Abstract
Cancer immunotherapies have significantly improved the prognosis of cancer patients. Despite the clinical success of targeting inhibitory checkpoint receptors, including PD-1 and/or CTLA-4 on T cells, only a minority of patients derive benefit from these therapies. New strategies to improve cancer immunotherapy are therefore needed. Combination therapy of checkpoint inhibitors with targeted agents has promisingly shown to increase the efficacy of immunotherapy. Here, we analyzed the immunomodulatory effects of the multi-receptor tyrosine kinase inhibitor axitinib and its efficacy in combination with immunotherapies. In different syngeneic murine tumor models, axitinib showed therapeutic efficacy that was not only mediated by VEGF–VEGFR inhibition, but also through the induction of anti-cancer immunity. Mechanistically, a significant reduction of immune-suppressive cells, including a decrease of tumor-promoting mast cells and tumor-associated macrophages was observed upon axitinib treatment. Inhibition of mast cells by axitinib as well as their experimental depletion led to reduced tumor growth. Of note, treatment with axitinib led to an improved T cell response, while the latter was pivotal for the therapeutic efficacy. Combination with immune checkpoint inhibitors anti-PD-1 and anti-TIM-3 and/or agonistic engagement of the activating receptor CD137 resulted in a synergistic therapeutic efficacy. This demonstrates non-redundant immune activation induced by axitinib via modulation of myeloid and mast cells. These findings provide important mechanistic insights into axitinib-mediated anti-cancer immunity and provide rationale for clinical combinations of axitinib with different immunotherapeutic modalities.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2136-x) contains supplementary material, which is available to authorized users.
Keywords: Cancer immunology, Hematopoietic stem cell, Mast cell, Tumor-associated macrophage, Tyrosine kinase inhibitor
Introduction
Cancer immunotherapies, which include antibodies that block inhibitory receptors on T cells, have permanently changed oncological practice [1–3]. Monotherapy with blocking antibodies against inhibitory receptors or ligands, including PD-1, PD-L1 and CTLA-4 have achieved durable responses in cancer patients. However, the majority of patients experiences only a transient benefit or even no objective response. Therefore, new approaches are clearly warranted to increase the percentage of long-term responders amongst patients receiving cancer immunotherapy. One possibility to further improve the clinical outcome is the combination of different checkpoint inhibitors [1, 3, 4] or combinations of checkpoint inhibitors with alternative immunostimulatory agents that target co-stimulatory receptors on T cells such as CD137 (4-1BB) or CD27 [3]. Another possibility is the combination of checkpoint blockade with immunomodulatory, targeted agents such as tyrosine kinase inhibitors (TKI) [1, 5]. The latter offer the opportunity to alter the cancer microenvironment to become less immunosuppressive or even support anti-cancer immunity.
TKIs are a class of targeted agents, which specifically interfere with the kinase activity of key signaling enzymes, e.g., involved in cancer cell growth, survival or neo-vascularization. In metastatic renal cell carcinoma (mRCC), the latter class of TKIs that target cancer neo-angiogenesis are successfully used to mitigate HIF-1α-mediated VEGF signaling [6]. Currently, pazopanib, sunitinib, cabozantinib, sorafenib and axintinib are approved TKIs for the treatment of metastatic mRCC [6]. While TKIs such as pazopanib have a rather narrow spectrum of kinase inhibition, axitinib does not only inhibit VEGF receptors but also kinases including fms-like tyrosine kinase 3 (FLT-3), PDGF receptors, and CD117 (cKIT) [7, 8]. Thus far, in cancer patients with metastatic RCC the clinical development of combination therapies composed of antibodies inhibiting PD-1 and (neo)-vascular targeting TKIs has been hampered by increased toxicity in early clinical trials. For instance, the combination of pazopanib with nivolumab or pembrolizumab had to be discontinued due to severe hepatotoxicity [9]. Noteworthy, avelumab concomitantly administered with axitinib appears more tolerable, which makes the latter an interesting TKI for combination strategies [10]. Equally important, immunostimulatory properties have been attributed to axitinib [11–13], while normalization of cancer blood vessels by VEGF-targeting seems to enhance recruitment of intratumoral T cells when combined with atezolizumab [14]. The detailed mechanisms, however, and the potential for synergism with other types of immunotherapies, including alternative immune checkpoint inhibitors or the stimulation of T cell co-receptors, such as CD137, have not been extensively explored to date.
Here, we analyzed the effects of axitinib on different cellular immune compartments in syngeneic, murine tumor models. Previously, effects on myeloid cells have been observed in mice treated with axitinib. Furthermore several of the receptors targeted by axitinib are expressed on myeloid cell, mast cells and their progenitors [11, 15]. Myeloid and mast cells have been described to exert profound immune suppression in the tumor microenvironment and are an interesting target for cancer immunotherapy [15, 16]. We therefore dissected the effects of axitinib on the myeloid cell compartment, mast cells and its crosstalk with T cells. Further building on these findings, combination regimens of checkpoint inhibitors and/or CD137 agonists with axitinib were explored.
Materials and methods
Mice
Mice were bought from Charles Rivers or bred in the animal facility of the Department of Biomedicine, University of Basel, Switzerland. For all experiments C57Bl/6 were used. Perpendicular tumor diameters were measured with calipers, and tumor volume was calculated according to the following formula: tumor volume (mm3) = d2 × D/2, where D and d are the longest and shortest diameters of the tumor (in millimeters), respectively. Mas-TRECK mice for conditional deletion of mast cells in mice were kindly provided by Masato Kubo and described previously [17].
Cells and culture
The C57Bl/6 murine tumor cell line MC38 were provided by Mark Smyth (Peter MacCallum Cancer Centre, Melbourne, AU). 3LL-Thy1.1-OVA cells from C57Bl/6 mice (referred to as LLC1) were provided by Douglas T. Fearon (Cancer Research UK Centre, Cambridge, UK). Both cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with Glutamax (Gibco) supplemented with 10% heat-inactivated FBS, sodium pyruvate, penicillin/streptomycin, l-glutamine mix, MEM, nonessential amino acids, ciproxin and 0.05 mM 2-mercaptoethanol. MC38 tumor cells secrete VEGF-A, but are not known to express VEGF receptors [18]. LLC1 tumor cells are also not known to express VEGF receptors [19]. Murine bone marrow mast cells were generated as follows. Bone marrow cells were harvested from femurs of mice and cultured in RPMI 1640 supplemented with 10% FBS, 2 mM l-glutamine, 1 mM sodium pyruvate, 1 mM HEPES, 50 µM 2-ME, 100 U/ml penicillin, and 100 µg/ml streptomycin. The cells were cultured in the presence of IL-3 and stem cell factor (SCF, 10 ng/ml each, PeproTech), and the non-adherent cells were passaged every 3 days. 4 weeks later, the purity of mast cells was assessed by FACS staining (CD45+ CD117+ ST1/2+ and FCεR1α+). Only those preparations containing > 95% mast cells were used in our studies and referred to as bone marrow-derived mast cells (BMMCs). For the adoptive transfer of BMMCs, 2 doses of 3 × 106 cells were injected intravenously once after 14 and 18 days post tumor cell injection.
In vivo tumor models and therapeutic treatments
For the transplantable MC38 or LLC1 tumor models, 500,000 or 250,000 tumor cells were, respectively, injected subcutaneously in C57Bl/6 mice. Mice were treated daily with axitinib, administered i.p., for 7 days at the indicated dose levels. Treatment was initiated after 12 (LLC1) and 16 (MC-38) days of tumor cell injection either alone or in combination with different blocking and stimulating antibodies (see below). For the majority of the experiments, axitinib was used at 25 mg/kg/day. Blocking of VEGF-receptor 2 (VEGR-2) with the DC101 clone (Bio X Cell) was performed with 3 doses at 40 mg/kg given i.p on day 16/19/22 post tumor cell injection or for the duration of the entire experiment twice per week (see Fig. 1). VEGF was neutralized with an anti-VEGF antibody (kindly provided by Roche) given i.p. twice per week at 5 mg/kg per dose for the duration of the experiment. Similarly, CD117 was targeted with the ACK2 antibody (Bio X Cell) given i.p on day 16/19/22 at 5 mg/kg per dose. CC-chemokine ligand 2 (CCL2) was neutralized with 10 mg/kg anti-CCL2 antibody (Bio X Cell) i.p. on day 16/18/20/22. Treatment with PD-1and/or TIM-3 (10 mg/kg) blocking antibodies or CD137 stimulating antibodies (5 mg/kg) (all from Bio X Cell) was started after 14 (LLC1) or 18 (MC-38) days after tumor cell injection. Anti-PD-1 and anti-Tim-3 were given on day 14/16/18/21/24/27/30/35/40 (LLC1) or 18/20/22/25/28/31/35/40 (MC-38). Anti-CD137 was given on day 14/16/18/21 (LLC1) or 18/20/22/25 (MC-38). Mast cells were depleted in Mas-TRECK mice by application of 12.5 ng/g diphtheria toxin (DT) at day 15 and 16 (Fig. 2 G/H) or as indicated on two consecutive days every 2 weeks (Fig. 2c–f) post-tumor cell injection. For T cell depletion, anti-CD4 (GK1.5; rat IgG2b, Bio X Cell) and anti-CD8 (53-6.72; rat IgG2a, Bio X Cell) depleting antibodies (5 mg/kg) were given at day 15/16/20 post tumor cell injection (for MC-38) followed by once per week until the end of the experiment. Mice were euthanized when tumors reached a volume of 1500 mm3. All surviving mice reflecting the tail of the curve remained tumor-free until completion of the experiments and euthanization.
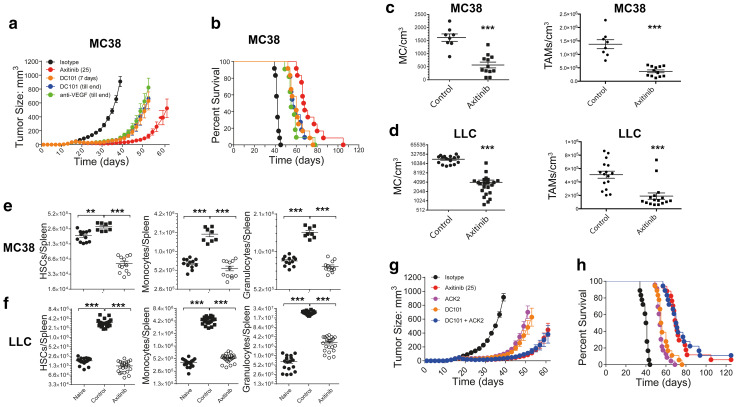
Fig. 1.
Axitinib inhibits cancer growth not only by targeting angiogenesis, but also by modulating myeloid cells. C57BL/6 mice bearing established MC38 tumors were treated with the indicated agents. Cumulative cancer growth (a) and the corresponding animal survival (b) are depicted as pooled data from two independent experiments (n = 11–12). Tumor resident mast cell densities and TAMs within the tumor-infiltrating immune cells are depicted for the MC38 (c) and the LLC1 (d) tumor models. Data are depicted as pooled data from four independent experiments (n = 8–12) for the MC38 tumor model (c) and as pooled data from two independent experiments (n = 17–22) for the LLC1 tumor model (d). MC38 (e) or LLC1 (f) tumor-bearing animals were treated with axitinib or carrier for 7 consecutive days. Naïve (untreated) animals were used as additional controls. Upon completion of the treatment animals were euthanized and spleen cell populations (HSCs, monocytes and granulocytes) analyzed using flow cytometry. Cell numbers/spleen are depicted as pooled data from four independent experiments (n = 17–22) for the MC38 cancer model (e) and as pooled data from two independent experiments (n = 8–12) for the LLC1 cancer model (f). C57BL/6 mice bearing established MC38 tumors were treated with the indicated agents. Cumulative tumor growth (g) and the corresponding animal survival (h) are depicted as pooled data from three independent experiments (n = 17–18). **p < 0.01, ***p < 0.001 determined by Student’s t test. Data are presented as mean ± SD
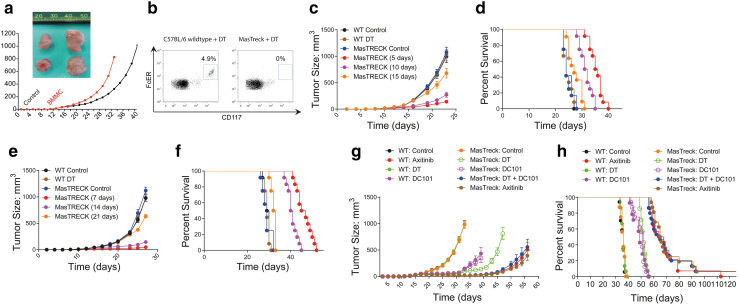
Fig. 2.
Axitinib inhibits tumor growth by targeting mast cells. C57BL/6 mice bearing established MC38 tumors were adoptively transferred with bone marrow-derived mast cells or left untreated. Cumulative tumor growth (a) and representative, isolated tumors from the respective treatment groups are depicted. b MasTreck mice and wild-type mice lacking mast cell selective expression of the diphtheria toxin receptor were treated with diphtheria toxin to deplete mast cells. Mast cell depletion was verified by flow cytometric analysis of the peritoneum. The effect of mast cell depletion (by diphtheria toxin) on tumor growth in MasTreck mice was analyzed in the LLC1 (c, d) as well as the MC38 (e, f) tumor models. Cumulative tumor growth and the corresponding animal survival are depicted as pooled data from three independent experiments (n = 14–16, a, b). Cumulative tumor growth (c, d) and the corresponding animal survival (d, f) are depicted as pooled data from two independent experiments (n = 11–12). g, h C57BL/6 wild type (WT) or MasTreck mice, bearing established MC38 tumors, were treated with diphtheria toxin (DT) and/or the indicated agents (n = 10–12). Data are presented as mean ± SD
Flow cytometric analysis of tumor cell infiltration
On day 8 after treatment initiation, mice were euthanized, and tumors were mechanically dissociated and digested with accutase (PAA), collagenase IV (Worthington), hyaluronidase (Sigma), and deoxyribonuclease type IV (Sigma). Single-cell suspensions were prepared and stained against the indicated markers for flow cytometric analysis. Dead cell exclusion was done with the live/dead fixable near-infrared dye (Invitrogen). For T cell staining (tumor) anti-CD45, anti-CD3, anti-CD8, anti-CD11b, anti-CD11c, anti-PD-1, anti-Ter119 and anti-TIM-3 antibodies were used. Splenic monocytes were determined with anti-CD45, anti-CD11b, anti-Ly-6C antibodies. For splenic granulocytes, anti-CD45, anti-CD11b and anti-Ly-6G antibodies were used. For the determination of tumor-associated macrophages (TAMs) anti-CD45, anti-CD11b, anti-F4/80 antibodies were used. Mast cells (tumor) were identified by staining with anti-CD45, anti-CD117, ST1/2 and anti-FcεR1α antibodies. Splenic hematopoietic stem cells (HSCs) were detected by staining with a lineage antibody cocktail (anti-B220 anti-CD19 anti-CD4 anti-CD8 anti-NK1.1 anti-CD90.2 anti-Ter119 anti-Ly-6G anti-CD127) and anti-CD117. HSCs were quantified by gating for Lin−, CD117+ cells. All FACS antibodies were purchased from Biolegend or BD Biosciences.
Quantitative PCR analysis
RNA was isolated from subcutaneous tumors and quantitative PCR performed with the following primers for indicated murine genes: Vegfa, AAAAACGAAAGCGCAAGAAA, TTTCTCCGCTCTGAACAAGG. Flt1 (VEGR-1), GGCCCGGGATATTTATAAGAAC, CCATCCATTTTAGGGGAAGTC. Kdr (VEGR-2), CAGTGGTACTGGCAGCTAGAAG, ACAAGCATACGGGCTTGTTT. Flt4 (VEGR-3), GAATGAGAGCCCCGGAAC, GGTCTCCAGACCAGCAACTC. MHC class I H2-K, ATACCTGAAGAAGGGAACG, TGATGTCAGCAGGGTAGAAGC.
Statistical analysis
Quantitative data were presented as mean plus or minus the standard deviation or standard error of the mean of three separate assays. Student’s t test was used to compare the mean values within the groups. p values less than 0.05 were considered statistically significant. Statistical analysis was performed with the GraphPad Prism Version 7.0.
Results
Axitinib inhibits tumor growth by modulation of splenic hematopoietic stem cells and myeloid cells in the tumor microenvironment
We observed a dose-dependent anti-tumor efficacy of axitinib in subcutaneous LLC1 tumors, comparing 10 and 25 mg/kg/day for 7 days. Both dosing regimens were well tolerated without any side effects (Supplementary Fig. 1a, b). A similar, pronounced tumor efficacy with axitinib at 25 mg/kg/day for 7 days was seen in MC38 tumors (Fig. 1a, b). To explore if axitinib has efficacy beyond inhibition of the VEGF–VEGFR axis, we compared animals treated either with axitinib or with the VEGFR-2 blocking antibody DC101 (short-term or continuously) and anti-VEGF neutralizing antibody. Both VEGF-directed antibodies led to a significant reduction in tumor growth (Fig. 1a, b), yet were significantly less efficacious compared to axitinib treatment. While axitinib increased VEGF-A mRNA in the tumor, no major effect was seen on the expression of VEGF receptors (Supplementary Fig. 1c).
We hypothesized that the additional effect of axitinib on tumor growth is due to the modulation of the immune system and induction of protective anti-tumor immunity. A previous report has demonstrated a reduction of immune-inhibiting innate immune cells—in particular immune-inhibitory myeloid cells—in the tumor microenvironment upon axitinib treatment [11]. This prompted us to study innate immune cells in the tumor microenvironment. Analysis of intratumoral innate immune cells showed a significant reduction mainly of tumor-promoting differentiated mast cells (CD45+, C117+, FcεR1α+) and TAMs (CD45+, CD11b+, F4/80+) in both subcutaneous MC38 (Fig. 1c) and LLC1 tumors (Fig. 1d). Intratumoral T cells and CD8 T cells were not significantly different (Supplementary Fig. 2). Axitinib decreased the frequency of regulatory T cells (Supplementary Fig. 2). As TAMs are dependent on splenic hematopoietic stem cells (HSCs) and also mast cells can derive from such precursors [20, 21], we analyzed the number of splenic HSCs and splenic myeloid cells of tumor-bearing mice after axitinib treatment (Fig. 1e, f). HSCs determined as CD45+ Lin−, CD117+, Sca-1+ cells were increased in spleens of MC38 tumor-bearing mice, the frequency of which was significantly reduced upon axitinib treatment (Fig. 1e, left panel). HSCs bearing CD117 have been demonstrated to be precursors of monocytes, which upon migration to the tumor differentiate into tumor-associated macrophages and other myeloid cells [20, 21]. Similarly, monocytes (CD45+, CD11b+, Ly-6G−, Ly6C+) and granulocytes (CD45+, CD11b+, Ly-6G+, Ly6C−) were decreased upon axitinib treatment within in the spleen of MC38 tumor-bearing animals (Fig. 1e, middle and right panel). Monocytes and granulocytes were also significantly reduced by axitinib treatment in the spleen in the LLC1 tumor model (Fig. 1f). Blocking of the recruitment of myeloid cells to the tumor microenvironment by CCL2 neutralization together with VEGF targeted therapy led to a comparable growth inhibition and survival prolongation as seen by axitinib alone, which shows that axitinib is effective as inhibitor of myeloid cell differentiation as well as migration and VEGF induced neoangiogenesis (Supplementary Fig. 3).
Since CD117 is expressed on HSCs and mast cells [11], we further explored the contribution of CD117 inhibition to the therapeutic efficacy of axitinib by using a blocking anti-CD117 antibody (clone ACK2) together with the VEGR-2 blocking antibody (clone DC101) (Fig. 1g, h). The combination of both antibodies was synergistic and equally effective in delaying the tumor growth by a similar rate as seen with axitinib alone (Fig. 1g). Accordingly, the survival was prolonged to a similar extent in mice treated with axitinib or ACK2 and DC101 (Fig. 1h), whereas neither of the individual treatments (ACK2 or DC101 alone) was able to complement for the therapeutic efficacy of axitinib on its own. This finding suggests that axitinib exerts its anti-cancer activity mainly by modulating the myeloid compartment, both in the periphery as well as within the tumor microenvironment.
Mast cells support cancer growth
Having documented a significant decrease in mast cell density upon treatment with axitinib, we wanted to further investigate the specific role of mast cells in cancer progression and axitinib-mediated tumor rejection. Mast cells have been implicated in shaping the immunosuppressive cancer microenvironment by supporting regulatory T cells and recruiting immature and therefore immunosuppressive myeloid cells [22]. Adoptive transfer of BMMCs led to an accelerated growth of subcutaneous MC38 tumors (Fig. 2a). To further study the role of mast cells, we used Mas-TRECK mice which allow the selective depletion of mast cells upon treatment with diphtheria toxin (DT) [17]. As previously demonstrated [17], administration of DT led to the rapid depletion of mast cells in Mas-TRECK mice but not in WT control mice (Fig. 2b). Next, we studied the impact of mast cells depletion on the growth of tumors and survival of tumor-bearing mice. To this end, we measured the growth of subcutaneously injected LLC1 and MC38 tumor cells in wild type (WT) control mice and Mas-TRECK mice treated with DT. Depletion of mast cells in Mas-TRECK mice led to an inhibition of tumor growth (Fig. 2c, e) as well as prolonged survival (Fig. 2d, f). Initiation of mast cell depletion within the early phase of tumor growth was more efficacious than depletion at later time points (Fig. 2c, e). To further understand the contribution of mast cells to the anti-tumor efficacy of axitinib, we assessed tumor growth and survival of MC38 bearing Mas-TRECK mice treated with DT and/or the anti-VEGFR2 antibody DC101 and compared it to axitinib treatment. We observed a similar delay in tumor growth and prolongation of survival in both conditions (Fig. 2g, h).
Axitinib-mediated cancer growth inhibition is dependent on T cells
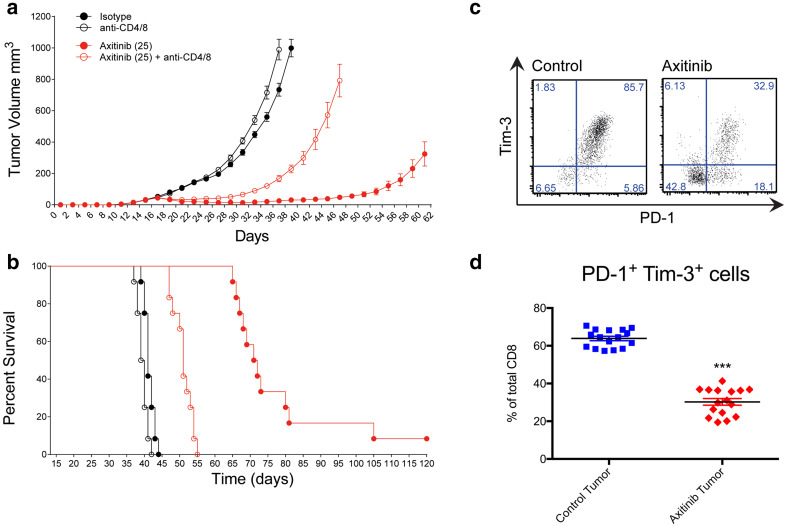
It has been suggested that myeloid cells and in particular mast cells impair T-cell-mediated immunity against cancer [15, 23]. We next assessed the requirement for T cells in the effectiveness of axitinib treatment against established MC38 tumors. To this end, we depleted both CD4+ and CD8+ T cells in mice bearing subcutaneous MC38 tumors. Depletion of T cells immediately prior to axitinib treatment severely abrogated the anti-tumor effect of the drug with significant loss of tumor growth suppression and survival benefit (Fig. 3a, b). Analysis of tumor-infiltrating lymphocytes (TILs) for the presence of exhaustion markers including PD-1 and TIM-3 revealed a significant decrease of PD-1 and TIM-3 double-positive tumor-infiltrating CD8+ T cells (Fig. 3c, d). This finding indicates local immunosuppression within the tumor, presumably mediated by myeloid cells and a reduction of T cell dysfunction. A similar observation has been previously made in humans [24].
Fig. 3.
Axitinib augments anti-cancer effects via adaptive T cell responses. a, b C57BL/6 mice bearing established MC38 tumors were T cell depleted using CD4 and CD8 specific antibodies or were treated with the corresponding isotype control antibodies. Two groups were further treated with axitinib or carrier for 7 consecutive days. Cumulative tumor growth (a) and the corresponding animal survival (b) are depicted as pooled data from two independent experiments (n = 12). CD8+ T cells from control and axitinib treated animals in tumors were stained for PD-1 as well as Tim-3 and analyzed by flow cytometry. A representative staining (c) and pooled data from three independent experiments (d, n = 16) are depicted. ***p < 0.001 determined by Student’s t test. Data are presented as mean ± SD
Combination of axitinib with checkpoint blockade and/or CD137 co-stimulation enhances anti-cancer immunity
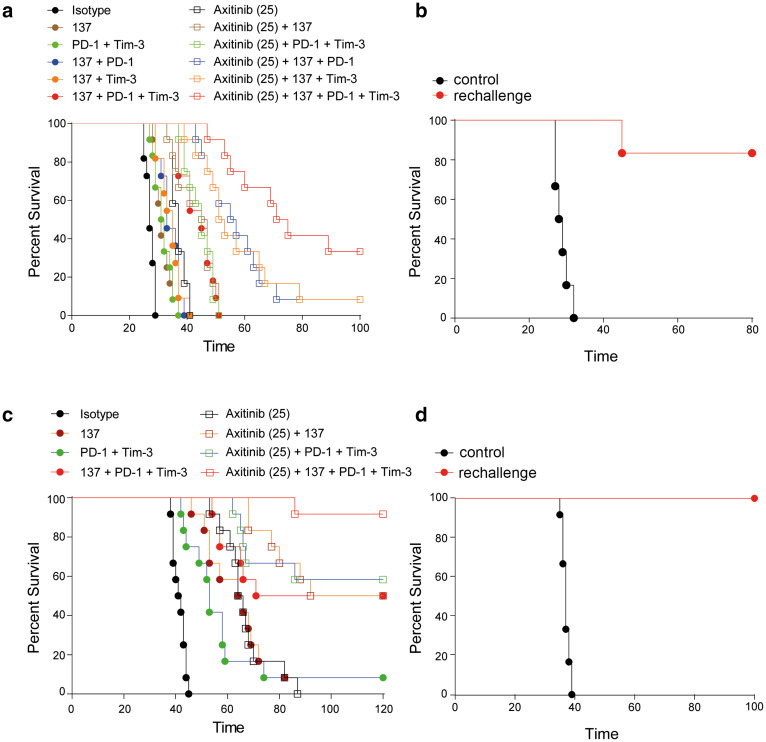
Our data support the pivotal contribution of T cells in outcomes of axitinib treatment. Thus, we asked whether combinations with T cell targeting antibodies can further improve the efficacy of axitinib. Recent analysis of T cell dysfunction in mRCC patients showed that co-expression of inhibitory checkpoint receptors PD-1 and TIM-3 on CD8+ T cells are associated with poor prognosis and these receptors are potential targets for combination therapy [25]. In addition, the co-immunostimulatory T cell receptor CD137 is known to be upregulated on T cells under cancer hypoxia [26]. We therefore explored the combination of axitinib treatment with anti-PD-1 and TIM-3 blocking as well as anti-CD137 stimulating antibodies. Combinations of these antibodies with axitinib significantly improved the survival of mice with established LLC1 tumors (Fig. 4a). In the combination group, tumors were rejected in 4 out of 12 animals (Fig. 4a). Animals that had rejected primary LLC1 tumors were protected in a re-challenge experiment with LLC1 cancer cells (Fig. 4b). We have observed an even better efficacy of this combination when treating established MC38 tumors where tumors were rejected in 11 out of 12 animals (Fig. 4c). All mice that had rejected primary MC38 tumors upon axitinib combination therapy also rejected MC38 tumors upon re-challenge (Fig. 4d). The different response behavior of LLC1 and MC38 tumors to immunotherapies is most likely due to differences in the immunogenicity of these two tumor cell lines. In particular, LLC1 is characterized by a very low MHC-I expression with no changes during axitinib treatment (Supplementary Fig. 4).
Fig. 4.
Axitinib synergizes with checkpoint inhibition and co-stimulatory molecules. a C57BL/6 mice bearing established LLC1 tumors were treated with the indicated agents. Animal survival is depicted as pooled data from two independent experiments (n = 11–12). b Cancer-free animals from a were re-challenged with the same tumor cells, injected into the contralateral flank. Naïve mice (n = 6) were used as control. c C57BL/6 mice bearing established MC38 tumors were treated with the indicated agents. Animal survival is depicted as pooled data from two independent experiments (n = 12). d Cancer-free animals from c were re-challenged with the same tumor cells into the contralateral flank. Naïve mice (n = 6) were used as control. Data are presented as mean ± SD
Discussion
The combination of antibody-based immunotherapies with targeted anti-cancer therapies, such as TKIs, can improve response rates as demonstrated in early clinical trials and there is considerable hope that this will lead to an increase in the number of cancer patients that experience durable remissions. Here we provide evidence that axitinib improves anti-cancer immunity when applied together with checkpoint inhibitors and/or CD137 agonistic antibodies by modulating anti-tumor immunity. Importantly, the combination induced long lasting anti-cancer immunity as well as protective memory formation, since re-challenge of tumor-free mice with the same tumor cell line led to rapid tumor rejection. Mechanistically, we link the immune-stimulatory function of axitinib to the alterations in the myeloid compartment. In particular, we found that the frequency of peripheral early myeloid progenitors as well as monocytes and granulocytes decreased upon axitinib treatment. In addition, we observed a significant reduction in tumor-infiltrating TAMs and mast cells. We also identified an essential role of axitinib in VEGFR and CD117 (cKIT) inhibition [7, 8], which is expressed on progenitors of myeloid cells (HSCs) and particularly on mast cells [15].
In agreement with previous work [27, 28], mast cells accelerated tumor growth in our tumor models. Indeed, depletion of mast cells in our models, either by experimental depletion or by depletion upon axitinib treatment led to inhibition of tumor growth. Mast cell infiltration has been associated with poor prognosis in multiple cancer types [15]. Mast cells can promote cancer progression by supporting tumor vascularization [27–29] as well as through the generation of immune-suppressive TAMs [15]. Early mast cell depletion resulted in more pronounced anti-tumor effects than late depletion, which most likely reflects a predominant immunosuppressive role of these cells during the early stage of tumor establishment, which is taken over by other cellular subsets during the later stages. We also noted a decrease in the number of TAMs upon axitinib treatment, either as consequence of reduction in mast cell numbers or through the direct effect of axitinib on HSC mobilization. Mast cells can directly or via TAMs increase the local production of IL-10 and TGFβ, which alter T cell frequency and function [29]. Our data demonstrates that axitinib increases T cell-mediated immunity by reversing myeloid and mast cell-derived immune suppression. Consequently, depletion of T cells significantly reduced the anti-cancer activity of axitinib.
Several groups including ours have demonstrated that intratumoral, tumor-specific T cells are dysfunctional and mimic chronic viral infections [30, 31]. This dysfunction is different from T cell anergy and senescence and is termed T cell exhaustion characterized by upregulation of inhibitory receptors [30]. We demonstrate a significant reduction of PD-1 and TIM-3 double-positive cells upon axitinib treatment (Fig. 3), which suggests that axitinib reinvigorates T cell responses and decreases T cell dysfunction (Fig. 3). It has been previously observed that tumor cell-derived VEGF-A is able to directly induce the expression of T cell exhaustion-associated inhibitory immune checkpoints on intratumoral CD8+ T cells [32]. Interestingly, the combination of bevacizumab and atezolizumab has recently shown to improve T cell-mediated immunity by increasing the expression of MHC-I and CD4+ helper T cell 1 cytokines [14]. Furthermore, the tumor neovasculature normalization upon VEGF/VEGFR inhibition facilitated improved infiltration of anti-tumor immune sub-populations within the tumor bed [14]. Similarly, axitinib could support other accessory mechanisms that involve T cell-mediated anti-cancer immunity. Tyrosine kinases that are involved in the maturation and generation of effector and memory T cells could be targeted and influenced by axitinib treatment. Reversal of T cell dysfunction by axitinib could lay also the ideal ground for a combination with immune checkpoint inhibitors to further enhance T cell-mediated anti-tumor immunity. While our in vivo data are suggestive, it remains to be determined how and if axitinib may be able to directly influence T cell dysfunction beyond VEGFR inhibition. It would also be interesting to compare different TKIs with regard to their capacity to influence and maybe revert T cell exhaustion to improve cancer immunotherapies.
Taken together, our results demonstrate that cancer control by axitinib is not only mediated by its anti-angiogenic effects, but is also mediated by its significant impact on anti-tumor immune-stimulatory effects. These data together with other published preclinical results and early clinical trials provide a strong rational for the clinical combination of axitinib and checkpoint inhibitors including blockers of PD-1 and TIM-3 as well as co-stimulatory agonists including CD137 engaging antibodies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Masato Kubo for providing the Mas-TRECK mice for our experiment. We also thank Petra Herzig and Béatrice Dolder Schlienger for their technical support.
Abbreviations
- BMMC
Bone marrow-derived mast cell
- CCL2
CC-chemokine ligand 2
- DT
Diphtheria toxin
- FLT-3
Fms-like tyrosine kinase 3
- mRCC
Metastatic renal cell carcinoma
- PDGF
Platelet-derived growth factor
- SCF
Stem cell factor
- TAM
Tumor-associated macrophage
- TKI
Tyrosine kinase inhibitor
- VEGFR
Vascular endothelial growth factor receptor
- WT
Wild type
Author contributions
PM planned and performed experiments. MB performed experiments. HL and PM evaluated the data and designed the figures. LD’ and ASK analyzed the results. HL, PM, ASK, AZ wrote the manuscript.
Funding
This work was supported by funding from a Grant from the Swiss Cancer League (KFS-3394-02-2014 to Alfred Zippelius), the Cancer League Basel (Krebsliga beider Basel, to Philipp Müller and Heinz Läubli), the Huggenberger Stiftung (to Heinz Läubli) as well as the Goldschmidt-Jacobson Foundation (to Heinz Läubli).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Animal source
Mice were bought from Charles Rivers or bred in the animal facility of the Department of Biomedicine, University of Basel, Switzerland. Mas-TRECK mice were kindly provided by Masato Kubo. All animals were housed under specific pathogen-free conditions and in accordance with Swiss federal regulations (approved by the local ethical committee of Basel Stadt).
Footnotes
Heinz Läubli and Philipp Müller have contributed equally.
Contributor Information
Heinz Läubli, Phone: +41 61 265 5074, Email: heinz.laeubli@unibas.ch.
Alfred Zippelius, Phone: +41 61 265 5074, Email: alfred.zippelius@usb.ch.
References
- 1.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 4.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melero I, Berman DM, Aznar MA, Korman AJ, Perez Gracia JL, Haanen J. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer. 2015;15:457472. doi: 10.1038/nrc3973. [DOI] [PubMed] [Google Scholar]
- 6.Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. 2017;376:354366. doi: 10.1056/NEJMra1601333. [DOI] [PubMed] [Google Scholar]
- 7.Hu-Lowe DD, Zou HY, Grazzini ML, et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res. 2008;14:7272–7283. doi: 10.1158/1078-0432.CCR-08-0652. [DOI] [PubMed] [Google Scholar]
- 8.Escudier B, Gore M. Axitinib for the management of metastatic renal cell carcinoma. Drugs R&D. 2011;11:113126. doi: 10.2165/11591240-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdhury S, McDermott DF, Voss MH, Hawkins RE, Aimone P, Voi M, Isabelle N, Wu Y, Infante JR. A phase I/II study to assess the safety and efficacy of pazopanib (PAZ) and pembrolizumab (PEM) in patients (pts) with advanced renal cell carcinoma (aRCC) J Clin Oncol. 2017;35:suppl; abstr 4506. doi: 10.1200/JCO.2017.35.6_suppl.212. [DOI] [Google Scholar]
- 10.Choueiri TK, Larkin JMG, Oya M, et al. First-line avelumab+axitinib therapy in patients (pts) with advanced renal cell carcinoma (aRCC): results from a phase Ib trial. J Clin Oncol. 2017;35:suppl; abstr 4504. [Google Scholar]
- 11.Du Four S, Maenhout SK, De Pierre K, Renmans D, Niclou SP, Thielemans K, Neyns B, Aerts JL. Axitinib increases the infiltration of immune cells and reduces the suppressive capacity of monocytic MDSCs in an intracranial mouse melanoma model. Oncoimmunology. 2015;4:e998107. doi: 10.1080/2162402X.2014.998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bose A, Lowe DB, Rao A, Storkus WJ. Combined vaccine+axitinib therapy yields superior antitumor efficacy in a murine melanoma model. Melanoma Res. 2012;22:236243. doi: 10.1097/CMR.0b013e3283538293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stehle F, Schulz K, Fahldieck C, Kalich J, Lichtenfels R, Riemann D, Seliger B. Reduced immunosuppressive properties of axitinib in comparison with other tyrosine kinase inhibitors. J Biol Chem. 2013;288:16334–16347. doi: 10.1074/jbc.M112.437962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallin JJ, Bendell JC, Funke R, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun. 2016;7:12624. doi: 10.1038/ncomms12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varricchi G, Galdiero MR, Loffredo S, Marone G, Iannone R, Marone G, Granata F. Are mast cells MASTers in cancer? Front Immunol. 2017;8:424. doi: 10.3389/fimmu.2017.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Investig. 2015;125:3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawaguchi M, Tanaka S, Nakatani Y, et al. Role of mast cells and basophils in IgE responses and in allergic airway hyperresponsiveness. J Immunol. 2012;188:1809–1818. doi: 10.4049/jimmunol.1101746. [DOI] [PubMed] [Google Scholar]
- 18.Arulanandam R, Batenchuk C, Angarita FA, et al. VEGF-mediated induction of PRD1-BF1/Blimp1 expression sensitizes tumor vasculature to oncolytic virus infection. Cancer Cell. 2015;28:210224. doi: 10.1016/j.ccell.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Stankevicius V, Vasauskas G, Bulotiene D, Butkyte S, Jarmalaite S, Rotomskis R, Suziedelis K. Gene and miRNA expression signature of Lewis lung carcinoma LLC1 cells in extracellular matrix enriched microenvironment. BMC Cancer. 2016;16:789. doi: 10.1186/s12885-016-2825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cortez-Retamozo V, Etzrodt M, Newton A, et al. Angiotensin II drives the production of tumor-promoting macrophages. Immunity. 2013;38:296–308. doi: 10.1016/j.immuni.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cortez-Retamozo V, Engblom C, Pittet MJ. Remote control of macrophage production by cancer. Oncoimmunology. 2013;2:e24183. doi: 10.4161/onci.24183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Z, Zhang B, Li D, Lv M, Huang C, Shen GX, Huang B. Mast cells mobilize myeloid-derived suppressor cells and Treg cells in tumor microenvironment via IL-17 pathway in murine hepatocarcinoma model. PLoS One. 2010;5:e8922. doi: 10.1371/journal.pone.0008922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalton DK, Noelle RJ. The roles of mast cells in anticancer immunity. Cancer Immunol Immunother. 2012;61:1511–1520. doi: 10.1007/s00262-012-1246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du Four S, Maenhout SK, Benteyn D, De Keersmaecker B, Duerinck J, Thielemans K, Neyns B, Aerts JL. Disease progression in recurrent glioblastoma patients treated with the VEGFR inhibitor axitinib is associated with increased regulatory T cell numbers and T cell exhaustion. Cancer Immunol Immunother. 2016;65:727740. doi: 10.1007/s00262-016-1836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Granier C, Dariane C, Combe P, et al. Tim-3 expression on tumor-infiltrating PD-1+ CD8+ T Cells correlates with poor clinical outcome in renal cell carcinoma. Cancer Res. 2017;77:1075–1082. doi: 10.1158/0008-5472.CAN-16-0274. [DOI] [PubMed] [Google Scholar]
- 26.Palazon A, Martinez-Forero I, Teijeira A, et al. The HIF-1alpha hypoxia response in tumor-infiltrating T lymphocytes induces functional CD137 (4-1BB) for immunotherapy. Cancer Discov. 2012;2:608623. doi: 10.1158/2159-8290.CD-11-0314. [DOI] [PubMed] [Google Scholar]
- 27.Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 29.Oldford SA, Marshall JS. Mast cells as targets for immunotherapy of solid tumors. Mol Immunol. 2015;63:113124. doi: 10.1016/j.molimm.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 30.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thommen DS, Schreiner J, Muller P, et al. Progression of lung cancer is associated with increased dysfunction of T cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol Res. 2015;3:1344–1355. doi: 10.1158/2326-6066.CIR-15-0097. [DOI] [PubMed] [Google Scholar]
- 32.Voron T, Colussi O, Marcheteau E, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212:139148. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.