Abstract
In many tumors, including prostate cancer, anti-apoptotic members of the Bcl-2 family are overexpressed and cause cell death resistance, which is a typical hallmark of cancer. Different therapeutic approaches, therefore, aim to restore the death mechanisms for enhanced apoptosis. Our recombinant immunotoxin D7(VL-VH)-PE40 is composed of the scFv D7(VL-VH) against the prostate-specific membrane antigen (PSMA) on the surface of prostate cancer cells and of the cytotoxic domain of the bacterial toxin Pseudomonas Exotoxin A (PE40). Since Pseudomonas Exotoxin A-based immunotoxins are known to preferentially inhibit the expression of the anti-apoptotic protein Mcl-1, the rationale was to test our immunotoxin in combination with the BH3 mimetic ABT-737, which specifically inhibits Bcl-2, Bcl-xl, and Bcl-w for enhanced induction of apoptosis in prostate cancer cells. The immunotoxin showed high and specific binding and cytotoxicity against PSMA expressing prostate cancer cells marked by a direct inhibition of Mcl-1. The combination of the immunotoxin with a subtoxic concentration of ABT-737 caused additive or even synergistic effects, which were based on an enhanced apoptosis induction as detected by poly(ADP-ribose) polymerase (PARP) and Caspase-3 cleavage in Western blot. Our study shows that the combination therapy of immunotoxin plus ABT-737 is a promising approach for the future treatment of advanced prostate cancer to improve therapeutic efficacy and to reduce adverse side effects.
Electronic supplementary material
The online version of this article (10.1007/s00262-017-2097-5) contains supplementary material, which is available to authorized users.
Keywords: Prostate cancer, PSMA, Immunotoxin, ABT-737, Bcl-2 proteins, Apoptosis
Introduction
With estimated 1.1 million new cases per year, prostate cancer remains the second-most frequently diagnosed cancer among men worldwide. Moreover, with expected 307,000 deaths, it represents the fifth leading cause of cancer deaths [1]. Whereas primary tumors can successfully be managed, there is no curative treatment for advanced stages. Therefore, new therapeutic options are urgently needed.
Tumor cells are characterized in that they hold cell death resistance, which is a typical hallmark of cancer [2]. Cell death resistance can be based on impaired apoptosis signaling marked by a disturbed balance between pro- and anti-apoptotic members of the B cell lymphoma 2 (Bcl-2) protein family. In many tumors including prostate cancer, the main anti-apoptotic members Bcl-2, B cell lymphoma extra-large (Bcl-xl), and myeloid cell leukemia sequence 1 (Mcl-1) are overexpressed and promote tumor development [3, 4].
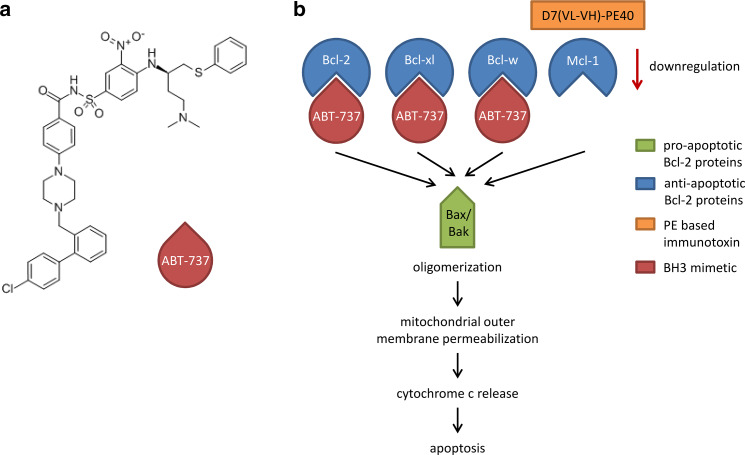
Different cancer therapeutic approaches aim to restore the death mechanisms for enhanced apoptosis and higher chemosensitivity [5]. Two main strategies to directly inhibit anti-apoptotic Bcl-2 family members comprise the use of antisense oligonucleotides and the application of BH3 (Bcl-2 homology domain 3) mimetics [6]. The latter are small molecules or modified peptides, which insert their BH3 motifs into the hydrophobic groove of the anti-apoptotic Bcl-2 proteins. This is followed by liberation of the pro-apoptotic members Bcl-2-associated X protein (Bax) and Bcl-2 antagonist/killer (Bak) and activation of the intrinsic apoptotic pathway [7]. The small-molecule BH3 mimetic ABT-737 (4-[4-[[2-(4-chlorophenyl)phenyl]methyl]piperazin-1-yl]-N-[4-[[(2R)-4-(dimethylamino)-1-phenylsulfanylbutan-2-yl]amino]-3-nitrophenyl] sulfonylbenzamide; Fig. 1a) was generated by structure-based design for binding to Bcl-xl [8]. Like the BH3-only protein Bcl-2 antagonist of cell death (BAD), ABT-737 selectively binds to Bcl-2, Bcl-xl, and Bcl-w with high affinity in the subnanomolar range, and is, therefore, also called a BAD-like BH3 mimetic (Fig. 1b) [8, 9]. Thus, in tumors with enhanced Mcl-1 expression, ABT-737 is less effective and combination with agents that degrade or neutralize Mcl-1 is preferred [10–13].
Fig. 1.
Chemical structure and mode of action of the BH3 mimetic ABT-737 in combination with the immunotoxin D7(VL-VH)-PE40. a Chemical structure of the BH3 mimetic ABT-737 (4-[4-[[2-(4-chlorophenyl)phenyl]methyl]piperazin-1-yl]-N-[4-[[(2R)-4-(dimethylamino)-1-phenylsulfanylbutan-2-yl]amino]-3-nitrophenyl] sulfonylbenzamide). b Induction of apoptosis by combination of ABT-737 and the immunotoxin D7(VL-VH)-PE40. ABT-737 inhibits the anti-apoptotic proteins Bcl-2, Bcl-xl, and Bcl-w, but not Mcl-1, by binding. The immunotoxin downregulates Mcl-1 by inhibition of protein biosynthesis. This leads to the induction of the intrinsic apoptotic pathway via oligomerization of the released pro-apoptotic proteins Bax and Bak, mitochondrial outer membrane permeabilization and cytochrome c release
In our laboratory, the recombinant immunotoxin D7(VL-VH)-PE40 was generated for the targeted treatment of prostate cancer. The binding domain consists of the scFv (single chain variable fragment) D7(VL-VH), which is specific for the prostate-specific membrane antigen (PSMA), a transmembrane protein on the surface of prostate cancer cells [14, 15]. The 40 kDa truncated form of Pseudomonas aeruginosa Exotoxin A (PE), called PE40, was used as the toxin domain. It enables the immunotoxin to permeate cellular compartments to reach the cytosol and to inhibit ribosomal protein biosynthesis by ADP-ribosylation of the eukaryotic elongation factor 2 (eEF-2). This is followed by induction of apoptosis of the target cell [16]. Since PE-based immunotoxins are known to preferentially inhibit the Mcl-1 expression [17–20], the rationale of the present study was to test the immunotoxin D7(VL-VH)-PE40 in combination with ABT-737 for enhanced induction of apoptosis in prostate cancer cells.
Materials and methods
Cell lines, immunotoxin and reagents
The PSMA-positive prostate cancer cell line LNCaP, its androgen-independent subline C4-2, and the PSMA-negative cell line DU 145 were purchased from ATCC (American Type Culture Collection, Manassas, VA, USA). Cells were propagated in RPMI 1640 medium (Gibco, Invitrogen, Karlsruhe, Germany) containing 10% fetal calf serum (Biochrom, Berlin, Germany) and penicillin/streptomycin (100 U/ml, 100 mg/l) at 37 °C and 5% CO2. Cell line identity was verified using short tandem repeat (STR) analysis (CLS GmbH, Eppelheim, Germany). The anti-PSMA immunotoxin D7(VL-VH)-PE40 was prepared as described earlier and stored at − 20 °C [14]. ABT-737 (Abcam, Cambridge, UK) was dissolved in DMSO at a stock solution of 20 mM and stored at 4 °C.
Flow cytometry
Flow cytometric analyses were performed to evaluate the cell binding of the immunotoxin as described previously [14]. In brief, prostate cancer cells were incubated with different concentrations of D7(VL-VH)-PE40 for 1 h on ice. After washing with ice-cold PBS, mouse anti-human c-myc (Avian myelocytomatosis virus oncogene cellular homolog) monoclonal antibody (Roche Diagnostics, Mannheim, Germany) and goat anti-mouse Ig-R-PE (Becton Dickinson, Mountain View, CA, USA) were each added for 40 min on ice. After resuspension in ice-cold PBS containing 3% FBS, 0.1% sodium azide and 2 µg/ml propidium iodide, MFI values of stained cells were determined with help of a FACSCalibur flow cytometer and the software CellQuest Pro (BD Biosciences, Heidelberg, Germany).
Cytotoxicity assay
The WST-1 (water-soluble tetrazolium salt) cell viability assay was used to examine cytotoxic effects (Roche Diagnostics, Indianapolis, IN, USA). For this, 1.5 × 104 cells/well were seeded in a 96-well plate and cultivated overnight. Then, the immunotoxin and ABT-737 were added either alone or in combination. After 24 and 48 h, WST-1 reagent was added and plates were incubated until the absorbance at 450 nm reached values of about 1.5–2.5 OD. IC50-values were defined as drug concentrations leading to a reduction of 50% cell viability. They were calculated by non-linear regression [log (inhibitor) vs. response (three parameters)] (GraphPad Prism 6 Software, Inc., San Diego, CA). Combination Index (CI) was determined for quantitative definition of synergism (< 1.0), additivity (1.0–1.2) or antagonism (> 1.2) of the two active substances [21].
Cell killing assay
Dead cells were determined via trypan blue assay (Logos Biosystems, Germany). 1.5 × 105 cells were seeded in a 6-well plate, incubated overnight and treated with subtoxic concentrations of the immunotoxin alone (50 pM for LNCaP and 190 pM for C4-2 and DU 145 cells), ABT-737 alone or with the combination of both. After 24 or 48 h, the cells were trypsinized and stained with 0.4% trypan blue solution. The populations of dead and viable cells were counted by LUNA Automated Cell Counter (Logos Biosystems, Germany) and represented as percent of dead cells. Significance of the data was estimated with Student’s t test (unpaired, parametric with Welch’s correlation, GraphPad Prism 6 Software).
Western blot analysis
Prostate cancer cells were treated with subtoxic immunotoxin concentrations (50 pM for LNCaP and 190 pM for C4-2 and DU 145 cells) alone or in combination with a subtoxic concentration of 100 nM ABT 737. After 4, 24 and 48 h, cells were harvested and incubated in lysis buffer containing 30 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1% Triton-X, 10% glycerol, 1 mM 1,4-dithiothreitol, 200 µM phenylmethylsulfonylfluorid supplemented with cOmplete™, EDTA-free Protease Inhibitor Cocktail (Roche Diagnostics) for 20 min on ice. Afterwards, cells were centrifuged at 4000 rpm and supernatants were collected for Western blot analysis. Protein concentration of the whole cell lysates was measured by BCA (Bicinchoninic Acid) protein assay kit (Thermo Scientific, Rockford, IL, USA).
150 µg of cell lysate per lane were separated by SDS–PAGE and blotted. Blot membranes were saturated in 5% low-fat milk/PBS-Tween 20 (0.05% v/v) for 1 h at room temperature (RT) and incubated with antibodies against human Bcl-2, Bcl-xl, Mcl-1 (Santa Cruz, Heidelberg, Germany), mouse anti-human caspase 3 (ECM Biosciences, Köln, Germany), or anti-human poly(ADP-ribose) polymerase (PARP; Cell Signaling Tech., Danvers, MA, USA) in 5% low-fat milk at 4 °C overnight. After washing with PBS-Tween 20, membranes were incubated with the secondary antibodies goat anti-mouse IgG-HRP or goat anti-rabbit IgG-HRP (Santa Cruz) in 5% low-fat milk at 4 °C overnight. β-actin was detected using a HRP-labeled mouse anti-human β-actin monoclonal antibody (Cell Signaling Tech.). Blots were developed with an enhanced chemiluminescence (ECL) system and protein bands were detected with help of Agfa device Curix 60 (Düsseldorf, Germany).
Results
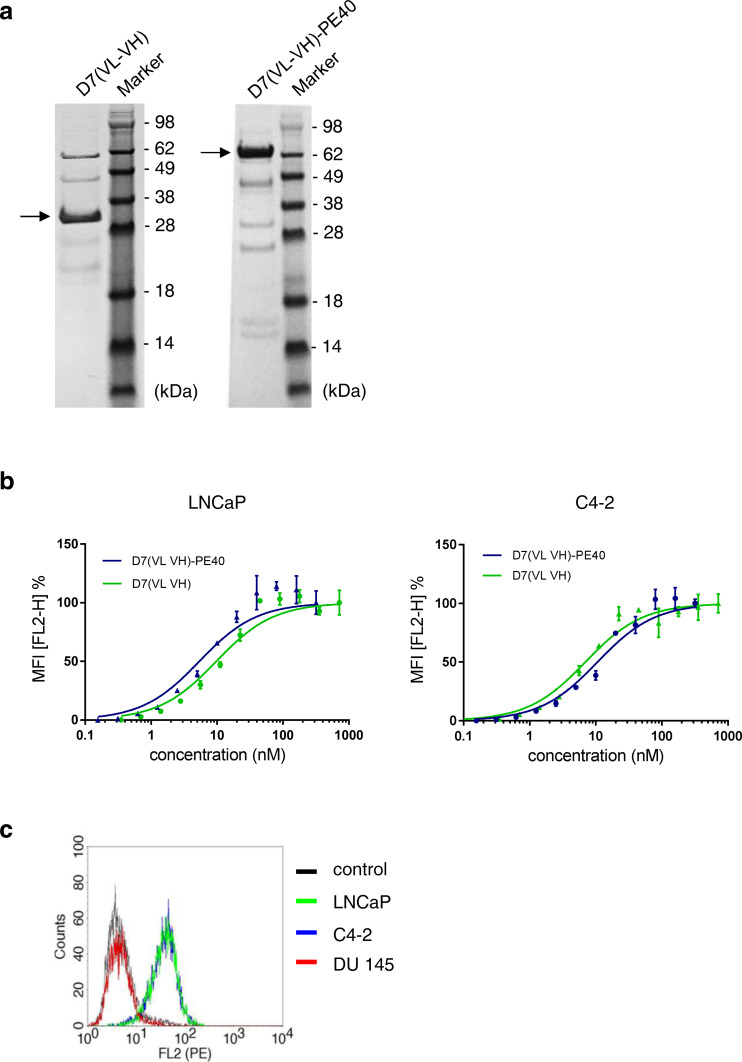
The purity of the 72 kDa anti-PSMA immunotoxin D7(VL-VH)-PE40 and the 32 kDa scFv D7(VL-VH) was proved by SDS-PAGE (Fig. 2a). To verify the specificity of the immunotoxin D7(VL-VH)-PE40, binding to PSMA-positive prostate cancer cells was tested by flow cytometry. The apparent binding affinity (K d), defined as the immunotoxin’s concentration leading to half-maximal-specific binding, was found to be 5.5 nM on LNCaP and 10.8 nM on C4-2 cells. Comparable binding affinities were calculated with the antibody fragment scFv D7(VL-VH) (LNCaP: K d = 10 nM and C4-2: K d = 6.1 nM), indicating that there was no influence of the PE40 toxin domain on PSMA binding (Fig. 2b). The immunotoxin was found not to bind to PSMA-negative DU 145 cells (Fig. 2c).
Fig. 2.
Purity and cell binding characteristics of the anti-PSMA scFv D7(VL-VH) and the immunotoxin D7(VL-VH)-PE40. a SDS-PAGE of the scFv D7(VL-VH) and the immunotoxin D7(VL-VH)-PE40. b Binding of the scFv D7(VL-VH) and the immunotoxin D7(VL-VH)-PE40 to PSMA expressing LNCaP and C4-2 prostate cancer cells as measured by flow cytometry. c Compared to LNCaP and C4-2 cells, no binding of D7(VL-VH)-PE40 was seen on PSMA-negative DU 145 cells at saturation concentration. Control: without immunotoxin
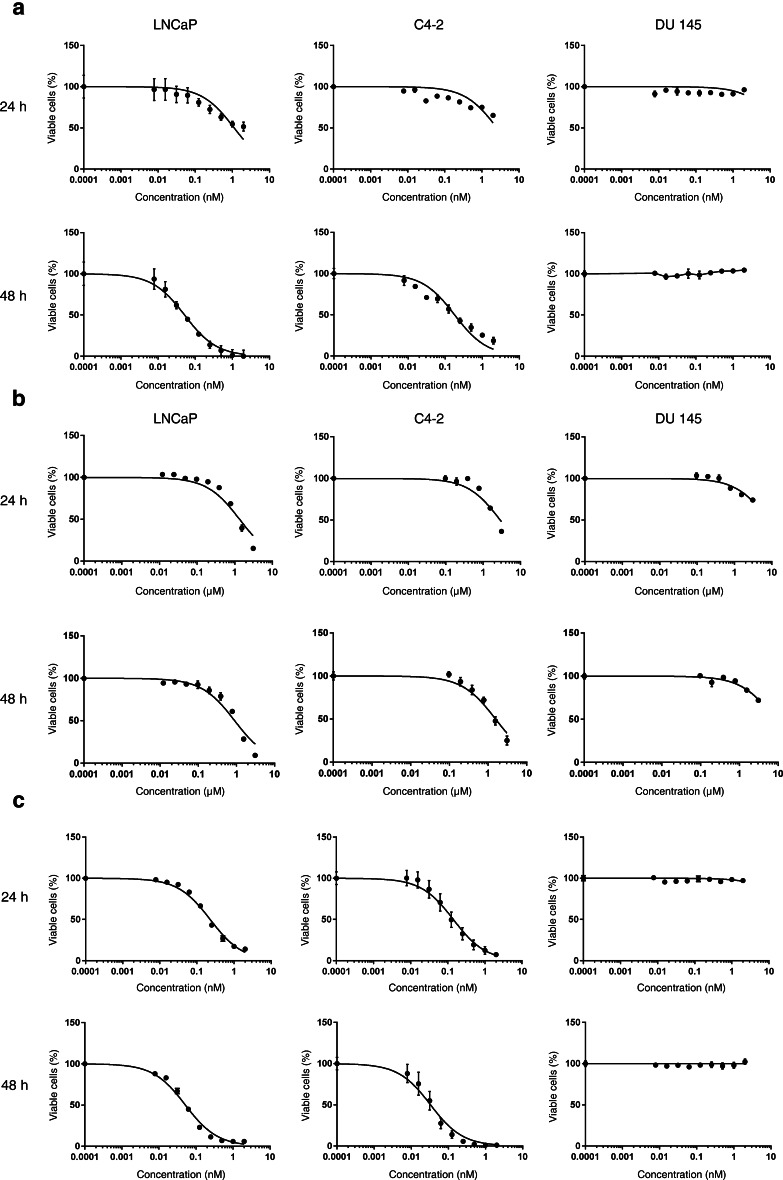
Next, we determined the cytotoxicity of the immunotoxin and ABT-737 to find suitable concentrations for further combinatorial testing. With D7(VL-VH)-PE40, IC50 values of 1.402 and 0.052 nM were reached on LNCaP cells after 24 and 48 h, respectively. On C4-2 cells, IC50 values of 2.56 and 0.191 nM in the same timeframes were about two to threefold higher than on LNCaP cells. No cytotoxicity was observed on DU 145 control cells (Fig. 3a; Table 1). ABT-737 was shown to elicit a 50% reduction of viable LNCaP cells at concentrations of 1.348 and 899 nM after 24 and 48 h, respectively. On C4-2 cells, IC50 values of 2.868 and 1.596 nM were reached. On DU 145 cells, only a weak cytotoxic activity was observed and IC50 values were not reached within our tested concentration range (Fig. 3b; Table 1).
Fig. 3.
Cytotoxicity of the anti-PSMA immunotoxin D7(VL-VH)-PE40 in combination with the BH3 mimetic ABT-737. a Anti-PSMA immunotoxin D7(VL-VH)-PE40, b ABT-737, and c D7(VL-VH)-PE40 in combination with 100 nM ABT-737 on prostate cancer cells was measured by WST-1 viability assay. The graphics represent mean values ± SEM of three independent experiments
Table 1.
IC50 values of D7(VL-VH)-PE40 and ABT-737 alone and in combination on different prostate cancer cells
| IC50 (nM) | ||||||
|---|---|---|---|---|---|---|
| LNCaP | C4-2 | DU145 | ||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| D7(VL-VH)-PE40 | 1.402 | 0.052 | 2.560 | 0.191 | > 2.0 | > 2.0 |
| ABT-737 | 1.348 | 899 | 2.868 | 1.596 | > 3.100 | > 3.100 |
| D7(VL-VH)-PE40 + 100 nM ABT-737 | 0.230 | 0.051 | 0.126 | 0.029 | > 2.0 | > 2.0 |
| D7(VL-VH)-PE40 + 200 nM ABT-737 | 0.210 | 0.032 | nd | nd | nd | nd |
| D7(VL-VH)-PE40 + 400 nM ABT-737 | 0.110 | 0.020 | nd | nd | nd | nd |
Mean values of 4–7 independent experiments in each case
nd not determinable
We tested different immunotoxin concentrations in combination with subtoxic concentrations of ABT-737. Compared to the immunotoxin alone, a 6.1-fold enhanced cytotoxicity after addition of 100 nM ABT-737 was determined already after 24 h on LNCaP cells (IC50 = 0.230 nM) (Fig. 3c; Table 1). Addition of 200 or 400 nM ABT-737 increased the cytotoxic effects of D7(VL-VH)-PE40 6.6-fold (IC50 = 0.210 nM) and 12.7-fold (IC50 = 0.110 nM). Calculation of the combination indices (CI) of 0.25, 0.32, and 0.40 evidenced that both agents acted with a strong synergism (Table 2). The combination of D7(VL-VH)-PE40 with 100, 200, or 400 nM ABT-737 led to additive effects of the two substances after 48 h on LNCaP cells (CI values between 0.97 and 1.2) (Table 2). The reason for a missing synergistic effect at that time was the strong cytotoxicity of the immunotoxin (IC50 = 0.052 nM), which predominated the effects of the BH3 mimetic.
Table 2.
Combination indices (CI) calculated for different concentrations of ABT-737 in combination with the immunotoxin D7(VL-VH)-PE40 according to Bijnsdorp et al. [21]
| Combination index (CI) | ||||
|---|---|---|---|---|
| LNCaP | C4-2 | |||
| 24 h | 48 h | 24 h | 48 h | |
| D7(VL-VH)-PE40 + 100 nM ABT-737 | 0.25 | 1.20 | 0.086 | 0.230 |
| D7(VL-VH)-PE40 + 200 nM ABT-737 | 0.32 | 0.97 | nd | nd |
| D7(VL-VH)-PE40 + 400 nM ABT-737 | 0.40 | 1.00 | nd | nd |
CI < 0.8 synergism; 0.8–1.2 additivity; > 1.2 antagonism
nd not determinable
A 20.3-fold increased cytotoxicity (IC50 = 0.126 nM) was observed on C4-2 cells by addition of 100 nM ABT-737 to the immunotoxin after 24 h. A CI of 0.086 indicated the synergism of both substances. After 48 h, an IC50 value of 0.029 nM was reached by combination, leading to a CI of 0.23 (Fig. 3c; Tables 1, 2). This showed that the synergistic effects of the immunotoxin and the BH3 mimetic continued until 48 h on the C4-2 line, representing the androgen-independent stage of prostate cancer and showing a higher resistance against the immunotoxin and ABT-737 than LNCaP cells.
Since no IC50 values were reached for both agents on DU 145 cells due to a missing or weak cytotoxicity, a CI calculation was not possible for this cell line (Fig. 3c; Table 1).
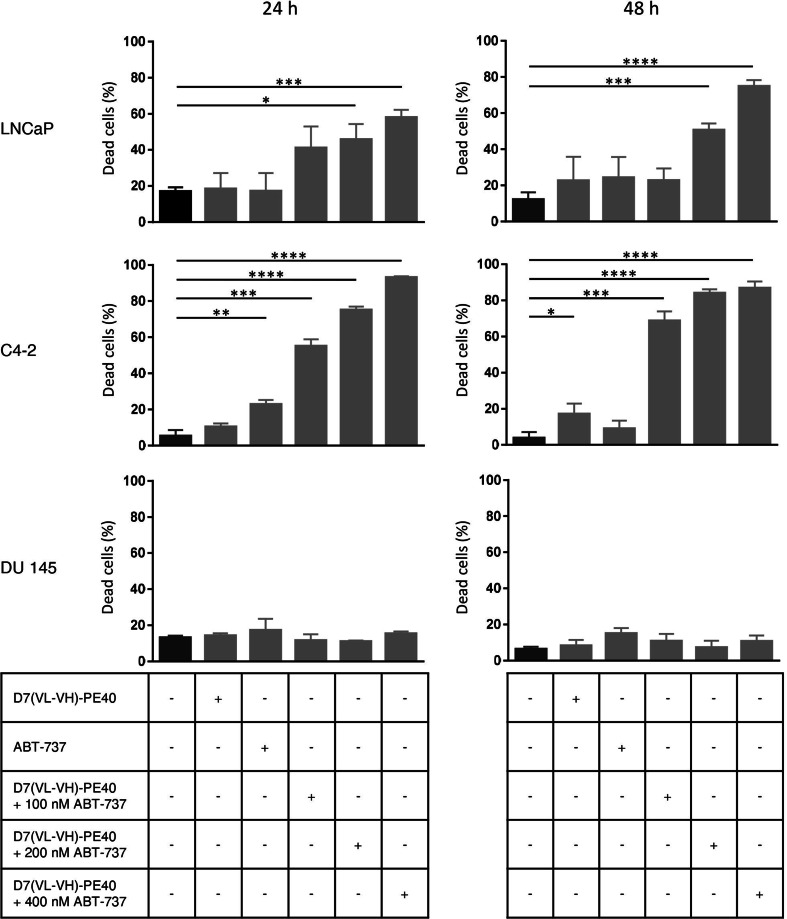
Since a reduction of cell viability can be rest upon inhibition of cell growth or cell killing, we examined the effects of D7(VL-VH)-PE40 in combination with ABT-737 in view of cell death via trypan blue assay. Addition of ABT-737 to subtoxic concentrations of immunotoxin D7(VL-VH)-PE40 significantly enhanced the percentage of dead LNCaP and C4-2 cells, whereas DU 145 control cells remained unaffected (Fig. 4). This proved that the reduction of cell viability by immunotoxin/ABT-737 combination therapy was based on the induction of cell death.
Fig. 4.
Killing of prostate cancer cells by the anti-PSMA immunotoxin D7(VL-VH)-PE40 in combination with the BH3 mimetic ABT-737. The cytotoxicity of the immunotoxin alone and in combination with 100, 200 and 400 nM ABT-737 after 24 and 48 h was determined by trypan blue assay (* p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001)
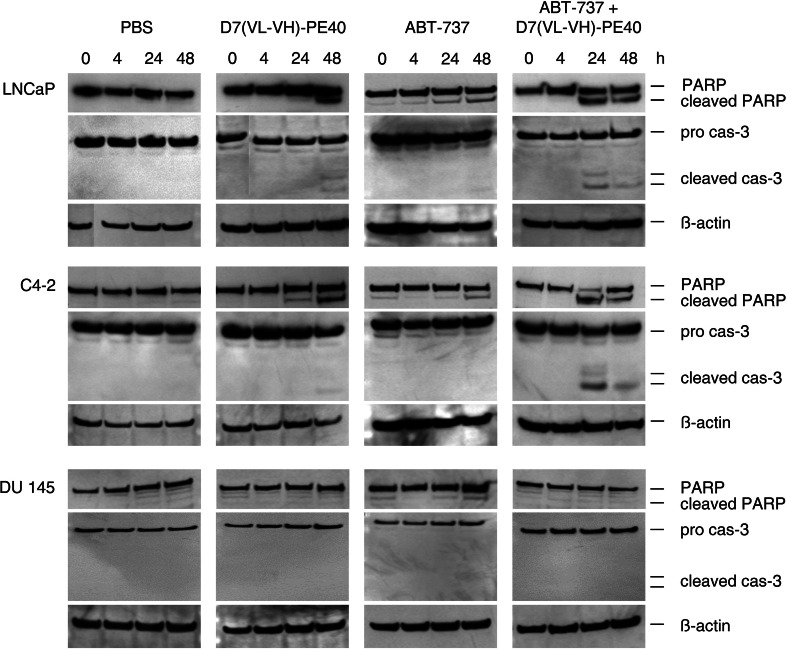
Next, Western blot analyses were performed to explore the molecular basics for cell death. As shown in Supplementary Fig. 1, the immunotoxin led to a reduced expression of the anti-apoptotic protein Mcl-1, whereas the levels of Bcl-2 and Bcl-xl remained unaffected. As a result, subtoxic concentrations of the immunotoxin alone only slightly elicited apoptosis in LNCaP and C4-2 cells characterized by PARP cleavage and Caspase-3 activation after 48 h. In contrast, the addition of 100 nM ABT-737 led to enhanced PARP and Caspase-3 cleavage already after 24 h. Both agents did not induce apoptosis either alone or in combination in DU 145 control cells (Fig. 5).
Fig. 5.
Induction of apoptosis by the anti-PSMA immunotoxin D7(VL-VH)-PE40 in combination with the BH3 mimetic ABT-737. Prostate cancer cells were treated with subtoxic concentrations of D7(VL-VH)-PE40 (50 pM for LNCaP cells and 190 pM for C4-2 and DU 145 cells) and 100 nM ABT-737 alone or in combination. Cleavage of poly(ADP-ribose) polymerase (PARP) and Caspase-3 (cas-3) was detected in cell lysates by Western blot. β-actin was used as loading control
Taken together, our results show that the combination of the anti-PSMA immunotoxin D7(VL-VH)-PE40 with ABT-737 led to a fast, synergistic cytotoxicity on PSMA expressing prostate cancer cells based on an enhanced induction of apoptosis.
Discussion
Cell death resistance in cancer cells is mainly based on the increased expression of anti-apoptotic members (Bcl-2, Bcl-xl, and Mcl-1) or on the decreased expression of pro-apoptotic members (Bax, Bak) of the Bcl-2 protein family. In an immunohistochemical study, Bcl-2 was shown to be present in 25% of cases with higher appearance in high-grade tumors (Gleason grade 8–10, 41%) and lymph node metastases (38%) than in lower-grade tumors (Gleason grade 2–7; 16%; p < 0.05) [4]. This is in accordance with a study of Anvari et al. in which Bcl-2 was present in 70.3% of locally advanced or metastatic tissues in association with higher Gleason scores and lower biochemical-free survival [22]. Bcl-xl and Mcl-1 were shown to be present in 100 and 86% of prostate tumor samples with more intensive staining of high grade tumors or metastases than of prostatic intraepithelial neoplasia (PIN) or low-grade tumors [4]. Investigations in prostate cancer cells showed evidence that Bcl-xl, in contrast to Bcl-2, plays a major cytoprotective role [23], which is comparable to the situation in other solid tumors [7, 24]. Mcl-1 was also shown to protect prostate cancer cells from chemotherapy and targeting of Mcl-1 was found to be promising in the treatment of the castration-resistant stage [25]. Interestingly, a decreased expression of pro-apoptotic proteins was not found in prostate cancer. Instead, Bax was shown to be expressed in most prostate cancers evaluated, with high percentages of immunopositive cells and strong immunointensity independently of tumor grade [4, 26]. Moreover, mutations of the Bak and Bax genes occur very infrequently in this tumor [26].
Taken together, inhibition of Bcl-xl and Mcl-1 should be a preferred strategy for the treatment of prostate cancer. Since Bax and Bak are usually present in a functional manner, the precondition is given that homo-oligomerization of these proteins and formation of mitochondrial pores can occur, which in turn results in mitochondrial outer membrane permeabilization, the point of no return in the commitment to apoptosis.
In our study, ABT-737 was found to induce death in prostate cancer cells with IC50 values in the low µM range in LNCaP and C4-2 cells, which is in line with earlier observations [27–30]. DU 145 cells were shown to be more resistant, which can be explained by the lack of the pro-apoptotic Bax protein in this cell line [28]. Since ABT-737 does not inhibit Mcl-1, the BH3 mimetic or its oral bioavailable compound of the same class, ABT-263, were combined with chemotherapeutics, 2-deoxyglucose, Pim kinase inhibitors or proteasomal inhibitors against prostate cancer cells and enhanced cytotoxic effects were noted [27–31]. However, these agents are not tumor cell specific. Therefore, to our knowledge, we examined for the first time a targeted molecule in combination with ABT-737 on prostate cancer cells.
Our anti-PSMA immunotoxin D7-(VL-VH)-PE40 showed an about 1000- to 18,000-fold higher cytotoxicity than ABT-737. An explanation for this big difference is that one immunotoxin molecule is able to catalyze the ADP-ribosylation of many eEF-2 molecules on the ribosomes, which in turn results in a fast inhibition of protein biosynthesis, especially of proteins with a short half-life. In contrast, one molecule ABT-737 can only inhibit one molecule of an anti-apoptotic protein.
We could demonstrate that subtoxic concentrations of the immunotoxin in combination with a subtoxic concentration of ABT-737 caused fast, additive or synergistic cytotoxic effects in PSMA-expressing prostate cancer cells. These effects reflect the complementary beneficial characteristics of the two agents. On the one side, the immunotoxin acts target-specific on PSMA, ensuring that the combination therapy is preferably restricted to prostate cancer cells, and markedly reduces the levels of the short-lived Mcl-1 protein by inhibition of protein biosynthesis. On the other side, ABT-737 is very effective in the specific inhibition of the Bcl-2, Bcl-xl and Bcl-w proteins. Since the therapeutic resistance of prostate cancer cells is mainly based on Mcl-1 and Bcl-xl overexpression [23, 25], both agents seem to be suitable for the future treatment of advanced prostate cancer. Moreover, the combination of both substances at subtoxic doses could lead to a reduction of side effects and to an enhanced therapeutic efficiency compared to monotherapy.
An improved cytotoxicity of ABT-737/ABT-263 in combination with PE-based immunotoxins against different target antigens was also demonstrated in cervical, melanoma, small cell lung and pancreatic cancer cells [17–20]. Moreover, combination therapy resulted in an enhanced anti-tumor activity in mice-bearing tumor xenografts [19, 20]. Future experiments will show if our combination of ABT-737 with D7(VL-VH)-PE40 will also be successful in animal models.
Conclusions
In the present study, we could show that the BH3 mimetic ABT-737, which has only limited antitumor activity as single agent, is able to lower the threshold for the induction of apoptosis by the anti-PSMA immunotoxin D7(VL-VH)-PE40 (Fig. 1b). This makes the combination therapy a promising approach for the future treatment of advanced prostate with improvement of efficacy and reduction of adverse side effects.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was funded by the Wilhelm Sander-Stiftung (Grant No. 2016.089.01 to Philipp Wolf).
Abbreviations
- ABT-737
4-[4-[[2-(4-chlorophenyl)phenyl]methyl]piperazin-1-yl]-N-[4-[[(2R)-4-(dimethylamino)-1-phenylsulfanylbutan-2-yl]amino]-3-nitrophenyl] sulfonylbenzamide
- Bak
Bcl-2 antagonist/killer
- Bax
Bcl-2-associated X protein
- BCA
Bicinchoninic acid
- Bcl-2
B cell lymphoma 2
- Bcl-w
Bcl-2 like 2
- Bcl-xl
B cell lymphoma extra-large
- BH3
Bcl-2 homology domain 3
- CI
Combination Index
- c-myc
Avian myelocytomatosis virus oncogene cellular homolog
- ECL
Enhanced chemiluminescence
- eEF-2
Eukaryotic elongation factor-2
- Mcl-1
Myeloid cell leukemia sequence 1
- PARP
Poly(ADP-ribose) polymerase
- PE
Pseudomonas aeruginosa Exotoxin A
- PSMA
Prostate-specific membrane antigen
- WST-1
Water-soluble tetrazolium salt
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Zhou CK, Check DP, Lortet-Tieulent J, Laversanne M, Jemal A, Ferlay J, Bray F, Cook MB, Devesa SS. Prostate cancer incidence in 43 populations worldwide: an analysis of time trends overall and by age group. Int J Cancer. 2016;138:1388–400. doi: 10.1002/ijc.29894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 4.Krajewska M, Krajewski S, Epstein JI, Shabaik A, Sauvageot J, Song K, Kitada S, Reed JC. Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. Am J Pathol. 1996;148:1567–1576. [PMC free article] [PubMed] [Google Scholar]
- 5.Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014;2014:150845. doi: 10.1155/2014/150845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Scarfo L, Ghia P. Reprogramming cell death: BCL2 family inhibition in hematological malignancies. Immunol Lett. 2013;155:36–9. doi: 10.1016/j.imlet.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Ashkenazi A, Fairbrother WJ, Leverson JD, Souers AJ. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat Rev Drug Discov. 2017;16:273–84. doi: 10.1038/nrd.2016.253. [DOI] [PubMed] [Google Scholar]
- 8.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 9.Zhai D, Jin C, Satterthwait AC, Reed JC. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ. 2006;13:1419–1421. doi: 10.1038/sj.cdd.4401937. [DOI] [PubMed] [Google Scholar]
- 10.van Delft MF, Wei AH, Mason KD, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams JM, Huang DC, Strasser A, et al. Subversion of the Bcl-2 life/death switch in cancer development and therapy. Cold Spring Harb Symp Quant Biol. 2005;70:469–77. doi: 10.1101/sqb.2005.70.009. [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–91. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 13.Lestini BJ, Goldsmith KC, Fluchel MN, Liu X, Chen NL, Goyal B, Pawel BR, Hogarty MD. Mcl1 downregulation sensitizes neuroblastoma to cytotoxic chemotherapy and small molecule Bcl2-family antagonists. Cancer Biol Ther. 2009;8:1587–1595. doi: 10.4161/cbt.8.16.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michalska M, Schultze-Seemann S, Bogatyreva L, Hauschke D, Wetterauer U, Wolf P. In vitro and in vivo effects of a recombinant anti-PSMA immunotoxin in combination with docetaxel against prostate cancer. Oncotarget. 2016;7:22531–22542. doi: 10.18632/oncotarget.8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf P, Alt K, Wetterauer D, Buhler P, Gierschner D, Katzenwadel A, Wetterauer U, Elsasser-Beile U. Preclinical evaluation of a recombinant anti-prostate specific membrane antigen single-chain immunotoxin against prostate cancer. J Immunother. 2010;33:262–71. doi: 10.1097/CJI.0b013e3181c5495c. [DOI] [PubMed] [Google Scholar]
- 16.Michalska M, Wolf P. Pseudomonas Exotoxin A: optimized by evolution for effective killing. Front Microbiol. 2015;6:963. doi: 10.3389/fmicb.2015.00963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antignani A, Sarnovsky R, FitzGerald DJ. ABT-737 promotes the dislocation of ER luminal proteins to the cytosol, including pseudomonas exotoxin. Mol Cancer Ther. 2014;13:1655–1663. doi: 10.1158/1535-7163.MCT-13-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollevoet K, Antignani A, Fitzgerald DJ, Pastan I. Combining the antimesothelin immunotoxin SS1P with the BH3-mimetic ABT-737 induces cell death in SS1P-resistant pancreatic cancer cells. J Immunother. 2014;37:8–15. doi: 10.1097/CJI.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattoo AR, FitzGerald DJ. Combination treatments with ABT-263 and an immunotoxin produce synergistic killing of ABT-263-resistant small cell lung cancer cell lines. Int J Cancer. 2013;132:978–87. doi: 10.1002/ijc.27732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Risberg K, Fodstad O, Andersson Y. Synergistic anticancer effects of the 9.2.27PE immunotoxin and ABT-737 in melanoma. PLoS One. 2011;6:e24012. doi: 10.1371/journal.pone.0024012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bijnsdorp IV, Giovannetti E, Peters GJ. Analysis of drug interactions. Method Mol Biol. 2011;731:421–34. doi: 10.1007/978-1-61779-080-5_34. [DOI] [PubMed] [Google Scholar]
- 22.Anvari K, Seilanian Toussi M, alantari M, et al. Expression of Bcl-2 and Bax in advanced or metastatic prostate carcinoma. Urol J. 2012;9:381–388. [PubMed] [Google Scholar]
- 23.Lebedeva I, Rando R, Ojwang J, Cossum P, Stein CA. Bcl-xL in prostate cancer cells: effects of overexpression and down-regulation on chemosensitivity. Cancer Res. 2000;60:6052–6060. [PubMed] [Google Scholar]
- 24.Amundson SA, Myers TG, Scudiero D, Kitada S, Reed JC, Fornace AJ., Jr An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000;60:6101–6110. [PubMed] [Google Scholar]
- 25.Reiner T, de Las Pozas A, Parrondo R, Palenzuela D, Cayuso W, Rai P, Perez-Stable C. Mcl-1 protects prostate cancer cells from cell death mediated by chemotherapy-induced DNA damage. Oncoscience. 2015;2:703–15. doi: 10.18632/oncoscience.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshino T, Shiina H, Urakami S, Kikuno N, Yoneda T, Shigeno K, Igawa M. Bcl-2 expression as a predictive marker of hormone-refractory prostate cancer treated with taxane-based chemotherapy. Clin Cancer Res. 2006;12:6116–6124. doi: 10.1158/1078-0432.CCR-06-0147. [DOI] [PubMed] [Google Scholar]
- 27.Cervantes-Gomez F, Lavergne B, Keating MJ, Wierda WG, Gandhi V. Combination of Pim kinase inhibitors and Bcl-2 antagonists in chronic lymphocytic leukemia cells. Leuk Lymphoma. 2015 doi: 10.3109/10428194.2015.1063141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parrondo R, de Las Pozas A, Reiner T, Perez-Stable C. ABT-737, a small molecule Bcl-2/Bcl-xL antagonist, increases antimitotic-mediated apoptosis in human prostate cancer cells. Peer J. 2013;1:e144. doi: 10.7717/peerj.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song JH, Kraft AS. Pim kinase inhibitors sensitize prostate cancer cells to apoptosis triggered by Bcl-2 family inhibitor ABT-737. Cancer Res. 2012;72:294–303. doi: 10.1158/0008-5472.CAN-11-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaguchi R, Janssen E, Perkins G, Ellisman M, Kitada S, Reed JC. Efficient elimination of cancer cells by deoxyglucose-ABT-263/737 combination therapy. PLoS One. 2011;6:e24102. doi: 10.1371/journal.pone.0024102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamaki H, Harashima N, Hiraki M, Arichi N, Nishimura N, Shiina H, Naora K, Harada M. Bcl-2 family inhibition sensitizes human prostate cancer cells to docetaxel and promotes unexpected apoptosis under caspase-9 inhibition. Oncotarget. 2014;5:11399–11412. doi: 10.18632/oncotarget.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.