Abstract
Glioblastoma is a highly prevalent and aggressive form of primary brain tumor. It represents approximately 56% of all the newly diagnosed gliomas. Macrophages are one of the major constituents of tumor-infiltrating immune cells in the human gliomas. The role of immunosuppressive macrophages is very well documented in correlation with the poor prognosis of patients suffering from breast, prostate, bladder and cervical cancers. The current study highlights the correlation between the tumor-associated macrophage phenotypes and glioma progression. We observed an increase in the pool of M2 macrophages in high-grade gliomas, as confirmed by their CD68 and CD163 double-positive phenotype. In contrast, less M1 macrophages were noticed in high-grade gliomas, as evidenced by the down-regulation in the expression of CCL3 marker. In addition, we observed that higher gene expression ratio of CD163/CCL3 is associated with glioma progression. The Kaplan–Meier survival plots indicate that glioma patients with lower expression of M2c marker (CD163), and higher expression of M1 marker (CCL3) had better survival. Furthermore, we examined the systemic immune response in the peripheral blood and noted a predominance of M2 macrophages, myeloid-derived suppressor cells and PD-1+ CD4 T cells in glioma patients. Thus, the study indicates a high gene expression ratio of CD163/CCL3 in high-grade gliomas as compared to low-grade gliomas and significantly elevated frequency of M2 macrophages and PD-1+ CD4 T cells in the blood of tumor patients. These parameters could be used as an indicator of the early diagnosis and prognosis of the disease.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02423-8) contains supplementary material, which is available to authorized users.
Keywords: Glioma, Tumor-associated macrophages, CD163, CCL3, PD-1
Introduction
Glioblastoma is the most frequent and highly lethal primary brain tumor of the central nervous system. Current therapeutic strategies against glioblastoma are surgical resection, radiotherapy and, chemotherapy (Temozolomide) [1, 2]. These therapies against gliomas are not specific and have several side effects and are not fully successful [3]. Therefore, despite an extensive investigation in designing therapies for glioblastoma, new strategies are urgently needed to improve the prognosis of the patients. In the last few decades, our better understanding of the tumor and immune cell interactions has reinvigorated the interest in improving the currently available therapies against gliomas [4]. Immune suppression induced by glial cancer cells is considered as one of the major factors responsible for poor prognosis and decreased survival of glioma patients. Accumulating evidence suggests that glial cancer cells release factors like TGF-β, IL-10 and prostaglandin E2, which inhibit anti-tumor immunity [5–7]. Macrophages are the most abundant innate immune cells found within the microenvironment of most tumors [8]. Macrophages can acquire distinct subtype from pro-inflammatory to anti-inflammatory depending on the microenvironment. Further, macrophages are subdivided into M1, M2a, M2b, and M2c functional phenotypes. M1 polarization requires stimulation by IFN-γ and LPS and M2a polarization requires IL-4 and IL-13 stimulation, whereas M2c polarization requires TGF-β and IL-10. The markers for M1 macrophages are CCL3, CCL5 and CD86 and for M2c macrophages, the markers are CD163 and IL-10R [9]. Tumor-associated macrophages (TAMs) are considered to acquire a pro-tumorous M2-like macrophage phenotype. Surprisingly, suppressed immune cells not only fail to regress tumors, but also interact intimately with glial cancer cells and support tumor progression [10]. Further, studies have shown that gliomas induce systemic immune suppression [11]. Hence, it is important to identify the mechanism and molecules associated with local and systemic immune suppression, which may provide a basis for diagnosis and novel therapeutics against gliomas.
To address this hypothesis, we studied the gene expression profiling and immune cell infiltration in different grades of gliomas and compared the changes with the adjacent normal tissues. We noticed an increased infiltration of TAMs in glioma microenvironment. Moreover, we identified TAMs phenotype as deactivated macrophages (M2c-like), as evidenced by the upregulation of genes specific for M2c phenotype. Further, a decrease in anti-tumorigenic macrophages (M1 macrophage phenotype) was observed with an increase in the glioma grade. Interestingly, glioma-induced suppression was not only restricted to the tumor microenvironment but also exhibited in the peripheral blood. Importantly, we identified the differential ratio of gene expression of CD163 and CCL3, which could be employed as a marker for the diagnosis and prognosis of the brain tumor patients.
Materials and methods
Clinical samples
Blood and brain tumor specimens were obtained from patients with informed consents and were reviewed and released by pathologist and surgeon. Pathologist classified the type and grade of the tumors in accordance with the WHO histological grading of central nervous system tumors. For microarray and immunohistochemistry, adjacent normal tissue was taken as a control. This part of the brain was excised during surgery to access the tumor and appeared normal on MRI. Further, we confirmed normal brain tissue through histopathology. Brain tissue from cadavers was used as control for qRT-PCR. Characteristics of healthy individuals, glioma patients and cadavers are shown in Figure S1.
Isolation of peripheral blood mononuclear cells
Whole blood was collected in heparin tubes and peripheral blood mononuclear cells (PBMCs) were separated by ficoll–hypaque density gradient centrifugation (BD, San Diego, CA). A total of 1 × 107 isolated PBMCs were stored in RNAlater® solution at − 80 °C for further analysis.
RNA isolation and cDNA synthesis
RNA was isolated from the PBMCs, fresh tumor and normal tissue using the RNeasy kit (Qiagen, Limburg, Netherlands), according to the manufacturer’s protocol as described previously [12]. RNA samples were incubated with amplification grade DNase (Sigma, St Louis, MO, USA) to remove DNA contamination. In brief, RNA was reverse-transcribed into cDNA using Maxima first-strand cDNA synthesis kit for qRT-PCR (Thermo Fisher Scientific, Waltham, MA). Gene expression was quantified using qRT-PCR analysis. The mRNA expression unit of the gene was calculated by the ΔCT method as described previously [13]. The ratio for CD163/CCL3 was calculated as: (mRNA expression unit of CD163/mRNA expression unit of CCL3). Primers used for qRT-PCR. GAPDH: FP, 5′-AGCCACATCGCTCAGACAC-3′; RP, 5′-GCCAATACGACCAAATCC-3′. CD163: FP, 5′-GCAAGTGGCCTCTGTAATCT-3′; RP, 5′-AGCACTTTCTTCTGGAATGG-3′. CCL3: FP, 5′-CAGAATCATGCAGGTCTCCAC-3′; RP, 5′-GCGTGTCAGCAGCAAGTG-3′. TGF- β: FP, 5′- CTGGAAACCCACAACGAAA-3′; RP, 5′- AACTTGAGCCTCAGCAGAC-3′. IL-10: FP, 5′- GAGAACAGCTGCACCCACT-3′; RP, 5′- CCCAGGTAACCCTTAAAGTCC-3′.
Gene expression microarray
Microarray experiments were done by iLife Discoveries, Pvt. Ltd. (Gurgaon, India). Total RNA was extracted from the fresh tumor and normal tissue using an RNeasy kit (Qiagen, Limburg, Netherlands), according to a manufacturer’s protocol. RNA purity and integrity were analyzed by BioAnalyser 2100 (Agilent Technologies, Santa Clara, CA). Gene expression profiling was performed using GeneChip® Human 2.0 ST Array Affymetrix (Santa Clara, CA). Target preparation for Affymetrix Gene ChipsTM was performed as per the manufacturer’s instructions. Hybridization, washing, staining and scanning of chips were carried out according to standard protocols (Affymetrix, Santa Clara, CA). The data were analyzed using softwares Gene Spring (Agilent Technologies, Santa Clara, CA) and Transcriptome Analysis Console (Affymetrix, Santa Clara, CA). Geo accession number: GSE117423.
Immunofluorescent staining
PBMCs isolated from glioma patients and healthy individuals were resuspended in FACS buffer (FCS-2%, 2-mM sodium azide in PBS). Cells were stained for extracellular markers following the same protocol as described earlier [14]. In brief, cells were incubated with Fc-binding inhibitor (eBiosciences, San Diego, CA) for 25 min at 4 °C. Later, cells were incubated with fluorochrome-conjugated Abs specific for human CD11b, CD14, CD163, CD206, CD4, PD-1 or isotype-matched control Abs, at a recommended dilution. The cells were fixed with 1× paraformaldehyde. The acquisition was done using FACS Aria and analyzed with BD DIVA software.
Immunohistochemistry
Four-μm-thick tissue sections were de-waxed in xylene and rehydrated through graded alcohols. Antigen retrieval was carried out using Dako PT link (Dako/Agilent Technologies, Santa Clara, CA). Anti-human CD68, CD206 and CD163 Abs were procured from Abcam, Cambridge, UK and anti-human Ki-67 Ab was purchased from (Dako/Agilent Technologies, Santa Clara, CA). Immunohistochemical staining of individual markers CD206 and Ki67 or double staining of CD68 and CD163 was performed using EnVision™ G|2 Doublestain System, rabbit/mouse (DAB/Permanent Red) kit (Dako/Agilent Technologies, Santa Clara, CA), according to the manufacturer’s instructions. Development of color was performed using permanent red dye. Development of brown color was performed using DAB (3,3′- diaminobenzidine). Sections were then counterstained with hematoxylin and mounted in an aqueous mounting medium. Six representative high-power fields (40× magnification) per tissue section were selected using an Olympus microscope (Olympus, Tokyo, Japan). For CD68+/CD163+ double-positive cell quantification, immunohistochemistry was analyzed by blinded manual scoring. The mean number of double-positive cells from 6 fields/tumor section was calculated. For CD206 brown color quantification, Fiji software was used.
Database
Freely accessible server, OncoLnc tool (URL: www.oncolnc.org) was used for analyzing the survival correlation of selected M1 macrophage marker, M2 macrophage marker and Pro-tumorigenic cytokines. OncoLnc tool generated the Kaplan–Meier plots for the studied genes using the low- and high-expressing LGG patient cohorts that are publically available in TCGA database. For assessing The RNA seq. expression level of CCL3, CD163, IL-10 and TGF-β1 in low-grade glioma and high-grade glioma, TCGA data set: cBioportal (URL: http://www.cbioportal.org/) was used to assess the dataset of LGG and glioblastoma from TCGA to explore the log fold change of the RNA seq. expression level in the patient population.
Statistics
All statistical calculations were conducted using Graph Pad Prism 5. For comparison between groups, statistical analysis was done using Student’s t test or Mann–Whitney test. For microarray, ‘Moderated t test’ was applied for assessing the statistically significant change in the differentially expressed genes between two groups. p values < 0.05 were considered as significant.
Results
Histological grading of gliomas
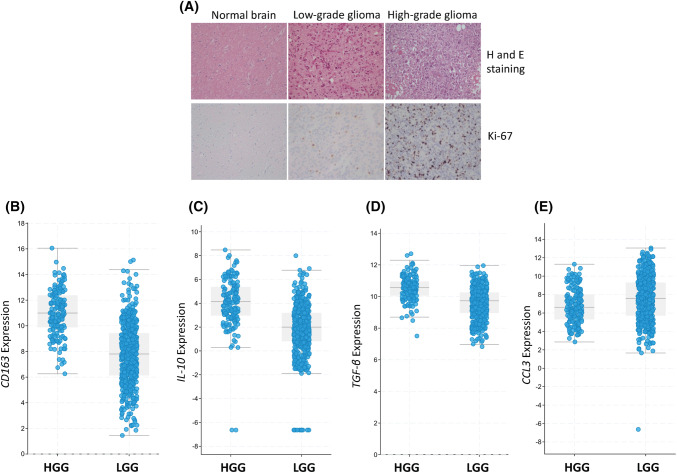
The characterization of different grades of gliomas was done by hematoxylin and eosin staining. Further, this was confirmed by Ki-67 immunostaining, which is a proliferation marker (Figs. 1a, S2). High-grade gliomas showed features like hypercellularity, nuclear pleomorphism, mitosis and necrosis. Likewise, features of low-grade gliomas were moderate cellularity, mild nuclear pleomorphism and no necrosis (Fig. 1a).
Fig. 1.
Grading of the gliomas. a Hematoxylin (H), eosin (E) and Ki67 immunostaining of histological sections of normal tissue, low and high-grade glioma tissues were performed. RNA seq. expression level of bCD163; cIL-10; dTGF-β and eCCL3 in low-grade glioma (LGG) and high-grade glioma (HGG) is accessed using TCGA data set (cBioportal)
Differential gene expression of human gliomas
It is a well-established fact that the cancer cells are evolved to evade the immune response by suppressing the immune system [15]. In human gliomas, macrophages are the major constituent of tumor-infiltrating immune cells [16, 17]. Therefore, we studied the global gene expression profiles by microarray in gliomas and adjacent normal tissue samples to (1) identify genes that were either overexpressed or underexpressed in the low-grade gliomas versus adjacent normal tissues, high-grade gliomas versus adjacent normal tissues and high-grade gliomas versus low-grade gliomas; (2) analyze the genes related to macrophage subtypes like M1, M2a and M2c. Differential gene expression for M1, M2a and M2c was observed in low- and high-grade gliomas, as compared to adjacent normal tissues. Interestingly, macrophages in glioma showed higher expression of CD163, IL10RA and CCL18 genes related to deactivated macrophages, i.e., M2c phenotype. In contrast, CCL3 and CCL5 genes related to M1 macrophages exhibited lower expression in gliomas, as compared to normal tissues (Fig. S3). These data suggest the suppression of anti-tumor immunity in human gliomas. It is important to note that there was a disparity in the expression level of genes among the gliomas and normal tissues, representing a heterogeneity within the gliomas and genetic variations among the studied subjects. To further validate the observation from our microarray data, we assessed the RNA seq. expression level of CCL3, CD163, IL-10 and TGF-β in LGG and HGG patients using TCGA public data set. We observed higher expression of CD163, IL-10 and TGF-β in HGG patients as compared to LGG patients (Fig. 1b–d). In contrast, the expression of CCL3 is less in HGG patients as compared to LGG patients (Fig. 1e).
Association of higher M2/M1 ratio with glioma progression
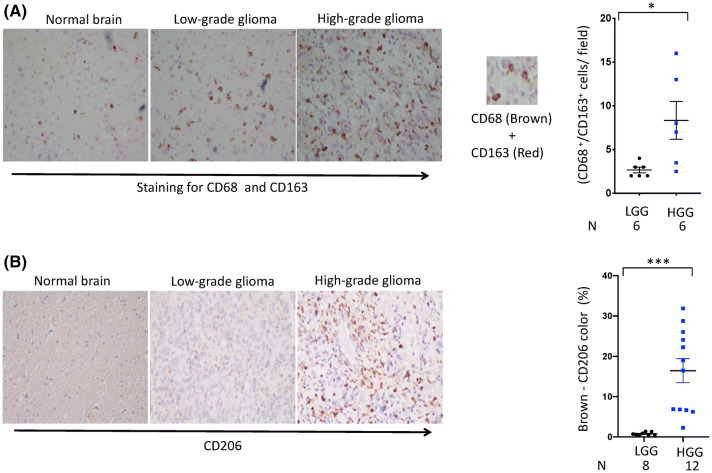
Most therapeutic strategies against glial tumor cells have failed. Interesting studies have shown that targeting glioma microenvironment cells, such as TAMs, significantly regresses tumors and increases survival [13, 18, 19]. Therefore, it would be imperative to study the phenotypes of TAMs in human glioma microenvironment. Expression of CD68 marker for TAM was examined in the gliomas by immunohistochemistry. We observed an increase in the expression of CD68, suggesting an elevated presence of TAMs in gliomas compared to normal brain tissue section (Fig. 2a). The polarization of M1 and M2 macrophages in gliomas and adjacent normal tissues was confirmed by analyzing the expression of various genes that are associated with M1/M2 phenotype and was identified through microarray, RNA seq. expression, immunohistochemistry and qRT-PCR. The CD163 and CD206 are the classical markers of M2 macrophages. It is noteworthy that, the expression of both CD206 and CD163 was upregulated in gliomas, as compared to tumor-adjacent normal tissues (Fig. 2a, b). Further, we observed significantly (p < 0.05) higher number of M2 macrophages (CD68 and CD163 double-positive) in high-grade gliomas, as compared to low-grade gliomas (Fig. 2a). RNA seq. analysis showed decreased expression of CCL3, which is a classical marker for M1 macrophages in high-grade gliomas, in comparison to low-grade gliomas (Fig. 1e). Further, we confirmed the expression of CD163 and CCL3 through qRT-PCR (Fig. 3a, b). Microarray data also showed an elevation in M2c-related genes CD163, CCL18 and IL10RA (Fig. S3). Interestingly, the expression of IL-10 (p < 0.01) and TGF-β was substantially higher in high-grade gliomas, as compared to low-grade gliomas (Fig. 3c, d). Both TGF-β and IL-10 are M2c-differentiating cytokines in glioma microenvironment [20]. Furthermore, we compared the expression of CD163 and CCL3 in normal brain tissues obtained from autopsy, low-grade gliomas and high-grade gliomas. In low-grade gliomas, CCL3 level was significantly higher (p < 0.05), as compared to high-grade gliomas. Finally, we calculated the CD163/CCL3 ratio that represents a balance between M2c and M1 TAMs. Intriguingly, CD163/CCL3 ratio was considerably higher (p < 0001) in high-grade gliomas than the low-grade gliomas (Fig. 3e). In essence, these data provide an indication concerning higher CD163/CCL3 ratio and its association with glioma progression. To further substantiate our observations, we used the survival data of glioma patient’s population publically available in the TCGA resource portal; we assessed the correlation between CCL3, CD163, and two pro-tumorigenic markers: IL-10, TGF-β1 with mortality in higher and lower expressing cohorts. The Kaplan–Meier survival plots indicate patients with lower expression of CD163 and higher expression of CCL3 correlated with better survival in LGG patients (Fig. 3f). Furthermore, cohorts expressing higher IL-10 and TGF-β1 demonstrated a significant decline in survival as compared to the cohorts expressing lower level of IL-10 and TGF-β1 (Fig. 3g).
Fig. 2.
Phenotypic analysis of M2 TAMs in gliomas. Representative double immunohistochemically stained specimens of low- and high-grade gliomas, and normal tissue adjacent to tumor for a CD68 (brown) and CD163 (red) (double staining); b CD206 (brown). Hematoxylin is used as a counterstain for nuclear staining (blue). Magnification: 40 × . The mean number shows double-positive cells from 6 fields/tumor section. Dot plot represents the number of CD68+/CD163+ double-positive cells per field (right panel). CD206-brown color quantification was done using Fiji software. Data shown as mean ± SEM. *p < 0.05. ‘N’ represents number of individuals
Fig. 3.
Higher M2/M1 ratio is associated with glioma progression. Vertical scatter plots signify the gene expression at mRNA level by RT-qPCR in low- and high-grade gliomas and normal brain tissues for the a CD163; b CCL3; c IL-10; d TGF-β. e Ratio of CD163/CCL3 is shown in gliomas and normal brain tissues. Each dot represents a single individual. Data expressed as arbitrary units after normalization are designated as mean ± SEM. NBC: normal brain control, LGG: low-grade glioma, HGG: high-grade glioma. *p < 0.05; **p < 0.01; ***p < 0.001. N represents number of individuals. Survival correlations of M1 and M2c macrophage markers in glioma patients; OncoLnc tool was used to explore the survival correlations of f CCL3 and CD163; g IL-10 and TGFβ1 in glioma patient data in TCGA portal. Kaplan–Meier plots show the survival correlation in glioma patients expressing the above genes, and the level of significance is represented by the log rank p value (< 0.05). Red and blue lines indicate the cohort of glioma patients with high and low expression of the subject genes, respectively
Systemic immunosuppression in human gliomas
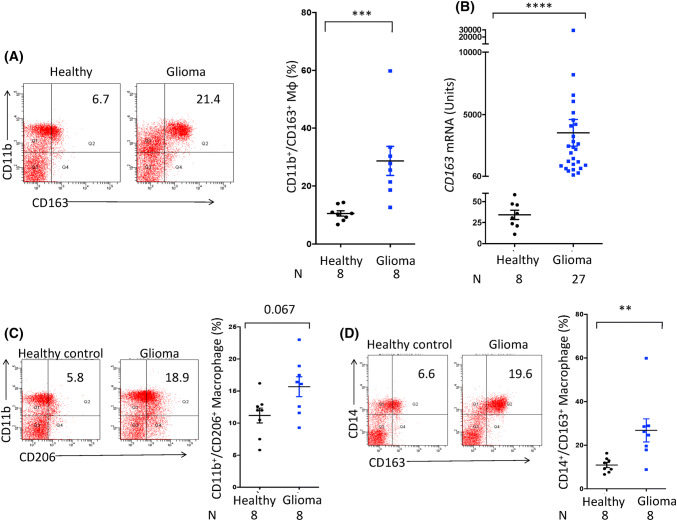
Studies have shown disturbed blood–brain barrier in human gliomas [21]. Hence, we were inquisitive to know whether glioma-inflicted immune suppression in the brain can influence systemic immunity. Therefore, we analyzed the immune phenotypes of peripheral blood cells collected from the newly diagnosed glioma patients before receiving any therapy. Immune phenotypes from healthy donors were used as controls. We investigated the presence of M2 macrophages in glioma patients and healthy individuals by flow cytometry. Interestingly, our data signify that there is a systemic immune suppression in the gliomas, as supported by significant upregulation (p < 0.001) in the frequency of CD11b+/CD163+ macrophages in the PBMCs of the glioma patients, as compared to healthy subjects (Fig. 4a). CD163 is a hallmark marker for M2 macrophages. Furthermore, the flow cytometry results were confirmed by gene expression at the mRNA level. Significant augmentation (p < 0.0001) in the expression of CD163 was noted in the glioma patients with respect to healthy volunteers (Fig. 4b). Simultaneously, in the same patients, we noticed an increase in the frequency of CD11b+/CD206+ macrophages (Fig. 4c). It is known that CD163 expression is largely restricted to the monocyte/macrophage lineage and expression of CD14 is associated with myeloid-derived suppressor cells, which are known to induce immune suppression in humans [22]. Therefore, we further assessed the expression of CD14+/CD163+ on peripheral monocytes. Intriguingly, noticeable (p < 0.01) upregulation of CD163 was noticed on the peripheral blood monocytes of the glioma patients than healthy controls (Fig. 4d).
Fig. 4.
Higher frequency of M2 macrophages is reflected in the peripheral blood of the glioma patients. The PBMCs obtained from the glioma patients and healthy subjects were monitored for the expression of CD163 by a flow cytometer on CD11b gated population and data expressed as dot plots; b RT-qPCR and data expressed as arbitrary units after normalization and denoted as mean ± SEM. The PBMCs were also evaluated for the expression of c CD11b and CD206; d CD14 and CD163. The data expressed as dot plots depict a percentage of double-positive cells. The vertical scatter plot on the right panel shows percentage of double-positive cells and expressed as mean ± SEM. Each dot represents single individual. *p < 0.05; **p < 0.01; ***p < 0.0001. ‘N’ represents number of individuals
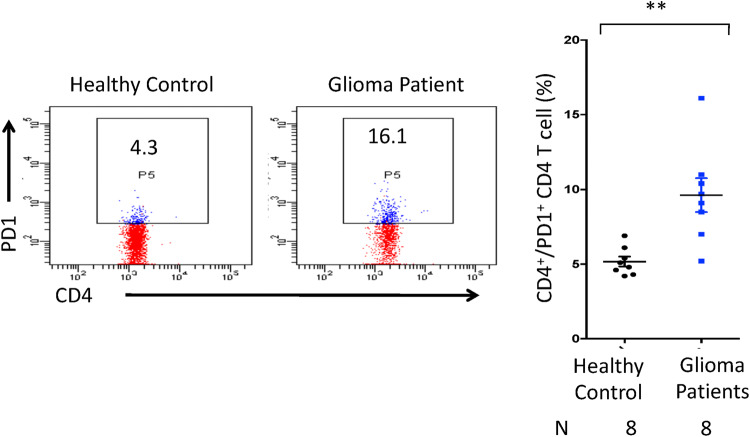
The suppressive macrophages are well known to skew CD4 T cells towards exhaustion; with lower effector cytokine secretion and higher expression of PD-1 and TIM-3 [23, 24]. Hence, we next assessed the expression of PD-1 on the peripheral T cells. Interestingly, the elevated pool of PD-1+/CD4+ T cells (p < 0.01) was observed in the PBMCs of glioma patients as compared to healthy controls (Fig. 5). Flow cytometry isotype controls are shown in Figure S4. These results designate that not only local immunity in the brain but also systemic immunity is suppressed in glioma patients.
Fig. 5.
The CD4 T cells of glioma patients show high expression of PD-1. The PBMCs obtained from glioma patients and healthy subjects were monitored by flow cytometer for the expression of PD-1 on CD4 T cells. The data are expressed as dot plots; depicting the percentage of CD4 T cells expressing PD-1. The vertical scatter plot on the right panel shows percentage of cells and the data are expressed as mean ± SEM. Each dot represents single individual. **p < 0.01. N represents number of individuals
Discussion
Most therapeutic strategies against glial cancer cells have failed due to genetic heterogeneity [25, 26]. In contrast, non-cancerous cells in the tumor microenvironment are genetically stable and can be exploited as therapeutic targets [27]. TAMs are the major constituents of the tumor microenvironment. Consequently, we thought that it would be imperative to study and characterize the phenotypes of TAMs. To quantify macrophages, we used CD68 marker. It is important to mention here that CD68 is expressed both on microglia and macrophages in the glioma tumor microenvironment [28, 29]. Therefore, in the present study, macrophages and microglia are considered in conjunction. In the initial phase of the study, increase in the recruitment of macrophages was noticed in the glioma microenvironment. These macrophages acquired the phenotype of deactivated macrophages (M2c), as evidenced by the upregulation of genes specific for M2c phenotype viz CD163, IL10RA, CXCL13, etc., in the gliomas. Increased expression of TGF-β and IL-10, which are differentiating factors for M2c macrophages, supports suppressive macrophages in the glioma microenvironment. These data suggest the suppression of anti-tumor immunity in human gliomas. Further, we demonstrated decreased M1 macrophage phenotype with an increase in glioma grade. Interestingly, for the first time, we have identified that a higher CD163/CCL3 ratio was associated with glioma progression, which can be considered as a diagnostic marker for gliomas. There has been a study suggesting the M0 macrophage phenotype in the gliomas [30]. This discrepancy might be due to the different marker selection to phenotype the TAMs. Glioma patients show signs of systemic immune suppression that might be responsible for the failure of conventional immunotherapies. An improved understanding of mechanisms accountable for systemic immune defects is important for designing novel therapies; which will lead to the improved prognosis of the patients. We identified elevated levels of suppressive population of M2 macrophages (CD11b and CD163 double positive) and myeloid-derived suppressor cells (CD14 and CD163 double positive) in peripheral blood of glioma patients, as compared to healthy controls.
To better understand the extent of systemic suppression, we also investigated the T cells. As cancer progresses, T cells are known to undergo exhaustion. A programmed cell death protein 1 (PD-1) is an important marker for T cell exhaustion [31]. It has been reported that the immune checkpoint suppressors directed towards PD-1 are shown to be greatly successful in the treatment of cancer [32, 33]. Therefore, we monitored the expression of PD-1 on the T cells obtained from the PBMCs of the glioma patients. Interestingly, as compared to healthy controls, we observed elevated frequency of exhausted CD4 T cells in glioma patients, as evidenced by the upregulation in the expression of PD-1. Our data demonstrate that there is not only local but also systemic immune suppression in human glioma patients, which might have a significant impact on the progression of tumors and needs further investigation. This could also increase the susceptibility of patients to other infections.
There are several potential clinical implications of these findings. First, glioma grade can be associated with CD163/CCL3 ratio. Second, we have identified the TAMs phenotype in human gliomas as M2c like. Consequently; this cell type can be one of the future therapeutic targets to regress tumors. Third, the Kaplan–Meier survival plots indicate that LGG patients with lower expression of M2c marker (CD163), and higher expression of M1 marker (CCL3) correlated with better survival. Fourth, the elevated levels of M2 macrophages, myeloid-derived suppressor cells and exhausted CD4 T cells in the peripheral blood of glioma patients can be correlated with other diagnostic techniques for early diagnosis of the gliomas. Additionally, specific targeting of elevated cell populations can be explored as a therapy to overcome immune suppression in glioma patients. In essence, these results signify that the gene expression of CD163 and CCL3 could be used as an important indicator for monitoring the prognosis of tumor patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Debajyoti Chatterjee, Gurpreet, Ishwar and Alka from Department of Histopathology, Postgraduate Institute of Medical Education and Research, Chandigarh, India for the helpful suggestions for doing immunohistochemistry.
Abbreviations
- Abs
Antibodies
- DAB
(3,3′-diaminobenzidine)
- FACS
Fluorescence-activated cell sorting
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- HGG
High-grade glioma
- HLA-DR
Human Leukocyte Antigen—DR isotype
- IFN-γ
Interferon gamma
- IL-10
Interleukin 10
- IL-10R
Interleukin-10 receptor
- LGG
Low-grade glioma
- LPS
Lipopolysaccharides
- PBMCs
Peripheral blood mononuclear cells
- PBS
Phosphate-buffered saline
- PD-1
Programmed cell death protein 1
- TAM
Tumor-associated macrophages
- TCGA
The Cancer Genome Atlas
- TGF-β
Transforming growth factor-beta
Author contributions
JNA and AV conceived the project. AV, TA and NK performed experiments. AV, TA, NK, JNA, BDR, MKT and DC analyzed data. AV, JNA, NK, MKT and SS wrote the manuscript. All authors assisted in editing the manuscript and approved its final version.
Funding
Authors are grateful to the Council of Scientific and Industrial Research (CSIR), Department of Biotechnology (DBT), Indian Council of Medical Research (ICMR), India for financial support. Aurobind Vidyarthi and Tapan Agnihotri received fellowships from the CSIR, Nargis Khan from DBT, and Sanpreet Singh from ICMR.
Compliance with ethical standards
Competing interests
All authors have declared no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee. The study was approved by the Postgraduate Institute of Medical Education and Research, Chandigarh, India ‘Institutional Ethical Committee’ (Ref. No. PGI/IEC/2012/1498-99) and ‘Institutional Biosafety Committee of the Institute of Microbial Technology’ (Ref. No. 1/IEC/1/9-2014), Chandigarh, India.
Informed consent
Written informed consent was obtained from all individual participants and close relative of the deceased (For cadavers) included in the study for using the specimens for research and publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Broekman ML, Maas SLN, Abels ER, Mempel TR, Krichevsky AM, Breakefield XO. Multidimensional communication in the microenvirons of glioblastoma. Nat Rev Neurol. 2018 doi: 10.1038/s41582-018-0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Preusser M, de Ribaupierre S, Wohrer A, Erridge SC, Hegi M, Weller M, Stupp R. Current concepts and management of glioblastoma. Ann Neurol. 2011;70:9–21. doi: 10.1002/ana.22425. [DOI] [PubMed] [Google Scholar]
- 3.Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol. 2015;11:504–514. doi: 10.1038/nrneurol.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arevalo AST, Erices JI, Uribe DA, Howden J, Niechi I, Munoz S, Martin RS, Monras CAQ. Current therapeutic alternatives and new perspectives in glioblastoma multiforme. Curr Med Chem. 2017;24:2781–2795. doi: 10.2174/0929867324666170303122241. [DOI] [PubMed] [Google Scholar]
- 5.Magana-Maldonado R, Chavez-Cortez EG, Olascoaga-Arellano NK, Lopez-Mejia M, Maldonado-Leal FM, Sotelo J, Pineda B. Immunological evasion in glioblastoma. Biomed Res Int. 2016;2016:7487313. doi: 10.1155/2016/7487313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ries CH, Cannarile MA, Hoves S, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Colegio OR, Chu NQ, Szabo AL, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 10.Sarkar S, Doring A, Zemp FJ, et al. Therapeutic activation of macrophages and microglia to suppress brain tumor-initiating cells. Nat Neurosci. 2014;17:46–55. doi: 10.1038/nn.3597. [DOI] [PubMed] [Google Scholar]
- 11.Gustafson MP, Lin Y, New KC, Bulur PA, O’Neill BP, Gastineau DA, Dietz AB. Systemic immune suppression in glioblastoma: the interplay between CD14 + HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol. 2010;12:631–644. doi: 10.1093/neuonc/noq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan N, Pahari S, Vidyarthi A, Aqdas M, Agrewala JN. Stimulation through CD40 and TLR-4 is an effective host directed therapy against mycobacterium tuberculosis. Front Immunol. 2016;7:386. doi: 10.3389/fimmu.2016.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vidyarthi A, Khan N, Agnihotri T, et al. TLR-3 stimulation skews M2 macrophages to M1 through IFN-alphabeta signaling and restricts tumor progression. Front Immunol. 2018;9:1650. doi: 10.3389/fimmu.2018.01650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan N, Vidyarthi A, Pahari S, Negi S, Aqdas M, Nadeem S, Agnihotri T, Agrewala JN. Signaling through NOD-2 and TLR-4 Bolsters the T cell priming capability of dendritic cells by inducing autophagy. Sci Rep. 2016;6:19084. doi: 10.1038/srep19084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paul S, Calmels B, Regulier E. Tumor-induced immunosuppression. Ann Biol Clin (Paris) 2002;60:143–152. [PubMed] [Google Scholar]
- 16.Kostianovsky AM, Maier LM, Anderson RC, Bruce JN, Anderson DE. Astrocytic regulation of human monocytic/microglial activation. J Immunol. 2008;181:5425–5432. doi: 10.4049/jimmunol.181.8.5425. [DOI] [PubMed] [Google Scholar]
- 17.Morantz RA, Wood GW, Foster M, Clark M, Gollahon K. Macrophages in experimental and human brain tumors. Part 2: studies of the macrophage content of human brain tumors. J Neurosurg. 1979;50:305–311. doi: 10.3171/jns.1979.50.3.0305. [DOI] [PubMed] [Google Scholar]
- 18.Fritz JM, Tennis MA, Orlicky DJ, et al. Depletion of tumor-associated macrophages slows the growth of chemically induced mouse lung adenocarcinomas. Front Immunol. 2014;5:587. doi: 10.3389/fimmu.2014.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan D, Kowal J, Akkari L, Schuhmacher AJ, Huse JT, West BL, Joyce JA. Inhibition of colony stimulating factor-1 receptor abrogates microenvironment-mediated therapeutic resistance in gliomas. Oncogene. 2017;36:6049–6058. doi: 10.1038/onc.2017.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 21.Schneider SW, Ludwig T, Tatenhorst L, Braune S, Oberleithner H, Senner V, Paulus W. Glioblastoma cells release factors that disrupt blood-brain barrier features. Acta Neuropathol. 2004;107:272–276. doi: 10.1007/s00401-003-0810-2. [DOI] [PubMed] [Google Scholar]
- 22.Buechler C, Ritter M, Orso E, Langmann T, Klucken J, Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000;67:97–103. doi: 10.1002/jlb.67.1.97. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Y, Li Y, Zhu B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015;6:e1792. doi: 10.1038/cddis.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber S, Hoffmann R, Muskens F, Voehringer D. Alternatively activated macrophages inhibit T-cell proliferation by Stat6-dependent expression of PD-L2. Blood. 2010;116:3311–3320. doi: 10.1182/blood-2010-02-271981. [DOI] [PubMed] [Google Scholar]
- 25.Turner NC, Reis-Filho JS. Genetic heterogeneity and cancer drug resistance. Lancet Oncol. 2012;13:e178–e185. doi: 10.1016/S1470-2045(11)70335-7. [DOI] [PubMed] [Google Scholar]
- 26.Sottoriva A, Spiteri I, Piccirillo SG, Touloumis A, Collins VP, Marioni JC, Curtis C, Watts C, Tavare S. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci USA. 2013;110:4009–4014. doi: 10.1073/pnas.1219747110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawasaki Y, Akiyama T. Tumor microenvironment: promising therapeutic target. Nihon Rinsho. 2015;73:1283–1287. [PubMed] [Google Scholar]
- 28.Fahrenhold M, Rakic S, Classey J, Brayne C, Ince PG, Nicoll JAR, Boche D, Mrc C. TREM2 expression in the human brain: a marker of monocyte recruitment? Brain Pathol. 2018;28:595–602. doi: 10.1111/bpa.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendrickx DAE, van Eden CG, Schuurman KG, Hamann J, Huitinga I. Staining of HLA-DR, Iba1 and CD68 in human microglia reveals partially overlapping expression depending on cellular morphology and pathology. J Neuroimmunol. 2017;309:12–22. doi: 10.1016/j.jneuroim.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Gabrusiewicz K, Rodriguez B, Wei J, et al. Glioblastoma-infiltrated innate immune cells resemble M0 macrophage phenotype. JCI Insight. 2016 doi: 10.1172/jci.insight.85841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arrieta O, Montes-Servin E, Hernandez-Martinez JM, Cardona AF, Casas-Ruiz E, Crispin JC, Motola D, Flores-Estrada D, Barrera L. Expression of PD-1/PD-L1 and PD-L2 in peripheral T-cells from non-small cell lung cancer patients. Oncotarget. 2017;8:101994–102005. doi: 10.18632/oncotarget.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang C, Cai X, Zhang H, Xia X, Zhang B, Xia L. Activity and immune correlates of a programmed death-1 blockade antibody in the treatment of refractory solid tumors. J Cancer. 2018;9:205–212. doi: 10.7150/jca.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stecher C, Battin C, Leitner J, Zettl M, Grabmeier-Pfistershammer K, Holler C, Zlabinger GJ, Steinberger P. PD-1 blockade promotes emerging checkpoint inhibitors in enhancing T cell responses to allogeneic dendritic cells. Front Immunol. 2017;8:572. doi: 10.3389/fimmu.2017.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.