Abstract
Background
Immune checkpoint inhibitors are now standard-of-care treatments for metastatic cutaneous melanoma. However, for rare sub-groups, such as mucosal melanomas, few published data are available, and with no established therapeutic guidelines. Our objective was to assess the response to anti-CTLA4 and anti-PD1 immunotherapy in patients with mucosal melanomas.
Methods
We performed a single-center, prospective cohort analysis of patients with non-surgical locally advanced and/or metastatic mucosal melanoma receiving anti-CTLA4 and/or anti-PD1 immunotherapy from 2010 to 2016.
Results
Forty-four patients were enrolled, including 18 (40.9%) with head and neck, 12 (27.3%) with vulvo-vaginal and 14 (31.8%) with ano-rectal primary tumours. Eleven (25%) patients had stage 3 disease, and 11 (25%) had distant metastases. The first-line immunotherapy was ipilimumab in 24 patients and pembrolizumab in 20. The objective response rate (ORR) was 8.2% (one complete response) for ipilimumab and 35% (four complete responses) for pembrolizumab. No significant difference was observed for primary tumour location. The median follow-up was 24 months (range 4–73). The median progression-free survival (PFS) in the first-line ipilimumab and pembrolizumab groups was 3 months [95% confidence interval (CI) 2.5–4.6] and 5 months (95% CI 2.6–33.1), respectively (p = 0.0147).
Conclusion
In the patients with unresectable and/or metastatic mucosal melanoma, we found ORR and PFS rates comparable to those in patients with cutaneous melanoma, with no significant differences in the types of mucosal surfaces involved. Anti-PD1 therapy has a more favorable benefit-risk ratio than ipilimumab and should be used preferentially.
Keywords: Mucosal melanoma, Immunotherapy, Ipilimumab, Pembrolizumab, Anti-PD1, Anti-CTLA4
Introduction
Immune checkpoint inhibitors (anti-CTLA4 and anti-PD1 antibodies) have recently significantly improved the outcomes of patients with metastatic cutaneous melanoma and are now considered a standard-of-care treatment [1–7]. However, for rare sub-groups, such as mucosal melanomas, few published data are available, and therapeutic guidelines have not been established yet.
Mucosal melanoma accounts for 0.7–4.3% of all melanomas [8–10]. The primary tumour locations are the head and neck (55%), ano-rectal region (23.8%) and vulvo-vaginal area (18%) [8]. The mean age at diagnosis is between 60 and 70 years [11]. Mucosal melanoma is known as an aggressive tumour with high risk of metastasis and local recurrence, but this can also result from late diagnosis because primary tumours are frequently not readily visible. Thus, the progression-free survival (PFS) is quite low, with poorer outcomes than stage-matched cutaneous melanomas [12].
At the metastatic stage, as in cutaneous melanoma, no conventional chemotherapy has shown a clear benefit, with a 5-year survival rate lower than 20% [11]. Moreover, the low rates of BRAF and c-KIT mutations in mucosal melanoma make the use of targeted therapies rarely suitable [11, 12]. Thus, immunotherapy is apparently the optimal therapeutic option. For rare melanoma sub-groups, such as mucosal melanomas, only few published data are available. These reports that have not taken into account the primary tumour location suggest a lower response rate of mucosal melanoma than of cutaneous melanoma [13–16].
The main objective of our study is to assess the efficacy of anti-CTLA4 (ipilimumab) and anti-PD1 (pembrolizumab) immunotherapies for non-resectable and/or metastatic mucosal melanoma. The secondary objectives are to identify prognostic factors for response to therapy, to evaluate the safety of this treatment, and to determine the efficacy of second-line immunotherapy after first-line treatment failure.
Materials and methods
We performed a prospective cohort analysis of patients with non-surgical locally advanced and/or metastatic mucosal melanoma who received anti-CTLA4 (ipilimumab) and/or anti-PD1 (pembrolizumab) immunotherapy at our institution between October 2010 and October 2016 as standard clinical practice after market authorization was obtained in France. Every histological sample was reviewed by expert pathologists to confirm this rare diagnosis.
Patients who received ≥ 1 dose of immunotherapy were evaluated. Pembrolizumab was delivered at 2 mg/kg every 3 weeks, while ipilimumab treatment was administered at 3 mg/kg every 3 weeks.
PFS, overall survival (OS) and duration of response (DOR) were assessed using the Kaplan–Meier method. Objective responses to immunotherapy were determined using the response evaluation criteria in solid tumours (RECIST) version 1.1. Indeed, when we started this study in 2010, immune-related response criteria (irRC) were not validated for mucosal melanoma (only for cutaneous melanoma). We, then, considered the use of immune RECIST (iRECIST) whose main advantage is the evaluation of pseudo-progressors. However, in our cohort, pseudo-progression was not a major issue. Thus, we decided to use only RECIST 1.1.
As previously described [17], tumour size (D) was defined as the sum of the longest diameters of the target lesions, as per the RECIST 1.1 criteria. Let t be the time expressed in months at the tumour evaluation. Assuming the tumour growth follows an exponential law, Vt, the tumour volume at time t, is equal to Vt = V0 exp(TG·t), where V0 is the volume at baseline, and TG represented tumour growth. We approximated the tumour volume (V) by V = 4 π R3/3, where R, the radius of the sphere, is equal to D/2. Consecutively, TG is equal to TG = 3 Log(Dt/D0)/t. Tumour growth rate (TGR) is expressed as a percentage increase in tumour volume over 1 month using the following transformation: TGR = 100 [exp(TG) − 1], where exp(TG) represents the exponential of TG. Pseudo-progressors were defined as patients who experienced an initial increase in the size of tumour lesions (according to the RECIST 1.1 criteria) followed by delayed clinical responses. Every CT-scan was reviewed retrospectively by the same radiologist so as to have a homogeneous analysis.
Oncological outcomes were assessed every 12 weeks for the sub-groups of patients treated with ipilimumab and pembrolizumab. We defined the objective response rate (ORR) as complete response (CR) plus partial response (PR), the disease control rate (DCR) as CR plus PR plus stable disease (SD), the DOR as the time from objective response to the first progression, PFS as the time from the initiation of treatment to the first progression or death, and OS as the time from the initiation of treatment to death from any cause.
Results
Patient and tumour characteristics (Table 1)
Table 1.
Patient and tumour characteristics
| Total | |
|---|---|
| Sex ratio (M/F) | 0.57 |
| Age | 63 [24; 88] |
| Primary tumour location | |
| Head and Neck (with sinonasal 15/18) | 18 (40.9%) |
| Vulvo-vaginal | 12 (27.3%) |
| Ano-rectal | 14 (31.8%) |
| ECOG before treatment | |
| 0 | 28 (63.6%) |
| 1 | 16 (36.3%) |
| Metastasis at diagnosis | 11 (25%) |
| Relapse after first treatment | 30 (68%) |
| Local | 11 (25%) |
| Metastatic | 19 (43%) |
| Previous surgery | 28 (64%) |
| Previous radiotherapy | 27 (61%) |
| Previous chemotherapy | 17 (39%) |
| Tumoural stage | |
| T1/T2 | 3 (7%) |
| T3/T4 | 27 (61%) |
| Tx | 14 (32%) |
| Nodal stage | |
| N0 | 25 (57%) |
| N + | 11 (25%) |
| Nx | 8 (18%) |
| Metastases | |
| M0 | 33 (75%) |
| M1 | 11 (25%) |
| UICC stage | |
| ½ | 25 (57%) |
| ¾ | 19 (43%) |
| Metastases | |
| Liver | 19 (43%) |
| Brain | 4 (9%) |
| Lung | 21 (48%) |
| Mutation | |
| KIT | 2 (5%) |
| NRAS | 3 (7%) |
Forty-four patients were enrolled. The median age was 63 years (range 24–88) with a sex ratio of 0.57. The primary tumour location was the head and neck in 18 patients (40.9%), ano-rectal region in 14 (31.8%), and vulvo-vaginal area in 12 (27.3%).
At inclusion, 11 (25%) patients had unresectable stage 3 disease, and 11 (25%) presented with distant metastases. Twenty-eight patients (64%) had previous surgery, 27 (61%) had previous radiotherapy, and 17 (39%) had received prior systemic therapy.
No BRAF mutations were found. We observed 2 c-KIT (N822Y in a rectal tumour and V560D in a vulvar tumour) and 3 NRAS (G12C in a nasal fossa tumour, G13V in a hard palate tumour, and K61R in a rectal tumour) mutated mucosal melanomas.
First-line immunotherapy was ipilimumab in 24 (55%) patients and pembrolizumab in 20 (45%) (Table 2). Eighteen patients who progressed after the first-line of immunotherapy received second-line immunotherapy, as described in Table 3.
Table 2.
First-line immunotherapy according to primary tumour location
| First-line pembrolizumab | First-line ipilimumab | |
|---|---|---|
| Head and neck (n = 18) | 9/18 (50%) | 9/18 (50%) |
| Vulvo-vaginal (n = 12) | 6/12 (50%) | 6/12 (50%) |
| Ano-rectal (n = 14) | 5/14 (28.5%) | 9/14 (71.5%) |
Table 3.
Repartition of the second line of immunotherapy among patients after the first-line failure (N = 18)
| First-line | Total | |||||
|---|---|---|---|---|---|---|
| Ipilimumab | Pembrolizumab | |||||
| N | % | N | % | N | % | |
| Second-line | ||||||
| Nivolumab | 3 | 17 | 1 | 6 | 4 | 22 |
| Pembrolizumab | 8 | 44 | 1 | 6 | 9 | 50 |
| Ipilimumab | 1 | 6 | 4 | 22 | 5 | 28 |
Response assessment
The ORR was 20% (95% confidence interval (CI) 8.1–31.8) overall, 8% (95% CI 2.8–18.8) for ipilimumab (one CR) and 35% (95% CI 14.0–55.9) for pembrolizumab (4 CRs) (Table 4). No significant difference was observed considering the primary tumour location (head and neck vs vulvo-vaginal vs ano-rectal).
Table 4.
Response to the first-line immunotherapy
| CR | PR | SD | PD | |
|---|---|---|---|---|
| Ipilimumab (n = 24) | 1 (4.1%) | 1 (4.1%) | 2 (8.2%) | 20 (83.3%) |
| Pembrolizumab (n = 20) | 4 (20%) | 3 (15%) | 2 (10%) | 11 (55%) |
CR complete response, PR partial response, SD stable disease, PD progressive disease
The DCR was 30% (95% CI 16.4–43.5) overall with 17% (95% CI 2.0–32.0) for the first-line ipilimumab and 45% (95% CI 23.1–66.8) for the first-line pembrolizumab.
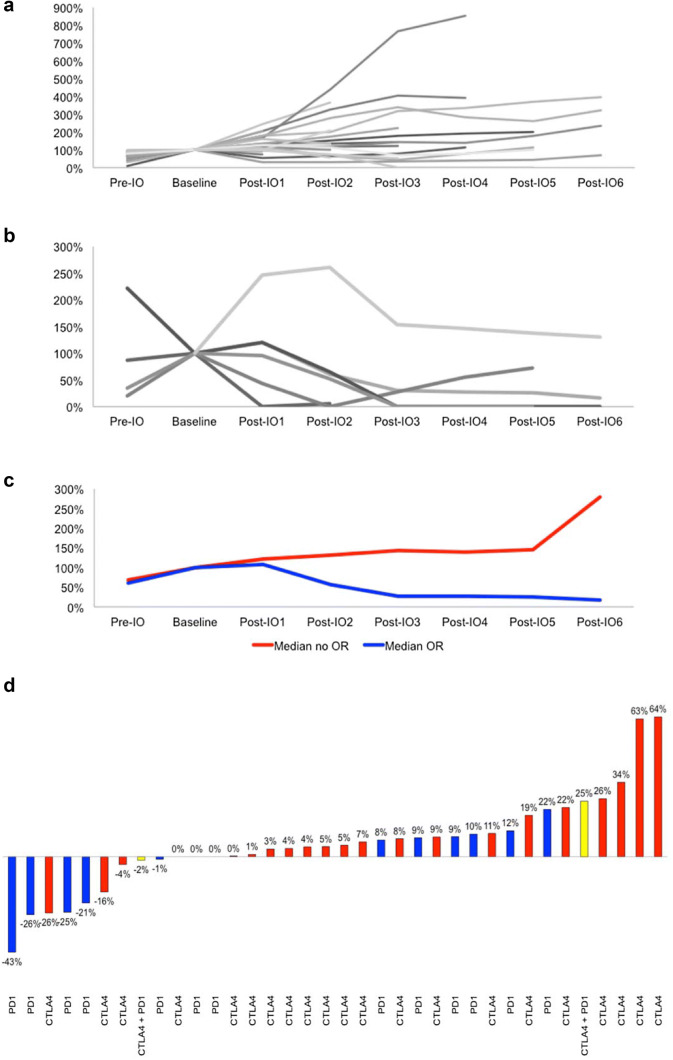
Considering the first-line immunotherapy, we observed three cases (7%) of pseudo-progression followed by a delayed clinical response from 3 to 6 months after the start of immunotherapy (Fig. 1).
Fig. 1.
Tumour growth rate variations before and during first-line immunotherapy in patients without (a) or with (b) objective radiological response (baseline is 100%). Median TGR variations are shown in c. Waterfall plot showing, for each patient, the objective response to anti-CTLA4 or anti-PD1 immunotherapy according to RECIST 1.1 in d. IO immunotherapy
Among patients with SD (four treated with ipilimumab and nine with pembrolizumab), three patients with late progression were observed: one at 51 months after pembrolizumab treatment and two at 49 and 52 months after ipilimumab treatment. Of the nine patients with complete or PR (seven with pembrolizumab and two with ipilimumab), no secondary progression was noted during the follow-up period.
In four patients, ipilimumab was used after pembrolizumab failure. No objective response was observed [one SD and three progressive diseases (PDs)]. Conversely, pembrolizumab was delivered in eight patients with ipilimumab failure with an ORR of 13% (one PR, three SDs and four PDs).
Oncological outcomes (Table 5)
Table 5.
Comparing OS and PFS at 1 and 2 years in ipilimumab and pembrolizumab sub-groups
| 1-year OS | 2-year OS | 6-month PFS | 1-year PFS | |
|---|---|---|---|---|
| Ipilimumab | 54% (CI 95% 32.2–71.2) | 34% (CI 95% 16.0–53.9) | 8% (CI 95% 1.4–23.3) | 8% (CI 95% 1.4–23.3) |
| Pembrolizumab | 57% (CI 95% 31.5–75.6) | 44% (CI 95% 20.8–65.2) | 43% (CI 95% 20.9–63.1) | 38% (CI 95% 16.9–58.1) |
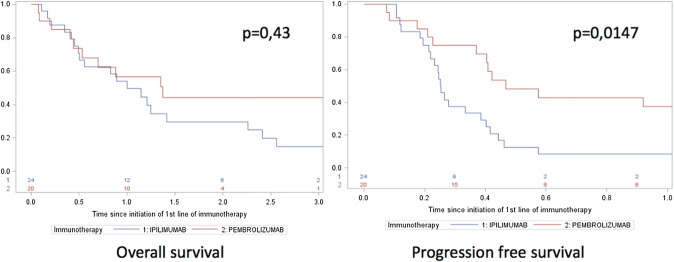
The median follow-up was 24 months (range 4–73). The median PFS in the first-line ipilimumab group and in the first-line pembrolizumab group was 3 months (95% CI 2.5–4.6) and 5 months (95% CI 2.6–33.1), respectively (p = 0.0147).
The median OS in the first-line ipilimumab group and in the first-line pembrolizumab group was 12 months (95% CI 5.9–26.9) and 16.2 months (95% CI 5.3–42.6), respectively (p = 0.43) (Fig. 2).
Fig. 2.
Oncologic outcomes (OS and PFS) in patients treated with ipilimumab or pembrolizumab
When pembrolizumab was used after ipilimumab failure, a median PFS of 8 months was noted.
Toxicity evaluation
There were no grade IV adverse events (AEs) in this cohort. Grade III AEs (namely, colitis) were reported in three (12.5%) patients treated with ipilimumab and one (5%) patient treated with pembrolizumab.
Discussion
As in cutaneous melanoma, we found that immunotherapy with immune checkpoint inhibitors had encouraging results for mucosal melanoma with comparable OR rates (approximately 10% for ipilimumab and 35% for pembrolizumab) and a similar pattern of response. Indeed, in our cohort, we observed durable anti-tumour activity as previously reported in cutaneous melanoma [5, 18]. Among the 13 patients with SD, only 3 presented with late progression (median, 51 months), while no secondary progression was noted in patients with CR or PR.
As reported previously, we confirmed that the benefit-risk ratio was superior with anti-PD1 immunotherapy than with anti-CTLA-4 immunotherapy suggesting that anti-PD1 should be used preferentially as the first-line therapy. However, we did not evaluate the response rate obtained with a combination of ipilimumab and nivolumab, which has yielded an approximately 55% ORR in patients with cutaneous melanoma [19, 20]. In our study, for anti-PD1 immunotherapy, we observed a statistically significant improvement in PFS but not in OS. We hypothesize that there may be bias considering that some patients with ipilimumab failure were treated with second-line pembrolizumab and showed some benefits, while no clinical response was noted for second-line immunotherapy after pembrolizumab failure.
Interestingly, in advanced cutaneous melanoma, ipilimumab is considered as a potential therapeutic option after anti-PD1 failure with an ORR of 15% [21] whereas, in our cohort of mucosal melanomas, no benefit was observed. Conversely, pembrolizumab allowed some clinical responses after anti-CTLA4 failure with an ORR of 13% as previously described in mucosal melanoma [15].
Response to checkpoint inhibitors has been correlated to mutational burden in several tumour types including melanoma [22]. Ultraviolet light is known to induce DNA damage resulting in high mutational burden in cutaneous melanoma with a predominant C > T nucleotide transition UV signature [23]. Recently, a particularly high ORR of 70% was reported in a cohort of 60 patients with desmoplastic melanoma, and it was suggested that this high ORR was due to the high tumour burden of this melanoma subtype [24]. Conversely, whole-genome sequencing of mucosal melanoma has shown a very low mutational burden, with 8193 somatic single-nucleotide variants (SNVs) per tumour, compared with an average of 86,495 SNVs per tumour in cutaneous melanoma [25, 26], and a totally different molecular profile unrelated to sun-exposure [27, 28] with rare BRAF and NRAS mutations whereas recurrent SF3B1 mutations and co-mutation of NF1/KIT were reported [28].
Uveal melanoma is another rare type of melanoma unrelated to UV exposure associated with a low mutation burden. Uveal melanomas have specific molecular profiles with monosomy of chromosome 3 often associated with an inactivating mutation (GNAQ or GNA11) in the tumour-suppressor gene BRCA1-associated protein 1 (BAP1) in high-risk sub-groups [29]. Recently, as in mucosal melanoma, SF3B1 mutations have been described in uveal melanoma [30]. As expected from the low mutation burden of uveal melanoma, the ORRs to immunotherapy (anti-CTLA4, anti-PD1 or anti-PDL1) are extremely poor, with less than 5% of patients responding [31, 32]. In a recent report, however, a patient with a uveal melanoma responded particularly well to anti-PD1. In this patient, a germline MBD4 mutation resulted in genetic instability and an exceptionally high mutational burden as compared to what is usually observed in this rare type of melanoma [33].
Concerning mucosal melanoma, it is this, therefore, intriguing, that we and others found a quite encouraging response rate to checkpoint inhibitors in spite of this low tumour mutation burden, and suggests that some critical tumour-associated antigens that remain to be discovered could be expressed by this rare melanoma subtype.
Previous small effective and retrospective studies have reported ORRs of mucosal melanoma of approximately 25% and 10% to anti-PD1 and ipilimumab treatment, respectively [13–16], without taking into account the primary tumour site. However, these data are of limited importance given that therapeutic strategy must be tailored to each primary location. Our study shows that anti-PD1 immunotherapy efficacy is not linked to the primary tumour site, allowing its use for all non-resectable and/or metastatic mucosal melanomas.
One limitation of our study is its retrospective design although data were collected prospectively. Considering the rarity of mucosal melanoma and the poor prognosis of this tumour, it is challenging to report on large cohorts of patients outside of prospective multicentric studies, which are difficult to organize for this type of tumour because it is managed by various medical specialists depending on its primary location. Nevertheless, our monocentric study provides a homogenous therapeutic strategy with lower selection bias and more reproducible data than previous studies. However, studies with longer follow-up are critical to confirm durable anti-tumoural responses.
Finally, the clinical benefit of anti-PD1 immunotherapy for mucosal melanoma and the high risk of local or distant relapse after surgery should lead us to consider the indications for this treatment as a neo-adjuvant and/or adjuvant therapy, as it is presently used for cutaneous melanoma.
Conclusion
Immunotherapy with immune checkpoint inhibitors has yielded encouraging results for mucosal melanoma with ORRs almost comparable or slightly less than those obtained for cutaneous melanoma and durable clinical responses despite the absence of a link to UV exposure and a significantly lower mutational burden in these tumours than in cutaneous melanoma. Anti-PD1 immunotherapy should be used preferentially due to its efficiency and lower toxicity than other therapies. The combination of ipilimumab and anti-PD1 might also be an interesting option to consider. Translational research is critical to identify prognostic factors, mechanisms of response and potential neoantigens involved in this particular sub-group of melanomas.
Abbreviations
- BRAF
B-Raf proto-oncogene, serine/threonine kinase
- BRCA1
Breast cancer 1
- cKIT
KIT proto-oncogene, receptor tyrosine kinase
- CR
Complete response
- DCR
Disease control rate
- DOR
Duration of response
- iRECIST
Immune response evaluation criteria in solid tumours
- irRC
Immune-related response criteria
- MBD4
Methyl-CpG-binding domain 4
- NF1
Neurofibromin 1
- NRAS
NRAS proto-oncogene, GTPase
- ORR
Objective response rate
- PD
Progressive disease
- SD
Stable disease
- SF3B1
Splicing factor 3b subunit 1
- SNV
Somatic nucleotide variants
- TG
Tumour growth
- TGR
Tumor growth rate
- V0
Tumour volume at baseline
- Vt
Tumour volume at time t
Author contributions
AM-P, FJ, AMME, LD and CR conceptualized the manuscript, harmonized and edited the text and references and produced the figures. CR and IG performed statistical analysis. RGHG, SA, SR, J-YS and SV were responsible for data collection and analysis. All the authors contributed to the writing and editing of the manuscript. All the authors approved the final version.
Funding
No relevant funding.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The translational research study NCT02105168 was approved by the Institutional Review Board at Gustave Roussy Cancer Campus on April 7, 2014. All the methods and procedures associated with this study were conducted in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
All the patients have given their written authorization to perform research on their tumour samples by signing the Institutional Tumor Bank form (before April 2014) or the form of the translational research study NCT02105168. All the patients provided written informed consent to the use of their anonymized data in scientific studies.
Footnotes
Moya-Plana A, Herrera Gómez RG, Rossoni C, Dercle L, Ammari S, Girault I et al. “Response assessment to anti-CTLA4 or/and anti-PD1 immunotherapy in mucosal melanomas”. American Society of Clinical Oncology (ASCO) Annual Meeting, June 2018 Chicago, USA. [Abstract #221719].
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 5.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 6.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin CC, Wu XC, Jemal A, Martin HJ, Roche LM, Chen VW. Incidence of noncutaneous melanomas in the U.S. Cancer. 2005;103:1000–1007. doi: 10.1002/cncr.20866. [DOI] [PubMed] [Google Scholar]
- 9.Patrick RJ, Fenske NA, Messina JL. Primary mucosal melanoma. J Am Acad Dermatol. 2007;56:828–834. doi: 10.1016/j.jaad.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 11.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83:1664–1678. doi: 10.1002/(SICI)1097-0142(19981015)83:8<1664::AID-CNCR23>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12.Kuk D, Shoushtari AN, Barker CA, Panageas KS, Munhoz RR, Momtaz P, et al. Prognosis of mucosal, uveal, acral, non acral cutaneous, and unknown primary melanoma from the time of first metastasis. Oncologist. 2016;21:848–854. doi: 10.1634/theoncologist.2015-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamazaki N, Takenouchi T, Fujimoto M, Ihn H, Uchi H, Inozume T, et al. Phase 1b study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced melanoma (KEYNOTE-041) Cancer Chemother Pharmacol. 2017;79:651–660. doi: 10.1007/s00280-016-3237-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Angelo SP, Larkin J, Sosman JA, Lebbé C, Brady B, Neyns B, et al. Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients With mucosal melanoma: a pooled analysis. J Clin Oncol. 2017;35:226–235. doi: 10.1200/JCO.2016.67.9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoushtari AN, Munhoz RR, Kuk D, Ott PA, Johnson DB, Tsai KK, et al. The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer. 2016;122:3354–3362. doi: 10.1002/cncr.30259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Vecchio M, Di Guardo L, Ascierto PA, Grimaldi AM, Sileni VC, Pigozzo J, et al. Efficacy and safety of ipilimumab 3 mg/kg in patients with pretreated, metastatic, mucosal melanoma. Eur J Cancer. 2014;50:121–127. doi: 10.1016/j.ejca.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23:1920–1928. doi: 10.1158/1078-0432.CCR-16-1741. [DOI] [PubMed] [Google Scholar]
- 18.Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol. 2018;36:1668–1674. doi: 10.1200/JCO.2017.75.6270. [DOI] [PubMed] [Google Scholar]
- 19.Wolchock JD, Chiaron-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558–1568. doi: 10.1016/S1470-2045(16)30366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmer L, Apuri S, Eroglu Z, Kottschade LA, Forschner A, Gutzmer R. Ipilimumab alone or in combination with nivolumab after progression on anti-PD1 therapy in advanced melanoma. Eur J Cancer. 2017;75:47–55. doi: 10.1016/j.ejca.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eroglu Z, Zaretsky JM, Hu-Lieskovan S, Kim DW, Algazi A, Johnson DB, et al. High response rate to PD-1 blockade in desmoplastic melanomas. Nature. 2018;553:347–350. doi: 10.1038/nature25187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furney SJ, Turajlic S, Stamp G, Nohadani M, Carlisle A, Thomas JM, et al. Genome sequencing of mucosal melanomas reveals that they are driven by distinct mechanisms from cutaneous melanoma. J Pathol. 2013;230:261–269. doi: 10.1002/path.4204. [DOI] [PubMed] [Google Scholar]
- 26.Furney SJ, Turajlic S, Stamp G, Thomas JM, Hayes A, Strauss D, et al. The mutational burden of acral melanoma revealed by whole-genome sequencing and comparative analysis. Pigment Cell Melanoma Res. 2014;27:835–838. doi: 10.1111/pcmr.12279. [DOI] [PubMed] [Google Scholar]
- 27.Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545:175–180. doi: 10.1038/nature22071. [DOI] [PubMed] [Google Scholar]
- 28.Hintzsche JD, Gorden NT, Amato CM, Kim J, Wuensch KE, Robinson SE, et al. Whole-exome sequencing identifies recurrent SF3B1 R625 mutation and comutation of NF1 and KIT in mucosal melanoma. Melanoma Res. 2017;27:189–199. doi: 10.1097/CMR.0000000000000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Engen-van Grunsven AC, Baar MP, Pfundt R, Rijntjes J, Küsters-Vandevelde HV, Delbecq AL, et al. Whole-genome copy-number analysis identifies new leads for chromosomal aberrations involved in the oncogenesis and metastastic behavior of uveal melanomas. Melanoma Res. 2015;25:200–209. doi: 10.1097/CMR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 30.Furney SJ, Pedersen M, Gentien D, Dumont AG, Rapinat A, Desjardins L, et al. SF3B1 mutations are associated with alternative splicing in uveal melanoma. Cancer Discov. 2013;2013(3):1122–1129. doi: 10.1158/2159-8290.CD-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heppt MV, Heinzerling L, Kähler KC, Forschner A, Kirchberger MC, Loquai C, et al. Prognostic factors and outcomes in metastatic uveal melanoma treated with programmed cell death-1 or combined PD-1/cytotoxic T-lymphocyte antigen-4 inhibition. Eur J Cancer. 2017;82:56–65. doi: 10.1016/j.ejca.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 32.Algazi AP, Tsai KK, Shoushtari AN, Munhoz RR, Eroglu Z, Piulats JM, et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer. 2016;122:3344–3353. doi: 10.1002/cncr.30258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodrigues M, Mobuchon L, Houy A, Fiévet A, Gardrat S, Barnhill RL, et al. Outlier response to anti-pd1 in uveal melanoma reveals germline MBD4 mutations in hypermutated tumors. Nat Commun. 2018;9(1):1866. doi: 10.1038/s41467-018-04322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]