Abstract
Patients with non-small cell lung cancer (NSCLC) and renal cell carcinoma (RCC) have shown benefit from anti-PD-1 therapies. However, not all patients experience tumor shrinkage, durable responses or prolonged survival, demonstrating the need to find response markers. In blood samples from NSCLC and RCC patients obtained before and after anti-PD-1 treatment, we studied leukocytes by complete blood cell count, lymphocyte subsets using flow cytometry and plasma concentration of nine soluble mediators, in order to find predictive biomarkers of response and to study changes produced after anti-PD-1 therapy. In baseline samples, discriminant analysis revealed a combination of four variables that helped differentiate stable disease-response (SD-R) from progressive disease (PD) patients: augmented frequency of central memory CD4+ T cells and leukocyte count was associated with response while increased percentage of PD-L1+ natural killer cells and naïve CD4+ T cells was associated with lack of response. After therapy, differential changes between responders and non-responders were found in leukocytes, T cells and TIM-3+ T cells. Patients with progressive disease showed an increase in the frequency of TIM-3 expressing CD4+ and CD8+ T cells, whereas SD-R patients showed a decrease in these subsets. Our findings indicate that a combination of immune variables from peripheral blood (PB) could be useful to distinguish response groups in NSCLC and RCC patients treated with anti-PD-1 therapy. Frequency of TIM-3+ T cells showed differential changes after treatment in PD vs SD-R patients, suggesting that it may be an interesting marker for monitoring progression during therapy.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02391-z) contains supplementary material, which is available to authorized users.
Keywords: Anti-PD-1 therapy, Nivolumab, Pembrolizumab, NSCLC, Renal cell carcinoma, TIM-3
Introduction
Cancer immunotherapies that target the programmed death 1 ligand (PD-L1)/PD-1 axis of immune regulation have shown remarkable efficacy, demonstrating improved progression-free survival (PFS) and overall survival (OS) in the treatment of many different tumor types [1–5]. In addition to clinical activity in cancers historically considered as immunogenic such as melanoma [6–9] and renal cell carcinoma (RCC) [4, 10–12], anti-PD-L1 and anti-PD-1 agents can induce anti-tumor responses in a growing list of malignancies, including non-small cell lung cancer (NSCLC) [1, 13–17]. However, not all patients treated with PD-L1/PD-1-blocking antibodies experience tumor shrinkage, durable responses, or prolonged survival. Predictive treatment response biomarkers were first evaluated in tumor cells and in surrounding immune-micro-environment [18]. Status of PD-L1, tumor-infiltrating lymphocytes, mutational burden, immune gene signatures, and others are associated with clinical outcomes for anti-checkpoint therapies. However, their utility is still under discussion because of inconsistent results, difficulty to obtain tumor samples, technical approach, and costs [19, 20]. On the other hand, information about peripheral blood (PB) markers associated with response to immune-checkpoint inhibition and changes in immune subsets induced by this treatment is currently limited. Determining PB changes that are associated with lack of response or acquired resistance could be very useful in the monitoring of patients during treatment.
Here, we studied peripheral immune cell populations and soluble mediators before and after therapy with PD-1 blocking antibodies, pembrolizumab or nivolumab, in NSCLC and RCC patients. We aimed to describe potential biomarkers of response to anti-PD-1 therapies and to identify variations in peripheral immune cell populations that reflect the response against the tumor.
Materials and methods
Patient samples
Twenty-five patients with NSCLC (n = 18) or RCC (n = 7) from the Department of Clinical Oncology of the Alexander Fleming Institute (Ciudad Autónoma de Buenos Aires, Argentina) provided samples for this study. Heparinized PB samples were collected before starting anti-PD-1 treatment (PRE sample), and either after 6 cycles of nivolumab or 4 cycles of pembrolizumab or at disease progression, depending on whichever occurred first (POST sample). Patients were treated with 3 mg per kg body weight (mg/kg) of nivolumab every 2 weeks or 2 mg/kg pembrolizumab every 3 weeks. Response was considered as the best clinical response in the course of treatment according to RECIST 1.1, with the stable disease-response (SD-R) group defined by patients displaying stable disease, partial or complete response, and the progressive disease (PD) group defined by patients showing disease progression characterized by a measurable increase in tumor size, the presence of new metastatic sites, or the requirement of secondary treatment such as radiotherapy. Patients who received less than 4 weeks of treatment were not considered for response assessment.
Immunohistochemistry (IHC) on tissue samples
PD-L1 IHC was carried out on 4 μm tumor sections, using the test corresponding to the anti-PD-1 treatment received (Dako PD-L1 IHC 28-8 pharmDx for nivolumab and Dako PD-L1 IHC 22C3 pharmDx for pembrolizumab).
Complete blood cell count (CBC) and flow cytometry analysis on PB
Neutrophils, lymphocytes, monocytes and eosinophils were evaluated in PB samples using an automated cell blood counter. Peripheral blood mononuclear cells (PBMC) were isolated through a Ficoll–Paque density gradient (GE Healthcare, UK). For T and NK cell phenotyping, 2.5 × 105 fresh PBMC were stained in PBS with Fixable viability Stain 510 for 15 min at room temperature, washed with washing buffer (PBS with 2% FCS), incubated with the appropriate mAbs (Supplementary Table 1) for 30 min at 4 °C, and then washed twice with washing buffer. 1.0 × 106 cells were used to stain regulatory T cells (Tregs), following manufacturer’s recommendations (BD Pharmingen™ FoxP3 Staining Kit, catalog 560132). Isotype-matched irrelevant mAbs were used as negative controls.
Lymphocytes were gated using forward scatter-area (FSC-A) vs side scatter-area (SSC-A) plot, single cells using FSC-A vs forward scatter-height (FSC-H), and viable cells (≥ 50,000 events) using Fixable Viability Stain 510 staining. Lymphocyte subsets were defined as CD3−CD56+ NK cells, CD3+CD56+ cells, CD4+ CD3+ T cells, CD8+ CD3+ T cells and CD4+CD25+FoxP3+ Tregs. A representative gating strategy is shown in Supplementary figure 1. Data acquisition was performed using a FACSCanto II cytometer and FACSDiva software (BD Biosciences). Data analysis was performed with FlowJo software.
CRP and cytokines evaluation
CRP was measured in patients’ plasma using the C-reactive protein assay on the ARCHITECT cSystem following manufacturer´s instructions (Abbott, Chicago, USA). IL-8, IL-1β, IL-6, IL-10, TNF-α, and IL-12p70 cytokines were assessed using BD™ Cytometric Bead Array (CBA) Human Inflammatory Cytokine Kit, catalog 551811. Plasmatic concentrations of the proteins were determined using FCAP Array software (BD).
Statistical analysis
Individual datapoints representing the measurement from each patient were graphed using GraphPad v7.00 (GraphPad Software, USA). The mean value or box and whisker plots are shown, while paired PRE and POST-treatment samples are displayed using connecting lines. The variation (Δ) of each marker was calculated as POST minus PRE value of individual patients. To test the difference between SD-R and PD patients, we used general linear mixed models in Infostat 2017 software [21]. Each marker (immune cell population or soluble mediator) was considered as a response variable and clinical benefit (SD-R or PD) as a fixed effect. To compare paired PRE and POST-samples, patient ID was introduced as a random effect. Homoscedasticity and normality of residuals were analyzed by visual assessment of plots. If homoscedasticity was not achieved, models were fitted using a constant variance function (VarIdent function) [22]. The best model was chosen by comparison of Akaike’s and Bayesian’s Information Criteria.
For multivariate analysis, partial least-squares discriminant analysis (PLS-DA) was used to identify the most important markers that help classify patients into both response groups (SD-R or PD), using mixOmics library in R [23]. Two analyses were done, the first one with markers at baseline and the second one with the variations of the markers after treatment (Δ: POST minus PRE values). PLS-DA can handle both, multicollinearity problem and datasets with the number of variables higher than that of samples [24]. To choose the optimal number of variables for each PLS-DA, we use the estimation of the misclassification error rate using leave-one-out or stratified cross-validation [23]. Correlation matrix was built using corrplot package in R [25].
Progression-free survival (PFS) was defined as the interval between the date of treatment start to the date of disease progression or death. Kaplan–Meier graphs were used to estimate PFS and univariate differences in PFS were compared with log rank test. 95% confidence intervals (95% CI) for the statistic of interest are informed. A p < 0.05 was considered to be statistically significant.
Results
Patient characteristics and clinical responses
Twenty-five patients were recruited, 18 with NSCLC and 7 with RCC. Clinical and treatment characteristics are described in Table 1. Patients were divided into two groups according to their best clinical response: the SD-R group comprised one patient with complete response (CR), four patients with partial response (PR) and seven patients with stable disease (SD), while the PD group comprised nine patients with progressive disease. Four patients were left out from the analysis since they received less than 4 weeks of treatment and were therefore not considered for response assessment. The median follow-up was 6.97 months and the median PFS was 5.17 months. POST-samples were obtained in 18 patients: in the 12 patients of the SD-R group after 6 cycles of nivolumab or 4 cycles of pembrolizumab (around 12 weeks of treatment), and in six out of nine patients of the PD group at the time of progression (between 8 and 12 weeks of treatment). In the other three patients of the PD group, POST sample could not be obtained.
Table 1.
Patients’ characteristics and clinical response to anti-PD-1 treatment
| All (n = 25) | NSCLC (n = 18) | RCC (n = 7) | |
|---|---|---|---|
| Age: median (IQR) | 60 (54–68) | 61 (54–68) | 59 (53–78) |
| Sex: n (%) | |||
| Male | 15 (60) | 9 (50) | 6 (85.7) |
| Female | 10 (40) | 9 (50) | 1 (14.3) |
| Histology: n (%) | |||
| Adenocarcinoma | 13 (52) | 13 (72.2) | – |
| Squamous cell carcinoma | 5 (20) | 5 (27.8) | – |
| Clear cell carcinoma | 7 (28) | – | 7 (100) |
| Stage at diagnosis: n (%) | |||
| I | 1 (4) | 1 (5.5) | 0 |
| II | 3 (12) | 3 (16.7) | 0 |
| III | 5 (20) | 2 (11.1) | 3 (42.9) |
| IV | 16 (64) | 12 (66.7) | 4 (57.1) |
| Surgery of primary: n (%) | 12 (48) | 6 (33.3) | 6 (85.7) |
| Previous radiotherapy: n (%) | 15 (60) | 11 (61.1) | 4 (57.1) |
| Previous lines of treatment: n (%) | |||
| 1 | 18 (72.0) | 13 (72.2) | 5 (71.4) |
| 2 | 7 (28.0) | 5 (27.8) | 2 (28.6) |
| Anti-PD-1: n (%) | |||
| Nivolumab | 18 (72) | 11 (61.1) | 7 (100) |
| Pembrolizumab | 7 (28) | 7 (38.9) | – |
| PD-L1: n/cases evaluated (%) | |||
| < 1% | 9/17 (52.9) | 6/13 (46.2) | 3/4 (75) |
| ≥ 1% | 8/17 (47.1) | 7/13 (53.8) | 1/4 (25) |
| ≥ 10% | 5/17 (29.4) | 5/13 (38.5) | 0/4 (0) |
| ≥ 50% | 3/17 (17.6) | 3/13 (23.1) | 0/4 (0) |
| Response: n (%) | |||
| Progressive disease (PD) | 9 (36) | 6 (33.3) | 3 (42.8) |
| Stable disease (SD) | 7(28) | 5 (27.8) | 2 (28.6) |
| Partial response (PR) | 4 (16) | 3 (16.7) | 1 (14.3) |
| Complete response (CR) | 1 (4) | 1 (5.5) | 0 |
| Response not evaluable | 4 (16) | 3 (16.7) | 1 (14.3) |
Response rates according to pathology are presented in Table 1. No association between clinical or pathological characteristics and anti-PD-1 treatment response was found. PD-L1 positivity was not associated with response (p = 0.29).
A combination of four variables in PRE-treatment samples helped differentiate SD-R and PD response groups
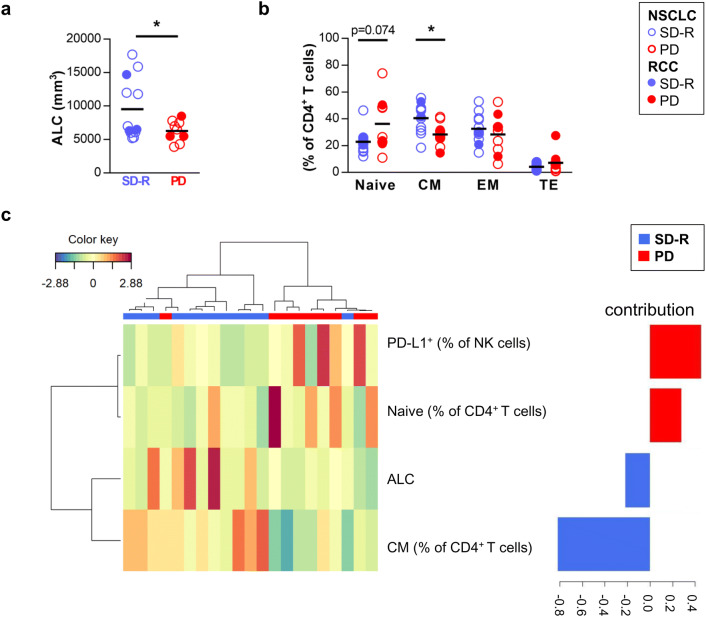
First, we studied markers in PRE-treatment PB samples that could be used as predictors of response by univariate analysis. We analyzed leukocytes by CBC, lymphocyte subsets by FACS, and the plasma concentration of 9 soluble mediators. We looked for differences between SD-R and PD patients. Among the results from CBC, PD patients presented a lower absolute leukocyte count (ALC) than SD-R patients (mean [95% CI]: 6277/mm3 [5059–7496] vs 9541/mm3 [6622–12,460]; p = 0.034) (Fig. 1a). We did not find differences in the relative number of the different leukocyte subsets nor in the neutrophil-to-lymphocyte ratio (NLR) (Supplementary figure 2).
Fig. 1.
Analysis of PRE-treatment samples. NSCLC and RCC patients were divided into two groups according to their best clinical response to anti-PD-1 therapy: stable disease-response (SD-R) and progressive disease (PD) patients. a Absolute leukocyte count (ALC) by complete blood cell count. b Frequency of memory CD4+ T cell subsets by FACS: CD45RO−CCR7+ naïve cells, CD45RO+CCR7+ central memory (CM) cells, CD45RO+CCR7− effector memory (EM) cells and CD45RO−CCR7− terminal effector (TE) cells. *p < 0.05. c Clustered image map showing the model that best classifies patients (represented in columns) into both groups (SD-R or PD) based on markers at baseline (represented in rows). Barplot on the right displays the loading weights associated to each marker, with colors indicating the response group with the maximum average value. Partial least-squares discriminant analysis (PLS-DA) was done
The analysis of FACS results showed no significant differences between SD-R and PD patients in the frequency of T, NK or CD3+CD56+ cells, CD4+ or CD8+ T cells, nor in the frequency of Tregs (Supplementary figure 3a). In addition, the assessment of activation and exhaustion markers on CD4+ and CD8+ T cells showed no differences between both response groups (Supplementary figure 3b). Meanwhile, PD patients exhibited a non-significant higher proportion of PD-L1+ NK cells than SD-R patients (p = 0.054) (Supplementary figure 3c).
Memory T cell subsets were studied by subdividing CD4+ and CD8+ T cells into CD45RO−CCR7+ naïve cells, CD45RO+CCR7+ central memory (CM) cells, CD45RO+CCR7− effector memory (EM) cells and CD45RO−CCR7− terminal effector (TE) cells (representative plots are shown in Supplementary figure 4a). The percentage of CM CD4+ T cells was higher in SD-R than in PD patients (mean [95% CI]: 40.5% [33.7–47.3] vs 28.3% [21.5–35.2]; p = 0.013) while a tendency towards a lower percentage of naïve CD4+ cells was observed in SD-R group (p = 0.074) (Fig. 1b). No differences were observed in memory CD8+ T cell compartment (Supplementary figure 4b).
We next performed a multivariate analysis including all immunological parameters assayed, which correlation is shown in Supplementary figure 5. A combination of four variables was optimal to differentiate both response groups (Fig. 1c): augmented frequency of CM CD4+ T cells and ALC was associated with SD-R patients while increased percentage of PD-L1+ NK cell and naïve CD4+ T cells was associated with PD group. The contribution of each variable to the discriminant analysis is displayed on the right, with frequency of CM CD4+ T cells presenting the highest contribution. In this model, two patients could not be properly classified.
Variations in leukocytes after anti-PD-1 therapy were associated with treatment response
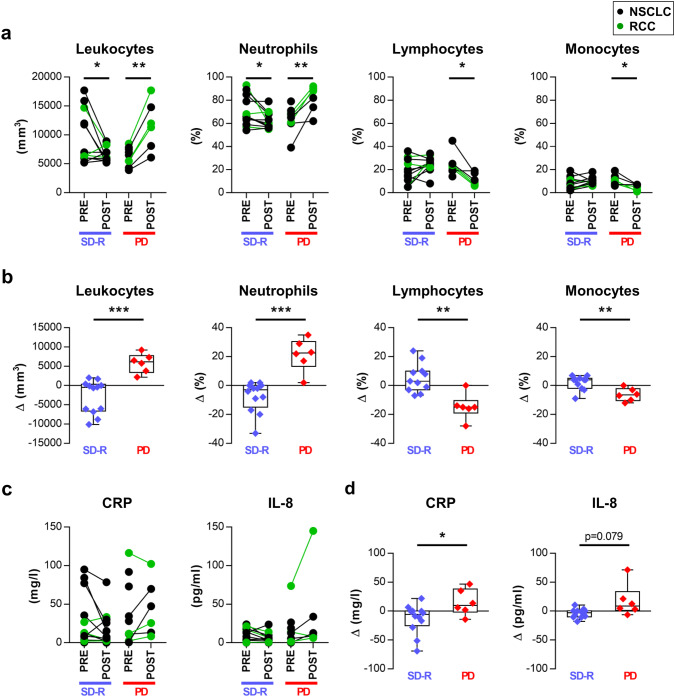
The same immunological parameters were studied in samples taken after several cycles of anti-PD-1 treatment (POST) and compared with PRE-treatment samples. CBC analysis revealed differential variations after treatment: SD-R patients showed a significant decrease in ALC and neutrophil percentage, while PD patients showed a significant increase in ALC and neutrophil percentage and a decrease in lymphocyte and monocyte percentage (Fig. 2a).
Fig. 2.
Variations in leukocytes and soluble mediators after anti-PD-1 therapy were associated with treatment response. a Absolute leukocyte count (ALC) and frequency of neutrophils, lymphocytes and monocytes from paired PRE and POST-treatment samples are shown for both response groups. b Comparison of the variation (Δ: POST minus PRE value) in these cell populations between both response groups. c C-reactive protein (CRP) and IL-8 plasma levels from paired PRE and POST-treatment samples. d Comparison of the variation (Δ) in the concentration of CRP and IL-8 between SD-R and PD patients. *p < 0.05, **p < 0.01, ***p < 0.001
We compared the variation (Δ: POST minus PRE value) of each marker between both response groups, and found significant differences in ALC (p < 0.001) and in neutrophil (p < 0.001), lymphocyte (p = 0.001) and monocyte percentage (p = 0.003) (Fig. 2b).
Changes in inflammatory proteins in plasma after treatment
Due to changes observed in leukocyte populations in SD-R and PD groups, we decided to evaluate soluble mediators associated with systemic inflammation in plasma: CRP and eight cytokines. Comparative analysis of the variation in concentration (Δ) showed significant differences between SD-R and PD patients in CRP (p = 0.035) and a tendency in IL-8 levels (p = 0.079) (Fig. 2c, d) but not in IL-6 (Supplementary figure 6a). IL-1β, IL-10, TNF-α and IL-12 were undetectable in plasma before and after treatment (data not shown).
Changes in lymphocyte populations after treatment
FACS analysis of the frequency of lymphocyte populations in POST vs PRE treatment samples revealed a statistically significant decrease in T cells in PD patients and an increase in NK cells both in PD and SD-R patients (Fig. 3a).
Fig. 3.
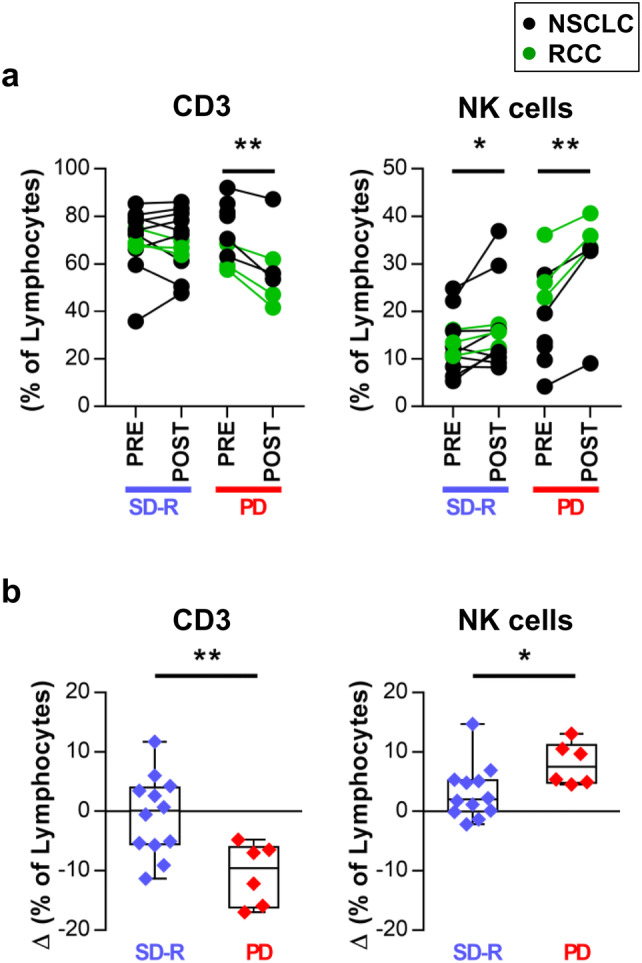
Changes in lymphocyte populations after anti-PD-1 treatment. a Frequency of T and NK cells within lymphocytes from paired PRE and POST-treatment samples are shown for both response groups. b Comparison of the variation (Δ: POST minus PRE value) in T and NK cell frequencies between SD-R and PD patients. *p < 0.05, **p < 0.01
Comparatively, we found significant differences in the variation (Δ) of T cells (p = 0.007) and NK cells (p = 0.040) between PD and SD-R patients (Fig. 3b).
The frequency of CD3+CD56+ cells, CD4+ and CD8+ T cells, and Tregs did not change after treatment in either response group (Supplementary figure 6b).
TIM-3 increased in PD patients after anti-PD-1 therapy
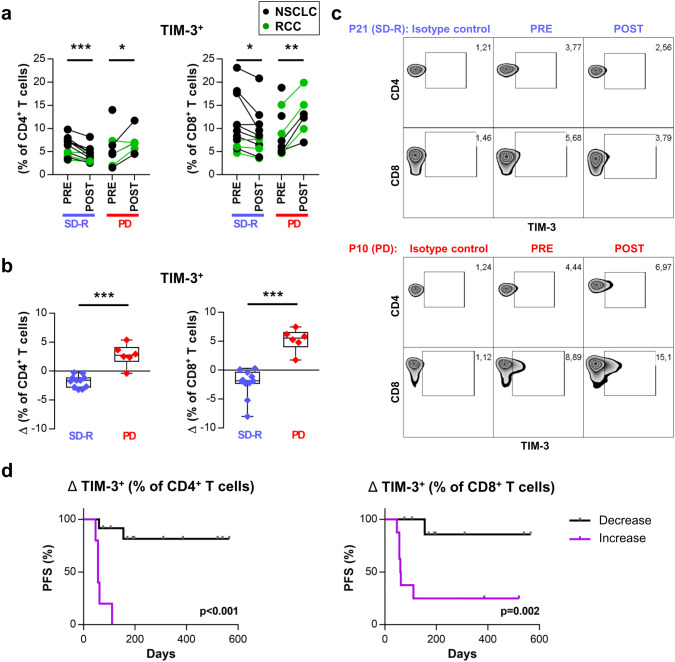
We also characterized TIM-3, CD69 activation marker, the inducible co-stimulatory molecule (ICOS), and PD-L1 through FACS in CD4+ and CD8+ T cells. Frequency of TIM-3+ T cells showed opposite changes after treatment in PD vs SD-R patients. PD patients showed an increase in TIM-3 expressing cells in CD4+ and CD8+ T cells whereas SD-R patients showed a decrease in these subsets (Fig. 4a). The variation (Δ) was statistically different between PD and SD-R patients (CD4+p < 0.001; CD8+p < 0.001) (Fig. 4b). Representative plots are shown in Fig. 4c. No differences were observed in the other markers studied (Supplementary figure 6c).
Fig. 4.
Frequency of TIM-3+ CD4+ and CD8+ T cells increased after treatment in PD but not in SD-R patients. a Frequency of TIM-3 expressing cells in CD4+ (left) and CD8+ (right) T cells from paired PRE and POST-treatment samples is shown for both groups of response. b Comparison of the variation (Δ: POST minus PRE value) in the frequency of TIM-3+ CD4+ (left) and CD8+ (right) T cells between both response groups. *p < 0.05, **p < 0.01, ***p < 0.001. c Representative density plots showing TIM-3 expression in CD4+ and CD8+ T cells from two patients. d PFS analysis with patients dichotomized as those with either an increase or a decrease in TIM-3 expressing cells after anti-PD-1 treatments. Kaplan–Meier curves are shown and p-values were determined by log-rank test
PFS analysis was carried out with patients dichotomized as those with either an increase or a decrease in TIM-3 expressing cells. As shown in Fig. 4d, PFS was lower in patients presenting an increase in TIM-3+ cells, either within CD4+ T cells (12-month PFS 0% vs 81.5%, p < 0.001) or within CD8+ T cells (12-month PFS 20.8% vs 85.7%, p = 0.002).
Multivariate analysis of changes after treatment revealed a combination of six variables that differentiated SD-R and PD patients
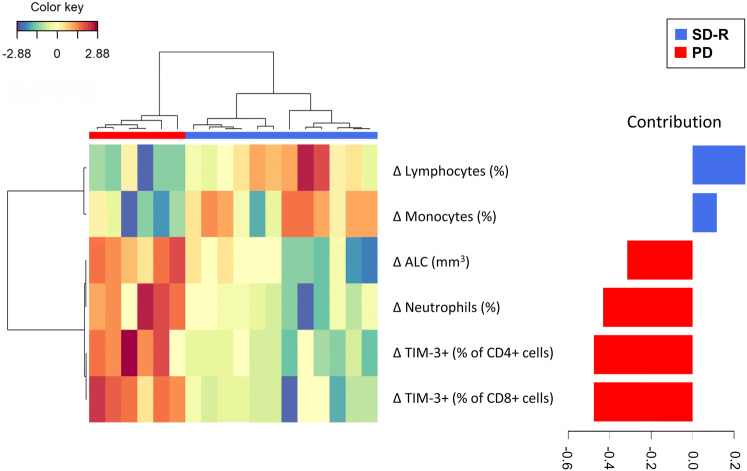
Finally, we performed a multivariate analysis including Δ values of all immunological parameters assayed, which correlation is shown in Supplementary figure 7, to identify the variables that best differentiated SD-R and PD groups taking into account both the PRE and POST-samples. A model with a combination of 6 variables was best to differentiate both response groups: an increase in ALC and in the frequency of neutrophils and TIM-3+ CD4+ and CD8+ T cells was associated with PD group, while an augmentation in the percentage of lymphocytes and monocytes was associated with SD-R patients. The changes in the frequency of TIM-3+ CD4+ and CD8+ T cells contributed the most to the discriminant analysis (Fig. 5).
Fig. 5.
Multivariate analysis of changes after treatment. Clustered image map showing the model that best classifies patients (represented in columns) into both groups (SD-R or PD) based on the variations (Δ: POST minus PRE-values) of the markers after treatment (represented in rows). Barplot on the right displays the loading weights associated to each marker, with colors indicating the response group with the maximum average value. Partial least-squares discriminant analysis (PLS-DA) was done
Discussion
The success of immunotherapy agents depends on the ability to select patients most likely to respond to treatment. In this work, we analyzed PB in the context of anti-PD1 therapy, looking for predictive markers and studying changes produced after treatment. We analyzed a cohort of 25 metastatic NSCLC or RCC patients, with PB samples taken before starting and after around 12 weeks of nivolumab or pembrolizumab treatment. In this cohort, PD-L1 positivity was not associated with response to anti-PD-1 therapy. This may be due to the size of the sample or to the fact that the cohort included RCC patients where this association seems weak [4, 26, 27]. Even in NSCLC, PD-L1 usefulness as a response predictor is under discussion [28, 29].
As univariate analysis did not allow for determination of a variable clearly associated with response, we performed a discriminant analysis. A combination of four variables helped differentiate SD-R and PD response groups: together with higher frequency of CM CD4+ T cells, augmented ALC was associated with response. On the other hand, higher frequency of naïve CD4+ T cells and PD-L1+ NK cells was associated with lack of response. Previous reports have linked memory T cells with response to anti-PD-1 treatment. Krieg et al. (2018) reported higher frequencies of CM CD8+ T cells in circulation before and after anti-PD-1 treatment in melanoma patients responding to therapy [30]. In addition, high circulating CM/effector T cell ratios were associated with tumor inflammation, increased PD-L1 expression at tumor site, and longer PFS in response to nivolumab treatment in NSCLC [31]. This shift of frequency from naïve to CM T cells is in line with the concept that anti-PD-1 treatment supports functionally activated T cells [32]. In fact, impaired formation of T-cell memory was described as a resistance mechanism to anti-PD-1 therapy [33, 34].
Different research groups have focused on increased NLR as a marker of chronic inflammation, which may lead to impaired immunity [35]. In this regard, some studies have shown that high NLR at baseline and during treatment is a poor prognostic factor in patients with advanced malignancies receiving PD-1/PD-L1 blockade [35, 36]. However, in our cohort, neither relative neutrophil count nor NLR were different between response groups. Moreover, we did not find differences in other subsets previously described associated with response to anti-PD-1 therapy such as eosinophil and lymphocyte counts [37] and frequency of monocytes [30].
Concerning NK cells, only PD-L1 was evaluated, so we cannot rule out the existence of other NK markers associated with response. In this regard, Subrahmanyam et al. (2018) reported differences in CD69 and MIP-1β expressing NK cells between responders and non-responders in anti-PD-1 melanoma-treated patients, suggesting that functionally active NK cells might play a role in the anti-tumor response triggered by anti-PD-1 [38]. These findings, together with ours, highlight the role of peripheral immune status in the prediction of anti-PD-1 therapy response. However, more studies with larger cohorts of patients and markers are needed to validate the results.
Additionally, we studied changes induced by treatment. The collection time of POST-samples was after at least 8 weeks of anti-PD1 treatment, contrary to other works that evaluate changes after one dose of the anti-PD-1 agent. This choice allowed us to evaluate the changes induced after various doses, near the time when most clinical responses are seen. The variations observed indicate an increase in systemic inflammation in patients with PD displayed by an increase in ALC, neutrophil percentage and a tendency to increase CRP and IL-8 levels. Meanwhile, variations in patients in SD-R group indicate a decrease in systemic inflammation, with a decrease in leucocyte count, neutrophil percentage and a tendency to lower CRP levels. In support, Sanmamed et al. (2018) reported a significant increase in IL-8 levels upon disease progression, and that early changes in serum IL-8 levels (2–4 weeks after treatment initiation) were strongly associated with response [39].
Expression of alternative co-inhibitory immune checkpoints (e.g., CTLA-4, TIM-3, LAG-3, and VISTA) has been associated with resistance to PD-1 blockade [40, 41]. TIM-3, the T cell immunoglobulin-3, was first identified as a cell surface molecule selectively expressed on IFN-γ-producing CD4+ T helper 1 (Th1) and CD8+ cytotoxic T cells, and marks dysfunctional or exhausted T cells in cancer [42]. Here, we found that TIM-3 expressing cells significantly increased in CD4+ and CD8+ T cells in PD patients but not in SD-R patients after anti-PD-1 therapy; and that this augmentation was associated with poorer PFS. The kinetics of TIM-3 variation would be interesting to evaluate in further studies, in order to identify if the increase detected in the PD group is observed at earlier times as well, and thus could be used as an early-on treatment-predictive marker. In regard to this, Kato et al. (2018) reported an opposite variation after one dose of nivolumab in advanced esophageal squamous cell carcinoma patients. %TIM-3+ CD4 cells increased in CR/PR patients, but not in SD/PD patients, together with an increase in the %OX-40+ CD4 cells, which could reflect an activation of anti-tumor response instead of an exhaustion of T cells at early times [43]. On the other hand, Koyama et al. (2015) identified up-regulation of TIM-3 as a marker of resistance to anti-PD-1 treatment in an animal model and in pleural effusions from two NSCLC patients [41]. The emergence of this potential mechanism of resistance promotes the feasibility of its therapeutic blockade as demonstrated by Koyama et al. [41]. Multiple cell line and murine models have shown the synergistic effect of anti-PD-1 and anti-TIM-3 antibodies in reducing tumor growth [44–46]. Nowadays, three antibodies against TIM-3 are being evaluated in phase I trials (Sym023 NCT03489343, TSR022 NCT02817633, MBG453 NCT02608268).
To take into account, the strengths of this study include the prospective recruitment of patients, with a careful collection and manipulation of blood samples and a rigorous patient follow-up. In addition, parallel analysis at different levels (leukocytes, lymphocyte subsets and plasma proteins) in pre- and after-treatment samples was done. On the other hand, the study design did not include taking an early sample after treatment, and the sample size was limited which implied a low chance of detecting those variables with small differences between SD-R and PD patients, particularly if occurred only in the RCC group. Regarding the joint analysis of patients with two different tumors, we first compared NSCLC and RCC patients in terms of all immunological variables assessed in PRE-treatment samples, and did not find any significant difference. Neither had we found a multivariate model able to separate patients from both tumor types (data not shown), meaning that they had a similar immunological profile. Second, we analyzed NSCLC patients separately looking for variables that differentiate SD-R and PD patients, and found similar tendencies than when studying all patients together (data not shown).
Our results indicate that a combination of PB variables rather than one alone was useful to differentiate both response groups, and are in line with previous reports indicating an association between the basal presence of a subset of memory T cells and response to treatment. This encourages further studies in larger cohorts and including other markers co-expressed in these populations. We also found differential variations after treatment in peripheral immune cells in SD-R vs PD patients. Noteworthy, patients with progressive disease showed an increased frequency of TIM-3 expressing T cells after therapy, which may be useful for monitoring response during therapy and warrant additional studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Holliday Cartar for her assistance in language correction. We thank Dr. Laura Noro and all the laboratory staff from Alexander Fleming Institute for their help in this study.
Abbreviations
- ALC
Absolute leukocyte count
- CM
Central memory
- CBC
Complete blood cell count
- CRP
C-reactive protein
- EM
Effector memory
- FSC-A
Forward scatter-area
- FSC-H
Forward scatter-height
- NLR
Neutrophil-to-lymphocyte ratio
- PD
Progressive disease
- RCC
Renal cell carcinoma
- SSC-A
Side scatter-area
- SD-R
Stable disease-response
- TIM-3
T cell immunoglobulin and mucin-domain containing-3
- TE
Terminal effector
Author contributions
EPJ collected, analyzed and interpreted the data, and wrote the manuscript. PM and EML analyzed and interpreted the data, and wrote the manuscript. MMR, FT and RL contributed with patients’ clinical data analysis. GRC contributed to the statistical analysis and performed discriminant analyses. AIB and WA performed IHC analysis. JM, CP and CM interpreted the data and revised the manuscript. All authors contributed to manuscript revision; read and approved the final manuscript version.
Funding
This work was supported by grants from Fundación Sales, Fundación Cáncer, Fundación Pedro F. Mosoteguy, Argentina. José Mordoh and Estrella Mariel Levy are members of Consejo Nacional de Investigaciones Científicas y Técnicas-CONICET. Estefanía Paula Juliá is a fellow from Consejo Nacional de Investigaciones Científicas y Técnicas-CONICET.
Compliance with ethical standards
Conflict of interest
Claudio Martín has served as speaker and advisory board member for Bristol Myers Squibb and Merck Sharp and Dohme. Carmen Pupareli has served as speaker and advisor board member for Merck Sharp and Dohme and as speaker for Bristol Myers Squibb. The authors declare that there is no other conflict of interest.
Ethical approval and ethical standards
All procedures involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Comité de Ética en Investigación del Instituto Alexander Fleming CEIAF, approval number: 616, 14th June 2016.
Informed consent
All samples were taken after patients gave written informed consent approved by Comité de Ética en Investigación del Instituto Alexander Fleming CEIAF. Patients consented to the use of their specimens and data for research and for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558–1568. doi: 10.1016/S1470-2045(16)30366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 6.Hamid O, Robert C, Daud A, Hodi FS, Hwu W-J, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -Naive Melanoma. J Clin Oncol. 2013;31:4311–4318. doi: 10.1200/JCO.2013.51.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 9.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2015;33:1430–1437. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDermott DF, Sosman JA, Sznol M, Massard C, Gordon MS, Hamid O, et al. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase ia study. J Clin Oncol. 2016;34:833–842. doi: 10.1200/JCO.2015.63.7421. [DOI] [PubMed] [Google Scholar]
- 13.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 15.Rizvi NA, Mazières J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 17.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 18.Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17:e542–e551. doi: 10.1016/S1470-2045(16)30406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada MRC. InfoStat versión 2017. Argentina: Grupo InfoStat, FCA, Universidad Nacional de Córdoba; 2017. [Google Scholar]
- 22.Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM. Mixed effects models and extensions in ecology with R. New York: Springer; 2009. [Google Scholar]
- 23.Rohart F, Gautier B, Singh A, Lê Cao KA. mixOmics: an R package for ‘omics feature selection and multiple data integration. PLoS Comput Biol. 2017;13:e1005752. doi: 10.1371/journal.pcbi.1005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trivittayasil V, Tsuta M, Kasai S, Matsuo Y, Sekiyama Y, Shoji T, et al. Classification of 1-methylcyclopropene treated apples by fluorescence fingerprint using partial least squares discriminant analysis with stepwise selectivity ratio variable selection method. Chemom Intell Lab Syst. 2018;175:30–36. doi: 10.1016/j.chemolab.2018.02.004. [DOI] [Google Scholar]
- 25.Wei T, Simko V (2017) R package “corrplot”: Visualization of a Correlation Matrix (Version 0.84). Available from https://github.com/taiyun/corrplot. Accessed Jan 2019
- 26.Callea M, Albiges L, Gupta M, Cheng S-C, Genega EM, Fay AP, et al. Differential expression of PD-L1 between primary and metastatic sites in clear-cell renal cell carcinoma. Cancer Immunol Res. 2015;3:1158–1164. doi: 10.1158/2326-6066.CIR-15-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jilaveanu LB, Shuch B, Zito CR, Parisi F, Barr M, Kluger Y, et al. PD-L1 expression in clear cell renal cell carcinoma: an analysis of nephrectomy and sites of metastases. J Cancer. 2014;5:166–172. doi: 10.7150/jca.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munari E, Zamboni G, Marconi M, Sommaggio M, Brunelli M, Martignoni G, et al. PD-L1 expression heterogeneity in non-small cell lung cancer: evaluation of small biopsies reliability. Oncotarget. 2017;8:90123–90131. doi: 10.18632/oncotarget.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casadevall D, Clavé S, Taus Á, Hardy-Werbin M, Rocha P, Lorenzo M, et al. Heterogeneity of tumor and immune cell PD-L1 expression and lymphocyte counts in surgical NSCLC samples. Clin Lung Cancer. 2017;18(682–691):e5. doi: 10.1016/j.cllc.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Krieg C, Nowicka M, Guglietta S, Schindler S, Hartmann FJ, Weber LM, et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med. 2018;24:144–153. doi: 10.1038/nm.4466. [DOI] [PubMed] [Google Scholar]
- 31.Manjarrez-Orduño N, Menard LC, Kansal S, Fischer P, Kakrecha B, Jiang C, et al. Circulating T cell subpopulations correlate with immune responses at the tumor site and clinical response to PD1 inhibition in non-small cell lung cancer. Front Immunol. 2018;9:1–9. doi: 10.3389/fimmu.2018.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 33.O’Donnell JS, Long GV, Scolyer RA, Teng MWL, Smyth MJ. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat Rev. 2017;52:71–81. doi: 10.1016/j.ctrv.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ameratunga M, Chénard-Poirier M, Moreno Candilejo I, Pedregal M, Lui A, Dolling D, et al. Neutrophil-lymphocyte ratio kinetics in patients with advanced solid tumours on phase I trials of PD-1/PD-L1 inhibitors. Eur J Cancer. 2018;89:56–63. doi: 10.1016/j.ejca.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Sacdalan DB, Lucero JA, Sacdalan D. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. Onco Targets Ther. 2018;11:955–965. doi: 10.2147/OTT.S153290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weide B, Martens A, Hassel JC, Berking C, Postow MA, Bisschop K, et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res. 2016;22:5487–5496. doi: 10.1158/1078-0432.CCR-16-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subrahmanyam PB, Dong Z, Gusenleitner D, Giobbie-Hurder A, Severgnini M, Zhou J, et al. Distinct predictive biomarker candidates for response to anti-CTLA-4 and anti-PD-1 immunotherapy in melanoma patients. J Immunother Cancer. 2018;6:18. doi: 10.1186/s40425-018-0328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanmamed MF, Perez-Gracia JL, Schalper KA, Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol. 2017;28:1988–1995. doi: 10.1093/annonc/mdx190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thommen DS, Schreiner J, Muller P, Herzig P, Roller A, Belousov A, et al. Progression of lung cancer is associated with increased dysfunction of T cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol Res. 2015;3:1344–1355. doi: 10.1158/2326-6066.CIR-15-0097. [DOI] [PubMed] [Google Scholar]
- 41.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kato R, Yamasaki M, Urakawa S, Nishida K, Makino T, Morimoto-Okazawa A, et al. Increased Tim-3 + T cells in PBMCs during nivolumab therapy correlate with responses and prognosis of advanced esophageal squamous cell carcinoma patients. Cancer Immunol Immunother. 2018;67:1673–1683. doi: 10.1007/s00262-018-2225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MWL, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-—mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540–3551. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 46.Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, et al. Coexpression of Tim-3 and PD-1 identifies a CD8 + T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.