Abstract
Background
Immunotherapy has raised the issue of appropriate treatment response evaluation, due to the unique mechanism of action of the immunotherapeutic agents. Aim of this analysis is to evaluate the potential role of quantitative analysis of 2-deoxy-2-(18F)fluoro-d-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) data in monitoring of patients with metastatic melanoma undergoing ipilimumab therapy.
Methods
25 patients with unresectable metastatic melanoma underwent dynamic PET/CT (dPET/CT) of the thorax and upper abdomen as well as static, whole body PET/CT with 18F-FDG before the start of ipilimumab treatment (baseline PET/CT), after two cycles of treatment (interim PET/CT) and at the end of treatment after four cycles (late PET/CT). The evaluation of dPET/CT studies was based on semi-quantitative (standardized uptake value, SUV) calculation as well as quantitative analysis, based on two-tissue compartment modeling and a fractal approach. Patients’ best clinical response, assessed at a mean of 59 weeks, was used as reference.
Results
According to their best clinical response, patients were dichotomized in those demonstrating clinical benefit (CB, n = 16 patients) and those demonstrating no clinical benefit (no-CB, n = 9 patients). No statistically significant differences were observed between CB and no-CB regarding either semi-quantitative or quantitative parameters in all scans. On contrary, the application of the recently introduced PET response evaluation criteria for immunotherapy (PERCIMT) led to a correct classification rate of 84% (21/25 patients).
Conclusion
Quantitative analysis of 18F-FDG PET data does not provide additional information in treatment response evaluation of metastatic melanoma patients receiving ipilimumab. PERCIMT criteria correlated better with clinical response.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2183-3) contains supplementary material, which is available to authorized users.
Keywords: Metastatic melanoma, Ipilimumab, 18F-FDG PET/CT, PERCIMT criteria
Introduction
Ipilimumab is a fully human recombinant monoclonal antibody, which acts by blocking the immune checkpoint pathway involving the CTLA-4. CTLA-4 exerts, when activated, an inhibitory effect on T cells [1]. Blocking of CTLA-4 by ipilimumab results in the ‘switching off’ of the T-cell inhibition and the subsequent potentiation of anti-tumor response through the interaction of T cells and cancer cells [2]. Ipilimumab has led to a median overall survival (OS) of 10.1 months and to a progression-free survival (PFS) of 4.4 months in metastatic melanoma, and has been the first immunotherapeutic agent approved by the Food and Drug Administration (FDA) and the European Medicines Agency in 2011 for the treatment of advanced melanoma in over a decade [3–5]. Meanwhile, it is mainly used in combination with the PD-1 antibody nivolumab [6].
Treatment monitoring is of great significance given the therapy-related side effects and its high cost [7]. In addition, timely recognition of non-responders can lead to an early treatment switch to more efficient treatment options. Conventional response criteria are based on changes in tumor volume, assessed by radiological methods. However, a tumor volume reduction may take place at a considerably late time point, even after an effective therapy. Moreover, these criteria were developed for treatment evaluation of conventional cytotoxic chemotherapy. Nevertheless, immunotherapy acts in a markedly different way than conventional chemotherapeutic agents and is partially accompanied by atypical response patterns [8].
18F-FDG PET/CT is the most powerful non-invasive tool for imaging metabolic processes in patients. Qualitative/visual evaluation and semi-quantitative measurements are mainly used for evaluating a therapeutic effect by means of PET. The most widely used method for quantification of PET data is the calculation of standardized uptake value (SUV), which represents tissue activity within a volume of interest (VOI) corrected for injected activity and body weight. SUV calculation requires only static imaging when the tracer has reached equilibrium. Nevertheless, the generally accepted method for accurate analysis of 18F-FDG metabolism and acquisition of quantitative data regarding tracer kinetics is a two-tissue compartment model [9]. A pre-requisite for this is the performance of full dynamic PET (dPET) studies for at least 60 min. Moreover, a non-compartment model can be applied leading to the estimation of fractal dimension (FD) for the time activity data, a parameter reflecting heterogeneity [10]. By applying compartmental and non-compartmental analysis to the dynamic data, the shape of the tracer time activity curve (TAC) is characterized and, therefore, additional valuable information concerning the influx and efflux of 18F-FDG, as well as phosphorylation and dephosphorylation rate can be obtained.
The key question, which remains open at the moment, is which is the appropriate approach for evaluation of 18F-FDG PET data for accurate prediction of response as well as for therapy monitoring in case of immunotherapy, since the so-far published data in this field remain limited [11–14]. In 2017, two different PET/CT-based immunotherapy response criteria systems developed by two different groups were proposed: the PET/CT Criteria for early prediction of Response to Immune checkpoint inhibitor Therapy (PECRIT) and the PET Response Evaluation Criteria for Immunotherapy (PERCIMT) [12, 14].
Aim of the present analysis was to evaluate the potential role of both the semi-quantitative (SUV) and quantitative/kinetic analysis of 18F-FDG PET data in melanoma lesions in treatment response evaluation to ipilimumab. For this reason, dPET/CT studies of the thorax and upper abdomen as well as whole body, static PET/CT studies were performed in a homogeneous group of unresectable, stage IV metastatic melanoma patients receiving ipilimumab before, during and after the end of treatment. These results were compared with the results derived after application of the PERCIMT criteria in the same patient group.
Materials and methods
Patients
A total of 41 patients with unresectable metastatic melanoma underwent dPET/CT of the thorax and upper abdomen with 18F-FDG before the start of ipilimumab treatment (baseline PET/CT), after two cycles of treatment (interim PET/CT) and at the end of treatment after four cycles (late PET/CT). Twenty-five patients (18 male, 7 female; mean age 58.8 years) with melanoma-indicative 18F-FDG avid thoracic/upper abdomen lesions in all three scans during the course of treatment were identified and included in the analysis. Ipilimumab was administered intravenously at a dose of 3 mg/kg every 3 weeks for a total of four doses. All patients had normal renal, hepatic and hematological function. The included patients had not received chemotherapy for at least 1 month prior to the initial PET/CT studies. Some patients of this cohort have already been evaluated in previous publications of our group, but with a different approach and analysis than in the here presented study [11, 14].
Data acquisition
Data acquisition consisted of two parts: the dynamic part (dPET/CT studies) and the static part (whole body PET/CT). dPET/CT studies were performed over the thorax and upper abdomen after intravenous administration of maximum 250 MBq 18F-FDG for 60 min using a 24-frame protocol (10 frames of 30 s, 5 frames of 60 s, 5 frames of 120 s and 4 frames of 600 s). Whole body, static imaging was performed in all patients with an image duration of 2 min per bed position for the emission scans after the end of the dynamic acquisition. A dedicated PET/CT system (Biograph mCT, S128, Siemens Co., Erlangen, Germany) with an axial field of view of 21.6 cm with TruePoint and TrueV, operated in a three-dimensional mode was used. A low-dose attenuation CT (120 kV, 30 mA) was used for attenuation correction of the dynamic emission PET data and for image fusion. All PET images were attenuation-corrected and an image matrix of 400 × 400 pixels was used for iterative image reconstruction. Iterative images reconstruction was based on the ordered subset expectation maximization (OSEM) algorithm with two iterations and 21 subsets as well as time of flight (TOF). The reconstructed images were converted to SUV images based on the formula: SUV = tissue concentration (Bq/g)/injected dose (Bq)/body weight (g) [15].
Data analysis
Data analysis consisted of visual (qualitative) analysis of the PET/CT scans, semi-quantitative evaluation based on SUV calculations, and quantitative analysis of the dynamic 18F-FDG PET data. For every lesion both semi-quantitative (SUV) and quantitative analysis were performed.
Qualitative analysis of the PET/CT scans was based on the identification of sites of non-physiological 18F-FDG uptake greater than the background or liver activity, indicating melanoma lesions. All foci of increased 18F-FDG uptake were correlated with the fused low-dose CT findings, to ensure a higher diagnostic accuracy of metastatic lesions. The assessment of PET/CT scans was performed by two nuclear medicine physicians (Christos Sachpekidis, Antonia Dimitrakopoulou-Strauss).
Semi-quantitative evaluation was based on volumes of interest (VOIs) and on subsequent calculation of SUVmean and SUVmax. VOIs were drawn with an isocontour mode (pseudo-snake) and were placed over melanoma-indicative lesions [16]. SUV measurement was performed at the 60-min post-injection frames.
Quantitative evaluation of the dynamic 18F-FDG PET/CT data of the thorax and upper abdomen were performed in the hottest, melanoma-indicative lesions in each patient, up to a maximum of five lesions total (and a maximum of two lesions per organ), which were present in all three scans. The analysis was performed using a dedicated software and based on a two-tissue compartment model, with methods already reported in literature and performed previously by our group [17–22]. Time activity curves (TACs) were created using VOIs. The application of a two-tissue compartment model leads to the extraction of the kinetic parameters K1, k2, k3 and k4 as well as influx (Ki) that describe specific molecular processes: K1 reflects the carrier-mediated transport of 18F-FDG from plasma to tissue while k2 reflects its transport back from tissue to plasma, and k3 represents the phosphorylation rate while k4 the dephosphorylation rate of the glucose analog. Influx (Ki) is derived from the equation = (K1 × k3)/(k2 + k3).
In addition to performing compartment analysis, a non-compartment model based on the fractal dimension (FD) for the time activity data was applied. FD is a parameter of heterogeneity based on the box counting procedure of chaos theory and was calculated in each individual voxel of a VOI. The values of FD vary from 0 to 2 showing the more deterministic or chaotic distribution of the tracer activity via time [10].
Response evaluation
The PERCIMT criteria classify tumor response by means of 18F-FDG PET/CT into four categories: progressive metabolic disease (PMD), stable metabolic disease (SMD), partial metabolic response (PMR), and complete metabolic response (CMR) (Table 1).
Table 1.
Summary of the PET response evaluation criteria for immunotherapy (PERCIMT) response criteria applied in the study
| PET findings | |
|---|---|
| CMR | Complete resolution of all pre-existing 18F-FDG avid lesions. No new, 18F-FDG avid lesions |
| PMR | Complete resolution of some pre-existing 18F-FDG avid lesions. No new, 18F-FDG avid lesions |
| SMD | Neither PMD nor PMR/CMR |
| PMD |
≥ 4 new lesions of less than 1 cm in functional diameter or ≥ 3 new lesions of more than 1.0 cm in functional diameter or ≥ 2 new lesions of more than 1.5 cm in functional diameter |
CMR complete metabolic response, PMR partial metabolic response, SMD stable metabolic disease, PMD progressive metabolic disease
Patients’ best clinical response was determined by the dermato-oncologists (Julia K. Winkler, Jessica C. Hassel) and was based on clinical follow-up, serum level of the tumor marker lactate dehydrogenase (LDH) and standard of care imaging (including follow-up brain MRI and 18F-FDG PET/CT studies). Best clinical response was assessed at a mean of 59 weeks (range 16–153 weeks) and used as reference. Patients were classified into four different groups of best clinical response: (1) complete response (CR), defined as resolution of all melanoma-indicative lesions on physical examination, complete resolution of all lesions on brain MRI and 18F-FDG PET/CT as well as no increase in serum levels of LDH, (2) partial response (PR) defined as a decrease in size or partial resolution of pre-existing melanoma-indicative lesions and no newly emerging lesions on physical examination, partial resolution of pre-existing lesions and no new lesions on brain MRI and 18F-FDG PET/CT, as well as no increase in serum levels of LDH, (3) progressive disease (PD) defined as the appearance of newly emerging suspicious lesions on physical examination or newly developed lesions or massive growth of pre-existing lesions on 18F-FDG PET/CT or newly developed lesions or growth of pre-existing lesions on brain MRI, or major increase in the levels of serum LDH, and (4) stable disease (SD) defined by exclusion of CR, PR and PD. Given the fact that stable disease demonstrates a satisfactory result to immunotherapy, since it can be, on contrary to conventional chemotherapy, durable and exhibits survival rates comparable to those of response [23–26], patients were classified according to their clinical response as patients with clinical benefit (CB), involving those with SD, PR and CR, and as patients with no clinical benefit (no-CB), involving those with PD. It has to be noted that PET-based response evaluation according to PERCIMT was blinded from the patient’s best clinical response.
We compared the baseline semi-quantitative and quantitative parameters of the CB and no-CB groups in search of statistically significant differences that could be predictive of the final clinical outcome. We, moreover, evaluated these parameters during treatment in search of statistically significant changes as response to ipilimumab.
Statistical analysis
Up to five lesions were evaluated per patient. The hottest lesions, based on SUV calculations, were included in the analysis. The lesions were not aggregated but kept as individual lesions, keeping track of which patient they belong to. Measurements on the same patient are more correlated than between different patients, hence linear mixed model analysis was performed to account for the correlation structure in the data. For the parameters SUVmean, SUVmax, K1, k3, influx (Ki) and FD, the following comparisons were made: for every PET measurement, parameters were compared between the CB and no-CB groups using linear mixed model analysis with fixed factor group. Separately for both patient groups, it was tested whether a significant change in parameters between PET measurements could be observed and, finally, the change in parameters was compared between both patient groups. P-values were corrected for multiplicity of testing by the Bonferroni–Holm method. Calculations were performed in R (R version 3.3.1, The R Foundation for Statistical Computing) and linear mixed model analysis was performed using R package nlme.
Results
Of the 25 patients included in the analysis, 16 were classified as showing CB and 9 as showing no-CB. Baseline SUVmean, SUVmax, k3, influx and FD were in average higher in the no-CB group, while baseline K1 was in average higher in the CB group. However, linear mixed model analysis revealed no statistically significant differences between CB and no-CB groups regarding both semi-quantitative and quantitative baseline parameters.
We also studied the parameters’ changes during the whole course of ipilimumab administration and found that no significant changes took place in each group as response to treatment, with the exception of K1 which was different for the third PET compared to baseline (raw p value 0.0287) in the CB group. We have to note, however, that Bonferroni–Holm correction for multiplicity of testing adjusts this p value to 1. Moreover, there were no significant between-group differences regarding the parameters’ changes. The results of this analysis are presented in Tables 2 and 3. Figure 1 presents the box plots of parameters SUVmean (Fig. 1a) and SUVmax (Fig. 1b) in the groups CB and no-CB during ipilimumab treatment. Respectively, Supplementary Figs. 1 and 2 present the box plots of parameters influx (Ki) and FD in the groups CB and no-CB during ipilimumab treatment.
Table 2.
Median values (minimum and maximum given in brackets) for the 18F-FDG semi-quantitative and quantitative parameters before, after two cycles and after four cycles of ipilimumab in the CB group
| Parameter | First PET/ CT (baseline) | Second PET/CT (interim) | Third PET/CT (late) |
|---|---|---|---|
| SUVmean | 5.0 (1.8–13.3) | 5.0 (1.7–11.1) | 5.2 (1.4–19.0) |
| SUVmax | 8.1 (2.0-20.7) | 7.8 (2.1–24.7) | 8.5 (1.9–26.1) |
| K 1 | 0.17 (0.02–0.72) | 0.14 (0.03–0.55) | 0.15 (0.02–0.37) |
| k 3 | 0.09 (0.01–0.90) | 0.13 (0.02–0.41) | 0.11 (0.01–0.37) |
| Influx (Ki) | 0.02 (0.003–0.07) | 0.02 (0.01–0.08) | 0.03 (0.005–0.09) |
| FD | 1.20 (0.85–1.66) | 1.22 (0.90–1.30) | 1.21 (0.87–1.33) |
The values are derived from the hottest, melanoma-indicative lesions, up to a maximum of five lesions total. The units of parameters K1, k3 and influx are 1/min
SUVmean SUVmax and FD have no unit
Table 3.
Median values (minimum and maximum given in brackets) for the 18F-FDG semi-quantitative and quantitative parameters before, after two cycles and after four cycles of ipilimumab in the no-CB group
| Parameter | First PET/CT (baseline) | Second PET/CT (interim) | Third PET/CT (late) |
|---|---|---|---|
| SUVmean | 6.0 (1.4–33.6) | 7.6 (1.5–33.0) | 6.6 (1.5–17.0) |
| SUVmax | 8.2 (1.6–43.4) | 12.1 (1.6–44.2) | 8.6 (2.0-23.9) |
| K 1 | 0.15 (0.06–0.67) | 0.16 (0.01–0.99) | 0.16 (0.03–0.29) |
| k 3 | 0.15 (0.03–0.54) | 0.12 (0.03–0.99) | 0.14 (0.03–0.99) |
| Influx (Ki) | 0.04 (0.004–0.13) | 0.06 (0.002–0.15) | 0.04 (0.01–0.10) |
| FD | 1.23 (0.87–1.41) | 1.27 (0.89–1.43) | 1.24 (0.88–1.40) |
The values are derived from the hottest, melanoma-indicative lesions, up to a maximum of five lesions total. The units of parameters K1, k3 and influx are 1/min
SUVmean SUVmax and FD have no unit
Fig. 1.
Box plots of SUVmean (a) and SUVmax (b) measurements during the course of treatment in the two groups of CB and no-CB. Single dots represent observed values outside the range determined by the whiskers. No statistically significant differences between CB and no-CB patients were regarding both SUVmean and SUVmax. Moreover, there were no significant SUV changes during the whole course of ipilimumab administration as response to treatment in mixed linear model analysis
We, further, applied the PERCIMT criteria in both interim and late PET/CT, and compared response evaluation according to these criteria with patients’ best clinical response. Similarly to the best clinical response-based classification of patients into CB and no-CB, a PET-based dichotomisation of the studied population into patients demonstrating metabolic benefit (MB) including those with SMD, PMR and CMR, and patients demonstrating no metabolic benefit (no-MB) including those with PMD was performed. Based on this classification, in the CB group 15/16 patients demonstrated MB and 1/16 patients no-MB on interim PET/CT, while 14/16 patients showed MB and 2/16 patients no-MB on late PET/CT, according to the PERCIMT criteria. Respectively, in the no-CB group 6/9 patients showed no-MB and 3/9 patients showed MB on interim PET/CT, while 7/9 patients demonstrated no-MB and 2/9 patients demonstrated MB on late PET/CT. Based on this analysis, 21/25 were correctly classified with PERCIMT applied on interim and late PET/CT leading to a correct classification rate (CCR) of 84%. The results of this analysis are presented in Supplementary Table 1.
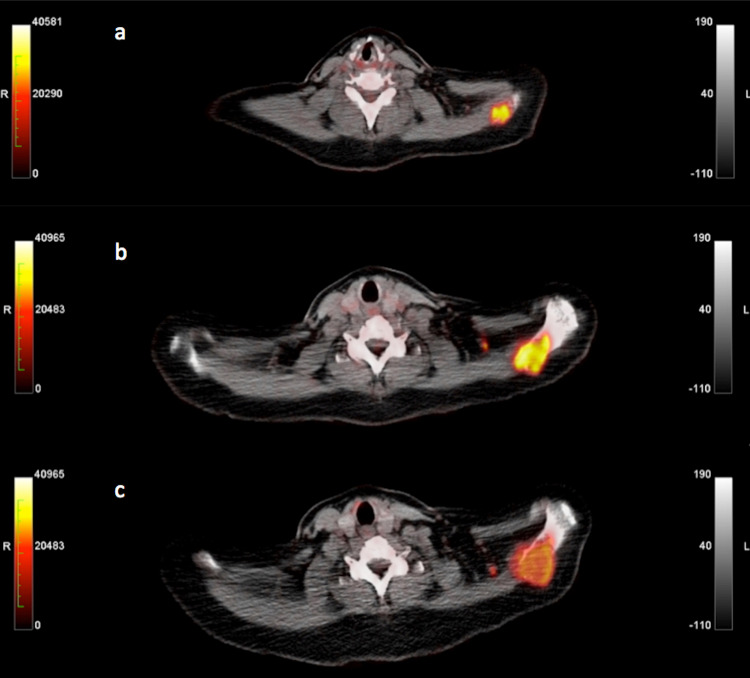
An example of a CB patient correctly classified with PERCIMT is provided in Figs. 2 and 3. The figures demonstrate the PET/CT studies of a 52-year-old female, stage IV melanoma patient during the whole course of ipilimumab treatment. Baseline PET/CT before onset of treatment demonstrated 18F-FDG avid lesions in the right hilus (SUVmean = 10.9, SUVmax = 20.1, influx = 0.05) and in the left scapula (SUVmean = 7.8, SUVmax = 14.0, influx = 0.04), corresponding to metastases (Figs. 2a, 3a). Interim PET/CT after two cycles demonstrated a decrease in tracer uptake and influx of the right hilar metastasis (SUVmean = 4.5, SUVmax= 9.5, influx = 0.01) and at the same time an increase in tracer uptake of the scapula metastasis (SUVmean = 11.1, SUVmax = 24.7, influx = 0.04). Two small, newly emerging, 18F-FDG avid, left supraclavicular lymph nodes as well as an intramuscular lesion in the right thigh were demonstrated. According to PERCIMT, the patient was characterized as SMD (MB) (Figs. 2b, 3b). PET/CT performed shortly after the end of the four-cycle treatment demonstrated in comparison with interim PET/CT an increase in tracer uptake and influx of the right hilar metastasis (SUVmean =7.7, SUVmax =15.0, influx = 0.04) and a decrease in tracer uptake of the scapular metastasis (SUVmean = 7.7, SUVmax = 13.9, influx = 0.04), and complete metabolic remission of one supraclavicular lymph node as well as of the intramuscular lesion in the right thigh; hence the patient was still characterized as SMD (MB) according to PERCIMT, in comparison with the baseline scan (Figs. 2c, 3c). Based on her clinical status, the patient was characterized as demonstrating SD, thus, clinical benefit (CB) as response to ipilimumab. PET/CT assessed by PERCIMT proved to be predictive of the patient’s clinical response. On the other hand, changes in 18F-FDG uptake and kinetics were rather inconclusive of the patient’s clinical outcome. The enhanced FDG uptake in the colon is a common finding following ipilimumab immunotherapy (Fig. 2b, c).
Fig. 2.
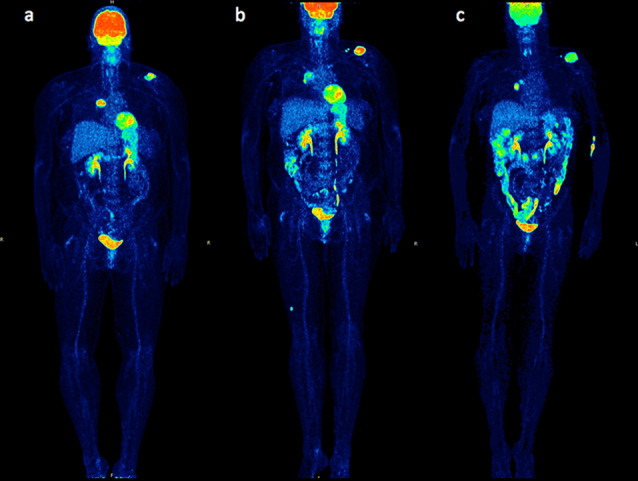
Maximum intensity projection (MIP) 18F-FDG PET/CT images of a stage IV melanoma patient before onset of treatment (a), after two cycles of ipilimumab (b) and shortly after the end of the four-cycle treatment (c). The patient was characterized as SMD (MB) according to PERCIMT. Based on her clinical status, the patient was characterized as demonstrating SD, thus, clinical benefit (CB). PET/CT assessed by PERCIMT proved to be predictive of the patient’s clinical response. On the other hand, changes in 18F-FDG uptake and kinetics were rather inconclusive of the patient’s clinical outcome
Fig. 3.
Transaxial 18F-FDG PET/CT images at the supraclavicular level of the same patient as in Fig. 2 before onset of treatment (a), after two cycles of ipilimumab (b) and shortly after the end of the four-cycle treatment (c)
Discussion
Consistent with its unique mechanism of action, ipilimumab is associated with four distinct patterns of response: (1) response in baseline/index lesions, that is a ‘chemotherapy-like’ response, (2) a slow, steady decline of tumor burden, (3) response after an initial increase in tumor burden, that is, after initially progressive disease by standard response criteria (pseudoprogression), and (4) response in baseline and new lesions accompanied by the appearance of other new lesions [27]. The latter two are considered atypical response patterns by means of standard criteria. These unusual response patterns, the wide variety of immune-related adverse effects associated with immunotherapeutic agents, as well as the time required for the ipilimumab-induced tumor responses to reach their full potential, render assessment of immunotherapy anti-tumor efficacy challenging [28, 29]. In this setting, the application of conventional response criteria may misinterpret the effectiveness of immunotherapy. To avoid such misinterpretations and to assess the atypical response patterns, approximately 200 oncologists, immunotherapists and regulatory experts introduced in 2009 the immune-related response criteria (irRC) using data from ipilimumab phase II clinical trials in patients with advanced melanoma. The core novelty of these criteria was the incorporation of measurable new lesions into total tumor burden and comparison of this variable to baseline measurements [23]. Most recently in 2017, the revised Response Evaluation Criteria In Solid Tumors (RECIST) criteria for evaluation of immunotherapy response (iRECIST) were published taking also into account the atypical response patterns to immune therapy. These revised criteria introduce the term unconfirmed progressive disease (iUPD) and suggest tumor reassessment between 4 and 8 weeks after iUPD. If iUPD is confirmed, then the patient is characterized as iCPD (confirmed progressive disease). On the other hand, if progression is not confirmed, the patient is classified as iCR, iPR or iSD and reassessment should continue as originally planned [30].
The role of 18F-FDG PET in immunotherapy response assessment is unclarified, since the published data in the field are still limited. Our group has previously shown that PET/CT, performed after the end of the first two ipilimumab cycles and assessed by means of European Organization for Research and Treatment of Cancer (EORTC) criteria, could predict the treatment outcome in 18 of 22 stage IV melanoma patients, using as reference the results of PET/CT performed after the end of the four-cycle treatment. Moreover, patients showing stable metabolic disease (SMD) on PET demonstrated significantly longer OS and PFS than patients with progressive metabolic disease (PMD). However, interim PET/CT could not predict the two patients who finally demonstrated partial metabolic response (PMR) [11]. Cho et al. compared the performance of the RECIST 1.1, irRC, PET Response Criteria in Solid Tumors (PERCIST) and EORTC response criteria, applied early in the course of treatment, in predicting best overall response at ≥ 4 months in a sample size of 20 melanoma patients treated with ipilimumab, BMS-936,559 (anti-PD-L1) and nivolumab (anti-PD-1). The authors found that a combination of changes in lesional dimensions along with 18F-FDG uptake changes is more accurate predictor of eventual response, and proposed the PECRIT criteria, based on a combination of RECIST and PERCIST. These criteria predicted eventual response to immunotherapy with 100% sensitivity, 93% specificity and 95% accuracy [12]. However, the study of Cho et al. has several limitations, like the inhomogenous cohort of patients who received three different treatments for immunotherapy, the limited number of patients and the fact that only the hottest lesion was used as an indicator of therapy response in patients classified as SD according to RECIST 1.1, which seems questionable because it is known that tumors are very heterogeneous and metastases may demonstrate an equivocal response to therapy.
18F-FDG PET is a modality with unparalleled reported levels of sensitivity and specificity in detecting melanoma metastatic disease [31–39]. In an attempt to deepen our understanding of the role of 18F-FDG PET/CT in immunotherapy response assessment of malignant melanoma, we evaluated in the present analysis the kinetic behavior of 18F-FDG during the course of ipilimumab treatment in a homogeneous group of stage IV melanoma patients. The evaluations were performed in the hottest, melanoma-indicative lesions of the thorax and upper abdomen, where dynamic PET/CT took place, up to a maximum of five lesions total (and a maximum of two lesions per organ). This evaluation strategy was based on the PERCIST concept, which characterizes treatment response according to tracer uptake changes in the same number of tumor lesions with the highest uptake (target lesions). This is the first study to assess 18F-FDG metabolism by applying dynamic PET/CT in melanoma patients undergoing immunotherapy. Our analysis revealed no significant differences between the CB and no-CB groups based on a single parameter analysis regarding both semi-quantitative and quantitative parameters. In particular, the baseline exams revealed that the degree of 18F-FDG uptake in the melanoma lesions (SUVmean, SUVmax), the phosphorylation rate of the tracer (k3), its influx (Ki), and the fractal dimension (FD) demonstrated higher values for the no-CB group, while the carrier-mediated transport of the tracer from plasma to tissue (K1) was higher in the CB group. However, the results were not significantly different between CB and no-CB. Moreover, no single parameter showed a significant change which correlated with the clinical response to ipilimumab in both groups and there were also no between-group differences regarding the parameters’ changes.
Our results imply a limited utility of studying 18F-FDG metabolism by means of two-tissue compartment modeling in clinical response prediction and evaluation of immunotherapy in melanoma. Given the poor performance of quantitative analysis of 18F-FDG data, other approaches in 18F-FDG PET need to be evaluated. Most recently, our group has developed a more accurate and easily applicable approach of 18F-FDG PET data evaluation as response to immunotherapy, derived from a cohort of 41 metastatic melanoma patients including the ones involved in the present analysis. This approach, is based on the application of a cut-off of four newly merging melanoma lesions, as detected on PET/CT after completion of the ipilimumab treatment, for the characterization of progressive disease (PERCIMT criteria) [14]. Indeed, the application of these criteria, both on interim and late PET/CT, in the present cohort of patients correlated closely with clinical response to immunotherapy (CCR = 84%). Of course, these results warrant further validation in larger cohorts of patients, which is the topic of another ongoing project of our group. In particular, we will focus on a combined evaluation of both kinetic data and the number of newly emerging lesions to enhance prediction to several immunotherapeutic agents with 18F-FDG PET/CT.
Another approach could be the application of more specific imaging biomarkers than 18F-FDG, currently under investigation. On a clinical level, 18F-FLT, the most studied cellular proliferation PET agent with uptake directly related to that of DNA synthesis [40], has been evaluated in combination with 18F-FDG in 12 patients with metastatic melanoma undergoing treatment with another CTLA-4 inhibitor, tremelimumab. Nevertheless, apart from a SUVmax increase in the spleen occurring both in responders and non-responders, probably due to the T-cell lymphoproliferative effect of the anti-CTLA4-antibody, no significant SUV changes occurred after treatment [41]. On a preclinical level, several radiopharmaceuticals, labeled with different isotopes such as 64Cu, 18F, 68Ga and 89Zr-desferrioxamine are being currently tested as potential surrogate markers of response to checkpoint inhibitors either by tracking the PD-L1 pathway or the CD8+ T-cell response [42–47]. Further research is, however, required for performance evaluation of these imaging agents.
The lack of histological validation of the vast majority of the PET/CT positive findings is a limitation of our analysis; this is, however, usually not possible in the clinical setting. Moreover, despite the fact that a two-bed position protocol, which allows the study of a relatively large field of view of 44 cm, was used, the dynamic PET/CT acquisition was confined only in the anatomic area of the thorax/upper abdomen [48]. However, whole-body dynamic studies cannot be yet performed. The advent of new PET/CT scanners, which allow dynamic studies over several bed positions using a continuous bed movement, will facilitate the use of dynamic PET protocols and reduce the whole acquisition time.
Conclusion
Our results show a poor performance of single semi-quantitative (SUVmean, SUVmax) and quantitative (compartmental, non-compartmental) parameters in predicting clinical benefit to ipilimumab. On the other hand, the application of a cut-off of four newly emerging 18F-FDG avid lesions, as proposed by the recently introduced PET response evaluation criteria for immunotherapy (PERCIMT), correlates better with clinical response.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- 18F-FDG
2-Deoxy-2-(18F)fluoro-d-glucose
- CB
Clinical benefit
- CMR
Complete metabolic response
- CR
Complete response
- CT
Computed tomography
- dPET/CT
Dynamic positron emission tomography/computed tomography
- FD
Fractal dimension
- iCPD
Confirmed progressive disease
- irRC
Immune-related response criteria
- iUPD
Unconfirmed progressive disease
- MB
Metabolic benefit
- MIP
Maximum intensity projection
- No-CB
No clinical benefit
- No-MB
No metabolic benefit
- PD
Progressive disease
- PECRIT PET/CT
Criteria for early prediction of response to immune checkpoint inhibitor therapy
- PERCIMT
PET response evaluation criteria for immunotherapy
- PERCIST
PET response criteria in solid tumors
- PET
Positron emission tomography
- PET/CT
Positron emission tomography/computed tomography
- PMD
Progressive metabolic disease
- PMR
Partial metabolic response
- PR
Partial response
- SD
Stable disease
- SMD
Stable metabolic disease
- SUV
Standardized uptake value
- TAC
Time activity curve
- VOI
Volume of interest
Authors’ contributions
CS performed the PET/CT studies, carried out the PET/CT data analysis and drafted the manuscript. HA performed the PET/CT studies. JKW performed the ipilimumab therapies. AKS was responsible for the statistical analysis of the study. LL contributed to draft the manuscript. UH participated in the design of the study. JCH was responsible for the selection of the patients who received the ipilimumab therapy. ADS was responsible for the PET-CT study design and the data evaluation, coordinated the project and contributed to the manuscript.
Funding
This study was supported in part by the German Cancer Aid under the project with the title “Therapy monitoring of ipilimumab” based on the quantification of F-18-FDG kinetics with 4D PET/CT (dPET-CT) in patients with melanoma (stage 4). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Compliance with ethical standards
Conflict of interest
Christos Sachpekidis reports travel grants from Janssen-Cilag outside the submitted work. Julia Winkler received speakers honoraria from Merck Sharp & Dohme (MSD), and travel support from AMGEN, Bristol-Myers Squibb (BMS), MSD, Philochem and Roche. Jessica C. Hassel received honoraria for talks and travel expenses from BMS, MSD, Roche, Novartis, Pfizer and is a member of an advisory board for MSD and Amgen. The other authors declare that they have no conflict of interest.
Ethical approval
Patients gave written informed consent to participate in the study and to have their medical records released. The study was approved by the Ethical Committee of the University of Heidelberg and the Federal Agency for Radiation Protection (Bundesamt für Strahlenschutz).
Informed consent
Informed consent was obtained from all individual participants included in the study. The patients presented on Figs. 2 and 3 agreed on the publication of these figures. This study does not contain any studies with animals performed by any of the authors.
References
- 1.Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily—CTLA-4. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 2.Schneider H, Downey J, Smith A, et al. Reversal of the TCR Stop Signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 3.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 5.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 8.Gilardi L, Grana CM, Paganelli G. Evaluation of response to immunotherapy: new challenges and opportunities for PET imaging. Eur J Nucl Med Mol Imaging. 2014;41:2090–2092. doi: 10.1007/s00259-014-2848-x. [DOI] [PubMed] [Google Scholar]
- 9.Phelps ME, Huang SC, Hoffman EJ, et al. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-d-glucose: validation of method. Ann Neurol. 1979;6:371–388. doi: 10.1002/ana.410060502. [DOI] [PubMed] [Google Scholar]
- 10.Dimitrakopoulou-Strauss A, Strauss LG, Burger C, et al. On the fractal nature of positron emission tomography (PET) studies. World J Nucl Med. 2003;4:306–313. [Google Scholar]
- 11.Sachpekidis C, Larribere L, Pan L, et al. Predictive value of early 18F-FDG PET/CT studies for treatment response evaluation to ipilimumab in metastatic melanoma: preliminary results of an ongoing study. Eur J Nucl Med Mol Imaging. 2014;42:386–396. doi: 10.1007/s00259-014-2944-y. [DOI] [PubMed] [Google Scholar]
- 12.Cho SY, Lipson EJ, Im H-J, et al. Prediction of response to immune checkpoint inhibitor therapy using early-time-point 18F-FDG PET/CT imaging in patients with advanced melanoma. J Nucl Med. 2017;58:1421–1428. doi: 10.2967/jnumed.116.188839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seith F, Forschner A, Schmidt H, et al. 18F-FDG-PET detects complete response to PD1-therapy in melanoma patients two weeks after therapy start. Eur J Nucl Med Mol Imaging. 2018;45:95–101. doi: 10.1007/s00259-017-3813-2. [DOI] [PubMed] [Google Scholar]
- 14.Anwar H, Sachpekidis C, Winkler J, et al. Absolute number of new lesions on 18F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur J Nucl Med Mol Imaging. 2018;45:376–383. doi: 10.1007/s00259-017-3870-6. [DOI] [PubMed] [Google Scholar]
- 15.Strauss LG, Conti PS. The applications of PET in clinical oncology. J Nucl Med. 1991;32:623–648. [PubMed] [Google Scholar]
- 16.PMOD Technologies. http://www.pmod.com/files/download/v31/doc/pbas/4729.htm. Accessed 20 October 2017
- 17.Sokoloff L, Smith CB. Basic principles underlying radioisotopic methods for assay of biochemical processes in vivo. In: Greitz T, Ingvar DH, Widén L, editors. The metabolism of the human brain studied with positron emission tomography. New York: Raven Press; 1983. pp. 123–148. [Google Scholar]
- 18.Ohtake T, Kosaka N, Watanabe T, et al. Noninvasive method to obtain input function for measuring tissue glucose utilization of thoracic and abdominal organs. J Nucl Med. 1991;32:1432–1438. [PubMed] [Google Scholar]
- 19.Miyazawa H, Osmont A, Petit-Taboué MC, et al. Determination of 18F-fluoro-2-deoxy-d-glucose rate constants in the anesthetized baboon brain with dynamic positron tomography. J Neurosci Methods. 1993;50:263–272. doi: 10.1016/0165-0270(93)90033-N. [DOI] [PubMed] [Google Scholar]
- 20.Burger C, Buck A. Requirements and implementation of a flexible kinetic modeling tool. J Nucl Med. 1997;38:1818–1823. [PubMed] [Google Scholar]
- 21.Mikolajczyk K, Szabatin M, Rudnicki P. A JAVA environment for medical image data analysis: initial application for brain PET quantitation. Med Inform (Lond) 1998;23:207–214. doi: 10.3109/14639239809001400. [DOI] [PubMed] [Google Scholar]
- 22.Sachpekidis C, Mai EK, Goldschmidt H, et al. (18)F-FDG dynamic PET/CT in patients with multiple myeloma: patterns of tracer uptake and correlation with bone marrow plasma cell infiltration rate. Clin Nucl Med. 2015;40:e3007. doi: 10.1097/RLU.0000000000000773. [DOI] [PubMed] [Google Scholar]
- 23.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 24.Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci. 2008;105:3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 26.Hersh EM, O’Day SJ, Powderly J, et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naïve patients with advanced melanoma. Invest New Drugs. 2011;29:489–498. doi: 10.1007/s10637-009-9376-8. [DOI] [PubMed] [Google Scholar]
- 27.Pennock GK, Waterfield W, Wolchok JD. Patient responses to ipilimumab, a novel immunopotentiator for metastatic melanoma: how different are these from conventional treatment responses? Am J Clin Oncol. 2012;35:606–611. doi: 10.1097/COC.0b013e318209cda9. [DOI] [PubMed] [Google Scholar]
- 28.Tirumani SH, Ramaiya NH, Keraliya A, et al. Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res. 2015;3:1185–1192. doi: 10.1158/2326-6066.CIR-15-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amin A, Lawson DH, Salama AKS, et al. Phase II study of vemurafenib followed by ipilimumab in patients with previously untreated BRAF-mutated metastatic melanoma. J Immunother Cancer. 2016;4:44. doi: 10.1186/s40425-016-0148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holder WD, White RL, Zuger JH, et al. Effectiveness of positron emission tomography for the detection of melanoma metastases. Ann Surg. 1998;227:764–769. doi: 10.1097/00000658-199805000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dietlein M, Krug B, Groth W, et al. Positron emission tomography using 18F-fluorodeoxyglucose in advanced stages of malignant melanoma: a comparison of ultrasonographic and radiological methods of diagnosis. Nucl Med Commun. 1999;20:255–261. doi: 10.1097/00006231-199903000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Eigtved A, Andersson AP, Dahlstrøm K, et al. Use of fluorine-18 fluorodeoxyglucose positron emission tomography in the detection of silent metastases from malignant melanoma. Eur J Nucl Med. 2000;27:70–75. doi: 10.1007/PL00006666. [DOI] [PubMed] [Google Scholar]
- 34.Mijnhout GS, Hoekstra OS, van Tulder MW, et al. Systematic review of the diagnostic accuracy of (18)F-fluorodeoxyglucose positron emission tomography in melanoma patients. Cancer. 2001;91:1530–1542. doi: 10.1002/1097-0142(20010415)91:8<1530::AID-CNCR1162>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 35.Swetter SM, Carroll LA, Johnson DL, Segall GM. Positron emission tomography is superior to computed tomography for metastatic detection in melanoma patients. Ann Surg Oncol. 2002;9:646–653. doi: 10.1007/BF02574480. [DOI] [PubMed] [Google Scholar]
- 36.Fuster D, Chiang S, Johnson G, et al. Is 18F-FDG PET more accurate than standard diagnostic procedures in the detection of suspected recurrent melanoma? J Nucl Med. 2004;45:1323–1327. [PubMed] [Google Scholar]
- 37.Strobel K, Skalsky J, Hany TF, et al. Small bowel invagination caused by intestinal melanoma metastasis: unsuspected diagnosis by FDG-PET/CT imaging. Clin Nucl Med. 2007;32:213–214. doi: 10.1097/01.rlu.0000255212.17086.e9. [DOI] [PubMed] [Google Scholar]
- 38.Xing Y, Bronstein Y, Ross MI, et al. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. J Natl Cancer Inst. 2011;103:129–142. doi: 10.1093/jnci/djq455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Danielsen M, Højgaard L, Kjær A, Fischer BM. Positron emission tomography in the follow-up of cutaneous malignant melanoma patients: a systematic review. Am J Nucl Med Mol Imaging. 2013;4:17–28. [PMC free article] [PubMed] [Google Scholar]
- 40.Peck M, Pollack HA, Friesen A, et al. Applications of PET imaging with the proliferation marker [18F]-FLT. Q J Nucl Med Mol Imaging. 2015;59:95–104. [PMC free article] [PubMed] [Google Scholar]
- 41.Ribas A, Benz MR, Allen-Auerbach MS, et al. Imaging of CTLA4 blockade-induced cell replication with (18)F-FLT PET in patients with advanced melanoma treated with tremelimumab. J Nucl Med. 2010;51:340–346. doi: 10.2967/jnumed.109.070946. [DOI] [PubMed] [Google Scholar]
- 42.Maute RL, Gordon SR, Mayer AT, et al. Engineering high-affinity PD-1 variants for optimized immunotherapy and immuno-PET imaging. Proc Natl Acad Sci. 2015;112:E6506–E6514. doi: 10.1073/pnas.1519623112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tavare R, McCracken MN, Zettlitz KA, et al. Engineered antibody fragments for immuno-PET imaging of endogenous CD8+ T cells in vivo. Proc Natl Acad Sci. 2014;111:1108–1113. doi: 10.1073/pnas.1316922111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tavaré R, Escuin-Ordinas H, Mok S, et al. An effective immuno-PET imaging method to monitor CD8-dependent responses to immunotherapy. Cancer Res. 2016;76:73–82. doi: 10.1158/0008-5472.CAN-15-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larimer BM, Wehrenberg-Klee E, Dubois F, et al. Granzyme B PET imaging as a predictive biomarker of immunotherapy response. Cancer Res. 2017;77:2318–2327. doi: 10.1158/0008-5472.CAN-16-3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayer AT, Natarajan A, Gordon SR, et al. Practical immuno-PET radiotracer design considerations for human immune checkpoint imaging. J Nucl Med. 2017;58:538–546. doi: 10.2967/jnumed.116.177659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guldbrandsen KF, Hendel HW, Langer SW, Fischer BM. Nuclear molecular imaging strategies in immune checkpoint inhibitor therapy. Diagnostics (Basel) 2017 doi: 10.3390/diagnostics7020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dimitrakopoulou-Strauss A, Pan L, Strauss LG. Quantitative approaches of dynamic FDG-PET and PET/CT studies (dPET/CT) for the evaluation of oncological patients. Cancer Imaging. 2012;12:283–289. doi: 10.1102/1470-7330.2012.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.