Abstract
Plasmacytoid dendritic cells (pDCs) are present in various primary and metastatic human neoplasms; however, their clinical significance in hepatocellular carcinoma (HCC) is unclear. In this study, we investigated the distribution, prognostic value, and potential function of pDCs in HCC patients undergoing curative resection. We performed immunohistochemical analyses of whole tumor sections from 224 patients to assess the expression of BDCA2, CD3, CD4, CD8, Foxp3, granzyme B, IL-17, and CD34. The findings were validated using tissue microarrays from another two independent cohorts totaling 841 HCC patients undergoing curative resection. Our results demonstrated that high numbers of BDCA2+ pDCs within tumors correlated with high alpha-fetoprotein levels, greater vascular invasion, advanced tumor-node-metastasis stage, shorter overall survival, and a higher recurrence rate. However, patient outcomes were not associated with pDCs in peritumoral stromal or nontumor tissues. Furthermore, an increase in intratumoral pDCs was associated with increased intratumoral infiltration of Foxp3+ regulatory T cells and IL-17-producing cells and correlated with tumor vascular density. Univariate and multivariate analyses revealed that the presence of intratumoral pDCs alone or in combination with regulatory T and/or IL-17-producing cells was an independent predictor of time to recurrence and overall survival. In conclusion, our study demonstrated that intratumoral infiltration by pDCs is a novel indicator for poor prognosis in patients with HCC, possibly through the induction of an immune tolerogenic and inflammatory tumor microenvironment comprising regulatory T and IL-17-producing cells. An assessment of the combination of these cells represents a superior predictor of patient outcome.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02355-3) contains supplementary material, which is available to authorized users.
Keywords: Plasmacytoid dendritic cells, Hepatocellular carcinoma, Treg cells, IL-17, Prognosis
Introduction
The incidence and mortality rates of hepatocellular carcinoma (HCC), one of the most prevalent tumor types, are increasing [1]. Despite innovations in operative procedures and disease management [2], high rates of recurrence and metastasis impede long-term patient survival [3]. Therefore, better predictive biomarkers of recurrence or metastasis are urgently needed.
A major hallmark of cancer is inflammation [4], and inflammatory responses have been shown over the past decade to be determinants of tumor stage and development, from tumor initiation, malignant conversion, and invasion to metastasis [4]. HCC is most often found in inflamed livers, such as with hepatitis B and C virus infections in Chinese and Western populations, respectively, indicating a link between inflammation and the development of HCC [5]. The immune/inflammatory cells and their secretory products may produce an environment that promotes tumor progression. Some aspects of the molecular communication between infiltrating immune cells and tumor cells have been described [6]. Moreover, the clinical outcomes of several cancer types have been linked to various characteristics of tumor-infiltrating immune cells, such as their types and densities, as well as their location within the tumor microenvironment [7, 8]. These associations indicate that immune responses surrounding the tumor could be used to predict clinical outcomes as well as to monitor responses to immunotherapeutic strategies. We previously showed that the prognosis of patients with HCC after resection is associated with the infiltration of regulatory T (Treg) cells [9], neutrophils [5, 10], and tumor-associated macrophages [11, 12]. These findings, together with evidence from additional relevant studies [13, clearly reveal the existence of crosstalk between host immunity and cancer in the liver.
Plasmacytoid dendritic cells (pDCs) were first identified in humans as a small subset of blood leukocytes that produce and secrete large amounts of type I interferon (IFN) [14]. These cells are thought to provide the host with an initial defense against viral infection [14]. As antigen-presenting cells, pDCs can also regulate adaptive immunity mediated by T cells and immunopathogenesis [15]. Recent evidence suggests that this link between innate and adaptive immunity by pDCs may play a role in cancer immunity [15, 16]. Indeed, various neoplasms, including cancers of the head and neck, breast, ovaries, lung, and skin, exhibit pDCs [17], which are in a nonactive state but generate and maintain an immunosuppressive tumor microenvironment. The presence of large numbers of pDCs in breast cancer tumors is correlated with tumor dissemination and relapse [18], whereas tumor growth and bone metastasis are inhibited by pDC depletion [19]. Immune tolerance via pDCs is also critical for the progression of ovarian cancer. pDCs can induce Treg cells but cannot activate T cells [20]. In addition to regulating the tumor immune microenvironment, pDCs have also been shown to directly contribute to tumor growth, survival, chemotaxis, and drug resistance in multiple myeloma [21].
Although infiltration of pDCs into neoplasms has been documented, the clinical significance of tumor-associated pDCs (TA-pDCs) remains largely unknown. In addition, the distribution of pDCs in different regions of the tumor and their potential crosstalk with other cells in the tumor microenvironment are largely unexplored [17]. Here, we examined the distribution and prognostic significance of pDCs in HCC. We also investigated the possible mechanisms linking TA-pDCs with the HCC immune microenvironment. We found that an intratumoral infiltration of pDCs predicts poor outcomes for patients undergoing curative resection for HCC, possibly due to the induction of an immune tolerogenic and inflammatory tumor microenvironment comprising Treg and IL-17+ cells, respectively. Thus, the combination of intratumoral pDCs and Treg and/or IL-17+ cells is a strong predictor of outcome in HCC patients.
Materials and methods
Patients and follow-up
Three separate cohorts totaling 1065 HCC patients that received curative resection for HCC in the department of liver surgery and transplantation of Zhongshan Hospital, Fudan University, were enrolled in this study. Patients receiving palliative surgeries or prior interventions (such as trans-hepatic artery embolization, chemotherapy, or radiotherapy) or with other primary malignancies and inflammatory diseases during the follow-up were excluded from the study. All tissue samples from the patients included in the study were consecutively collected from 2005 (cohort 3, n = 461), 2006 (cohort 2, n = 380), and 2007 (cohort 1, n = 224). Histopathological diagnoses were according to World Health Organization criteria, and tumor differentiation grading was according to the classification by Edmondson and Steiner [22]. The Child–Pugh scoring system was applied to assess liver function [23], and tumor-node-metastasis (TNM) grading was used to define tumor stage according to the 2010 International Union Against Cancer [23]. The patients were monitored after the surgery as previously described [24]. The duration from the time of the surgery until the death or the last observation of the patient (15 March 2013) was used to determine the overall survival (OS). The data from surviving patients were censored at the final follow-up. The duration from the time of the surgery to the date of intrahepatic recurrence or extrahepatic metastasis was diagnosed was used to determine the time to recurrence (TTR) [24]. Detailed information is provided in Supplementary Table 1.
Tissue microarray and immunohistochemistry
Tissue microarrays were performed with 380 cases in cohort 2 and with 461 cases in cohort 3. The paraffin blocks contained areas devoid of necrotic and hemorrhagic damage that were identified from hematoxylin and eosin-stained sections and two 1-mm-diameter biopsy cores containing intratumoral and nontumoral tissues.
Sections from the tissue blocks were rehydrated and underwent microwave antigen retrieval. Next, the sections were incubated overnight at 4 °C with polyclonal antibody against human blood dendritic cell antigen 2 (BDCA2, 1:200; Abnova), monoclonal antibodies against human CD3 (1:50, clone F7.2.38; DakoCytomation), CD4 (1:100, clone EPR6855; Epitomics), CD8 (1:50, clone C8/144B; DakoCytomation), granzyme B (1:25, clone GrB-7; DakoCytomation), Foxp3 (1:100, clone 236A/E7; Abcam), IL-17 (1:100, clone AF-317-NA; R&D Systems), and CD34 (1:200, clone EP373Y; Abcam) and then for 30 min at 37 °C with secondary antibodies (GK500705, Gene Tech, China). Immunoreactivity was visualized with 3,3′-diaminobenzidine according to the avidin–biotin–peroxidase complex method [25], and the sections were counterstained with Mayer’s hematoxylin. Slides in which the primary antibody was omitted served as negative controls, and rabbit IgG was used as the control for BDCA2. The controls indicated the immunohistochemistry used in the study was specific and robust (Supplementary Figure 1).
Three investigators who were blind to the characteristics of the patients each assessed the immunohistochemical staining, with any discrepancies among their findings resolved by consensus. The mean values for the numbers of cells positive for immunostaining in triplicate sections were determined (cells/mm2). In subsequent analyses, the medians were used as the cutoff values unless specified otherwise.
Statistical analysis
Statistical analyses were performed with SPSS 16.0 statistical software. Paired-sample t tests were used to assess differences in the densities of immunopositive cells (mean/median values) between subgroups. Chi square and Fisher’s exact tests were used to assess associations and Spearman’s rho coefficients were used to assess correlations between clinicopathologic features and results of immunohistochemistry as appropriate. The Kaplan–Meier method was used to determine OS and cumulative recurrence rates, which were analyzed by the log-rank test. The Cox proportional hazards regression model was used for univariate and multivariate analyses. To identify the cutoff for the optimal separation of patients according to TTR, the “minimum p value” approach was adopted [7]. A p value of < 0.05 was considered statistically significant.
Results
Distribution of pDCs in HCC and the correlations with clinicopathologic features
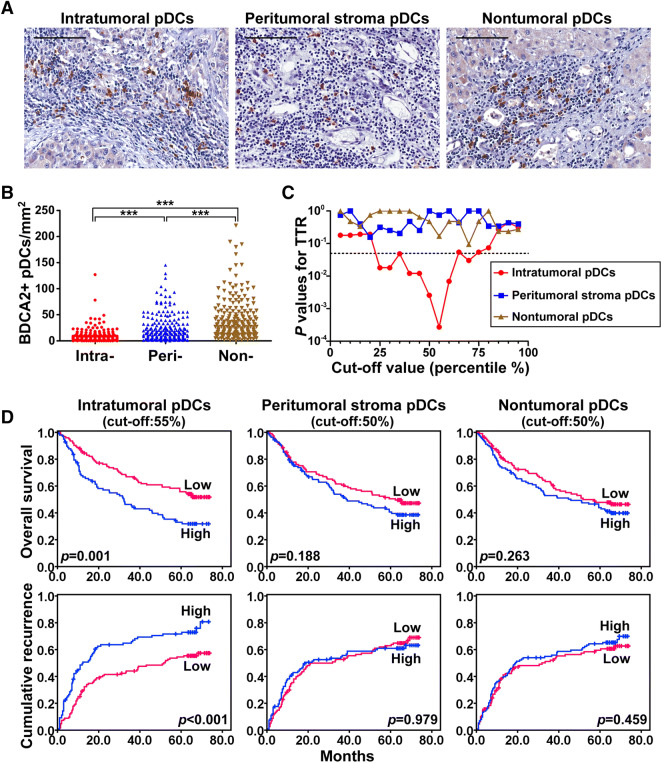
To assess the infiltration and distribution of pDCs in HCC, whole tumor sections from 224 patients (cohort 1) were immunostained for BDCA2. As shown in Fig. 1a, b and Supplementary Table 2, pDCs were present throughout the tissue sections but were predominantly found in the peritumoral stromal and nontumoral tissues rather than in the cancer nests (23.4 ± 25.4 and 38.6 ± 37.4 cells/mm2 vs. 8.9 ± 12.6 cells/mm2, respectively; p < 0.001). There were significant correlations between an increase in intratumoral pDCs and high alpha-fetoprotein (AFP) levels (p = 0.018), greater vascular invasion (p = 0.009), and advanced TNM stage (p = 0.047) (Table 1). An increased number of intratumoral pDCs also correlated with larger tumor size (p = 0.073) and the absence of a tumor capsule (p = 0.092), although the effects were less pronounced. The number of pDCs in the nontumoral tissues also correlated with TNM stage. However, none of the clinical characteristics correlated with the number of pDCs in peritumoral stromal tissue. We also investigated the correlation between the ratio of intratumoral pDCs and/or pDCs of nontumor tissues and peritumor stromal tissues with clinical characteristics. The results demonstrated that an increase in the ratio of intratumoral to peritumor stromal pDCs correlated with advanced TNM stage (p = 0.042) (Supplementary Table 3).
Fig. 1.
Distribution of pDCs in HCC and its correlation with prognosis. a Representative HCC tumor samples showing BDCA2+ pDCs in tumoral, peritumoral stroma, and nontumoral tissues. Scale bar = 100 μm. b pDCs were distributed throughout the tissue but were predominantly in the peritumoral stroma and nontumoral tissues, not within the cancer nests. c A minimum p value approach generated cutoffs for intratumoral BDCA2 (25–75%) in terms of TTR. d Prognostic value of pDCs in tumoral, peritumoral stroma, and nontumoral tissues of HCC patients
Table 1.
Correlation between plasmacytoid dendritic cells in different areas of whole tumor sections and clinicopathologic characteristics in HCC (cohort 1, n = 224)
| Clinicopathological indexes | Intratumoral pDCs (cutoff 55%) |
Peritumoral stromal pDCs (cutoff 50%) |
Nontumoral pDCs (cutoff 50%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Low | High | P | Low | High | P | Low | High | P | |
| Age (year) | |||||||||
| ≤ 50 | 54 | 41 | 0.701 | 51 | 44 | 0.547 | 51 | 44 | 0.289 |
| > 50 | 70 | 59 | 64 | 65 | 60 | 69 | |||
| Sex | |||||||||
| Female | 16 | 9 | 0.356 | 11 | 14 | 0.436 | 10 | 15 | 0.393 |
| Male | 108 | 91 | 104 | 95 | 104 | 95 | |||
| HBsAg | |||||||||
| Negative | 25 | 17 | 0.547 | 18 | 24 | 0.222 | 22 | 20 | 0.684 |
| Positive | 99 | 83 | 97 | 85 | 89 | 93 | |||
| HCV | |||||||||
| Negative | 122 | 100 | 0.504† | 155 | 107 | 0.236† | 111 | 111 | 0.498† |
| Positive | 2 | 0 | 0 | 2 | 0 | 2 | |||
| AFP (ng/ml) | |||||||||
| ≤ 20 | 59 | 32 | 0.018 | 48 | 43 | 0.727 | 43 | 48 | 0.569 |
| > 20 | 65 | 68 | 67 | 66 | 68 | 65 | |||
| GGT (U/L) | |||||||||
| ≤ 54 | 47 | 36 | 0.769 | 40 | 43 | 0.470 | 42 | 41 | 0.810 |
| > 54 | 77 | 64 | 75 | 66 | 69 | 72 | |||
| Liver cirrhosis | |||||||||
| No | 17 | 13 | 0.877 | 16 | 14 | 0.814 | 18 | 12 | 0.219 |
| Yes | 107 | 87 | 99 | 95 | 93 | 101 | |||
| Tumor size (cm) | |||||||||
| ≤ 5 | 67 | 42 | 0.073 | 54 | 55 | 0.600 | 49 | 60 | 0.180 |
| > 5 | 57 | 58 | 61 | 54 | 62 | 53 | |||
| Tumor number | |||||||||
| Single | 104 | 88 | 0.380 | 97 | 95 | 0.548 | 90 | 102 | 0.050 |
| Multiple | 20 | 12 | 18 | 14 | 21 | 11 | |||
| Vascular invasion | |||||||||
| Absence | 89 | 55 | 0.009 | 76 | 68 | 0.563 | 68 | 76 | 0.349 |
| Present | 35 | 45 | 39 | 41 | 43 | 37 | |||
| Tumor encapsulation | |||||||||
| Complete | 60 | 43 | 0.421 | 50 | 53 | 0.440 | 50 | 53 | 0.780 |
| None | 64 | 57 | 65 | 56 | 61 | 60 | |||
| Tumor differentiation | |||||||||
| I + II | 90 | 62 | 0.092 | 78 | 74 | 0.992 | 71 | 81 | 0.216 |
| III + IV | 34 | 38 | 37 | 35 | 40 | 32 | |||
| TNM stage | |||||||||
| I | 76 | 48 | 0.047 | 63 | 61 | 0.859 | 54 | 70 | 0.045 |
| II + III | 48 | 52 | 52 | 48 | 57 | 43 | |||
The boldfaced numerals indicate P value < 0.05
AFP alpha-fetoprotein, GGT gamma glutamyl transferase, TNM tumor-node-metastasis
†Fisher’s exact tests; Chi square tests for all the other analyses
Accumulation of intratumoral pDCs predicts poor prognosis in HCC patients
At the final follow-up (March 2013), 62.1% (139/224) of the patients in cohort 1 had suffered a recurrence and 54.2% (121/224) had died. For this cohort, the 1-, 3-, and 5-year OS rates were 78.2%, 57.5%, and 45.9%, and the 1-, 3-, and 5-year cumulative recurrence rates were 37.1%, 53.5%, and 62.0%.
An increased number of intratumoral pDCs indicated a worse patient outcome. The significant cutoff values for TTR of intratumoral pDCs ranged from 25 to 75% (Fig. 1C). The greatest minimum was at a cutoff of 55%, which was used to classify patients into two groups: intratumoral pDCslow (≤ 6 cells/mm2) and intratumoral pDCshigh (> 6 cells/mm2). The 1-, 3-, and 5-year OS rates were significantly higher in the intratumoral pDCslow group than in the intratumoral pDCshigh group (86.2% vs. 68.2%, 66.9% vs. 45.5%, and 55.8% vs. 33.2%, respectively; Fig. 1D). HCC patients in the intratumoral pDCshigh group had poorer prognoses, with higher cumulative recurrence rates at 1, 3, and 5 years than those in the intratumoral pDCslow group (48.2% vs. 28.3%, 65.7% vs. 44.1%, and 71.5% vs. 54.5%, respectively; Fig. 1d). By contrast, OS and cumulative recurrence rates were not associated with either peritumoral stroma or nontumoral pDCs (Fig. 1d). However, intratumoral pDCs were associated with OS and cumulative recurrence rates in patients with early-stage (Barcelona Clinic Liver Cancer stage 0 + A) HCC (n = 74) and normal AFP levels (≤ 20 ng/ml, n = 91) (Supplementary Figure 2).
In a univariate analysis, a decrease in intratumoral pDCs, as well as clinicopathologic factors, predicted prolonged TTR and OS (Table 2). These significant factors were then included in the multivariate analysis with the Cox proportional hazards regression. The results revealed that, together with gamma-glutamyltransferase, tumor size, and tumor differentiation, the infiltration of intratumoral pDCs was an independent prognostic factor for OS (p < 0.001, hazard ratio [HR] = 1.654) and TTR (p = 0.002, HR = 1.715; Table 2).
Table 2.
Univariate and multivariate analyses of prognostic factors in the whole tumor sections of HCC (cohort 1, n = 224)
| Variable | TTR | OS | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Univariate analysis | ||||
| Age, year (≤ 50 vs. > 50) | 1.239 (0.880–1.746) | .220 | 1.415 (0.973–2.059) | .070 |
| Sex (female vs. male) | 1.205 (0.843–1.722) | .306 | 1.230 (0.692–2.188) | .480 |
| HBsAg (negative vs. positive) | 1.173 (0.760–1.808) | .471 | 0.948 (0.606–1.482) | .815 |
| AFP, ng/ml (≤ 20 vs. > 20) | 1.473 (1.041–2.085) | .029 | 1.616 (1.109–2.355) | .013 |
| GGT, U/L (≤ 54 vs. > 54) | 1.512 (1.063–2.150) | .021 | 1.893 (1.280–2.799) | .001 |
| Liver cirrhosis (no vs. yes) | 1.713 (0.986–2.978) | .056 | 1.406 (0.791–2.500) | .246 |
| Tumor size, cm (≤ 5 vs. > 5) | 1.662 (1.186–2.329) | .003 | 2.573 (1.766–3.749) | .000 |
| Tumor number (single vs. multiple) | 1.364 (0.878–2.119) | .168 | 1.145 (0.709–1.850) | .580 |
| Vascular invasion (no vs. yes) | 1.633 (1.160–2.299) | .005 | 1.962 (1.366–2.817) | .000 |
| Tumor encapsulation (complete vs. none) | 1.170 (0.837–1.635) | .357 | 1.392 (0.968–2.001) | .074 |
| Tumor differentiation (I + II vs. III + IV) | 1.695 (1.199–2.398) | .003 | 1.890 (1.313–2.720) | .001 |
| TNM stage (I vs. II III) | 1.687 (1.208–2.357) | .000 | 1.915 (1.338–2.740) | .000 |
| Intra-pDCs (low vs. high) | 1.837 (1.315–2.566) | .000 | 1.803 (1.379–2.358) | .000 |
| Peri-pDCs (low vs. high) | 1.004 (0.720–1.402) | .979 | 1.270 (0.889–1.815) | .190 |
| Non-pDCs (low vs. high) | 1.133 (0.812–1.582) | .462 | 1.002 (0.998–1.007) | .271 |
| Intra-pDCs and IL-17 + cells (both low vs. both high) | 2.242 (1.529–3.288) | .000 | 2.416 (1.596–3.658) | .000 |
| Intra-pDCs and Treg cells (both low vs. both high) | 2.276 (1.525–3.398) | .000 | 2.442 (1.585–3.764) | .000 |
| Intra- pDCs and IL-17 + , and Treg cells (all low vs. all high) | 2.702 (1.615–4.510) | .000 | 3.014 (1.831–4.962) | .000 |
| Multivariate analysis | ||||
| AFP, ng/ml (≤ 20 vs. > 20) | 1.310 (0.921–1.863) | .133 | 1.444 (0.984–2.119) | .060 |
| GGT, U/L (≤ 54 vs. > 54) | 1.449 (0.991–2.118) | .055 | 1.569 (1.027–2.397) | .037 |
| Tumor size, cm (≤ 5 vs. > 5) | 1.313 (0.915–1.883) | .139 | 1.935 (1.291–2.902) | .001 |
| Vascular invasion (no vs. yes) | 1.225 (0.853–1.759) | .272 | 1.395 (0.956–2.035) | .084 |
| Tumor differentiation (I + II vs. III + IV) | 1.561 (1.086–2.243) | .016 | 1.607 (1.104–2.340) | .013 |
| Intra-pDCs (low vs. high) | 1.715 (1.218–2.416) | .002 | 1.654 (1.150–2.378) | .007 |
| Intra-pDCs and IL-17 + cells (both low vs. both high) | 2.075 (1.395–3.087) | .000 | 1.957 (1.274–3.006) | .002 |
| Intra-pDCs and Treg cells (both low vs. both high) | 2.253 (1.494–3.398) | .000 | 2.326 (1.494–3.621) | .000 |
| Intra-pDCs and IL-17 + , and Treg cells (all low vs. all high) | 2.693 (1.677–4.326) | .000 | 2.702 (1.615–4.520) | .000 |
The boldfaced numerals indicate P value < 0.05
Cox proportional hazards regression model
AFP alpha-fetoprotein, GGT gamma glutamyl transferase, TNM tumor-node-metastasis, HR hazard ratio, CI confidential interval
Increased infiltration of intratumoral pDCs is associated with increased intratumoral Treg and IL-17+ cells
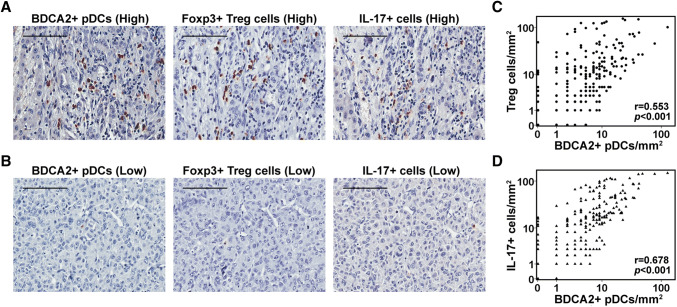
As pDCs act as antigen-presenting cells to regulate T cell-mediated adaptive and innate immune responses, we explored the relationship between the intratumoral infiltration of pDCs and T lymphocytes in HCC. Representative immunohistochemistry images of intratumoral T lymphocytes are shown in Fig. 2a, b. The number of intratumoral pDCs significantly correlated with the numbers of Foxp3+ Treg cells (p < 0.001, R = 0.553, Fig. 2c) and IL-17+ cells (p < 0.001, R = 0.678, Fig. 2d). However, there was no significant correlation between intratumoral pDCs and other types of T lymphocytes, such as CD3+, CD4+, CD8+, and granzyme B+ lymphocytes (Supplementary Table 4). Furthermore, we observed that an increased number of intratumoral Treg cells correlated with greater vascular invasion (p = 0.049). An increased number of intratumoral IL-17 + cells correlated with larger tumor size (p = 0.035), greater vascular invasion (p = 0.013), and advanced TNM stage (p = 0.02) (Supplementary Table 5).
Fig. 2.
Number of intratumoral pDCs is significantly associated with the numbers of intratumoral Treg cells and IL-17+ cells. High (a) and low (b) infiltration of intratumoral pDCs, Treg cells, and IL-17+ cells. Scale bar = 100 μm. Scatterplots showing significant positive correlations between BDCA2+ pDCs and Treg cells (c) and IL-17+ cells (d) in cancer tissues
Number of intratumoral pDCs is associated with microvessel density
Previous studies attributed the grim outcome of patients with HCC to the high vascularity of the disease [26.] The Treg and IL-17+ cells present in the tumor can promote growth by supporting angiogenesis [13, 27.] Therefore, we assessed the microvessel density (MVD) along with the density of intratumoral pDCs. Paraffin-embedded tissues were immunohistochemically stained with an antibody against CD34. The MVD was higher in tumor tissues with high numbers of infiltrated pDCs than in tissues with low numbers of infiltrated pDCs (p < 0.001; Fig. 3).
Fig. 3.
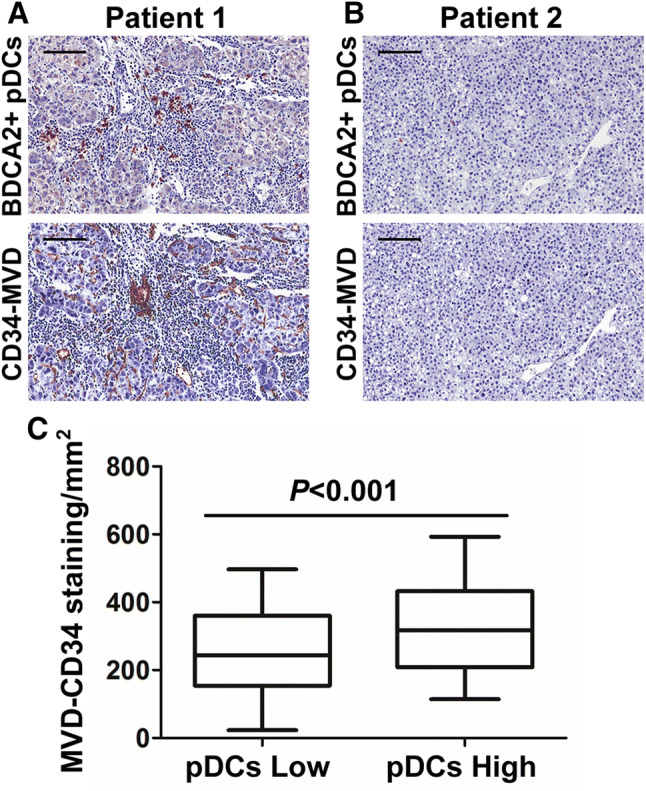
High infiltration of intratumoral pDCs is associated with increased MVD. a High infiltration of intratumoral pDCs and high MVD. b Low infiltration of intratumoral pDCs and low MVD. Scale bar = 100 μm. c Association between intratumoral pDC frequency and MVD. Data represent the mean values ± SDs and are representative of three independent experiments
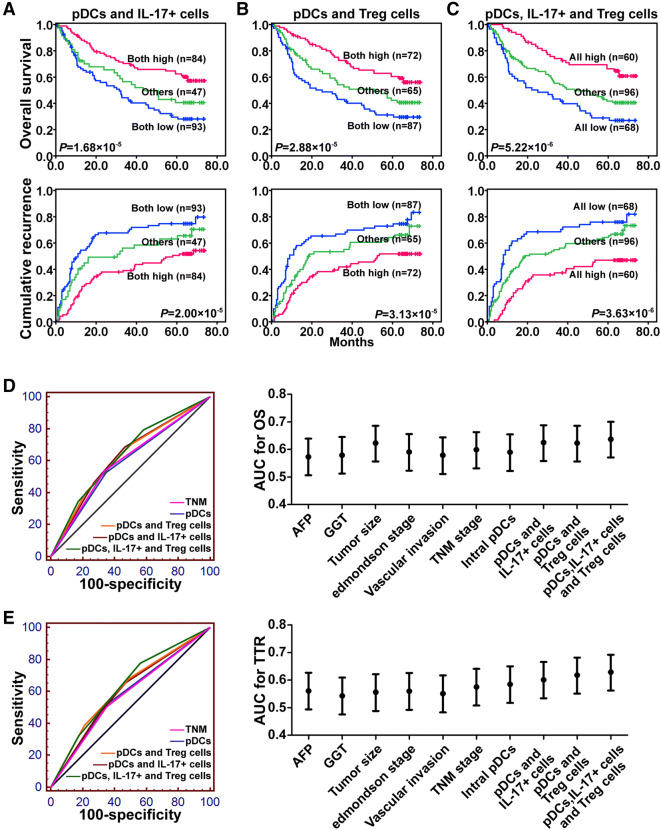
Combination of intratumoral pDCs and Treg and/or IL-17+ cells is a superior predictor of patient outcome for HCC
The prognostic value of the combined presence of intratumoral pDCs with Treg cells and/or IL-17+ cells in HCC patients was assessed. We directly compared the prognoses of HCC patients among three subgroups: intratumoral pDCslow/Tregslow, intratumoral pDCslow/Tregshigh or intratumoral pDCshigh/Tregslow, and intratumoral pDCshigh/Tregshigh. In the survival analysis, the 1-, 3-, and 5-year OS rates for the intratumoral pDCshigh/Tregshigh patients were 62.0%, 43.2%, and 31.2%, respectively, which were significantly lower than those for intratumoral pDCslow/Tregslow patients (91.9%, 72.6%, and 60.4%, respectively; Fig. 4a). The 1-, 3-, and 5-year cumulative recurrence rates for the intratumoral pDCshigh/Tregshigh patients were 54.9%, 66.6%, and 72.8%, respectively, which were significantly higher than those for the intratumoral pDCslow/Tregslow patients (24.2%, 42.0%, and 51.8%, respectively; Fig. 4a). Moreover, we obtained similar results in the prognostic analysis for combined intratumoral pDCs with IL-17+ cells or intratumoral pDCs combined with Treg and IL-17+ cells (Fig. 4b, c). The areas under the curves for intratumoral pDCs combined with Treg and IL-17+ cells were 0.637 for OS and 0.629 for TTR. Compared with other clinical indices, this was the strongest factor predicting OS and TTR in HCC (Fig. 4d, e).
Fig. 4.
The prognostic values of intratumoral pDCs combined with IL-17+ cells (a), with Treg cells (b), and with Treg cells and IL-17+ cells (c) in HCC patients. The predictive ability for OS (d) and TTR (e) of pDCs, pDCs and IL-17+ cells, pDCs and Treg cells, and pDCs, IL-17+, and Treg cells compared with that of other clinical parameters by receiver operating characteristic curves for 224 HCC patients. The area under the curve with 95% confidence interval is shown
Independent validation
The prognostic value of intratumoral pDCs alone and in combination with Treg cells and/or IL-17+ cells was validated in two additional cohorts of 841 HCC patients by immunohistochemical staining of tissue microarrays. Univariate and multivariate analyses revealed that intratumoral pDCs and co-indices (intratumoral pDCs/Treg cells, intratumoral pDCs/IL-17+ cells, or intratumoral pDCs/Treg/IL-17+ cells) were independent prognostic factors for both OS and TTR (Supplementary Tables 6 and 7).
Discussion
The pronounced role of tumor and immune cell interactions in tumor development is well established, with immune cell abundance linked to the prognoses of a number of cancers [6, 8]. Here, we examined the clinical importance of pDC infiltration in a large cohort of patients who underwent surgical resection for HCC. To our knowledge, this is the first report identifying intratumoral pDCs as an independent factor for predicting poor outcomes in patients with HCC after curative resection. Patients with high levels of intratumoral pDCs had significantly lower 5-year OS and higher cumulative recurrence rates than those with lower infiltration. Therefore, patients exhibiting increased intratumoral pDC infiltration should be more carefully monitored after surgery.
Dendritic cells are critical for sensing pathogens and triggering adaptive immune responses [15]. pDCs have recently been identified as a novel subclass of dendritic cells distinguishable by their phenotypic surface, tissue distribution, secretion of cytokines, and ability to present antigens [15]. Those localized at tumor sites, the TA-pDCs, were detected using various strategies in primary carcinomas (in breast, ovary, head and neck, lung, skin, and cervix), cutaneous melanoma, and lymphomas [17]. Many immunohistochemical studies utilized frozen sections, limiting further analyses of large cancer cohorts and hindering the evaluation of the clinical significance of TA-pDCs. In addition, the anti-CD123 antibody commonly used as a marker of pDCs also recognizes macrophages and endothelial and myeloid cells [17]. More recently, the study of human pDCs has been facilitated by the identification of several pDC-specific markers, such as BDCA2, a member of the C-type lectin family of transmembrane glycoproteins [28, 29]. The highly specific and sensitive anti-BDCA2 monoclonal antibody labels pDCs in formalin-fixed paraffin-embedded sections, such as diagnostic tumor specimens [17, 28, 29]. However, a precise quantitative analysis of TA-pDCs and their clinical significance is still lacking [17]. In this study, we used this antibody to detect TA-pDCs in samples from a large HCC patient cohort. We also identified colocalization of BDCA2 and CD123 on most cell surfaces (Supplementary Figure 3), which further suggested that BDCA2 is specific to TA-pDCs in HCC. By immunostaining, we observed that the number of BDCA2+ TA-pDCs that infiltrated the intratumoral area correlated with many clinicopathologic features, including tumor size, vascular invasion, and TNM stage. More importantly, we found that the presence of TA-pDCs is a predictor of OS and TTR in HCC, emphasizing the importance of tumor compartmental evaluation.
Dendritic cells from the bloodstream infiltrate the tumor microenvironment where they reside as immature cells and can present tumor-associated antigens to naive T cells. Therefore, dendritic cells were proposed to initiate an antitumor immune response [30]. pDCs, one of the two major subsets of dendritic cells, were initially recognized as specialized effectors of immunity against viruses by their production of large amounts of IFN. However, pDCs in tumors cannot sustain the production of IFN required to eliminate cancer cells [17]. This may be because the tumor microenvironment lacks the stimuli to stimulate the pDCs or to suppress their activation [17]. Nevertheless, increasing evidence suggests that pDCs play regulatory functions in the tumor microenvironment [31]. The tolerogenic role of pDCs has been shown by their in vivo induction of Tregs in the periphery and their capacity to induce the differentiation and expansion of Treg cells in vitro [31, 32]. In line with these observations, we observed that the presence of intratumoral pDCs in HCC was highly correlated with the intratumoral infiltration of Treg cells but not of CD8+ or granzyme B+ cells. Intratumoral infiltration of Treg cells is associated with the invasiveness and prognosis of HCC [9] and is thought to contribute to immune tolerance in the tumor microenvironment [8]. Our data showed associations between intratumoral pDCs and the numbers of Treg cells and prognosis, suggesting that the tumor microenvironment conditions HCC-resident pDCs to favor the immune tolerance contributing to HCC progression and recurrence.
Recently, a population of IDO-expressing pDCs was identified in human melanoma sentinel nodes [33]. These IDO+ pDCs mediate active immunosuppression in vitro and create a profound local T cell anergy in vivo through the direct activation of mature Tregs [34]. IDO was also reported to be a key immunosuppressive factor, conferring T cell-suppressor activity on pDCs, which facilitates tumor progression in skin chronically exposed to carcinogens [35]. In our study, we observed that the number of IDO+ cells was positively correlated with the number of pDCs (Supplementary Figure 4). This result suggested that in HCC, pDCs may express IDO, which would contribute to their tolerogenic role in immune evasion.
The results from this study also showed a correlation between intratumoral pDCs and intratumoral infiltration of IL-17+ in HCC. IL-17 is the predominant cytokine secreted by proinflammatory Th17 cells, which facilitate inflammation and autoimmune diseases [36]. Recently, IL-17 was shown to act as a proinflammatory cytokine promoting the growth of HCC, and the presence of IL-17+ cells correlates with poor survival in patients with HCC [13]. Several studies have indicated that pDCs promote and modify the differentiation of Th17 cell after TLR7 stimulation by synthetic or natural ligands in vitro [37] and control the homeostasis of Th17 cells in vivo [38]. In the present study, we found that intratumoral infiltration of pDCs correlates with intratumoral infiltration of IL-17+ cells. In addition to the newly revealed prognostic role of intratumoral pDCs in HCC, increased intratumoral infiltration of IL-17+ cells was also found to predict poor survival in HCC patients, consistent with a previously reported study [13]. These results suggest that, in addition to inducing immune tolerance, TA-pDCs might also redirect the proinflammatory immune response to promote tumor growth and progression in HCC.
The ability to predict recurrence after curative resection is critical for managing HCC. At the present time, there are no excellent biomarkers for recurrence of HCC. Although AFP is widely used, it is not a sensitive marker for the presence of HCC and its prognostic value is also limited, especially in early-stage HCC [39]. It is therefore important to find another predictor to identify which of these patients are at risk for recurrence. Of note, some cases of early-stage HCC relapse unexpectedly just after curative resection. These patients may have a better outcome if recurrence can be predicted early and prevented. Our data suggest that levels of pDCs may serve as a predictor to identify these patients. Specifically, patients with high levels of intratumoral pDCs but normal AFP levels or early-stage HCC need to be carefully monitored after surgery, as they are more likely to experience tumor recurrence.
In conclusion, we demonstrate, for the first time, that intratumoral infiltration of pDCs is predictive of a poor prognosis after curative resection of HCC. Intratumoral pDCs may contribute to HCC progression and recurrence through the induction of immune tolerance by Treg cells and an inflammatory tumor microenvironment by IL-17+ cells. A combination of intratumoral pDCs and Treg cells and/or IL-17+ cells may therefore be a superior predictor of outcome. These findings provide a rationale for clinical therapies targeting the anti-immune tolerogenic and anti-inflammatory responses induced by TA-pDCs in HCC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- AFP
Alpha-fetoprotein
- BDCA2
Blood dendritic cell antigen 2
- HCC
Hepatocellular carcinoma
- HR
Hazard ratio
- MVD
Microvessel density
- OS
Overall survival
- pDCs
Plasmacytoid dendritic cells
- TA-pDCs
Tumor-associated pDCs
- TNM
Tumor-node-metastasis
- Treg
Regulatory T
- TTR
Time to recurrence
Author contributions
Z-JZ, H-YX, and JL performed the experiments; S-LZ and Z-JZ analyzed the data; Z-QH, and C-BL provided the samples; S-LZ and Z-JZ wrote the paper; S-LZ obtained funding and designed the research.
Funding
This study was jointly supported by the National Key R&D Program of China (No. 2018YFA0109400), the National Natural Science Foundation of China (No. 81773069), Shanghai Rising-Star Program (18QA1401200), and Municipal Human Resources Development Program for Outstanding Young Talents in Medical and Health Sciences in Shanghai (2018YQ14).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
This study was approved by the Research Ethics Committee of Zhongshan Hospital on February 26th, 2017.
Informed consent
All patients in all three cohorts gave written informed consent to the treatment and the use of their specimens and data for research and for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zheng-Jun Zhou, Hao-Yang Xin, Jia Li have contributed equally to this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Zhou S, Tan C, Dai Z, et al. Tacrolimus enhances the invasion potential of hepatocellular carcinoma cells and promotes lymphatic metastasis in a rat model of hepatocellular carcinoma: involvement of vascular endothelial growth factor-C. Transpl Proc. 2011;43:2747–2754. doi: 10.1016/j.transproceed.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312–1327. doi: 10.1002/hep.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantovani A. Cancer: inflaming metastasis. Nature. 2009;457:36–37. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 5.Zhou SL, Dai Z, Zhou ZJ, Wang XY, Yang GH, Wang Z, Huang XW, Fan J, Zhou J. Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology. 2012;56:2242–2254. doi: 10.1002/hep.25907. [DOI] [PubMed] [Google Scholar]
- 6.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 8.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 9.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 10.Zhou SL, Zhou ZJ, Hu ZQ, et al. Tumor-associated neutrophils recruit macrophages and t-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology. 2016;150(1646–58):e17. doi: 10.1053/j.gastro.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 11.Zhu XD, Zhang JB, Zhuang PY, et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26:2707–2716. doi: 10.1200/JCO.2007.15.6521. [DOI] [PubMed] [Google Scholar]
- 12.Zhou SL, Hu ZQ, Zhou ZJ, Dai Z, Wang Z, Cao Y, Fan J, Huang XW, Zhou J. miR-28-5p-IL-34-macrophage feedback loop modulates hepatocellular carcinoma metastasis. Hepatology. 2016;63:1560–1575. doi: 10.1002/hep.28445. [DOI] [PubMed] [Google Scholar]
- 13.Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 14.Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234:142–162. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lande R, Gilliet M. Plasmacytoid dendritic cells: key players in the initiation and regulation of immune responses. Ann N Y Acad Sci. 2010;1183:89–103. doi: 10.1111/j.1749-6632.2009.05152.x. [DOI] [PubMed] [Google Scholar]
- 16.Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol. 2015;15:471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vermi W, Soncini M, Melocchi L, Sozzani S, Facchetti F. Plasmacytoid dendritic cells and cancer. J Leukoc Biol. 2011;90:681–690. doi: 10.1189/jlb.0411190. [DOI] [PubMed] [Google Scholar]
- 18.Treilleux I, Blay JY, Bendriss-Vermare N, et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res. 2004;10:7466–7474. doi: 10.1158/1078-0432.CCR-04-0684. [DOI] [PubMed] [Google Scholar]
- 19.Sawant A, Hensel JA, Chanda D, Harris BA, Siegal GP, Maheshwari A, Ponnazhagan S. Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. J Immunol. 2012;189:4258–4265. doi: 10.4049/jimmunol.1101855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labidi-Galy SI, Sisirak V, Meeus P, et al. Quantitative and functional alterations of plasmacytoid dendritic cells contribute to immune tolerance in ovarian cancer. Cancer Res. 2011;71:5423–5434. doi: 10.1158/0008-5472.CAN-11-0367. [DOI] [PubMed] [Google Scholar]
- 21.Chauhan D, Singh AV, Brahmandam M, et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell. 2009;16:309–323. doi: 10.1016/j.ccr.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wittekind C. Pitfalls in the classification of liver tumors. Pathologe. 2006;27:289–293. doi: 10.1007/s00292-006-0834-1. [DOI] [PubMed] [Google Scholar]
- 23.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 24.Zhou ZJ, Dai Z, Zhou SL, Fu XT, Zhao YM, Shi YH, Zhou J, Fan J. Overexpression of HnRNP A1 promotes tumor invasion through regulating CD44v6 and indicates poor prognosis for hepatocellular carcinoma. Int J Cancer. 2013;132:1080–1089. doi: 10.1002/ijc.27742. [DOI] [PubMed] [Google Scholar]
- 25.Zhou SL, Dai Z, Zhou ZJ, et al. CXCL5 contributes to tumor metastasis and recurrence of intrahepatic cholangiocarcinoma by recruiting infiltrative intratumoral neutrophils. Carcinogenesis. 2014;35:597–605. doi: 10.1093/carcin/bgt397. [DOI] [PubMed] [Google Scholar]
- 26.Semela D, Dufour JF. Angiogenesis and hepatocellular carcinoma. J Hepatol. 2004;41:864–880. doi: 10.1016/j.jhep.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Facciabene A, Peng X, Hagemann IS, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 28.Dzionek A, Sohma Y, Nagafune J, et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boiocchi L, Lonardi S, Vermi W, Fisogni S, Facchetti F. BDCA-2 (CD303): a highly specific marker for normal and neoplastic plasmacytoid dendritic cells. Blood. 2013;122:296–297. doi: 10.1182/blood-2013-05-500413. [DOI] [PubMed] [Google Scholar]
- 30.Lin A, Schildknecht A, Nguyen LT, Ohashi PS. Dendritic cells integrate signals from the tumor microenvironment to modulate immunity and tumor growth. Immunol Lett. 2010;127:77–84. doi: 10.1016/j.imlet.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Demoulin S, Herfs M, Delvenne P, Hubert P. Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: insight into the molecular mechanisms. J Leukoc Biol. 2013;93:343–352. doi: 10.1189/jlb.0812397. [DOI] [PubMed] [Google Scholar]
- 32.Matta BM, Castellaneta A, Thomson AW. Tolerogenic plasmacytoid DC. Eur J Immunol. 2010;40:2667–2676. doi: 10.1002/eji.201040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerlini G, Di Gennaro P, Mariotti G, Urso C, Chiarugi A, Pimpinelli N, Borgognoni L. Indoleamine 2,3-dioxygenase + cells correspond to the BDCA2 + plasmacytoid dendritic cells in human melanoma sentinel nodes. J Invest Dermatol. 2010;130:898–901. doi: 10.1038/jid.2009.307. [DOI] [PubMed] [Google Scholar]
- 34.Sharma MD, Baban B, Chandler P, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller AJ, Sharma MD, Chandler PR, et al. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc Natl Acad Sci USA. 2008;105:17073–17078. doi: 10.1073/pnas.0806173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu CF, Peng WM, Oldenburg J, et al. Human plasmacytoid dendritic cells support Th17 cell effector function in response to TLR7 ligation. J Immunol. 2010;184:1159–1167. doi: 10.4049/jimmunol.0901706. [DOI] [PubMed] [Google Scholar]
- 38.Takagi H, Fukaya T, Eizumi K, et al. Plasmacytoid dendritic cells are crucial for the initiation of inflammation and T cell immunity in vivo. Immunity. 2011;35:958–971. doi: 10.1016/j.immuni.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 39.Farinati F, Marino D, De Giorgio M, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006;101:524–532. doi: 10.1111/j.1572-0241.2006.00443.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.