Abstract
Background
Merkel cell carcinoma (MCC) is an aggressive skin cancer in which PD-1/PD-L1 blockade has shown remarkable response rates. However, a significant proportion of patients shows primary or secondary resistance against PD-1/PD-L1 inhibition, with HLA class-I downregulation and insufficient influx of CD8+ T cells into the tumor as possible immune escape mechanisms. Histone deacetylase inhibitors (HDACi) have been demonstrated to reverse low HLA class-I expression caused by epigenetic downregulation of the antigen machinery (APM) in vitro and in pre-clinical models in vivo.
Case presentations
We report four cases of patients with metastatic MCC who did not respond to immunotherapy by PD-1/PD-L1 blockade. Two of the patients received, subsequently, the HDACi panobinostat in combination with PD-1/PD-L1 blockade. Tumor biopsies of the patients were analyzed for cellular and molecular markers of antigen processing and presentation as well as the degree of T-cell infiltration.
Results and conclusion
Low expression of APM-related genes associated with low HLA class-I surface expression was observed in all MCC patients, progressing on PD-1/PD-L1 blockade. In one evaluable patient, of the two treated with the combination therapy of the HDACi, panobinostat and PD-1/PD-L1 blockade, reintroduction of HLA class-I-related genes, enhanced HLA class-I surface expression, and elevated CD8+ T-cell infiltration into the MCC tumor tissue were observed; however, these changes did not translate into a clinical benefit. Our findings suggest that HDACi may be useful to overcome HLA class-I downregulation as a resistance mechanism against anti-PD-1/PD-L1 antibodies in MCC patients. Prospective clinical trials are needed to evaluate this notion.
Keywords: Merkel cell carcinoma, HDAC inhibitor, Immunotherapy, HLA class-I, Infiltration
Introduction
Merkel cell carcinoma (MCC) is a rare type of skin cancer with increasing incidence. MCC cells express both epithelial as well as neuroendocrine markers, and are either associated with the presence of Merkel cell polyomavirus (MCPyV) or a history of chronic UV-light exposure. MCC is a very aggressive type of cancer and 40% of the patients diagnosed used to die from the disease within 5 years [1]. Until recently, the only therapeutic option for metastatic MCC was chemotherapy, although its efficacy is rather low in terms of a prolongation of overall survival. Due to the high immunogenicity of MCC owing to its virus or UV-light association, the immune modulation by PD-1/PD-L1 blocking antibodies shows remarkable clinical activity. Indeed, the anti-PD-L1 antibody avelumab was recently approved in the US, EU, Japan, and Australia for inoperable or metastatic MCC [2]. Treatment with avelumab could achieve response rates of 33% in chemotherapy-pretreated patients and 62% in treatment-naïve patients. However, a substantial number of patients either primarily did not respond to PD-1/PD-L1 checkpoint inhibition, or later on experienced a progression or relapse of the disease due to secondary resistance [3, 4]. Recent findings suggest a possible immune evasion strategy of MCC by downregulation of the antigen processing machinery (APM) in general and HLA class-I in particular, as observed in MCC tumor tissues and cell lines [5, 6]. This downregulation is supposed to be a major cause for the low frequency of CD8+ T cells in a significant number of MCCs associated with limited response to anti-PD-1/PD-L1 checkpoint inhibitor immunotherapy. Since the expression of molecules associated with the APM in cancer cells is often altered by epigenetic mechanisms, treatment with epigenetic modulators like histone deacetylase inhibitors (HDACi) is supposed to reverse the downregulation of APM components and lead to an increased immunological visibility of tumor tissues [6, 7].
These findings lead to the rationale for a therapeutic increase of the response to anti-PD-1/PD-L1 checkpoint inhibition by a combination with epigenetic modulators like HDACi.
Patients, materials, and methods
Immunohistochemistry
Immunohistochemistry was performed on formalin-fixed paraffin-embedded (FFPE) tissue sections as previously described [5, 6, 8]. After deparaffinization in xylene, sections were rehydrated with 100%, 96%, 70%, and 50% ethanol for 5 min each, and finally rinsed with demineralized water. Antigens were retrieved with a citrate buffer at pH 6 in a steamer at 100 °C for 45 min. After cooling for 20 min and three additional washing steps in PBS, sections were blocked with peroxidase blocking solution (3% H2O2 in MetOH) followed by incubation for 1 h with a CD8-specific antibody (clone C8/144B, Zytomed, Berlin, Germany), diluted 1:100 in 3% BSA in PBS. Subsequently, sections were washed three times and incubated with an HLA-A specific antibody (clone EP1395Y, Abcam, Cambridge, UK), diluted 1:100 in 3% BSA in PBS. After three washing steps, sections were incubated with HRP polymer anti-mouse (Zytomed) for 30 min followed by three washing steps. Sections were further incubated with alkaline phosphatase polymer anti-rabbit (Zytomed) for 30 min, and after additional washing steps, sections were incubated with HRP green (Zytomed) for 10 min. After washing steps, sections were incubated for 20 min with permanent alkaline phosphatase red (Zytomed). Intratumoral infiltration of CD8+ T cells (IT-CD8+) was scored semi-quantitatively using six bins (IT-CD8+ scores ranging from 0 to 5) as previously described by two independent observers blinded to patient information [9].
DNA isolation and MCPyV detection
Genomic DNA was isolated from FFPE sections using a DNA Isolation Kit (Qiagen, Hilden, Germany). The samples were analyzed for the presence of MCPyV using a TaqMan assay specific for the MCPyV T antigen gene. Taqman primer and probe for T antigen were described previously [10]. Long interspersed nuclear element (LINE) 1, a highly repetitive DNA element, served as DNA control. The relative presence of the MCPyV genome in the samples was determined by the comparative cycle threshold (Ct) method ( method) where the DNA of a MCPyV-positive cell line with at least two copies of MCPyV genome served as calibrator allowing an estimation of copy numbers.
Quantitative real-time-PCR (qRT-PCR)
RNA of FFPE fixed tissue sections adjacent to those used for immunohistochemistry was isolated using the AllPrep DNA/RNA FFPE kit (Qiagen) and transcribed using SuperScript IV reverse transcriptase [Invitrogen, Carlsbad, CA)] according to the manufacturer’s instructions. qRT-PCR were performed using SYBR green or TaqMan PCR master mix (Sigma-Aldrich, St. Louis, MO) on the CFX96 Real-Time System (Bio-Rad, Hercules, CA) as previously described [6]. RPLP0 served as endogenous control and was detected with the sense primer: 5′-CCA TCA GCA CCA CAG CCT TA-3′, the antisense-primer: 5′-GGC GAC CTG GAA GTC CAA CT, and the probe ATC TGC TGC ATC TGC TTG GAG CCC A-3′. The mRNA expression of HLA-ABC, β2 microglobulin (B2M), transporter associated with antigen processing (TAP) 1, TAP2, low-molecular-weight proteins (LMP) 2, and LMP7 was detected using SYBR green assays with specific primers listed in Table 1. Cycle threshold (Ct) values were normalized to RPLP0. The comparative Ct method ( method) was used to calculate changes in gene expression as a relative fold difference between the experimental sample and calibrator sample, i.e., the expression of the respective genes in MKL-2 cells.
Table 1.
Primers qRT-PCR
| Forward | Reverse | |
|---|---|---|
| HLA-ABC | GCGGCTACTACAACCAGAGC | GATGTAATCCTTGCCGTCGT |
| B2 M | TCTCTGCTGGATGACGTGAG | TAGCTGTGCTCGCGCTACT |
| TAP1 | TCAGGGCTTTCGTACAGGAG | TCCGGAAACCGTGTGTACTT |
| TAP2 | ACTGCATCCTGGATCTCCC | TCGACTCACCCTCCTTTCTC |
| LMP2 (PSMB9) | TCAAACACTCGGTTCACCAC | GGAGAAGTCCACACCGGG |
| LMP7 (PSMB8) | CATGGGCCATCTCAATCTG | TCTCCAGAGCTCGCTTTACC |
Case presentations
Patient 1
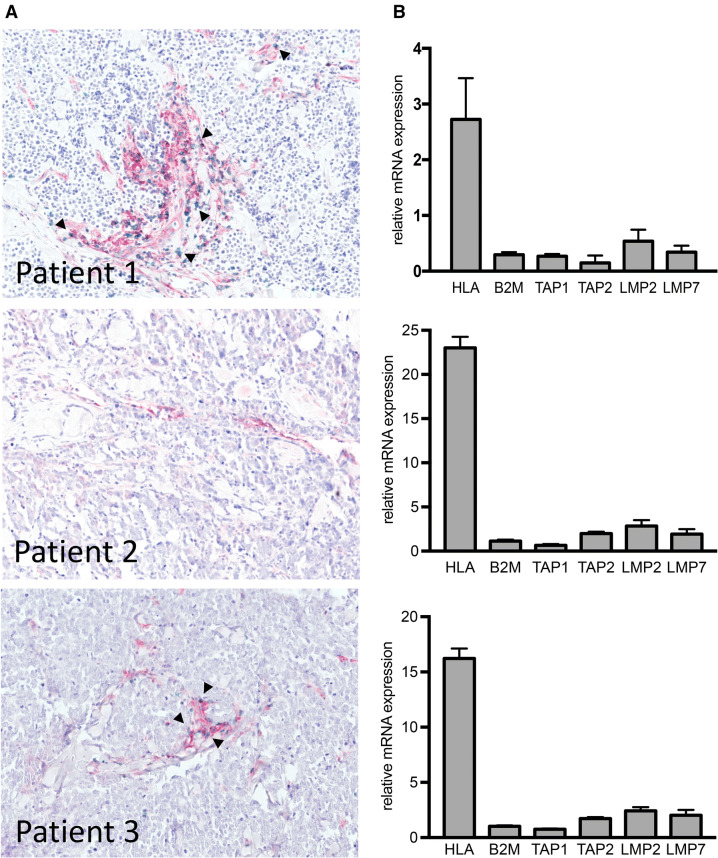
A 68-year-old woman was diagnosed with a MCC of the left upper arm. She had a history of rheumatoid arthritis, lupus erythematosus, and Hashimoto’s thyroiditis. The MCC was completely excised. The MCPyV status was positive. The patient further received adjuvant radiation of the left upper arm and tumor bed. Nevertheless, she developed cutaneous metastases of the left arm and the left thorax, which were treated by radiation. Since further metastatic spread occurred shortly thereafter, a systemic treatment with the anti-PD-L1 antibody avelumab (10 mg/kg every 2 weeks) was started. Still the patient developed new intramammary, axillary, peritoneal, perirenal, and perilienal lesions, and was progressive for the previous cutaneous lesions. Molecular analysis of the progressive tumor lesions revealed a low expression of APM-related genes. HLA class-I surface expression, visualized by a red membranous staining, was restricted to stromal cells, whereas tumor cells were not stained; CD8+ T cells were flagged by a green membranous staining and were mostly restricted to the stromal areas; intratumoral infiltration was rarely observed (Fig. 1). The IT-CD8+ score was 1 with less than 20 cells/mm2. Treatment was changed to chemotherapy with carboplatin plus etoposide, which resulted in a mixed response with a regression of some preexisting lesions and parallel development of new metastases in the pericardia and bone as well as an intraabdominal mass.
Fig. 1.
Molecular and immunohistochemical characterization of MCC tumor lesions refractory to prior PD-1/PD-L1 blockade. a FFPE sections of tumor lesions from three individual MCC patients were stained using an HLA-A-specific antibody (red) and a CD8-specific antibody (green); magnification × 10. bHLA-ABC, B2 M and APM components’ (TAP1, TAP2, LMP2, LMP7) mRNA expression was determined by qRT-PCR in triplicates using specific primers; Ct values were normalized to RPLP0. The comparative Ct method ( method) was used to calculate changes in gene expression as a relative fold difference between the experimental sample and calibrator sample, i.e., the expression of the respective genes in MKL-2 cells. Relative mRNA expression is depicted as mean + SD
Patient 2
An 81-year-old woman with a history of a systemically treated non-Hodgkin lymphoma and a surgically resected and irradiated anal carcinoma presented with a primary MCC of the upper lip. The lesion was excised and sentinel lymph-node biopsy was done without detection of micrometastases. The MCPyV status was negative. Within 3 months, the patient developed three submental lymph-node metastases. Subsequent positron-emission tomography and computed tomography (PET-CT) imaging revealed additional cervical, supraclavicular and mediastinal lesions. Biopsy of these lymph nodes revealed MCC metastases and ruled out a recurrence of the non-Hodgkin lymphoma. Based on these findings, the patient was treated with the anti-PD-1 antibody nivolumab 3 mg/kg every 2 weeks. PD-1 blockade had to be interrupted because of an autoimmune pneumonitis grade 2, but could be re-induced after 4 months. The patient showed a stable disease for a duration of 6 months. Thereafter, a disease progression, predominantly of the cervical lymph nodes, was detected. Analysis of biopsied tumor tissue from the cervical lymph nodes demonstrated a low expression of the APM-related genes, low HLA class-I surface expression by the tumor cells, but high expression by the stromal cells, as well as an almost absent CD8+ T-cell infiltration (Fig. 1). The IT-CD8+ score was 1 with less than 5 cells/mm2.
Patient 3
A 64-year-old man was diagnosed with a MCC of the left forearm. The tumor was tested positive for MCPyV; PD-L1-stained negative (positivity < 1% of tumor cells). The patient received adjuvant radiotherapy of the primary tumor region. 3 months after primary diagnosis, he developed macroscopic lymph-node involvement of the left axilla, with complete lymph-node dissection revealing 2 of 17 nodes positive for MCC. 10 months later distant metastases were detected in the mediastinum, together with a relapse of axillary lymph-node metastases. Due to inoperable disease, treatment with nivolumab 3 mg/kg every 2 weeks was started, leading to a partial response after 6 months of treatment. At 14 months of nivolumab, a relapse was detected in the left axilla, which was excised and histologically confirmed as MCC metastasis. After surgery, nivolumab treatment was paused and the patient followed by regular CT scans. 12 months later, a multifocal relapse occurred with growing tumor masses of the left axilla, mediastinum, and thoracic wall. Nivolumab therapy was re-induced, but this time did not achieve disease control. Due to tumor progression after 3 months of nivolumab, an additional radiation of metastatic lesions of the axilla and thoracic wall was performed. At 6 months of nivolumab, tumor growth was still ongoing. Tumor tissue biopsies revealed a low HLA class-I expression and very limited intratumoral T-cell infiltration (Fig. 1). The IT-CD8+ score was 1 with less than 10 cells/mm2. Thus, after careful consideration of therapy options, the patient received the HDAC inhibitor panobinostat (20 mg p.o. on d1, d3, d5, d8, d10, and d12 every 3 weeks) in combination with nivolumab (3 mg/kg q3w). Besides nausea and vomiting, this combination therapy was rather well tolerated. After 3 months of this combination therapy, a strong and multifocal disease progression was observed. Thus, treatment was stopped and the patient was switched to palliative care. The patient did not agree to an additional tumor biopsy upon disease progression.
Patient 4
A 71-year-old man presented with a histologically confirmed small-cell-type MCC with a primary lesion at the right wrist. The lesion stained positive for CK20, and showed the presence of both frequent apoptotic cells and high mitotic activity. The MCPyV status was positive. The patient rapidly developed histologically confirmed cutaneous, subcutaneous, and lymph-node metastases of the right axilla and the right upper arm, which were treated by complete excision and subsequent adjuvant radiation. 3 months later, however, the patient showed a tumor recurrence with lymph-node metastases of 5 cm diameter of the right axilla and new cutaneous metastases of the right upper arm. Again, radiation therapy induced a partial response within the radiation field, but disease progression occurred at several new locations including the right upper and lower arm, as well as mediastinal lymph nodes. The patient then started treatment with the anti-PD-1 antibody pembrolizumab (2 mg/kg every 3 weeks), which, after 3 months, induced a partial response lasting for 9 months. Thereafter, computed tomography (CT) imaging revealed new retrocrural lymph-node metastases which were treated with radiation therapy (30 Gray total dose) and ongoing doses of pembrolizumab. Despite this treatment, the patient developed further cutaneous and subcutaneous MCC lesions as well as new metastases of 8 cm diameter (cytokeratin 20 and MCPyV positive) of the scrotum, indicating a fulminant secondary resistance against PD-1 blockade. Immunohistochemical staining of tumor tissue obtained from these PD-1 resistant metastases revealed no HLA class-I expression by tumor cells, whereas tumor stromal cells displayed a membranous red staining. CD8+ T-cell infiltration was low and largely restricted to stromal areas (Fig. 2a, b). The IT-CD8+ score was 1 with less than 20 cells/mm2. The patient’s treatment thereafter was changed to the HDACi panobinostat (20 mg p.o. on d1, d3, d5, d8, d10, and d12 every 3 weeks) plus the anti-PD-L1 antibody avelumab (10 mg/kg every 2 weeks). This combination therapy was very well tolerated. Molecular analysis by quantitative real-time-PCR for expression of HLA-ABC as well as the APM-related genes B2 M, TAP1, TAP2, LMP2, and LMP7 before and at 8 weeks after initiation of HDACi treatment revealed a stronger mRNA expression of these genes, particularly LMP2 and LMP7 (Fig. 2c). Immunohistochemical analysis of this tumor tissue demonstrated an elevated CD8+ T-cell infiltration as well as increased HLA class-I expression likely to be caused by a marked upregulation of APM-related genes (Fig. 2d, e). The IT-CD8+ score markedly increased to 3 with almost 500 cells/mm2. After a short disease stabilization lasting only for 3 months, however, the patient developed further metastatic lesions and died from MCC at 4 months after onset of avelumab plus panobinostat.
Fig. 2.
HDAC inhibitor treatment restored APM gene and HLA-A surface expression as well as CD8+ T-cell infiltration. a–d FFPE tumor sections of patient 4 before (a, b) and after (c, d) treatment with the HDACi panobinostat plus PD-1 blockade were stained using an HLA-A-specific antibody (red) and a CD8-specific antibody (green); magnification × 10 (a, c) and × 40 (b, d). e Increased mRNA expression of HLA-ABC, B2 M, and APM components (TAP1, TAP2, LMP2, and LMP7) is depicted as the ratio of mRNA expression (+SD) determined before and after treatment with panobinostat by qRT-PCR in triplicates using specific primers
Discussion
In this small case series, we describe four MCC patients, which were progressing with their disease under PD-1/PD-L1 blockade therapy. All four patients were characterized by low HLA class-I surface expression on tumor cells and a low intratumoral CD8+ T-cell infiltration with IT-CD8+ scores of 1. Molecular analysis by quantitative real-time-PCR for expression of HLA-ABC as well as the APM-related genes B2 M, TAP1, TAP2, LMP2, and LMP7 revealed a low expression of all of the APM-related genes in all of the patients. This observation was in line with our previous report of an impaired expression of APM-related genes in a subset of MCC cell lines [6]. Based on our pre-clinical studies, demonstrating that treatment with HDACi resulted in an upregulation of APM-related genes and the subsequent expression of HLA class-I on the surface of MCC cell lines [6], two of the patients secondarily refractory to immune checkpoint blockade were treated with the HDACi panobinostat in combination with PD-1/PD-L1 blockade in a compassionate use setting. Immunohistological and molecular analyses of tumor tissue of one of these patients after 8 weeks of treatment revealed a restoration of the expression of APM-associated genes, an increased HLA class-I surface expression, as well as an elevated infiltration of CD8+ T cells with an IT-CD8+ score of 3 suggesting a higher susceptibility to anti-PD-1/PD-L1 immunotherapy. While this finding supports the rationale for using HDACi as a re-inducer of HLA class-I related genes and enhancer of CD8+ T-cell infiltration, unfortunately, neither of the patients experienced a relevant clinical benefit.
Immune checkpoint inhibitors have emerged as a promising treatment option inducing durable control of many solid tumor entities. Especially in immunogenic skin cancers like melanoma, squamous cell carcinoma and MCC, PD-1/PD-L1 blocking antibodies have shown remarkable results [11, 12]. However, primary or secondary resistances to PD-1/PD-L1 inhibitors are still problematic and not yet solved. Latest research has identified impaired immunological visibility as one of the reasons for poor response to immunotherapy [13]. Here, we extended our previous observations that limited intratumoral infiltration by CD8+ T cells and low MHC class-I expression was associated with an impaired prognosis to the therapeutic situation [5, 8]. Furthermore, we have demonstrated that low surface expression of HLA class-I was due to downregulation of APM-related genes [6]. The translation of these findings into the clinical setting demonstrated that, in patients refractory to PD-1/PD-L1 blockade, the respective tumor lesions were characterized by low expression levels of APM-related genes and HLA class-I, as well as poor intratumoral CD8+ T-cell infiltration. Moreover, similar to the pre-clinical findings, we observed in one of the patients that re-induction of APM-related genes by HDACi resulted in a strong upregulation of HLA class-I expression and, thus, likely bypassed the immunological camouflage of tumor tissues, hereby allowing infiltration of intratumoral CD8+ T cells.
It should be noted that, in our previous studies, efficient HLA class-I induction by the HDACi vorinostat required co-treatment with mithramycin A to boost its effect [6]. In the presented cases, however, we used panobinostat as it is registered for multiple myeloma in Germany. The strong increase in HLA expression induced by panobinostat suggests that it is more effective at altering the epigenetic landscape of the MCC cells than vorinostat. Indeed, while both agents are hydroxamic acids and act as pan-HDACi, panobinostat has a much longer half-life than vorinostat, i.e., 37 vs. 2 h, and a different tissue distribution [14, 15]. An alternative explanation is that histone deacetylase (HDAC) inhibition in vivo in immunocompetent hosts may reverse some T-cell dysfunction, resulting in enhanced migration into the tumor. These ‘reactivated’ T cells are likely to secrete inflammatory cytokines known to increase antigen presentation via HLA class-I [5].
This proof-of-principle of HDACi-mediated upregulation, however, has been demonstrated in only one of the patients of our case series and on the molecular basis only. For the second patient treated with panobinostat in combination with nivolumab, we could not analyze post-treatment tumor lesions as the patient did not want to undergo this additional procedure. The absence of a clinical response after onset of HDACi plus PD-1/PD-L1 inhibition is an obvious limitation for further conclusions from this case series. However, the late onset of the combination therapy in patients with extensively widespread disease could be an explanation for the contrast seen between the molecular and the clinical treatment efficacy observed. Still, the induction of MHC class-I expression and CD8+ T-cell infiltration by addition of HDACi to immune checkpoint blocking antibodies in refractory patients is an encouragement to proceed this approach into further clinical development. In addition, the combination treatment was well tolerated with no severe or unexpected additional side effects. Besides CD8+ infiltration, the attraction of other favorable immune cell subpopulations to the tumor tissue is to be investigated, as NK cells, gamma/delta T cells, and Th1 cells play important roles in tumor surveillance [16, 17]. In line with this, upregulation of HLA class-II-related genes could give new insights into immuno-oncology.
These findings fuel the rationale for combining different immuno-modulating treatment approaches, as limited expression of HLA molecules by tumor cells as well as low infiltration of effector cells are likely limiting factors for the efficacy of immune modulating therapies [17]. Our case series highlights the potential benefit of epigenetic priming by HDACi for MCC patients refractory to PD-1/PD-L1 blockade. However, further investigation and clinical trials are needed to confirm these observations on the molecular level as well as in the clinical setting.
Acknowledgements
We thank the patients and their families for participation in this research. Writing of the manuscript was supported by 4SC, Planegg-Martinsried (Germany).
Abbreviations
- APM
Antigen processing machinery
- B2 M
β2 microglobulin
- Ct
Cycle threshold
- CT
Computed tomography
- FFPE
Formalin-fixed paraffin-embedded
- HDAC
Histone deacetylase
- HDACi
Histone deacetylase inhibitor
- IT-CD8+
Intratumoral CD8+ T cells
- LINE
Long interspersed nuclear element
- LMP
Low-molecular-weight proteins
- MCC
Merkel cell carcinoma
- MCPyV
Merkel cell polyomavirus
- PET-CT
Positron-emission tomography and computed tomography
- qRT-PCR
Quantitative real-time-PCR
- TAP
Transporter associated with antigen processing
Method, comparative Ct method
Author contributions
SU and JCB designed experiments, acquired, analyzed and interpreted the data, and wrote and revised the manuscript. IS, AC, AM, and CR performed experiments, analyzed, and interpreted the data. JW, CD, AS, LZ, and DS participated in the design of experiments, interpretation of the data, and writing of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the German Cancer Consortium (Deutsches Konsortium für Translationale Krebsforschung, DKTK).
Compliance with ethical standards
Conflict of interest
Selma Ugurel declares research support from medac and Bristol-Myers Squibb, speakers and advisory board honoraria from Bristol-Myers Squibb, Merck Sharp & Dome, and Roche, and travel support from Bristol-Myers Squibb, medac, Merck Sharp & Dome, and Roche. Jonas Wohlfarth is an intern at 4SC. Lisa Zimmer has served as consultant or/and has received honoraria from Roche, Bristol-Myers Squibb, Merck Sharp & Dome, GlaxoSmithKline, Novartis, Merck Serono, and travel support from Merck Sharp & Dome, Bristol-Myers Squibb, Amgen and Novartis. Dirk Schadendorf has received speaker honoraria from Roche, Novartis, Bristol-Myers Squibb, Merck Sharp & Dome, Amgen, Merck Serono and Pierre-Fabre, advisory board honoraria from Roche, Novartis, Bristol-Myers Squibb, Merck Sharp & Dome, Amgen, Incyte, Merck Serono and Pierre-Fabre as well as research funding from Novartis and Bristol-Myers Squibb. Jürgen C. Becker has received speaker honoraria from Amgen, Merck Serono, and Pfizer, advisory board honoraria from Amgen, CureVac, eTheRNA, Lytix, Merck Serono, Novartis, Rigontec, and Takeda as well as research funding from Alcedis, Boehringer Ingelheim, Bristol-Myers Squibb, and Merck Serono; he also received travel support from 4SC and Incyte. The authors declare that there is no other conflict of interest.
Ethical approval
The investigational procedures were approved by the institutional review board/ethic committee (17-7539-BO; Ethics Committee of the University Duisburg-Essen).
Informed consent
All patients provided informed consent for the scientific work up of the tumor biopsies as well as for the publication of the results in an anonymized way.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Becker JC, Stang A, Decaprio JA, Cerroni L, Lebbé C, Veness M, Nghiem P. Merkel cell carcinoma. Nat Rev Dis Prim. 2017;3:17077. doi: 10.1038/nrdp.2017.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terheyden P, Becker JC. New developments in the biology and the treatment of metastatic Merkel cell carcinoma. Curr Opin Oncol. 2017 doi: 10.1097/CCO.0000000000000363. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman HL, Russell JS, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, Shih KC, Lebbé C, Milella M, Brownell I, Lewis KD, Lorch JH, von Heydebreck A, Hennessy M, Nghiem P. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥ 1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer. 2018;6:7. doi: 10.1186/s40425-017-0310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, Berry S, Chartash EK, Daud A, Fling SP, Friedlander PA, Kluger HM, Kohrt HE, Lundgren L, Margolin K, Mitchell A, Olencki T, Pardoll DM, Reddy SA, Shantha EM, Sharfman WH, Sharon E, Shemanski LR, Shinohara MM, Sunshine JC, Taube JM, Thompson JA, Townson SM, Yearley JH, Topalian SL, Cheever MA. PD-1 Blockade with pembrolizumab in advanced Merkel cell carcinoma. N Engl J Med. 2016;374:2542–2552. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paulson KG, Tegeder A, Willmes C, Iyer JG, Afanasiev OK, Schrama D, Koba S, Thibodeau R, Nagase K, Simonson WT, Seo A, Koelle DM, Madeleine M, Bhatia S, Nakajima H, Sano S, Hardwick JS, Disis ML, Cleary MA, Becker JC, Nghiem P. Downregulation of MHC-I expression is prevalent but reversible in Merkel cell carcinoma. Cancer Immunol Res. 2014;2:1071–1079. doi: 10.1158/2326-6066.CIR-14-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ritter C, Fan K, Paschen A, Reker Hardrup S, Ferrone S, Nghiem P, Ugurel S, Schrama D, Becker JC. Epigenetic priming restores the HLA class-I antigen processing machinery expression in Merkel cell carcinoma. Sci Rep. 2017;7:1. doi: 10.1038/s41598-017-02608-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llopiz D, Ruiz M, Villanueva L, Iglesias T, Silva L, Egea J, Lasarte JJ, Pivette P, Trochon-Joseph V, Vasseur B, Dixon G, Sangro B, Sarobe P. Enhanced anti-tumor efficacy of checkpoint inhibitors in combination with the histone deacetylase inhibitor belinostat in a murine hepatocellular carcinoma model. Cancer Immunol Immunother. 2018 doi: 10.1007/s00262-018-2283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paulson KG, Iyer JG, Tegeder AR, Thibodeau R, Schelter J, Koba S, Schrama D, Simonson WT, Lemos BD, Byrd DR, Koelle DM, Galloway DA, Leonard JH, Madeleine MM, Argenyi ZB, Disis ML, Becker JC, Cleary MA, Nghiem P. Transcriptome-wide studies of Merkel cell carcinoma and validation of intratumoral CD8+ lymphocyte invasion as an independent predictor of survival. J Clin Oncol. 2011;29:1539–1546. doi: 10.1200/JCO.2010.30.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paulson KG, Iyer JG, Simonson WT, Blom A, Thibodeau RM, Schmidt M, Pietromonaco S, Sokil M, Warton EM, Asgari MM, Nghiem P. CD8+ lymphocyte intratumoral infiltration as a stage-independent predictor of merkel cell carcinoma survival. Am J Clin Pathol. 2014;142:452–458. doi: 10.1309/AJCPIKDZM39CRPNC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrama D, Peitsch WK, Zapatka M, Kneitz H, Houben R, Eib S, Haferkamp S, Moore PS, Shuda M, Thompson JF, Trefzer U, Pföhler C, Scolyer RA, Becker JC. Merkel cell polyomavirus status is not associated with clinical course of Merkel cell carcinoma. J Investig Dermatol. 2011;131:1631–1638. doi: 10.1038/jid.2011.115. [DOI] [PubMed] [Google Scholar]
- 11.Maio M, Coukos G, Ferrone S, Fox BA, Fridman WH, Garcia PL, Lahn M, Provendier O, Russo V, Rüttinger D, Shalabi A, Trajanoski Z, Viallet J, Wolchok JD, Ibrahim R. Addressing current challenges and future directions in immuno-oncology: expert perspectives from the 2017 NIBIT Foundation Think Tank, Siena, Italy. Cancer Immunol Immunother. 2018 doi: 10.1007/s00262-018-2285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdin S, Zaher D, Arafa E-S, Omar H. Tackling cancer resistance by immunotherapy: updated clinical impact and safety of PD-1/PD-L1 inhibitors. Cancers. 2018;10:32. doi: 10.3390/cancers10020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai L, Michelakos T, Yamada T, Fan S, Wang X, Schwab JH, Ferrone CR, Ferrone S. Defective HLA class I antigen processing machinery in cancer. Cancer Immunol Immunother. 2018;67:999–1009. doi: 10.1007/s00262-018-2131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckschlager T, Plch J, Stiborova M, Hrabeta J. Histone deacetylase inhibitors as anticancer drugs. Int J Mol Sci. 2017;18(7):e1414. doi: 10.3390/ijms18071414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suresh PS, Devaraj VC, Srinivas NR, Mullangi R. Review of bioanalytical assays for the quantitation of various HDAC inhibitors such as vorinostat, belinostat, panobinostat, romidepsin and chidamine. Biomed Chromatogr. 2017 doi: 10.1002/bmc.3807. [DOI] [PubMed] [Google Scholar]
- 16.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, Bruneval P, Fridman WH, Becker C, Pagès F, Speicher MR, Trajanoski Z, Galon J. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Chen DS, Mellman I. Elements of cancer immunity and the cancer–immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]