Abstract
Intrahepatic cholangiocarcinoma (ICC) is a rare malignancy with poor prognosis. The evaluation of recurrence risk after liver resection is of great importance for ICCs. We aimed to assess the prognostic value of intra- and peritumoral immune infiltrations and to establish a novel histopathology-related immunoscore (HRI) associated with ICC recurrence. A total of 280 ICC patients who received curative resection between February 2005 and July 2011 were enrolled in our study. Patients were randomly assigned to the derivation cohort (n = 176) or the validation cohort (n = 104). Sixteen immune biomarkers in both intra- and peritumoral tissues were examined by immunohistochemistry. The least absolute shrinkage and selection operator (LASSO) Cox model was used to establish the HRI score. Cox regression analysis was used for multivariate analysis. Nine recurrence-related immune features were identified and integrated into the HRI score. The HRI score was used to categorize patients into low-risk and high-risk groups using the X-tile software. Kaplan–Meier analysis presented that the HRI score showed good stratification between low-risk and high-risk groups in both the derivation cohort (P < 0.001) and the validation cohort (P = 0.014), respectively. Multivariate analysis demonstrated that serum γ-glutamyl transpeptidase, carbohydrate antigen 19-9, lymphoid metastasis, tumor numbers, and the HRI score were independent risk factors associated with recurrence-free survival (RFS). The combination of Shen’s model and HRI score provided better performance in recurrence prediction compared with traditional staging systems. The HRI score might serve as a promising RFS predictor for ICC with prognostic values.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02371-3) contains supplementary material, which is available to authorized users.
Keywords: Intrahepatic cholangiocarcinoma, Recurrence, Immunoscore, Prognosis
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common type of liver cancer, accounting for 10 ~ 20% of all primary liver malignancies [1, 2]. Liver resection is the only curative treatment for ICCs and enables long-term survival for selected patients. However, over 50% of patients recur after curative resection within the first 12 months, and the early recurrence has been a major obstacle in clinical practice [3, 4]. Therefore, enhancing the prediction accuracy for recurrence risk may facilitate postoperative management for ICC patients. Currently, traditional staging systems [including the American Joint Committee on Cancer (AJCC) and Liver Cancer Study Group of Japan (LCSGJ) staging systems] and the nomogram model (Shen’s model) [5] were all constructed based on clinicopathological features, which may provide useful but insufficient information for more accurate recurrence prediction. Due to its heterogeneity, ICC within the same stage might comprise different subsets with different molecular features and a large variation in prognosis. Thus, there is an urgent need for novel biomarkers or strategies to improve the accuracy of recurrence prediction and facilitate treatment selection afterwards.
Histopathological evidence suggests that variable numbers of infiltrating immune cells can be found in different tumors of the same type at different locations in or around a tumor. Recently, it was found that the integration of infiltrating immune cells into immunoscore could effectively predict recurrence and patient survival [4, 6, 7], and it may even act as a new component of a TNM-immune classification for cancer patients. The new classification was based on the density and location of CD3- and CD8-positive cells instead of the function of T lymphocytes, implying that infiltrating immune cells could serve as a new immune parameter for the individualized prediction of tumor prognosis. Previously, we observed that the infiltration of tumor-associated macrophages (TAMs) [8], regulatory T cells (Tregs) [9] and CD20+ B cells [10] were associated with poor prognosis of hepatocellular carcinoma (HCC) after liver resection. We also observed that CCL2+ and CCL17+ tumor-associated neutrophils could recruit macrophages and Tregs to promote the tumor progression [11], and the effector function of γδT cells was substantially impaired in the tumor microenvironment [12]. Though the potential clinical relevance of the density of infiltrating cells has already been evaluated in our previous study [13] and other studies [14, 15], the prognostic values of immune cells and programmed cell death 1 (PD1)/programmed death ligand-1 (PD-L1) for ICC are still controversial. Therefore, developing an individualized histopathology-related immunoscore (HRI) is necessary and may provide a better prediction performance.
In this study, we examined the density and location of 32 immune features using tissue microarray and immunohistochemistry, and developed the HRI score using the least absolute shrinkage and selection operator (LASSO) Cox method. We evaluated and validated the combined performance of the HRI score and Shen’s model by comparing with three traditional staging systems using the receiver operating characteristic (ROC) curve analysis.
Materials and methods
Patients
Two hundred and eighty ICC patients who received liver resection at Zhongshan Hospital between February 2005 and July 2011 were enrolled in this study. The inclusion criteria were histopathological diagnosis of ICC, Child–Pugh class A, complete resection of tumors, no history of anticancer therapy previously, and no history of other types of cancer. The exclusion criteria were Child–Pugh class C, hilar or extrahepatic cholangiocarcinoma, distant or intrahepatic metastasis, or histopathologically proven combined hepatocellular cholangiocarcinoma. The pathological diagnosis of ICC was defined according to the World Health Organization criteria [16].
Tissue microarray, immunohistochemistry and evaluation of immune cells
In the present study, sixteen prognostic immune markers were chosen because of their close involvement in tumor growth (CD66b and PD1) [11, 17], tumor prognosis (CD3, CD4, CD8, CD57, CD68, Foxp3 and PD-L1) [8–10, 14, 18–21], antitumor function (CD14) [22] and local immune response (CD20, CD27 and CD45RO) [23, 24] in liver cancer. CXCR5 was selected due to its role in the pathogenesis of primary biliary cirrhosis [25], while altered expression of CD45RA [12] and CD103 [26] has been observed in liver malignancy, but their prognostic values remain unclear. Tissue microarray (TMA) was conducted as previously described [27]. The immunohistochemistry was performed in accordance with the two-step protocol (Novolink Polymer Detection System, Novocastra) according to the manufacturer’s instructions. Detailed information of antibody dilution is provided in the Supplementary Table 1.
To evaluate the levels of immune infiltrations, three most independent and representative areas were selected and photographed (at ×200 magnification) with an Olympus digital camera in intra- and peritumoral tissues, respectively. Identical settings were used for each photograph, and high-resolution spot-images were obtained and stored. Using Image-pro plus 6.0 software (Media Cybernetics Inc.), the numbers of positive staining cells were counted automatically and recorded as previously described [28], and mean values were used for statistical analysis [9]. Three representative spots presented good homogeneity of stained cells in each tumor and peritumor area.
Statistical analysis
Demographic, clinical and tumor characteristics are presented as percentages or median values. Pearson’s χ2 test or Fisher’s exact test was used for the analysis of categorical variables, and Wilcoxon test or Student’s t test for continuous variables. RFS curves were plotted using the Kaplan–Meier method and analyzed with the log-rank test. On the basis of RFS, the HRI score was established from the derivation cohort, and the weights for HRI represented the coefficient derived from the LASSO Cox analysis. The “glment” package was used as described [29]. The HRI score was validated with an internal dataset. According to the highest χ2 value defined by Kaplan–Meier curve analysis and log-rank tests, the X-tile software was used to identify the optimum cutoff value for the HRI score. Univariate and multivariate regression analyses were performed. The performance of staging systems and Shen’s model were compared with the rcorrp.cens package in Hmisc [30] and evaluated with ROC curve. Statistical analysis was performed using R version 3.1.0 software (R Foundation, Vienna, Austria). A P value < 0.05 was considered statistically significant.
Results
Clinicopathologic characteristics of patients
A total of 280 ICC patients were randomly assigned to the derivation group (n = 176) or the validation group (n = 104). The demographic and clinicopathologic characteristics are summarized in Table 1. In the derivation cohort, 42.1% of patients (74/176) were infected with HBV and 1.14% (2/176) with HCV. The median tumor diameter was 6.0 (4.0, 8.0) cm and 6.5 (4.9, 9.0) cm in the derivation and validation cohorts, respectively. Despite significant difference between the alanine aminotransferase (ALT) levels of the two cohorts, serum ALT values were still within the normal range and would not influence the liver function of ICC patients.
Table 1.
Demographic, clinical, and tumor characteristics of patients with intrahepatic cholangiocarcinoma
| Patient demographics | Derivation cohort (n = 176) | Validation cohort (n = 104) | P value |
|---|---|---|---|
| Age, year | |||
| < 60 | 99 (56.3%) | 54 (51.9%) | 0.56 |
| ≥ 60 | 77 (43.8%) | 50 (48.1%) | |
| Sex (male), n (%) | 101 (57.4%) | 72 (69.2%) | 0.07 |
| Etiology | |||
| HBV | 74 (42.1%) | 44 (42.3%) | 0.89 |
| HCV | 2 (1.1%) | 2 (1.9%) | |
| Others | 100 (56.8%) | 58 (55.8%) | |
| Liver cirrhosis, yes (%) | 37 (21.0%) | 33 (31.73%) | 0.06 |
| AFP, ng/mL | 2.7 (1.8, 4.6) | 2.8 (2.0, 5.0) | 0.31 |
| CEA, μg/mL | 2.3 (1.4, 4.6) | 2.4 (1.4, 3.7) | 0.94 |
| CA19-9, U/ml | 37.7 (13.4, 372.2) | 37.9 (17.3, 137.6) | 0.86 |
| Albumin, g/L | 43.0 (40.0, 45.0) | 43.5 (39.8, 46) | 0.79 |
| Bilirubin, μmol/L | 11.7 (8.8, 15.3) | 12.1 (9.2, 16.5) | 0.45 |
| ALT, IU/L | 19.0 (13.8, 32.0) | 25.0 (16.0, 42.3) | 0.02 |
| GGT, U/L | 47.0 (29.0, 92.0) | 55.0 (33.8, 107.0) | 0.38 |
| Platelets, 103/μL | 177.5 (141.5, 218.0) | 190 (137.8, 216.3) | 0.79 |
| Tumor nodularities, n (%) | |||
| 1 | 149 (84.7%) | 81 (77.9%) | 0.16 |
| 2 | 14 (8.0%) | 8 (7.7%) | |
| ≥ 3 | 13 (7.4%) | 15 (14.4%) | |
| Tumor diameter, cm | 6.0 (4.0, 8.0) | 6.5 (4.9, 9.0) | 0.29 |
| Tumor differentiation | |||
| I–II | 121 (68.8%) | 70 (67.3%) | 0.91 |
| III–IV | 55 (31.3%) | 34 (32.3%) | |
| Vascular invasion (yes), n (%) | 8 (4.6%) | 6 (5.8%) | 0.86 |
| Lymphoid metastasis (yes), n (%) | 8 (4.6%) | 9 (8.7%) | 0.26 |
| Direct invasion and local extrahepatic metastasis (yes), n (%) | 2 (1.1%) | 0 (0.0%) | 0.53 |
| Resection type, n (%) | |||
| Minor resection | 105 (59.7%) | 61 (58.7%) | 0.48 |
| Major resection | 71 (40.3%) | 43 (41.3%) | |
| Bleeding volume, ml | 200 (100, 400) | 200 (100, 400) | 0.57 |
| Occlusion, min | |||
| < 15 min | 115 (65.3%) | 66 (63.5%) | 0.85 |
| ≥ 15 min | 61 (34.7%) | 38 (36.5%) | |
HBV hepatitis B virus, HCV hepatitis C virus, AFP α-fetoprotein, CEA carcino-embryonic antigen, CA19-9 carbohydrate antigen 19-9, ALT alanine aminotransferase, GGT γ-glutamyl transpeptidase, VI vascular invasion
Values are presented as no. (%) or median (Q1, Q3)
The study was censored on April 12, 2014. The median overall survival was 27.6 months (range, 1–100.5 months) and 31.2 months (range, 1–99.5 months) in the derivation and validation cohorts, respectively. During the follow-up period, 71.6% (126/176) of patients developed recurrence in the derivation cohort and 71.2% (74/104) in the validation cohort; 63.1% (111/176) and 61.5% (64/104) of patients died in the derivation and validation cohorts, respectively.
Construction of HRI
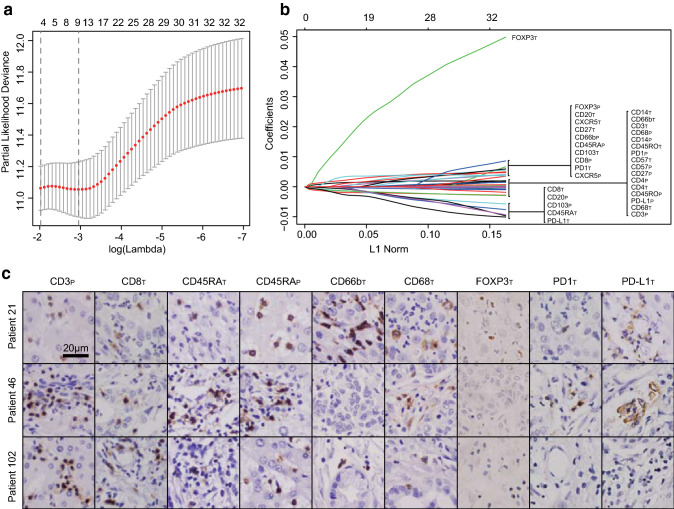
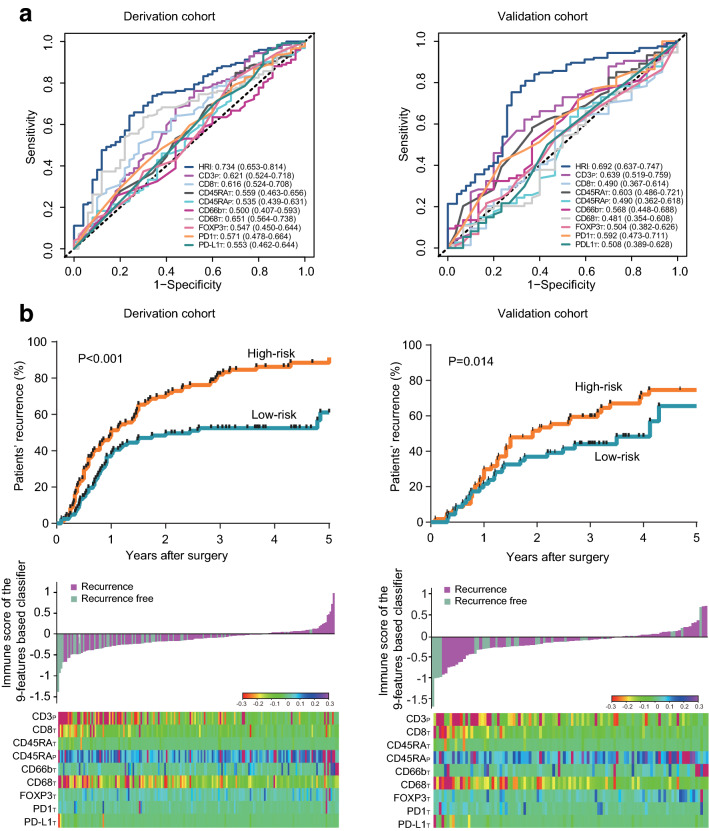
Using the LASSO Cox analysis, 9 prognostic predictors were selected out of 32 immune features on the basis of RFS in the derivation cohort (Fig. 1a, b). Figure 1c presents the histological expression of nine immune features in intra- or peritumoral tissues, including CD3peritumoral (p), CD8intratumoral (T), CD45RAT, CD45RAP, CD66bT, CD68T, Foxp3T, PD1T and PD-L1T. The individualized HRI score was constructed, and the calculation formula is (1.6081 × the levels of CD45RAP − 1.4985 × the levels of CD3P − 0.9289 × the levels of CD8T − 0.3987 × the levels of CD45RAT + 1.0428 × the levels of CD66bT − 1.0879 × the levels of CD68T + 8.5375 × the levels of Foxp3T + 1.6075 × the levels of PD1T − 1.3851 × the levels of PD-L1T) × 10−3. Figure 2a indicates that the area under curve (AUC) values of HRI score (derivation cohort, 0.734; validation cohort, 0.692) were better than those of selected immune features (derivation cohort, 0.500–0651; validation cohort, 0.481–0.639) in both cohorts.
Fig. 1.
Selection of immune features using the LASSO regression analysis in intrahepatic cholangiocarcinoma patients. a, b Nine immune features were selected by LASSO method. Left panel a: the two dotted vertical lines were drawn at the optimal values by minimum criteria and 1-s.e. criteria. Right panel b: LASSO coefficient profiles of the 32 immune features. c The expression pattern of selected immune features, including CD3P, CD8T, CD45RAT, CD45RAP, CD66bT, CD68T, FOXP3T, PD1T and PD-L1T in three different patients. Bar, 20 μm. LASSO least absolute shrinkage and selection operator
Fig. 2.
a ROC curves for the HRI score and nine selected immune features in the derivation cohort and the validation cohort. b Kaplan–Meier survival curves, the discrimination performance and the HRI distribution of the nine immune features in the derivation and the validation cohort, respectively. Upper panel: the Kaplan–Meier survival curves showing high-risk and low-risk groups in both cohorts. Middle panel: HRI distribution of the nine-immune-feature-based classifier and patient recurrence status. Lower panel: heat map presenting density of the nine immune features in ICC patients. ROC receiver operating characteristic, HRI histopathology-related immunoscore
The X-tile software was used to determine the optimum cutoff value of HRI score [31]. Patients were classified into two groups according to their HRI score: low-risk group (≤ − 0.14) and high-risk group (>− 0.14), with the AUC values 0.691 (95%CI, 0.617–0.766) and 0.676 (95%CI, 0.568–0.784) in the derivation and validation cohorts, respectively. The 1-, 3- and 5-year recurrence rates in the high-risk group were worse than those in the low-risk group in both cohorts (derivation cohort: low vs. high, P < 0.001; validation cohort: low vs. high, P = 0.014) (Fig. 2b).
Prognostic predictors
The results of both univariate analysis and multivariate analysis using Cox regression model are listed in Table 2 and Supplementary Table 2. Factors with P < 0.1 in univariate analysis, including carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), ALT, γ-glutamyl transpeptidase (GGT), HRI score, lymphoid metastasis, tumor number, and diameter, were enrolled in multivariate analysis. The multivariate analysis revealed that CA19-9, GGT, tumor number, lymphoid metastasis, and the HRI score remained independent risk factors for recurrence and overall survival after liver resection. The highest hazard ratios (HRs) were observed in three factors, HRI score, lymphoid metastasis and tumor numbers (≥ 3 nodules).
Table 2.
Cox proportional hazards regression model showing the association of variables with RFS
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P value | HR | 95%CI | P value | |
| CEA (≥ 5/< 5, ng/mL) | 1.007 | 0.999–1.016 | 0.09 | |||
| CA19-9 (≥ 37/< 37, U/mL) | 1.001 | 1.000–1.001 | <0.001 | 1.001 | 1.000–1.001 | < 0.001 |
| ALT (≥ 35/< 35, U/L) | 1.005 | 1.001–1.010 | 0.02 | |||
| GGT (≥ 40/< 40, U/L) | 1.004 | 1.002–1.005 | <0.001 | 1.003 | 1.000–1.005 | 0.03 |
| Tumor numbers | ||||||
| 1 nodule | 1.110 | 1.052–1.170 | < 0.001 | |||
| 2 nodules | 0.897 | 0.466–1.123 | 0.75 | 0.88 | 0.445–1.743 | 0.71 |
| ≥ 3 nodules | 2.100 | 1.123–3.927 | 0.02 | 3.029 | 1.543–5.947 | 0.001 |
| Tumor diameter, cm | 1.087 | 1.030–1.147 | 0.003 | |||
| Lymphoid metastasis (yes/no) | 1.997 | 0.928–4.297 | 0.08 | 2.763 | 1.179–6.473 | 0.02 |
| HRI score (low/high) | 7.787 | 3.714–16.328 | < 0.001 | 2.511 | 1.683–3.748 | < 0.001 |
CEA carcino-embryonic antigen, CA19-9 carbohydrate antigen 19-9, ALT alanine aminotransferase, GGT γ-glutamyl transpeptidase, HRI histopathology-related immunoscore, CI confidence interval
Integration of HRI score and Shen’s model
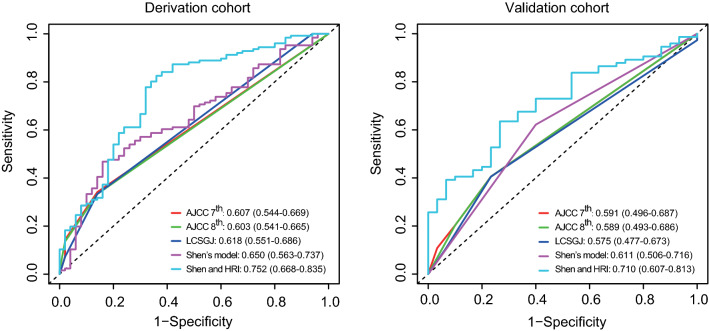
To compare the performance of the AJCC 7th, AJCC 8th, and LCSGJ staging systems, and Shen’s model in recurrence prediction, the ROC analysis was performed. In the derivation cohort, the AUC values of the AJCC 7th, AJCC 8th, LCSGJ, and Shen’s model were 0.607, 0.603, 0.618 and 0.650, respectively. Similar AUC values were observed in the validation cohort (AJCC 7th, 0.591; AJCC 8th, 0.589; LCSGJ, 0.575; Shen’s model, 0.611).
To evaluate the prognostic value of the HRI score in combination with the current prognostic system, the HRI score was integrated with Shen’s model. As shown in Fig. 3, the performance of the combination of the HRI score and Shen’s model (AUC, derivation cohort: 0.752, 95%CI, 0.688–0.835; validation cohort: 0.710, 95%CI, 0.607–0.813) was superior to other staging systems (AUC, derivation cohort, 0.603–0.650; validation cohort, 0.575–0.611) in both datasets.
Fig. 3.
ROC curves for three staging systems, Shen’s model, and the combination of HRI and Shen’s model in the derivation cohort and the validation cohort (Liver Cancer Study Group of Japan [LCSGJ]; American Joint Committee on Cancer [AJCC] 7th edition; AJCC 8th edition). ROC receiver operating characteristic
Discussion
Growing evidence indicated that the type, density, and location of immune cells within a tumor sample may strongly influence the evolution of various cancers and may provide prognostic information [9, 32, 33]. In the present study, we developed a novel histopathology-related immunoscore based on ICC TMA, which was independently associated with patients’ recurrence and overall survival. In stage I–III ICC patients, the combination of HRI score and Shen’s model provided better performance in recurrence prediction, suggesting that the HRI score could contribute prognostic information and act as an individual immune signature in prognostic prediction systems.
For the construction of the HRI score, 32 immune features, examined histologically in peri- and intratumoral tissues, were reduced to nine potential predictors with the LASSO method. Apart from the advantage in predictor selection, this method can also enable the panel of selected immune features to be combined into the HRI score. Recently, multimarker analyses have been widely used in studies of various tumors, such as a 5-gene signature in colorectal cancer [34], 5-gene score in hepatocellular carcinoma [35], and immunoscore signature in gastric cancer [36]. In the present study, the novel HRI score was developed on the basis of 32 immune features and presented good discrimination in the derivation cohort (AUC, 0.734) and validation cohort (AUC, 0.692), respectively.
We herein showed that the nine immune feature-based immunoscore was significantly correlated with RFS. The corpus of data strongly suggested that tumor behavior may result from the balance between the tumor invasion process and the response of the host of which the local immune reaction in a major component [37, 38]. Inconsistent with Galon’s study [39], Li’s study [36], and other studies [33, 40], two peritumoral- and seven intratumoral-specific immune markers were identified in intrahepatic cholangiocarcinoma patients, suggesting that the distribution and function of immune cells also vary in different tumor types. We observed that the densities of peritumoral CD3+ and intratumoral CD8+ T cells were associated with patients’ recurrence after partial hepatectomy. Previously, CD3+ and CD8+ T cells were reported to be correlated with cancer recurrence and overall survival in patients with colorectal cancer [41] and HCC [20], implying that CD3+ and CD8+ cell populations may contribute to the antitumor immune response. Evidence in mice revealed that the upregulation of PD-L1 and recruitment of Foxp3+ Tregs in tumor microenvironment were dependent on the presence of CD8+ lymphocytes [42]. CD45RA, an immune marker of naïve T cells, was found to exhibit reduced sensitivity to oxidative stress-induced cell death and maintained their suppressive function, a phenomenon that may be attributed to their observed high anti-oxidative capacity [43]. In our study, we observed that the peritumoral and intratumoral densities of CD45RA-positive cells were associated with the prognosis of intrahepatic cholangiocarcinoma patients. Consistent with our previous studies [13, 18], the presence of intratumoral CD66b+ (the marker of neutrophil) cells was observed as a poor prognostic predictor for ICC patients. Mechanistic evidence in mice indicated that neutrophils could facilitate liver metastasis by expressing substantial fibroblast growth factor-2 [44]. Inconsistent with a previous study on TAMs in liver cancer [45], we revealed that a low density of intratumoral macrophages was correlated with tumor progression and poor postoperative prognosis. Evidence in gastric cancer [46] indicated that TAM-derived CXCL8 could contribute to the immunosuppressive microenvironment by inducing PD-L1+ macrophages, providing a new opportunity for cancer immunotherapy. The HRI score is an individualized signature integrated with recurrence-related immune features. In the multivariate Cox analysis, the performance of HRI score in predicting recurrence was statistically significant when adjusted for other covariates.
Our clinicopathological analysis revealed that CA19-9, GGT, tumor number, and lymphoid metastasis were also indicative of poor RFS in stage I-III ICCs. Of note, CA19-9 was identified as a prognostic tumor marker in our previous studies [47, 48], in which elevated CA19-9 was associated with advanced TNM stages and poor prognosis. It is widely perceived that the prognosis of liver cancer relies on tumor stage and underlying liver function [49]. GGT is a relatively sensitive indicator of malignant liver disease and has been reported to be associated with the risk of liver cancer [50]. Consistently, GGT was reported to be associated with RFS in our study. Also, previous reports [5, 48] have revealed that the presence of multiple nodules may affect patient survival independently. Also, increased tumor numbers may be indicative of satellite nodules or intrahepatic metastasis. Based on these clinical and immunological predictors, the combination of HRI score and Shen’s model provided better performance in survival prediction.
Our study remains to be improved in several aspects. First, the prognostic model was established based on the data from a single liver center in China. Further external validation is needed for the HRI score. Second, in the present study, HBV-infected patients accounted for 42.1% of all patients, and only patients with resectable ICC were enrolled. It is necessary to validate our results in other geographic regions to extend our results and to increase sample variability. Third, the biologic mechanisms of the candidate markers such as CD3P, CD8T, CD45RAT, CD45RAP, CD66bT, CD68T, Foxp3T, PD1T, and PD-L1T require to be further explored and elucidated.
In conclusion, the HRI score, established on the basis of nine immune features, might be a potential prognostic predictor for recurrence in stage I–III ICC patients. The combination of Shen’s model with the HRI score may help the risk evaluation of individual recurrence and facilitate the clinical management for patients with intrahepatic cholangiocarcinoma.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- AJCC
American Joint Committee on Cancer
- ALT
Alanine aminotransferase
- AUC
Area under curve
- CA19-9
Carbohydrate antigen 19-9
- CEA
Carcino-embryonic antigen
- GGT
γ-Glutamyl transpeptidase
- HRI
Histopathology-related immunoscore
- ICC
Intrahepatic cholangiocarcinoma
- LASSO
Least absolute shrinkage and selection operator
- LCSGJ
Liver Cancer Study Group of Japan
- PD1
Programmed cell death 1
- PD-L1
Programmed death ligand-1
- RFS
Recurrence-free survival
- ROC
Receiver operating characteristic curve
- TAM
Tumor-associated macrophage
- TMA
Tissue microarray
- Tregs
Regulatory T cells
Author contributions
MXT, YFZ, WFQ, JF, and YHS performed and analyzed the experiments. WRL, LJ, XFJ, HW, CYT, PYZ, YF, ZBD, and YFP collected the clinical data. MXT, YFZ, WFQ, JZ, JF, and YHS designed the clinical trial protocol and experiments. All the authors approved the final version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81472674, 81502486, 81773067 and 81800790), the National Key Sci-Tech Project (2012ZX10002011-002), and a China Postdoctoral Science Foundation funded project (2018M640343). The work was sponsored by the Shanghai Sailing Program (19YF1407800), and the Shanghai Committee of Science and Technology, China (No. 16JC1404000).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
The study was approved by the institutional review board of Zhongshan Hospital on 31/1/2018 and was conducted in accordance with the standards of the Declaration of Helsinki.
Informed consent
Written informed consents for the use of their specimens and anonymous clinical data were obtained from all individual participants in the study prior to hepatectomy.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Meng-Xin Tian, Yu-Fu Zhou and Wei-Feng Qu contributed equally to this work.
References
- 1.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24(2):115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 2.Massarweh NN, El-Serag HB. Epidemiology of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Control. 2017;24(3):1073274817729245. doi: 10.1177/1073274817729245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, D’Angelica M, DeMatteo RP, Fong Y, Schwartz L, Kemeny N, O’Reilly E, Abou-Alfa GK, Shimada H, Blumgart LH, Jarnagin WR. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248(1):84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 4.Nepal C, O’Rourke CJ, Oliveira DV, Taranta A, Shema S, Gautam P, Calderaro J, Barbour A, Raggi C, Wennerberg K, Wang XW, Lautem A, Roberts LR, Andersen JB. Genomic perturbations reveal distinct regulatory networks in intrahepatic cholangiocarcinoma. Hepatology. 2017 doi: 10.1002/hep.29764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, Wan X, Liu G, Wu D, Shi L, Lau W, Wu M, Shen F. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31(9):1188–1195. doi: 10.1200/JCO.2012.41.5984. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Y, Zhang Q, Hu Y, Li T, Yu J, Zhao L, Ye G, Deng H, Mou T, Cai S, Zhou Z, Liu H, Chen G, Li G, Qi X. ImmunoScore Signature: a prognostic and predictive tool in gastric cancer. Ann Surg. 2018;267(3):504–513. doi: 10.1097/SLA.0000000000002116. [DOI] [PubMed] [Google Scholar]
- 7.Adam R, Bhangui P, Vibert E, Azoulay D, Pelletier G, Duclos-Vallee JC, Samuel D, Guettier C, Castaing D. Resection or transplantation for early hepatocellular carcinoma in a cirrhotic liver: does size define the best oncological strategy? Ann Surg. 2012;256(6):883–891. doi: 10.1097/SLA.0b013e318273bad0. [DOI] [PubMed] [Google Scholar]
- 8.Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, Wu WZ, Wang L, Tang ZY, Sun HC. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26(16):2707–2716. doi: 10.1200/JCO.2007.15.6521. [DOI] [PubMed] [Google Scholar]
- 9.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25(18):2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 10.Shi JY, Gao Q, Wang ZC, Zhou J, Wang XY, Min ZH, Shi YH, Shi GM, Ding ZB, Ke AW, Dai Z, Qiu SJ, Song K, Fan J. Margin-infiltrating CD20(+) B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clin Cancer Res. 2013;19(21):5994–6005. doi: 10.1158/1078-0432.CCR-12-3497. [DOI] [PubMed] [Google Scholar]
- 11.Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z, Chen EB, Fan J, Cao Y, Dai Z, Zhou J. Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology. 2016;150(7):1646–1658.e1617. doi: 10.1053/j.gastro.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 12.Yi Y, He HW, Wang JX, Cai XY, Li YW, Zhou J, Cheng YF, Jin JJ, Fan J, Qiu SJ. The functional impairment of HCC-infiltrating gammadelta T cells, partially mediated by regulatory T cells in a TGFbeta- and IL-10-dependent manner. J Hepatol. 2013;58(5):977–983. doi: 10.1016/j.jhep.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Gu FM, Gao Q, Shi GM, Zhang X, Wang J, Jiang JH, Wang XY, Shi YH, Ding ZB, Fan J, Zhou J. Intratumoral IL-17(+) cells and neutrophils show strong prognostic significance in intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2012;19(8):2506–2514. doi: 10.1245/s10434-012-2268-8. [DOI] [PubMed] [Google Scholar]
- 14.Sabbatino F, Villani V, Yearley JH, Deshpande V, Cai L, Konstantinidis IT, Moon C, Nota S, Wang Y, Al-Sukaini A, Zhu AX, Goyal L, Ting DT, Bardeesy N, Hong TS, Fernandez-del Castillo C, Tanabe KK, Lillemoe KD, Ferrone S, Ferrone CR. PD-L1 and HLA class I antigen expression and clinical course of the disease in intrahepatic cholangiocarcinoma. Clin Cancer Res. 2016;22(2):470–478. doi: 10.1158/1078-0432.CCR-15-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghesquiere B, Wong BW, Kuchnio A, Carmeliet P. Metabolism of stromal and immune cells in health and disease. Nature. 2014;511(7508):167–176. doi: 10.1038/nature13312. [DOI] [PubMed] [Google Scholar]
- 16.IARC, Bosman FT, Carneiro F, Hruban RH, Theise ND (2010) WHO classification of tumours of the digestive system, Fourth Edition. World Health Organization
- 17.Li H, Li X, Liu S, Guo L, Zhang B, Zhang J, Ye Q. Programmed cell death-1 (PD-1) checkpoint blockade in combination with a mammalian target of rapamycin inhibitor restrains hepatocellular carcinoma growth induced by hepatoma cell-intrinsic PD-1. Hepatology. 2017;66(6):1920–1933. doi: 10.1002/hep.29360. [DOI] [PubMed] [Google Scholar]
- 18.Li YW, Qiu SJ, Fan J, Zhou J, Gao Q, Xiao YS, Xu YF. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol. 2011;54(3):497–505. doi: 10.1016/j.jhep.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 19.Fu J, Zhang Z, Zhou L, Qi Z, Xing S, Lv J, Shi J, Fu B, Liu Z, Zhang JY, Jin L, Zhao Y, Lau GK, Zhao J, Wang FS. Impairment of CD4 + cytotoxic T cells predicts poor survival and high recurrence rates in patients with hepatocellular carcinoma. Hepatology. 2013;58(1):139–149. doi: 10.1002/hep.26054. [DOI] [PubMed] [Google Scholar]
- 20.Gabrielson A, Wu Y, Wang H, Jiang J, Kallakury B, Gatalica Z, Reddy S, Kleiner D, Fishbein T, Johnson L, Island E, Satoskar R, Banovac F, Jha R, Kachhela J, Feng P, Zhang T, Tesfaye A, Prins P, Loffredo C, Marshall J, Weiner L, Atkins M, He AR. Intratumoral CD3 and CD8 T-cell densities associated with relapse-free survival in HCC. Cancer Immunol Res. 2016;4(5):419–430. doi: 10.1158/2326-6066.CIR-15-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Q, Zhou J, Wang XY, Qiu SJ, Song K, Huang XW, Sun J, Shi YH, Li BZ, Xiao YS, Fan J. Infiltrating memory/senescent T cell ratio predicts extrahepatic metastasis of hepatocellular carcinoma. Ann Surg Oncol. 2012;19(2):455–466. doi: 10.1245/s10434-011-1864-3. [DOI] [PubMed] [Google Scholar]
- 22.Asai A, Tsuchimoto Y, Ohama H, Fukunishi S, Tsuda Y, Kobayashi M, Higuchi K, Suzuki F. Host antitumor resistance improved by the macrophage polarization in a chimera model of patients with HCC. Oncoimmunology. 2017;6(4):e1299301. doi: 10.1080/2162402X.2017.1299301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garnelo M, Tan A, Her Z, Yeong J, Lim CJ, Chen J, Lim KH, Weber A, Chow P, Chung A, Ooi LL, Toh HC, Heikenwalder M, Ng IO, Nardin A, Chen Q, Abastado JP, Chew V. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut. 2017;66(2):342–351. doi: 10.1136/gutjnl-2015-310814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takata Y, Nakamoto Y, Nakada A, Terashima T, Arihara F, Kitahara M, Kakinoki K, Arai K, Yamashita T, Sakai Y, Yamashita T, Mizukoshi E, Kaneko S. Frequency of CD45RO + subset in CD4 + CD25(high) regulatory T cells associated with progression of hepatocellular carcinoma. Cancer Lett. 2011;307(2):165–173. doi: 10.1016/j.canlet.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Sun Y, Zhang Z, Jia Y, Zou Z, Ding J, Li Y, Xu X, Jin L, Yang T, Li Z, Sun Y, Zhang JY, Lv S, Chen L, Li B, Gershwin ME, Wang FS. CXCR25 + CD4 + T follicular helper cells participate in the pathogenesis of primary biliary cirrhosis. Hepatology. 2015;61(2):627–638. doi: 10.1002/hep.27306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang HH, Mei MH, Fei R, Liao WJ, Wang XY, Qin LL, Wang JH, Wei L, Chen HS. Regulatory T cell depletion enhances tumor specific CD8 T-cell responses, elicited by tumor antigen NY-ESO-1b in hepatocellular carcinoma patients, in vitro. Int J Oncol. 2010;36(4):841–848. doi: 10.3892/ijo_00000561. [DOI] [PubMed] [Google Scholar]
- 27.Liu WR, Tian MX, Jin L, Yang LX, Ding ZB, Shen YH, Peng YF, Zhou J, Qiu SJ, Dai Z, Fan J, Shi YH. High expression of 5-hydroxymethylcytosine and isocitrate dehydrogenase 2 is associated with favorable prognosis after curative resection of hepatocellular carcinoma. J Exp Clin Cancer Res CR. 2014;33:32. doi: 10.1186/1756-9966-33-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S, Xu F, Li H, Zhang J, Zhong A, Huang B, Lai M. S100A8(+) stroma cells predict a good prognosis and inhibit aggressiveness in colorectal carcinoma. Oncoimmunology. 2017;6(1):e1260213. doi: 10.1080/2162402X.2016.1260213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang JX, Song W, Chen ZH, Wei JH, Liao YJ, Lei J, Hu M, Chen GZ, Liao B, Lu J, Zhao HW, Chen W, He YL, Wang HY, Xie D, Luo JH. Prognostic and predictive value of a microRNA signature in stage II colon cancer: a microRNA expression analysis. Lancet Oncol. 2013;14(13):1295–1306. doi: 10.1016/S1470-2045(13)70491-1. [DOI] [PubMed] [Google Scholar]
- 30.Frank E, Harrell J Hmisc: Harrell Miscellaneous. R Package version 3.9-2. http://CRAN.Rproject.org/package=Hmisc
- 31.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 32.Mina M, Boldrini R, Citti A, Romania P, D’Alicandro V, De Ioris M, Castellano A, Furlanello C, Locatelli F, Fruci D. Tumor-infiltrating T lymphocytes improve clinical outcome of therapy-resistant neuroblastoma. Oncoimmunology. 2015;4(9):e1019981. doi: 10.1080/2162402X.2015.1019981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geissler K, Fornara P, Lautenschlager C, Holzhausen HJ, Seliger B, Riemann D. Immune signature of tumor infiltrating immune cells in renal cancer. Oncoimmunology. 2015;4(1):e985082. doi: 10.4161/2162402X.2014.985082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang W, Gao X, Han Y, Du Y, Liu Q, Wang L, Tan X, Zhang Q, Liu Y, Zhu Y, Yu Y, Fan X, Zhang H, Zhou W, Wang J, Fu C, Cao G. Gene expression profiling-derived immunohistochemistry signature with high prognostic value in colorectal carcinoma. Gut. 2014;63(9):1457–1467. doi: 10.1136/gutjnl-2013-305475. [DOI] [PubMed] [Google Scholar]
- 35.Nault JC, De Reynies A, Villanueva A, Calderaro J, Rebouissou S, Couchy G, Decaens T, Franco D, Imbeaud S, Rousseau F, Azoulay D, Saric J, Blanc JF, Balabaud C, Bioulac-Sage P, Laurent A, Laurent-Puig P, Llovet JM, Zucman-Rossi J. A hepatocellular carcinoma 5-gene score associated with survival of patients after liver resection. Gastroenterology. 2013;145(1):176–187. doi: 10.1053/j.gastro.2013.03.051. [DOI] [PubMed] [Google Scholar]
- 36.Jiang Y, Zhang Q, Hu Y, Li T, Yu J, Zhao L, Ye G, Deng H, Mou T, Cai S, Zhou Z, Liu H, Chen G, Li G, Qi X. ImmunoScore Signature: a prognostic and predictive tool in gastric cancer. Ann Surg. 2016;10:15. doi: 10.1097/sla.0000000000002116. [DOI] [PubMed] [Google Scholar]
- 37.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 39.Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, Zatloukal K, Trajanoski Z, Berger A, Fridman WH, Galon J. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27(35):5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 40.Wen T, Wang Z, Li Y, Li Z, Che X, Fan Y, Wang S, Qu J, Yang X, Hou K, Zhou W, Xu L, Li C, Wang J, Liu J, Chen L, Zhang J, Qu X, Liu Y. A four-factor immunoscore system that predicts clinical outcome for stage II/III gastric cancer. Cancer Immunol Res. 2017;5(7):524–534. doi: 10.1158/2326-6066.CIR-16-0381. [DOI] [PubMed] [Google Scholar]
- 41.Angell H, Galon J. From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25(2):261–267. doi: 10.1016/j.coi.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5(200):200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mougiakakos D, Johansson CC, Kiessling R. Naturally occurring regulatory T cells show reduced sensitivity toward oxidative stress-induced cell death. Blood. 2009;113(15):3542–3545. doi: 10.1182/blood-2008-09-181040. [DOI] [PubMed] [Google Scholar]
- 44.Gordon-Weeks AN, Lim SY, Yuzhalin AE, Jones K, Markelc B, Kim KJ, Buzzelli JN, Fokas E, Cao Y, Smart S, Muschel R. Neutrophils promote hepatic metastasis growth through fibroblast growth factor 2-dependent angiogenesis in mice. Hepatology. 2017;65(6):1920–1935. doi: 10.1002/hep.29088. [DOI] [PubMed] [Google Scholar]
- 45.Rava M, D’Andrea A, Doni M, Kress TR, Ostuni R, Bianchi V, Morelli MJ, Collino A, Ghisletti S, Nicoli P, Recordati C, Iascone M, Sonzogni A, D’Antiga L, Shukla R, Faulkner GJ, Natoli G, Campaner S, Amati B. Mutual epithelium-macrophage dependency in liver carcinogenesis mediated by ST18. Hepatology. 2017;65(5):1708–1719. doi: 10.1002/hep.28942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin C, He H, Liu H, Li R, Chen Y, Qi Y, Jiang Q, Chen L, Zhang P, Zhang H, Li H, Zhang W, Sun Y, Xu J. Tumour-associated macrophages-derived CXCL8 determines immune evasion through autonomous PD-L1 expression in gastric cancer. Gut. 2019;10:15. doi: 10.1136/gutjnl-2018-316324. [DOI] [PubMed] [Google Scholar]
- 47.Shi RY, Yang XR, Shen QJ, Yang LX, Xu Y, Qiu SJ, Sun YF, Zhang X, Wang Z, Zhu K, Qin WX, Tang ZY, Fan J, Zhou J. High expression of Dickkopf-related protein 1 is related to lymphatic metastasis and indicates poor prognosis in intrahepatic cholangiocarcinoma patients after surgery. Cancer. 2013;119(5):993–1003. doi: 10.1002/cncr.27788. [DOI] [PubMed] [Google Scholar]
- 48.Jiang W, Zeng ZC, Tang ZY, Fan J, Sun HC, Zhou J, Zeng MS, Zhang BH, Ji Y, Chen YX. A prognostic scoring system based on clinical features of intrahepatic cholangiocarcinoma: the Fudan score. Ann Oncol. 2011;22(7):1644–1652. doi: 10.1093/annonc/mdq650. [DOI] [PubMed] [Google Scholar]
- 49.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O’Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Inarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mok Y, Son DK, Yun YD, Jee SH, Samet JM. gamma-Glutamyltransferase and cancer risk: the Korean cancer prevention study. Int J Cancer. 2016;138(2):311–319. doi: 10.1002/ijc.29659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.