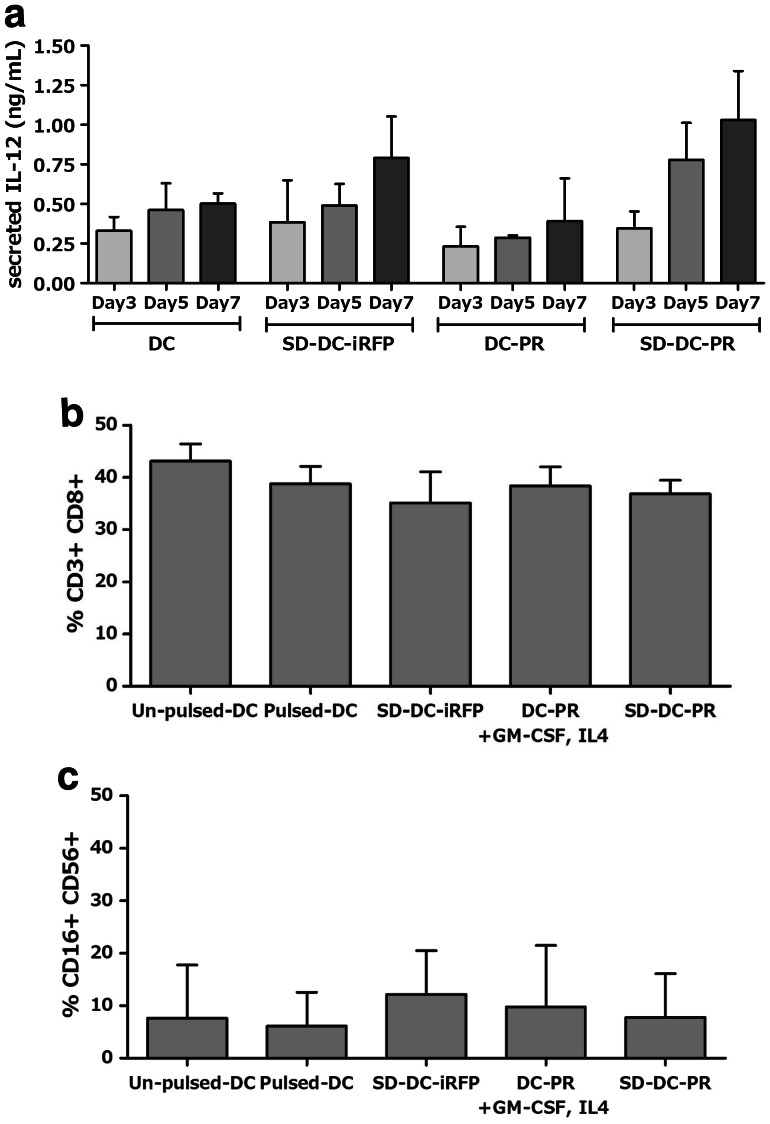
To evaluate the immunologic potency of SD-DC-PR, we examined the production of IL-12 (a T-cell stimulating factor) in the culture supernatant at days 3, 5, and 7 by ELISA. At day 7 post-transduction, the production of IL-12 in the culture supernatant of SD-DC-PR was ~ 1 ng/mL, which was higher than the production of IL-12 in the culture supernatant of conventional DCs (Fig. 4a). Importantly, that amount of IL-12 was sufficient to activate T cells [8]. The ability of DCs to prime autologous effector T cells was further studied. NK cells (CD3−, CD16+, and CD56+) and cytotoxic CD8+ T cells (CD3+ and CD8+) were compared among the DCs, the conventional DCs which were transduced with LV-PR (DC-PR), SD-DC-iRFP and SD-DC-PR. The results showed no significant differences among these four groups of DCs (Fig. 4b, c).
Fig. 4.
Efficiency of SD-DC-PR to activate effector T cells. a Ability of DCs to produce IL-12 in culture supernatant after activation. b Proportions of NK cells. c Proportions of cytotoxic CD8+ T cells after activation with DCs generated by different methods
Killing ability of effector T cells stimulated with SD-DC-PR
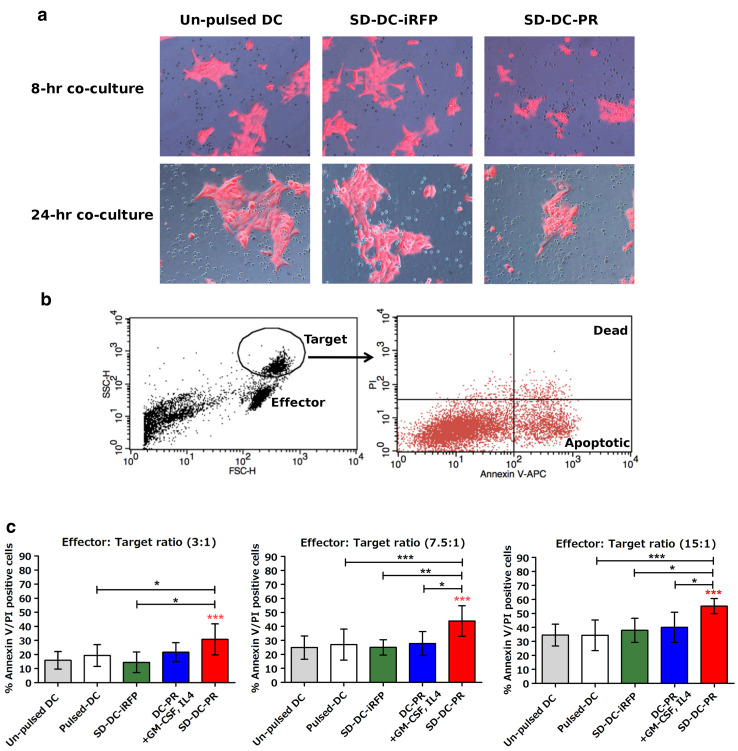
To examine whether the effector T cells stimulated with SD-DC-PR could kill target CCA cells or not, the stimulated effector T cells were co-cultured with target KKU-213 cells. After co-culturing for 8 h, the effector T cells stimulated with SD-DC-PR surrounded the target cells, but this finding was not observed in the co-culturing between the T cells stimulated with control unpulsed-DC or SD-DC-iRFP and the target cells (Fig. 5a). After co-culturing for 24 h, remarkable morphological changes in the target cells were observed (Fig. 5a). The numbers of dead cells were then analyzed by Annexin V/PI staining. The population of tumor cells were discriminated from effector cells by gating strategy (forward and side scatter signal-intensity), and further analyzed for Annexin V/PI-positive cells (Fig. 5b). The results showed that the effector T cells stimulated with SD-DC-PR caused greater apoptosis in target KKU-213 cells than that caused in effector T cells stimulated with DC derived by conventional DCs or DC-PR or SD-DC-iRFP at different effector-to-target (E:T) ratios (Fig. 5c). The cell killing activities correlated in a dose-dependent manner with the E:T ratios. At the highest E:T ratio (15:1), effector T cells stimulated with SD-DC-PR caused 60% cell death, which is approximately twofold greater than the cell death caused by T cells stimulated with the control unpulsed-DC and the other three conditions.
Fig. 5.
Efficiency of effector T cells for killing CCA (KKU-213) cells after activation by DC generated by different methods. Effector T cells after activation for 10 days by DCs generated by different methods were determined for their cytotoxic activity relative to the killing KKU-213 cells. a Attraction of effector T cells to KKU-213 (red) was compared under a fluorescence microscope among effector T cells activated by control DC (unpulsed), SD-DC-iRFP, or SD-DC-PR. b Gating strategy and Annexin V/PI staining used during flow cytometry to analyze dead tumor cells. c Percentages of target cell death were monitored by Annexin V/PI staining after incubation for 24 h with effector T cells at E:T ratios of 3:1, 7.5:1, and 15:1
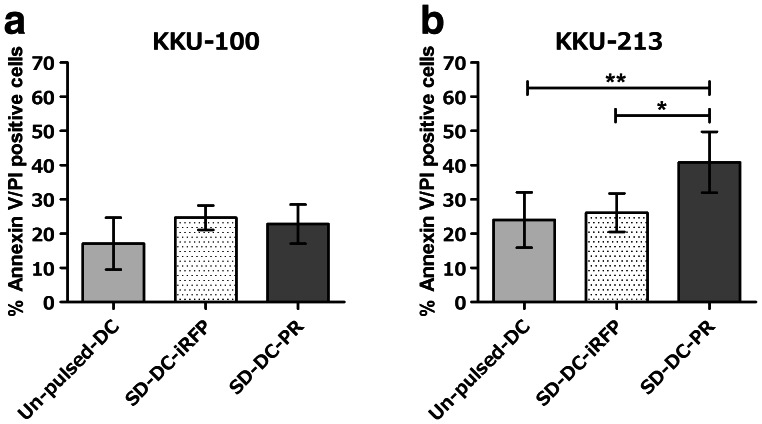
Killing ability of effector T cells associated with intracellular PRKAR1A levels
Since PRKAR1A expression was higher in KKU-213 cells than that in KKU-100 cells (Fig. 1b), we set forth to test association between the killing ability of effector T cells and the level of expression of intracellular PRKAR1A. Co-culturing of each of these target cells with effector T cells stimulated with SD-DC-PR was conducted. The results showed that at an E:T ratio of 7.5:1, effector T cells caused greater cell death in the co-culture with KKU-213 cells than in the co-culture with KKU-100 cells (Fig. 6a, b).
Fig. 6.
Cytotoxic activity of effector T cells, and PRKAR1A expression in CCA cells. Cytotoxic activities of activated effector T cells for killing CCA cells were examined. a KKU-100 cells expressing low level of PRKAR1A. b KKU-213 cells expressing high level of PRKAR1A. The E:T ratio was 3:1
Discussion
Adoptive T-cell transfer is a promising cellular immunotherapy for treatment of CCA, because it was previously shown that a postoperative vaccination with autologous tumor lysate-pulsed DCs plus ex vivo-activated T-cell transfer is an effective treatment for prevention of recurrence, and the achievement of long-term survival in patients with intrahepatic cholangiocarcinoma (ICC) [9]. To further develop this therapeutic approach for CCA, we generated self-differentiated dendritic cells with high expression of PRKAR1A (SD-DC-PR) by lentivirus transduction to enhance the cytotoxic activity of effector T cells against CCA. PRKAR1A is a protein that is overexpressed in CCA that plays an important role in carcinogenesis and tumor growth [7, 10]. By immunohistochemistry study, we confirmed the overexpression of PRKAR1A in CCA tissues, and its low expression in normal bile duct tissues (Fig. 1a). PRKAR1A was also found to be highly expressed in the KKU-213 and KKU-055 cell lines, but to be far less expressed in the KKU-100 cell line (Fig. 1b). This finding indicates variable PRKAR1A expression in CCA, and this suggests that PRKAR1A expression may need to be evaluated prior to cellular immunotherapy. TAA that is presented by DC is usually processed via the proteasome complex [11]. Indeed, PRKAR1A is one of the proteins that is targeted by proteasome [12]. In this study, we demonstrated that PRKAR1A in CCA cells was degraded via proteasome (Fig. 2b), which strongly suggests the possibility of using PRKAR1A as the TAA to be presented to activate effector T cells. In addition to PRKAR1A, several TAAs with high expression rates in CCA and that represent promising targets for the generation of SD-DC-TAA have recently been reviewed, including cancer–testis antigens (CTA), centrosome related protein TCC52, melanoma-associated antigen (MAGE), Forkhead box M1 (FOXM1), cancer–testis antigen NY-ESO-1, and mesothelin [13].
The SD-DC-PR and SD-DC-iRFP that we generated could drive the differentiation of monocytes to DC for more than 80%, which was evident by monocyte marker CD14 staining (Fig. 3a, d), which suggests the efficiency of GM-CSF and IL-4 production in culture supernatant (Fig. 3b, c), and their activation capacities. Immunophenotypes of SD-DC-PR and SD-DC-iRFP at day 5 were the same as those of the conventional DC generated by exogenous GM-CSF and IL-4, but some variations were observed among different donors (Fig. 3e-i). Additional knowledge of the kinetics of costimulatory molecules would be helpful for determining phenotype characteristics of DC over time. We have also demonstrated the ability of SD-DC-PR to stimulate effector T cells to kill CCA cell line (Fig. 5a). The effector T cells stimulated by SD-DC-PR had greater cytotoxic activity than conventional DC and other conditions at different effector-to-target (E:T) ratios (Fig. 5c), although the ability of SD-DC-PR to prime cytotoxic CD8+ T cells (CD3+ and CD8+) and NK cells (CD3−, CD16+, and CD56+) was not different when compared to the other groups (Fig. 4b, c). These results indicated that SD-DC-PR has more effect on the function of cytotoxic CD8+ T cells than on their numbers, which was in accordance with the higher level of IL-12 observed in SD-DC-PR in the culture supernatant (Fig. 4a), and also in accordance with the higher increased levels of IFN-γ, granzyme, and perforin expression in the cytotoxic T cells activated by SD-DC-PR (Supplemental Fig. a–c). The advantage of lentivirus transduction to transfer TAA to antigen-presenting cells (APC) has been reported to sustain transgene expression and to promote TAA-specific T-cell recognition [14]. Interestingly, the CD8+ T-cell population could recognize lentivirus-expressing antigens in MHC class I presentation [15]. The persistent expression of GM-CSF and IL-4 during the co-culture of DC and effector T cells may be another possibility underlying the higher cytotoxicity of effector T cells stimulated by SD-DC-PR. GM-CSF can regulate survival, activation, and differentiation of various hematopoietic cell linages. However, its effect on T-cell function remains poorly understood. Study in GM-CSF-deficient mice showed diminished response of either Th1 or Th2 cells to antigenic stimulation [16], suggesting the critical role of GM-CSF in the regulation of T cells. The involvement of IL-4 in the activation of CD8+ T cells has been reported for its effect on the quantity and quality of the CD8+ T-cell pool. Treatment of IL-4 in T-cell culture effectuated the upregulation of IFN-γ synthesis via the MAPK and PI3K signaling pathway [17]. Furthermore, the effect of IL-4 on CD8+ T-cell expansion was reported to strongly promote via IL-4 signaling, and their proliferation was stimulated in an MHC class I-dependent manner [18].
A personalized peptide vaccine designed to induce immune response sufficient enough to promote long-term survival in patients with CCA was recently reported, which suggests the efficiency of using TAA from personalized data [19]. Unlike the aforementioned personalized therapy, the PRKAR1A used in the present study was selected based on pooled clinical data from Thai patients with CCA to demonstrate the benefit of using SD-DC-PR as an off-the-shelf technology to provide a broad therapeutic approach for Thai patients with CCA. This may also foreshadow the use of the SD-DC system with other overexpressed TAAs that were previously reported. The SD-DC system may decrease the requirement for exogenous cytokines, and reduce the number of steps needed for the production of quality DC that can effectively activate effector T cells. This system should be safe, because the lentiviral constructs are only used to generate SD-DC for ex vivo activation of the effector T cells to be used in adoptive T-cell therapy. Additional studies using patient PBMCs should be conducted before moving forward to clinical trials. This method will work in a clinical setting by generation of a larger quantity of effector T cells in compliance with good manufacturing practices for clinical use.
In conclusion, we report here the development of adoptive T-cell therapy for CCA via the generation of SD-DC-PR, which demonstrated ability to stimulate and enhance effector T cells for killing CCA cells. This finding may help to accelerate the development of novel therapies for treating CCA.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors gratefully acknowledge Dr. Naravat Poungvarin of the Clinical Molecular Pathology Laboratory, Department of Clinical Pathology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand for providing lentivirus vectors. The authors also thank Kevin Jone for editing.
Abbreviations
- CCA
Cholangiocarcinoma
- CHX
Cycloheximide
- CTA
Cancer–testis antigens
- DAB
Diaminobenzidine
- FOXM1
Forkhead box M1
- MAGE
Melanoma-associated antigen
- PBMC
Peripheral blood mononuclear cell
- PI
Propidium iodide
- PRKAR1A
cAMP-dependent protein kinase type I-alpha regulatory subunit
- SD-DC
Self-differentiated monocyte-derived dendritic cell
- TAA
Tumor-associated antigen
- TRP2
Tyrosinase-related protein 2
Author contributions
AP conceived the study, designed and conducted experiments, and drafted and revised the manuscript. CT optimized DC culture protocol and conducted experiments. NS designed and conducted experiments. JS designed and conducted experiments. NP conducted experiments. MJ developed and optimized DC culture protocol, and conducted experiments. SW acquired human CCA tissues, and isolated and cultured CCA cells. PY conceived the study, managed the research team, designed experiments, and edited and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was financially supported by Mahidol University (Grant no. R016010006), the Thailand Research Fund (TRF) (Grant no. IRG5980006), the TRF-International Research Network (TRF-IRN) (Grant no. IRN58W001), and the Newton Fund-Office of Higher Education Commission (OHEC) Institutional Links Grant. Chutamas Thepmalee was supported by a TRF-Royal Golden Jubilee (TRF-RGJ)-Ph.D. Scholarship (Scholarship no. PHD/0044/2556). Nattaporn Phanthaphol was supported by a TRF-IRN Scholarship (Scholarship no. IRN5801PHDW03). Mutita Junking was supported by a TRF Grant for New Researcher (Grant no. TRG5780173) and a Siriraj Chalermprakiat Grant. Pa-thai Yenchitsomanus was supported by a Siriraj Chalermprakiat Grant.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval and ethical standards
For the use of human tissues from cancer patients, written informed consent was obtained in accordance with the approval of the Human Research Committee, Khon Kaen University (Approval number: #HE471214). For the use of blood samples from healthy donors, written informed consent was obtained in accordance with the approval of the Siriraj Institutional Review Board (SIRB), Faculty of Medicine Siriraj Hospital, Mahidol University (Approval number: Si 517/2016).
References
- 1.Senzolo M, T MS, Rossetto V, et al. Prospective evaluation of anticoagulation and transjugular intrahepatic portosystemic shunt for the management of portal vein thrombosis in cirrhosis. Liver Int. 2012;32(6):919–927. doi: 10.1111/j.1478-3231.2012.02785.x. [DOI] [PubMed] [Google Scholar]
- 2.Blechacz BR, Gores GJ. Cholangiocarcinoma. Clin Liver Dis. 2008;12(1):131–150. doi: 10.1016/j.cld.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 4.Koya RC, Kimura T, Ribas A, et al. Lentiviral vector-mediated autonomous differentiation of mouse bone marrow cells into immunologically potent dendritic cell vaccines. Mol Ther. 2007;15(5):971–980. doi: 10.1038/mt.sj.6300126. [DOI] [PubMed] [Google Scholar]
- 5.Pincha M, Sundarasetty BS, Salguero G, et al. Identity, potency, in vivo viability, and scaling up production of lentiviral vector-induced dendritic cells for melanoma immunotherapy. Hum Gene Ther Methods. 2012;23(1):38–55. doi: 10.1089/hgtb.2011.170. [DOI] [PubMed] [Google Scholar]
- 6.Loilome W, Yongvanit P, Wongkham C, et al. Altered gene expression in Opisthorchis viverrini-associated cholangiocarcinoma in hamster model. Mol Carcinog. 2006;45(5):279–287. doi: 10.1002/mc.20094. [DOI] [PubMed] [Google Scholar]
- 7.Loilome W, Juntana S, Namwat N, et al. PRKAR1A is overexpressed and represents a possible therapeutic target in human cholangiocarcinoma. Int J Cancer. 2011;129(1):34–44. doi: 10.1002/ijc.25646. [DOI] [PubMed] [Google Scholar]
- 8.Bright JJ, Sriram S. TGF-beta inhibits IL-12-induced activation of Jak–STAT pathway in T lymphocytes. J Immunol. 1998;161(4):1772–1777. [PubMed] [Google Scholar]
- 9.Shimizu K, Kotera Y, Aruga A, et al. Clinical utilization of postoperative dendritic cell vaccine plus activated T-cell transfer in patients with intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2012;19(2):171–178. doi: 10.1007/s00534-011-0437-y. [DOI] [PubMed] [Google Scholar]
- 10.Loilome W, Juntana S, Pinitsoontorn C, et al. Suppression of PRKAR1A expression enhances anti-proliferative and apoptotic effects of protein kinase inhibitors and chemotherapeutic drugs on cholangiocarcinoma cells. Asian Pac J Cancer Prev. 2012;13(Suppl):143–147. [PubMed] [Google Scholar]
- 11.Neefjes J, Jongsma ML, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. 2011;11(12):823–836. doi: 10.1038/nri3084. [DOI] [PubMed] [Google Scholar]
- 12.Patronas Y, Horvath A, Greene E, et al. In vitro studies of novel PRKAR1A mutants that extend the predicted RIalpha protein sequence into the 3′-untranslated open reading frame: proteasomal degradation leads to RIalpha haploinsufficiency and Carney complex. J Clin Endocrinol Metab. 2012;97(3):E496–E502. doi: 10.1210/jc.2011-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hochnadel I, Kossatz-Boehlert U, Jedicke N, et al. Cancer vaccines and immunotherapeutic approaches in hepatobiliary and pancreatic cancers. Hum Vaccines Immunother. 2017;13(12):2931–2952. doi: 10.1080/21645515.2017.1359362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lizee G, Gonzales MI, Topalian SL. Lentivirus vector-mediated expression of tumor-associated epitopes by human antigen presenting cells. Hum Gene Ther. 2004;15(4):393–404. doi: 10.1089/104303404322959542. [DOI] [PubMed] [Google Scholar]
- 15.Zarei S, Leuba F, Arrighi JF, Hauser C, Piguet V. Transduction of dendritic cells by antigen-encoding lentiviral vectors permits antigen processing and MHC class I-dependent presentation. J Allergy Clin Immunol. 2002;109(6):988–994. doi: 10.1067/mai.2002.124663. [DOI] [PubMed] [Google Scholar]
- 16.Ohta K, Yamashita N, Tajima M, et al. Diesel exhaust particulate induces airway hyperresponsiveness in a murine model: essential role of GM-CSF. J Allergy Clin Immunol. 1999;104(5):1024–1030. doi: 10.1016/S0091-6749(99)70084-9. [DOI] [PubMed] [Google Scholar]
- 17.Oliver JA, Stolberg VR, Chensue SW, King PD. IL-4 acts as a potent stimulator of IFN-gamma expression in CD8+ T cells through STAT6-dependent and independent induction of eomesodermin and T-bet. Cytokine. 2012;57(1):191–199. doi: 10.1016/j.cyto.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris SC, Heidorn SM, Herbert DR, et al. Endogenously produced IL-4 nonredundantly stimulates CD8+ T cell proliferation. J Immunol. 2009;182(3):1429–1438. doi: 10.4049/jimmunol.182.3.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loffler MW, Chandran PA, Laske K, et al. Personalized peptide vaccine-induced immune response associated with long-term survival of a metastatic cholangiocarcinoma patient. J Hepatol. 2016;65(4):849–855. doi: 10.1016/j.jhep.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.