Abstract
Programmed cell death-1 (PD-1) and programmed cell death-ligand-1 (PD-L1) inhibitors have been highlighted in the field of cancer treatment. The interaction between PD-1 and PD-L1 is thought to play an important role in the regulation of the self-immune tolerance mechanism, so blocking these molecules may cause serious immune-related adverse events (IrAE), including fulminant insulin-dependent (type 1) diabetes. Here, we describe a patient with fulminant type 1 diabetes induced by nivolumab, an anti-PD-1 antibody. The patient, a 78-year-old man, was being treated with nivolumab as a third-line treatment for squamous cell carcinoma of the lung. After three cycles, he experienced an abrupt flare-up of the blood glucose within half a day. His blood glucose further increased without clinical symptoms until his hospital visit. Laboratory data showed the complete exhaustion of intrinsic insulin and the elevation of serum antibody titer to glutamic acid decarboxylase (GAD). Although the patient was previously diagnosed with non-insulin-dependent (type 2) diabetes, his disease activity had been well controlled with oral medication and low-dose insulin therapy until just before the flare-up. Because of the laboratory findings and the extremely rapid onset of hyperglycemia, a diagnosis of fulminant, rather than the rapid onset, type 1 diabetes related to nivolumab therapy was strongly suspected. Our case study indicates that fulminant hyperglycemia can occur extremely rapidly. The blood glucose of patients receiving PD-1 antibody therapy should be closely monitored.
Keywords: Fulminant type 1 diabetes, PD-1 antibody, Nivolumab, Immune-related adverse event
Introduction
Several immune checkpoint inhibitors, including antibodies against programmed cell death-1 (PD-1) and programmed cell death-ligand 1 (PD-L1), have recently emerged as a novel and effective anti-tumor therapy for patients with various types of solid tumors [1], melanoma, and Hodgkin lymphoma. Although anti-PD-1 or anti-PD-L1 antibodies appear to have a tolerable toxic profile and a lower incidence of adverse effects than other cytotoxic agents, several immune-related adverse events (IrAEs) have been reported, such as thyroid dysfunction, hypophysitis, pneumonitis, colitis, myositis, nephritis, hepatitis, adrenal insufficiency, and uveitis [2]. Without prompt medical management, some of these events can develop into serious and fatal complications. It is thought that the inhibition of T-cell function augments these autoimmune reactions. Notably, the fulminant onset of insulin-dependent (type 1) diabetes, which is potentially life-threatening if unrecognized, can very occasionally occur in patients treated with immune checkpoint inhibitors, although its rarity means that there have been no reports describing the manner of its onset and its subsequent blood glucose dynamics. Although most IrAEs caused by immune checkpoint inhibition are treatable with the administration of glucocorticoids or other immunosuppressive agents, the usefulness of steroid therapy for type 1 diabetes has not been demonstrated. Here, we describe a patient who developed fulminant hyperglycemia within half a day during nivolumab therapy.
Case report
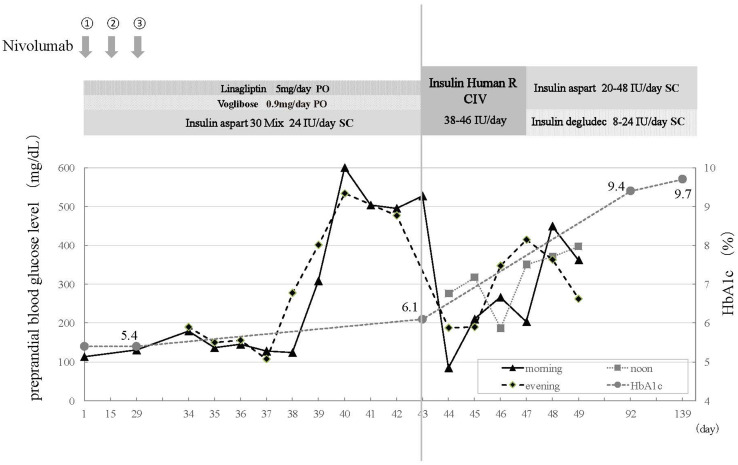
A 78-year-old man was referred to our department with suspected lung cancer. After a diagnostic work-up, he was diagnosed with squamous non-small cell lung cancer (NSCLC) with contralateral lung and mediastinal metastases. He received first-line chemotherapy with carboplatin [5 area under the curve (AUC)] and nab-paclitaxel, and achieved a partial response with good performance status. The first-line chemotherapy was continued for five cycles. Three months later, he experienced disease progression and second-line chemotherapy with vinorelbine was started. After two cycles, imaging showed exacerbation of the lung cancer. Consequently, nivolumab (3 mg/kg every 2 weeks) was administered as third-line chemotherapy. Before the nivolumab therapy, he was diagnosed with type 2 diabetes and was treated with combination therapy of dipeptidyl peptidase-4 (DPP-4) inhibitor (linagliptin 5 mg/day) and alpha-glucosidase inhibitor (voglibose 0.9 mg/day) and conventional insulin therapy with mixed-type insulin (insulin aspart 30 mix 24 units/day, twice a day). He had a good glycemic control with normal HbA1c (5.4%) 2 weeks before the flare-up (Fig. 1). On the ninth day of the third cycle of nivolumab, his preprandial blood glucose abruptly increased to 277 mg/dL in the evening, despite being 130 mg/dL, as usual, that morning. Prior to this flare-up, his morning and evening preprandial blood glucose levels, self-monitored at home, had been stable and consistently < 140 mg/dL (Table 1). No increased dietary volume or other potential factors or causes had been observed. His blood glucose level increased further to > 600 mg/dL in 2 days after the flare-up and did not return to baseline; however, he was unaware of any symptoms related to hyperglycemia.
Fig. 1.
Clinical course of the patient. After 3 cycles of nivolumab, abrupt elevation of hyperglycemia was noted without prominent hyperglycemic symptom. Serum and urinary C-peptide excretion was significantly decreased, whereas HbA1c remained in normal range
Table 1.
Preprandial blood glucose level (mg/dL)
| Day | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 |
|---|---|---|---|---|---|---|---|---|---|
| Morning | 136 | 145 | 128 | 124 | 308 | > 600 | 504 | 495 | 504 |
| Evening | 150 | 156 | 107 | 277 | 401 | 534 | − | 477 |
*Day from the initiation of nivolumab
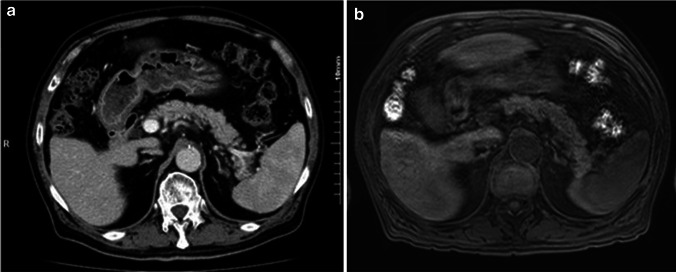
He attended the outpatient department 5 days later for his next cycle of nivolumab, as scheduled. He was neurologically normal with an alert mental status. Physical examination showed no specific findings. His skin and tongue were not so dry. His height and weight was 156 cm and 56.5 Kg, respectively. His blood pressure was 122/76 mm Hg and his heart rate was 78 bpm. Although neither ketoacidosis nor ketonuria were observed, his clinical course was suspicious for the development of fulminant type 1 diabetes. He was admitted for the management and further evaluation of the hyperglycemia. On the day of admission, his random blood glucose was elevated to 527 mg/dL, but glycated hemoglobin (HbA1c) remained in the normal range (6.1%; normal range 4.7–6.2%). Laboratory tests performed on admission are summarized in Table 2; the tests showed a remarkable decrease in serum C-peptide to less than 0.1 ng/ml and urinary C-peptide to less than 0.3 µg/day. Serum titers showed elevated anti-glutamic acid decarboxylase (GAD) antibody [Enzyme immunoassay (EIA), GAD Ab, Cosmic, Tokyo, Japan]; however, all other anti-pancreatic islet antibodies were negative. Serum chemistry and electrolytes levels were as follows: Na 136 mEq/L, K 3.6 mEq/L, Cl 98 mEq/L, Ca 9.2 mEq/L, BUN 9.7 mg/dL; Creatinine 0.91 mg/dL and total protein 7.6 mg/dL. A complete blood cell (CBC) count showed a hemoglobin level of 12.7 g/dL with 37% hematocrit, a WBC count of 67 × 102/µL with 54.6% neutrophils, and a plate count of 13.7 × 104/mm3. From these findings and the rapid clinical course, we finally diagnosed fulminant onset type 1 diabetes, suspecting nivolumab to be the agent responsible because there were no other factors associated with glucose metabolism. Human leukocyte antigen (HLA) typing did not identify any specific alleles known to be closely related to the onset of type 1 diabetes. After admission, the nivolumab therapy was suspended and intensive insulin therapy with the combination of rapid and long-acting insulin was started. Before and during his hospitalization, the patient experienced no abdominal or upper airway symptoms which are commonly observed with typical acute-onset type 1 diabetes. A combination of abdominal computed tomography (CT) with contrast and Magnetic Resonance Imaging (MRI) without gadolinium eliminated pancreatic complications (Fig. 2). He was discharged after the titration of insulin dose and was in stable disease for up to 7 months from the initiation of nivolumab therapy even though no radiological response had been observed. At the 6-month follow-up, he was negative for anti-GAD antibody; however, the diabetes had not improved and insulin requirement was not reduced. Nivolumab was initially administered for three cycles (6 weeks) and suspended for 7 months after hyperglycemic event. At the time when anti-GAD antibody disappeared, nivolumab was being suspended. Shortly after the confirmation of being negative for anti-GAD antibody, nivolumab was resumed without further exacerbation of diabetes or other endocrine disorders. However, no improvement of hyperglycemia was noted and insulin requirement was not reduced when the anti-GAD antibody titer decreased.
Table 2.
Laboratory data on admission
| Complete blood count | |
| White blood cell (WBC) | 67 × 102/µL |
| Red blood cell (RBC) | 375 × 104/µL |
| Hemoglobin | 12.7 g/dL |
| Hematocrit | 37.0% |
| MCV | 99 fL |
| MCH | 33.9 pg |
| MCHC | 34.3 g/dL |
| Platelet (PLT) | 13.7 × 104/µL |
| Biochemistry | |
| Total protein | 7.6 g/dL |
| Albumin | 3.7 g/dL |
| AST | 38 IU/L |
| ALT | 23 IU/L |
| Total bilirubin | 0.6 mg/dL |
| Alkaline phosphatase (ALP) | 597 IU/L |
| γ-glutamyltransferase (γ-GTP) | 295 IU/L |
| C-reactive protein (CRP) | 1.35 mg/dL |
| Plasma glucose | 527 mg/dL |
| HbA1c (NGSP) | 6.1% |
| Blood urea nitrogen (BUN) | 9.7 mg/dL |
| Creatinine | 0.91 mg/dL |
| Na | 136 mEq/L |
| K | 3.6 mEq/L |
| Cl | 98 mEq/L |
| Total ketone body | 101 µmol/L |
| Serum C-peptide | < 0.1 ng/mL |
| Urinary C-peptide | < 0.3 µg/day |
| ACTH | 27.6 pg/mL |
| Cortisol | 13.0 µg/dL |
| TSH | 4.45 µIU/mL |
| FT3 | 2.5 pg/mL |
| Urinalysis | |
| Sugar | (4+) |
| Protein | (±) |
| Occult blood | (−) |
| Urinary ketone body | (−) |
| Islet autoantibodies | |
| GAD antibody (< 5.0 U/ml) | 41.1 U/mL |
| IA-2 antibody | (−) |
| ZnT8 antibody | (−) |
| HLA DNA typing | |
| DRB1*0301-DQB1*0803 | |
| DRB1*0601-DQB1*1406 | |
ACTH adrenocorticotrophic hormone, AST aspartate aminotransferase, ALT alanine transaminase, FT3 free tri-iodothyronine, GAD glutamic acid decarboxylase, HLA human leukocyte antigen, IA-2 islet-associated antigen-2, MCV mean corpuscular volume, MCH mean corpuscular hemoglobin, MCHC mean corpuscular hemoglobin concentration, NGSP National Glycohemoglobin Standardization Program, TSH thyroid-stimulating hormone, ZnT8 zinc transporter 8
Fig. 2.
Enhanced CT (a) and MRI (b) imaging. Significant abnormal findings of pancreas were not noted
Discussion
With the rise of immunotherapy, the concept of anti-cancer treatment has been rapidly changing, and much of the recent therapeutic progression with immune checkpoint inhibitors has been encouraging. Nivolumab is a fully human IgG4 monoclonal antibody immune checkpoint inhibitor, which targets PD-1 and inhibits binding to its ligands, PD-L1 and PD-L2. It is currently approved for the treatment of NSCLC [3], malignant melanoma [4], renal cell carcinoma [5], head and neck cancer [6], and Hodgkin lymphoma [7], and it is expected to be approved soon for the treatment of some of gastrointestinal cancer [8, 9].
PD-1 is expressed on the surface of activated and regulatory T-cells, whereas PD-L1 is primarily expressed on tumor cells. The interaction between these is known to play an important role in the regulation of the immune tolerance mechanism, preventing the excessive self-immunity associated with the functional loss of T-cell inhibition. Conversely, the inhibition of immune checkpoint molecules such as PD-1, PD-L1, and cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) not only reinforces the anti-cancer mechanism through T-cell activation but also dysregulates the self-immune tolerance system responsible for autoimmune activation in various organs that can result in severe IrAEs [2]. Endocrine complications are also assumed to be types of IrAE, although their pathological mechanisms remain to be elucidated, unlike those of other IrAEs. It has been postulated that activated T-cells triggered by PD-1 inhibition infiltrate into normal tissue, generating autoimmune toxicities. Hypothyroidism is considered to be the most frequent endocrine disorder caused by anti-PD-1 antibodies, but other endocrine disorders, such as hypophysitis and adrenal insufficiency, have been reported [10]. Type 1 diabetes has also been identified as a serious adverse event provoked by anti PD-1 and PD-L1 antibodies [11–13]. However, the incidence of type 1 diabetes triggered by immune checkpoint inhibitors is extremely low compared with that of other IrAEs, although there have been several reports of patients developing fulminant type 1 diabetes during treatment with anti-PD-1 antibodies, as in our case [14–16].
Fulminant type 1 diabetes has only recently been discovered. It is an important subtype, especially in East Asia, and accounts for approximately 20% of acute-onset type 1 diabetes cases in Japan [17]. It is defined by the abrupt onset of hyperglycemia and ketoacidosis around a week and is mostly negative for islet-related antibodies such as islet-cell and anti-GAD antibodies [18]. On the other hand, several reports have remarkably demonstrated a case of the onset of fulminant type 1 diabetes with a presence of anti-GAD antibody [19–21]. Although the precise pathogenesis of fulminant type 1 diabetes is unclear, genetic background and viral infection both appear to be important factors [18]. There have been no reports on record regarding dramatic onset of hyperglycemia in an extremely short duration during PD-1 or PD-L1 treatment. Our case is very similar to that of fulminant type 1 diabetes in the manner of remarkably abrupt onset although it does not meet all requirements for the diagnostic criteria. The sudden increase in hyperglycemia in our patient may have occurred as a consequence of the rapid destruction of pancreatic islet beta cells, which leads to a lack of endogenous insulin secretion. It could be postulated that T-lymphocytes activated by immune checkpoint inhibitors infiltrated into pancreatic tissue, causing damage to islet beta cells; however, the precise mechanism remains unexplored. As already noted, ketoacidosis is a major diagnostic criterion of fulminant type 1 diabetes. The reason why ketoacidosis and other typical symptoms described in a previous case study [14] were not observed in our patient could be because he had continued self-injection of insulin as prescribed, preventing severe symptoms, even though the dose of insulin was insufficient to control his blood glucose. In this case, the anti-GAD antibody titer was elevated at the diagnosis of type 1 diabetes. It is uncertain whether anti-GAD antibodies were present or absent prior to the treatment with nivolumab, because our patient did not undergo a pre-treatment evaluation for this. In that regard, Lowe et al. reported the case of anti-GAD antibody which turned to be positive with the onset of diabetes after the combination therapy of nivolumab and ipilimumab [22]. A previous report classified a specific subgroup of diabetes as slowly progressive insulin-dependent type 1 diabetes (SPIDDM), in which anti-GAD and/or islet-cell antibodies are positive [23]. SPIDDM, which shows similar characteristics to type 2 diabetes, does not present ketosis or ketoacidosis at the time of onset and there is no requirement for urgent insulin treatment after diagnosis. In our patient, the presence of anti-GAD antibodies and a history of type 2 diabetes made it difficult to distinguish the fulminant exacerbation of SPIDDM from new-onset type 1 diabetes. It could be supposed that he already had SPIDDM before commencing nivolumab. Conversely, the anti-GAD antibodies may have been produced by the mechanism of PD-1 inhibition after treatment with nivolumab. Human leukocyte antigen (HLA) -typing analysis of the patient did not show a high-risk HLA haplotype that predisposed to fulminant type 1 diabetes. Regardless of the presence of anti-GAD antibodies, the mechanism for the fulminant hyperglycemia in our patient does not appear to have been mediated by humoral immunity because antibody-mediated type 1 diabetes, typically defined as acute-onset type 1 diabetes, usually has an onset within several weeks and shows significantly elevated HbA1c, in contrast to our case. Therefore, although it is clear that complete destruction of the pancreatic islet beta cells occurred, the mechanisms of this phenomenon still remain unclear. The pathogenesis of fulminating onset in our case appeared to be associated with cellular immunity driven by T-cells to some extent. Interestingly, a recent report demonstrated positive anti-thyroid peroxidase antibody titers after the initiation of pembrolizumab in some melanoma patients [24]. These results could imply that PD-1 inhibition also modulates the mechanism of humoral immunity, but details have not been investigated. In that regard, previous report showed that inhibition of the PD-1 and PD-L1 axis promoted B-cell proliferation and interleukin-6 production [25]. Previously reported cases of diabetes as IrAE with autoantibodies are summarized (Table 3). The time to diagnosis of diabetes in each case was not uniform and there was no report describing the precise period until hyperglycemia had occurred. Only one case was diagnosed with fulminant diabetes [32]. However, unlikely our case, HbA1c level at onset was elevated and it was assumed that it would take time for the progression of diabetes. GAD antibody was the most common antibody among reported cases. Of interest, besides this case, no Asian patients with autoantibody-positive diabetes provoked by immunotherapy have been reported in contrast to autoantibody-negative fulminant diabetes. Ethnic differences may influence this phenomenon but details remain obscure.
Table 3.
Case review of diabetic onset with autoantibodies during the treatment with PD-1/L1 antibody
| Authors | Age | Gender | Type of cancer | Relevant history | Agent | Time to onset (weeks) | HbA1c (%) | C-peptide (ng/mL) | Glucose (g/dL) | Symptoms | Autoantibodies | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Hughes et al. [26] | 83 | Female | NSCLC | None | Nivolumab | 4 | 7.7 | < 0.1 | 300 | DKA | GAD |
| 2 | Hughes et al | 63 | Male | RCC | None | Nivolumab | 16 | 8.2 | 1.3 | 200 | NR | GAD/ICA/IA-2 |
| 3 | Hughes et al | 58 | Male | SCLC | Type 2 DM | Nivolumab | 1 | 9.7 | < 0.1 | 719 | DKA | GAD |
| 4 | Hansen et al. [13] | 58 | Male | BRAF MT melanoma | NR | Pembrolizumab | 51 | 9.7 | NR | 400 | Polydipsia, polyuria | GAD |
| 5 | Martin-Liberal et al. [12] | 54 | Female | BRAF WT melanoma | Asthma | Pembrolizumab | 9 | NR | NR | NR | DKA | GAD |
| 6 | Mellati et al. [27] | 66 | Female | HNSCC | NR | Anti-PD-1Ab(*) | 8 | 9.4 | < 0.1 | 752 | NR | GAD |
| 7 | Hofmann et al. [28] | 58 | Female | Melanoma | NR | Pembrolizumab | 3 | NR | Low | NR | Thirsty, polyuria | GAD |
| 8 | Hofmann et al. | 78 | Female | Melanoma | NR | Nivolumab | 2 | NR | Low | NR | Vomiting, diarrhea | GAD |
| 9 | Chae et al. [29] | 76 | Male | NSCLC (AC) | NR | Pembrolizumab | 3 | NR | 0.81 | 600 | Asymptomatic | GAD/IA-2 |
| 10 | Kapke et al [30] | 83 | Male | HNSCC | Hypothyroidism | Nivolumab | 12 | 7.4 | 0.32 | 426 | DKA | GAD |
| 11 | Kapke et al | 63 | Male | Urothelial Carcinoma | Hypothyroidism | Atezolizumab | 24 | 7.8 | 0.02 | 801 | DKA | GAD |
| 12 | Godwin et al. [31] | 34 | Female | NSCLC | None | Nivolumab | 4 | 7.1 | < 0.1 | 739 | DKA | GAD/IA-2 |
| 13 | Gauci et al. [32] | 73 | Male | Melanoma | None | Nivolumab | 6 | 8.8 | Low | 504 | DKA | GAD/ZnT8 |
| 14 | This report | 78 | Male | NSCLC (SQCC) | Type 2 DM | Nivolumab | 5 | 6.1 | < 0.1 | 527 | Asymptomatic | GAD |
(*) Name unknown, Ab antibody, AC adenocarcinoma, BRAF MT B-RAF mutant type, BRAF WT B-RAF wild type, DKA diabetic ketoacidosis, GAD glutamic acid decarboxylase, HNSCC head and neck squamous cell carcinoma, IA-2 islet-associated antigen-2, ICA islet-cell autoantigen, NR not reported, NSCLC non-small cell lung cancer, RCC renal cell carcinoma, SCLC small cell lung cancer, SQCC squamous cell carcinoma, Type 2 DM type 2 diabetes mellitus, ZnT8 Zinc transporter 8
It is important to identify and manage patients who could suffer from serious IrAEs induced by immune checkpoint inhibitors. However, there is insufficient evidence to conclude that the presence of anti-GAD antibodies at baseline is a risk factor for type 1 diabetes in patients treated with anti-PD-1 antibodies. Further investigation is needed to identify the role of baseline measurement of anti-GAD antibodies as a predictive marker for fulminant hyperglycemia.
In conclusion, fulminant type 1 diabetes is a rare but a rapidly progressive and life-threatening adverse event. The present case emphasized that close and careful monitoring, as well as appropriate management, is essential for patients treated with immune checkpoint inhibitors.
Abbreviations
- AUC
Area under the curve
- CT
Computed tomography
- CTLA-4
Cytotoxic T-lymphocyte-associated protein-4
- DM
Diabetes mellitus
- EIA
Enzyme immunoassay
- GAD
Glutamic acid decarboxylase
- HbA1c
Hemoglobin A1c
- HLA
Human leukocyte antigen
- IrAE
Immune-related adverse event
- MRI
Magnetic resonance imaging
- NSCLC
Non-small cell lung cancer
- SPIDDM
Slowly progressive insulin-dependent diabetes mellitus
Author contributions
All the authors had full access to all the data in the study. Manuscript was prepared by NM and GK. Clinical data were prepared and interpreted by NM, GK, CK, SM, and Y T. HH, KK, and ME reviewed the paper and provided important advice.
Funding
This research did not receive any specific grant from any funding agencies in the public, commercial or not for profit sectors.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interests.
Informed consent
This report was approved by ethics committee of Yao-Tokushukai General Hospital. Written informed consent was obtained from the patient for this report.
Footnotes
Nobuko Matsuura and Genju Koh have contributed equally to this work.
Ken Kodama and Masanori Emoto are co-last authors.
References
- 1.De Velasco G, Je Y, Bosse D, et al. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res. 2017;5:312–318. doi: 10.1158/2326-6066.CIR-16-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17:1283–1294. doi: 10.1016/S1470-2045(16)30167-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kudo T, Hamamoto Y, Kato K, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol. 2017;18:631–639. doi: 10.1016/S1470-2045(17)30181-X. [DOI] [PubMed] [Google Scholar]
- 10.Costa RCB, Agulnik M, Rademaker AW, et al. Toxicity profile of approved anti-PD-1 monoclonal antibodies in solid tumors: a systematic review and meta-analysis of randomized clinical trials. Oncotarget. 2017;8:8910–8920. doi: 10.18632/oncotarget.13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hickmott L, De La Pena H, Turner H, et al. Anti -PD-L1 atezolizumab-induced autoimmune diabetes: a case report and review of the literature. Target. Oncol. 2017;12:235–241. doi: 10.1007/s11523-017-0480-y. [DOI] [PubMed] [Google Scholar]
- 12.Martin-Liberal J, Furness AJ, Joshi K, et al. Anti-programmed cell death-1 therapy and insulin-dependent diabetes: a case report. Cancer Immunol Immunother. 2015;64:765–767. doi: 10.1007/s00262-015-1689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen E, Sahasrabudhe D, Sievert L. A case report of insulin-dependent diabetes as immune-related toxicity of pembrolizumab: presentation, management and outcome. Cancer Immunol Immunother. 2016;65:765–767. doi: 10.1007/s00262-016-1835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong SH, Lee SY, Yang YS, Kim TM, Kwak SH. Anti-programmed cell death 1 therapy triggering diabetic ketoacidosis and fulminant type 1 diabetes. Acta Diabetol. 2016;53:853–856. doi: 10.1007/s00592-016-0872-y. [DOI] [PubMed] [Google Scholar]
- 15.Munakata W, Ohashi K, Yamauchi N, Tobinai K. Fulminant type I diabetes mellitus associated with nivolumab in a patient with relapsed classical Hodgkin lymphoma. Int J Hematol. 2017;105:383–386. doi: 10.1007/s12185-016-2101-4. [DOI] [PubMed] [Google Scholar]
- 16.Miyoshi Y, Ogawa O, Oyama Y. Nivolumab, an anti-programmed cell death-1 antibody, induces fulminant Type 1 Diabetes. Tohoku J Exp Med. 2016;239:155–158. doi: 10.1620/tjem.239.155. [DOI] [PubMed] [Google Scholar]
- 17.Imagawa A, Hnafusa T, Miyagawa J, Matsyzawa Y. A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence od diabetes-related antibodies. N Engl J Med. 2000;342:301–307. doi: 10.1056/NEJM200002033420501. [DOI] [PubMed] [Google Scholar]
- 18.Imagawa A, Hanafusa T. Fulminant type 1 diabetes–an important subtype in East Asia. Diabetes Metab Res Rev. 2011;27:959–964. doi: 10.1002/dmrr.1236. [DOI] [PubMed] [Google Scholar]
- 19.Iwaoka T. A case of fulminant type 1 diabetes with transiently positive anti-GAD antibodies. Endocrine J. 2003;50:225–231. doi: 10.1507/endocrj.50.225. [DOI] [PubMed] [Google Scholar]
- 20.Kahara T, Takamura T, Ando H, et al. Fulminating onset type 1 diabetes with positivity for anti-GAD antibody and elevated pancreatic exocrine enzyme concentrations. Intern Med. 2003;42:517–520. doi: 10.2169/internalmedicine.42.517. [DOI] [PubMed] [Google Scholar]
- 21.Makino SH, Iwata S, Fujiwara M. A case of abrupt onset autoimmune type 1 diabetes mimicking fulminant type 1 diabetes. Endocrine J. 2009;56:1113–1117. doi: 10.1507/endocrj.K09E-074. [DOI] [PubMed] [Google Scholar]
- 22.Lowe JR, Perry DJ, Salama AKS, et al. Genetic risk analysis of a patient with fulminant autoimmune type 1 diabetes mellitus secondary to combination ipilimumab and nivolumab immunotherapy. J Immunother Cancer. 2016;4:89. doi: 10.1186/s40425-016-0196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi T, Tanemoto T, Nakanishi K, et al. Immunogenetic and clinical characterization of slowly progressive IDDM. Diabetes Care. 1993;16:780–788. doi: 10.2337/diacare.16.5.780. [DOI] [PubMed] [Google Scholar]
- 24.Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017;28:583–589. doi: 10.1093/annonc/mdw640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thibult ML, Mamessier E, Gertner-Dardenne J, et al. PD-1 is a novel regulator of human B-cell activation. Int Immunol. 2013;25:129–137. doi: 10.1093/intimm/dxs098. [DOI] [PubMed] [Google Scholar]
- 26.Hughes J, Vudattu N, Sznol M, et al. Precipitation of autoimmune diabetes with anti PD-1 immunotherapy. Diabetes care. 2015;38:e55–e57. doi: 10.2337/dc15-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mellati M, Eaton KD, Brooks-Worrell BM, Hagopian WA, Martins R, Palmer JP, Hirsch IB. Anti-PD-1 and anti-PDL-1 monoclonal antibodies causing Type 1 Diabetes. Diabetes Care. 2015;38:e137–e138. doi: 10.2337/dc15-0889. [DOI] [PubMed] [Google Scholar]
- 28.Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190–209. doi: 10.1016/j.ejca.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 29.Chae YK, Chiec L, Mohindra N, et al. A case of pembrolizumab-induced type-1 diabetes mellitus and discussion of immune checkpoint inhibitor-induced type 1 diabetes. Cancer Immunol Immunother. 2017;66:25–32. doi: 10.1007/s00262-016-1913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapke J, Shaheen Z, Kilari D, et al. Immune checkpoint inhibitor associated type 1 diabetes mellitus: case series, review of literature and optimal management. Case Rep Oncol. 2017;10:897–909. doi: 10.1159/000480634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Godwin JL, Jaggi S, Sharda I, et al. Nivolumab-induced autoimmune diabetes mellitus presenting as diabetic ketoacidosis in a patient with metastatic lung cancer. J Immunother Cancer. 2017;5:40–46. doi: 10.1186/s40425-017-0245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gauci ML, Laly P, Vidal-Trecan T, et al. Autoimmune diabetes induced by PD-1 inhibitor-retrospective analysis and pathogenesis: a case report and literature review. Cancer Immunol Immunother. 2017;66:1399–1410. doi: 10.1007/s00262-017-2033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]