Abstract
Objective
Immunotherapy of cancer has the potential to be effective mostly in patients with a low tumour burden. Rising PSA (prostate-specific antigen) levels in patients with prostate cancer represents such a situation. We performed the present clinical study with dendritic cell (DC)-based immunotherapy in this patient population.
Materials and methods
The single-arm phase I/II trial registered as EudraCT 2009-017259-91 involved 27 patients with rising PSA levels. The study medication consisted of autologous DCs pulsed with the killed LNCaP cell line (DCVAC/PCa). Twelve patients with a favourable PSA response continued with the second cycle of immunotherapy. The primary and secondary objectives of the study were to assess the safety and determine the PSA doubling time (PSADT), respectively.
Results
No significant side effects were recorded. The median PSADT in all treated patients increased from 5.67 months prior to immunotherapy to 18.85 months after 12 doses (p < 0.0018). Twelve patients who continued immunotherapy with the second cycle had a median PSADT of 58 months that remained stable after the second cycle. In the peripheral blood, specific PSA-reacting T lymphocytes were increased significantly already after the fourth dose, and a stable frequency was detected throughout the remainder of DCVAC/PCa treatment.
Summary
Long-term immunotherapy of prostate cancer patients experiencing early signs of PSA recurrence using DCVAC/PCa was safe, induced an immune response and led to the significant prolongation of PSADT. Long-term follow-up may show whether the changes in PSADT might improve the clinical outcome in patients with biochemical recurrence of the prostate cancer.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-017-2068-x) contains supplementary material, which is available to authorized users.
Keywords: Immunotherapy, Dendritic cell, Biochemically recurrent prostate cancer, PSA doubling time
Introduction
Prostate cancer (PCa) is the most common cancer in men with an estimated 1.1 million new cases and 307,000 deaths in 2012 (Globocan 2012) [1]. The worldwide PCa burden is expected to grow to 1.7 million new cases and 499,000 deaths by 2030 simply due to the growth and ageing of the global population [2–4]. PCa can only be cured at the stage of localized organ-confined disease [5, 6]. Up to 40% of patients experience an isolated rise in the PSA (prostate-specific antigen) levels within 10 years after primary therapy, a so-called biochemical relapse (BCR) [7]. Due to the improved diagnosis, especially based on PSA screening, the patient population with PSA-recurrent PCa will grow in the near future and will concern younger men. Salvage radiotherapy (SRT) represents a treatment option for patients with BCR of PCa after radical prostatectomy (RP). If administered at the early signs of disease progression, preferably before PSA reaches the level of 0.5 ng/mL, SRT may lead to the long-term control of disease in patients with presumable local recurrence [8]. However, acute as well as chronic side effects associated with pelvis irradiation, including possible long-term sequelae (erectile dysfunction, post-radiation enteritis, cystitis, and higher risk of secondary neoplasia), represent the limits of this treatment modality, especially in young men with a long life expectancy. There is no effective treatment and no consensus on the optimal management of patients with a PSA rise after SRT before the evidence of metastatic disease. Possible strategies include watchful waiting and androgen-deprivation therapies. It has been repeatedly documented that the kinetics of the PSA doubling time (PSADT) determines the further fate of patients a shorter PSADT correlates with faster development of metastatic disease and higher cancer-specific death [9]. Any treatment leading to the stabilization of PSA may potentially impact the progression of the disease. However, as this stage of the disease is asymptomatic, the administered treatment needs to have a favourable safety profile.
From an immunological perspective, biochemical recurrence provides a unique opportunity for immunotherapeutic intervention in patients with cancer, as the immunosuppressive mechanisms (e.g., regulatory T cells and myeloid-derived suppressor cells) associated with an advanced tumour burden have not yet been fully developed [10, 11]. Among immune-based strategies to induce and boost the antitumour immune response, active immunization strategies might be of great interest for this particular patient population. Sipuleucel-T was approved for patients with advanced castrate-resistant metastatic PCa based on a 4.1-month survival benefit over placebo [12, 13] with a very favourable safety profile, similar to other active immunization strategies currently being tested in clinical trials.
Vaccination of dendritic cells (DCs) loaded with tumour antigens represents an additional extensively studied treatment modality in the setting of PCa [14–18]. A comprehensive review and meta-analysis of 29 clinical trials including 906 patients treated with DC-based immunotherapy including Sipuleucel-T for prostate and renal cancer has been recently published [15]. In this analysis, efficacy measured as a clinical benefit rate (counted as the combined percentage of the objective response and stable disease) was achieved in 54% of PCa patients. Based on the few side effects reported in these trials, DC-based immunotherapies are generally considered as a safe approach to cancer treatment [19].
The objective of this phase I/II clinical study was to evaluate the administration of DCVAC/PCa, an autologous DC-based cell therapy product, in patients with biochemical recurrence of PCa (EudraCT 2009-017259-91). DCVAC/PCa is an autologous active cellular immunotherapy (ACI) belonging to the group of advanced therapy medicinal products (ATMPs). It consists of autologous Poly I:C-activated DCs loaded with LNCaP PCa cells killed by UV irradiation. Recently, we published a study using the identical cell-based vaccine in metastatic castrate-resistant prostate cancer patients in combination with chemotherapy [20]. In the current study, DCVAC was administered as a monotherapy in clinically asymptomatic patients with rising serum PSA. This represents a unique opportunity to the assess safety of the tested product which was the primary endpoint of the study.
The primary endpoint of the study was safety, and the secondary endpoint was the monitoring of immune responses and PSA kinetics. We have also examined, in a suitable cohort of patients, the effect of long-term immunotherapy administration. We hypothesized that the repeated administration of DCVAC/PCa at an early stage of disease may induce an immune response that may control the proliferation of residual PCa cells reflected as the stabilization of PSA kinetics.
Materials and methods
Patient eligibility
The eligibility required histologically confirmed prostatic adenocarcinoma. Patients after RP and with a rising PSA serum concentration measured by ultrasensitive testing above the nadir within 2 years from RP or patients after SRT for PSA-recurrent PCa with at least two subsequent increases in the serum PSA concentration above the nadir after SRT were enrolled in the clinical trial. Additional eligibility requirements and exclusion criteria are listed in supplemental Table 1. All patients were also required to have at least three serum PSA values collected over at least a 3-month period prior to study entry, and all results were obtained from the same clinical laboratory and were measured by ultrasensitive testing, to determine the pre-treatment PSADT.
Study design and treatment
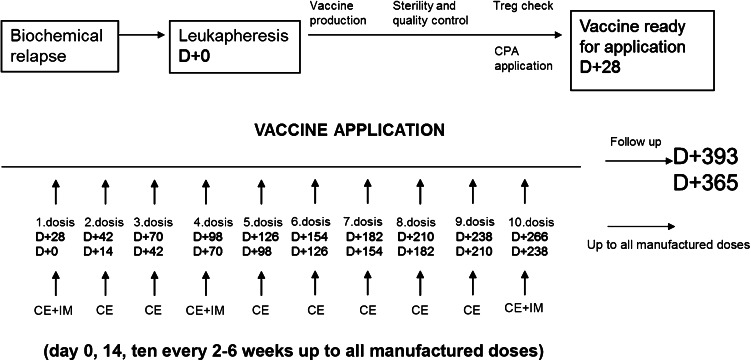
Twenty-seven patients were treated with DCVAC/PCa in the single-institution, single-arm, open-label phase I/II clinical trial (EudraCT 2009-017259-91). The treatment scheme is summarized in Fig. 1. Briefly, DCVAC/PCa consisted of, on average, 12 doses of 1 × 107 DCs injected s.c. at the axillary and inguinal areas (2.5 mL at each site). One leukapheresis yields a sufficient amount of DCVAC/PCa for approximately 1 year of treatment. By protocol amendment, a second or third leukapheresis procedure was performed, and the second/third cycle of DCVAC/PCa was administered to those patients in whom PSADT was significantly increased during the first cycle of immunotherapy and who were not indicated for another treatment. Immune monitoring was performed in two different time points during the treatment (before the first, fourth, and twelfth dose of treatment). Before the first dose of DCVAC/PCa, the patients received 1 week of cyclophosphamide in metronomic setting as published previously [21]. Imiquimod was applied in the place of vaccine application to support the accumulation of DCs. The primary endpoints of the study were the safety and feasibility of vaccine in BCR patients. The study was approved by the Institutional Review Board (IRB) and State Institute for Drug Control (SUKL).
Fig. 1.
Study design. The DCVAC/PCa treatment consisted of, on average, 12 doses of 1 × 107 DCs injected s.c. The treatment comprised an initial 7 days of metronomic cyclophosphamide administration. DCVAC/PCa was then administered every 2–6 weeks up to the maximum number of doses manufactured from one leukapheresis. Immunomonitoring (IM) was evaluated after the first, fourth, and twelfth dose or after the last dose of DCVAC/PCa if less than 12 doses were manufactured from 1 leukapheresis. Clinical evaluation was performed after every single DCVAC/PCa dose
Assessment of clinical activity and toxicity
Patients underwent CT, radiologic imaging, PET/CT, nuclear magnetic resonance (NMR) of the abdomen, pelvis and chest, bone scintigraphy, and laboratory tests. Serum PSA was evaluated before each vaccination by the ultrasensitive method using the chemiluminescence analyser Immulite 1000 (Siemens) at a certified clinical hospital laboratory. PSADT was calculated using all serum PSA values available from the pre-treatment period using a minimum of four PSA values and the formula ln(2)/b, where b denotes the least square estimator of the linear regression model of the log-transformed PSA values on time. If PSADT was negative (indicating that the curve was declining), an arbitrary value of 150 months was used for further statistical evaluation. For the pre-treatment with PSADT, all data available from the nadir were used, or at least a period of 6 months prior to the treatment, including day 1 of treatment. The treatment PSADT was determined using all PSA values from the first application of DCVAC/PCa to the twelfth dose or last dose before the introduction of another treatment (hormonal therapy, radiotherapy if indicated) provided that at least five doses of DCVAC/PCa were administered. According to the PSA kinetics, patients were classified as strong responders (PSADT on immunotherapy longer than 15 months), responders (PSADT on immunotherapy increased compared with the pre-treatment period but shorter than 15 months) and non-responders (PSADT on therapy did not differ from the pre-treatment period or decreased). For the statistical correlation of PSA kinetics with laboratory markers, we classified patients into responders (strong responders and responders) and non-responders.
DCVAC/PCa production
DCs were prepared under good manufacturing practice (GMP) conditions. UV-B irradiation was used to kill the LNCaP cells for further cultivation with immature DCs. Poly I:C (InvivoGen) was used for subsequent activation of DCs. The protocol was described in details previously [22, 23].
Measurement of the humoural and T cell response
Basic immunological and laboratory tests
The serum levels of immunoglobulin G, A, and M; C-reactive protein; autoantibodies; ANCA; RF; and anti-cardiolipin were monitored as described previously [20]. Lymphocytes subsets (CD3, CD8, CD4, CD19, CD16, and HLA-DR) were assessed by flow cytometry using the panel of monoclonal antibodies.
Detection of cellular and humoral antigen-specific immune response
The percentage of specific T cells against PSA, MAGE-1, and MAGE-3 tumour antigens was evaluated by flow cytometry at different time points during the treatments. The presence of tumour-specific antibodies was analysed in patient sera at different time points. Both protocols were described in details previously [20].
Quantitative PCR
The Low Density Array System (TaqMan® Array Human Immune Panel) was used to determine the immune expression profile of the patient’s PBMCs. The arrays were run on the Viia7 instrument (Applied Biosystems) using the TaqMan® Universal Master Mix II without UNG (Applied Biosystems). Four nanograms of cDNA per PCR reaction were used. The relative gene expression levels were calculated using the ∆∆Ct method and were normalized to the expression levels of reference genes selected by Normfinder.
Statistical analysis
Wilcoxon-signed-rank test was used to evaluate the changes in immune parameters during the course of treatment. Statistically significant differences in the gene expression of immune genes before and after DCVAC treatment and between responders and non-responders were evaluated using Student’s T-test.
Results
Patients characteristics
Twenty-seven patients with the median age of 63 (age range 49–77 years) were treated with DCVAC/PCa between 2010 and 2014. The patients’ baseline characteristics are shown in Table 1. Twenty-six patients received at least 12 doses of DCVAC/PCa. Two patients were excluded from the statistical analysis of PSA kinetics. Patient no. 203 underwent salvage radiotherapy because tumour residue was detected in the prostate bed on control CT scan performed at the beginning of the study and only three doses of DCVAC/PCa were received before SRT. Patient no. 227 did not fulfil the entry criteria of increased PSA above the nadir within 2 years after surgery and was classified as a protocol violation. However, because these patients consented to complete the immunotherapy protocol, they were included in the AE reports.
Table 1.
Patients’ baseline characteristics
| Total number of patients | 27 |
|---|---|
| Race | |
| Caucasian | 27 |
| Age (years)a | |
| Median | 63 |
| Mean | 64 |
| Range | 49–77 |
| ECOG performance statusa | |
| 0 | 27 |
| PSA, ng/mLa | |
| Median | 0171 |
| Mean | 0246 |
| Range | 0038–0983 |
| PSADT before immunotherapy, monthsa | n = 27 pts |
| Median | 5.67 |
| Mean | 5.7 |
| Range | 1.65–10.81 |
| PSADT after first cycle of IT, months | n = 25 pts |
| Median | 18.85 |
| Mean | 42.52 |
| Range | 2.67–150 |
| PSADT after second cycle of IT, months | n = 12 pts |
| Median | 58.03 |
| Mean | 76.93 |
| Range | 8.24–150 |
| Hemoglobin, g/dLa | |
| Median | 15.5 |
| Mean | 15.6 |
| Range | 13.8–17.4 |
| Lactate dehydrogenase, IU/La | |
| Median | 188.4 |
| Mean | 190.56 |
| Range | 141–340 |
| Alkaline phosphatase, IU/La | |
| Median | 66 |
| Mean | 66 |
| Range | 41–107 |
| Initial Gleason score, n (%) | |
| 5 | 5 (19) |
| 6 | 10 (37) |
| 7 | 8 (30) |
| 8 | 1 (3) |
| 9 | 3 (11) |
| Median | 6 |
| PSA levels at the time of initial dg. | |
| Median | 7.3 |
| Mean | 8.4 |
| Range | 2.9–19.6 |
| Prior treatment-surgery, n (%) | |
| Radical prostatectomy | 14 (52) |
| Radical prostatectomy and pelvic lymphadenectomy | 13 (48) |
| Salvage radiotherapy | 8 (30) |
ECOG Eastern Cooperative Oncology Group, PSA prostate-specific antigen, IT immunotherapy
a Values at the start of immunotherapy
Adverse events
An overview of all adverse events (AEs) that occurred during the treatment was summarized in Table 2. The most frequent AEs were local injection site reaction, fatigue, influenza like-illness, and mild infections. All recorded AEs were grade 1 and 2 and we did not observe any grade 4 AEs or treatment-related deaths. Moreover, we recorded eight serious adverse events (SAEs) but none of these was related to applied immunotherapy. No clinical signs of autoimmune disease were detected during the clinical trial. One patient had high titres of rheumatoid factor IgG, IgA, and IgM before and during immunotherapy without clinical signs of arthritis (anti-CCP was negative). All other autoantibodies detected in a few patients before or during the trial period were in low and stable titres. In summary, applied immunotherapy was well tolerated, and the overall safety profile remained favourable.
Table 2.
Cumulative summary tabulation of serious adverse events (SAEs) in the time period from March 2010 to March 2014
| System organ class | Active study drug (DCVAC/PCa) |
|---|---|
| Preferred term | |
| Study EudraCT number 2009-017259-91 | |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | |
| Thyroid cancer | 1 |
| Cardiac disorders | |
| Angina pectoris | 1 |
| Atrial fibrillation | 1 |
| Dyspnoea exertional | 1 |
| Renal and urinary disorders | |
| Haematuria | 1 |
| Urinary retention | 1 |
| Nervous system disorders | |
| Cerebrovascular accident | 1 |
| Injury, poisoning and procedural complications | |
| Tendon rupture | 1 |
| Total | 8 |
The Medical Dictionary for Regulatory Activities (MedDRA) version 15.1 was used for the coding of adverse events (AEs)
The summary tabulations of SAEs are arranged by the primary system organ class (SOC) and preferred term (PT) level
Clinical efficacy
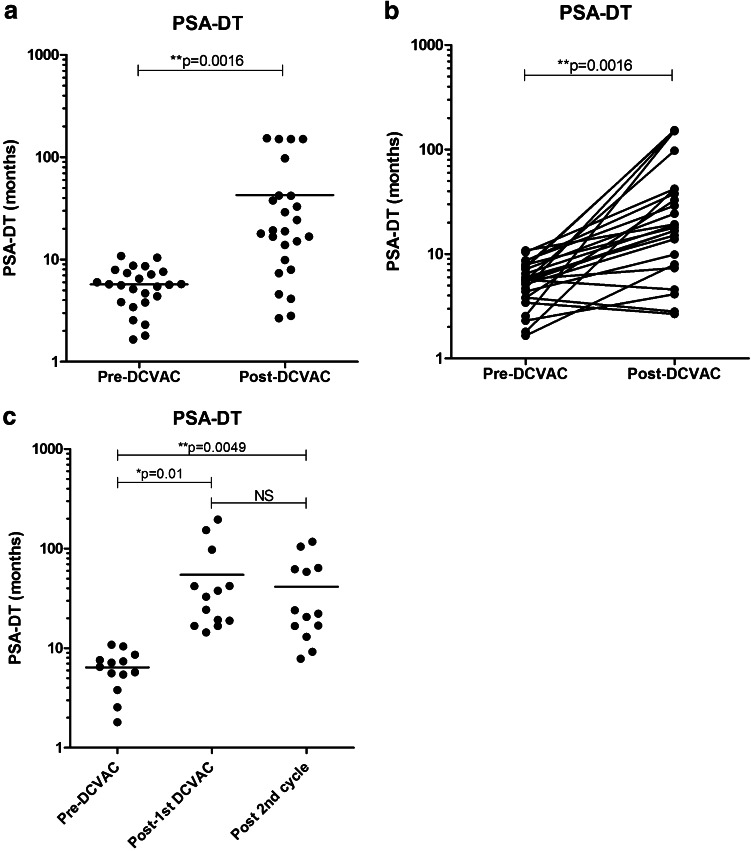
PSA kinetics expressed as PSADT was calculated in 25 patients who received at least five doses of DCVAC/PCa without any additional treatment modalities. Figure 2a and b summarizes PSADTs prior to and after the first course of immunotherapy. The median PSADT increased from 5.67 months prior to the treatment to 18.85 months after completing the first cycle of immunotherapy by DCVAC/PCa. There were 17 strong responders (PSADT longer than 15 months on immunotherapy, 5 responders (PSADT increased in comparison with the pre-treatment period but was shorter than 15 months) and three non-responders (PSADT on therapy did not differ from pre-treatment period or decreased). Twelve of 25 patients who had stable PSA levels during the treatment duration (median PSADT of 39.79 months after the first cycle of immunotherapy), consented with an additional cycle of DCVAC/PCa, and their PSADT remained stable during the additional cycle of treatment (median PSADT of 58.03 months; Fig. 2c; Supplemental Figure 1). None of the patients received additional treatment during the duration of the trial. Three patients underwent a third cycle with a median PSADT of 32.13 months after completion. No patient died during the study and follow-up, and none developed detectable metastases except for patient 202 (GS = 9) who had PSADT prior to and during the study of 3 months and in whom pelvic lymph node metastases were detected on 18 F-choline-PET-CT (after ten doses of DCVAC/PCa).
Fig. 2.
PSADT pre- and post-DCVAC/PCa treatment and in subsequent cycles of treatment for biochemical relapse prostate cancer patients (n = 25). The PSADT value for each patient pre- and post-treatment was plotted. PSADT was significantly prolonged; on average, there was a 3.32 times increase in the median PSADT after completion of the treatment. The median PSADT prior to immunotherapy was 5.67 months and increased to 18.85 months at the completion of the first cycle of immunotherapy (a, b). PSADT remained stable during the second cycle of treatment, with a median PSADT of 58.03 months (c)
Immunological response
T cell subsets in peripheral blood of treated patients were analysed pre- and post-vaccination using multicolor flow cytometry. We observed significant changes in the peripheral CD3+ T lymphocytes during the trial, (*p > 0.05) (Supplemental Figure 2a). The frequency of CD3+/HLA-DR+ cells, as well as CD4+ and CD8+, did not change (Supplemental Figure 2b–d). Additionally, we did not observe any changes in the percentage of circulating Tregs (Supplemental Figure 2g). The total levels of IgG and IgM also remained stable (Supplemental Figure 2e, f). In nine patients with stable PSA levels during the treatment duration who opted for additional cycles of DCVAC/PCa treatment, we observed stable frequencies of monitored immune parameters CD3+, CD3+HLA-DR+, CD4+, CD8+ (Supplemental Figure 3a–d), and stable levels of IgG and IgM (Supplemental Figure 3e, f). The frequency of Tregs in the peripheral blood was significantly decreased after the second cycle of DCVAC/PCa treatment (*p > 0.05) (Supplemental Figure 3g).
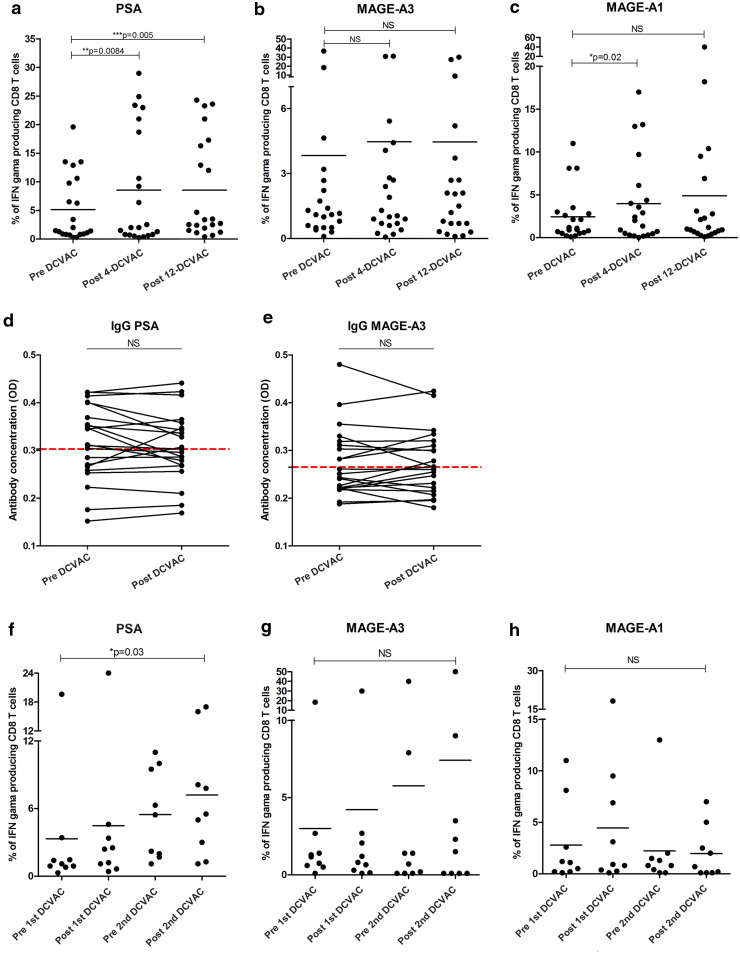
In all vaccinated patients, we assessed the cellular and humoral antigen-specific immune response. In 12 of 27 patients, the frequency of antigen-specific T cells against PSA was significantly higher compared to healthy donors (Supplemental Figure 4a). Similarly, the frequency of MAGE-A1 and MAGE-A3 tumour-specific T cells was significantly increased in 6 of 27 patients compared to healthy donors (Supplemental Figure 4b, c). Administration of DCVAC/PCa induced a significant increase in the frequency of antigen-specific T cells against PSA at both tested time points (DCVAC-4 and DCVAC-12) (*p < 0.05) (Fig. 3a) and against MAGE-A1 only in the first tested time point (DCVAC-4). We did not observe any changes in the percentage of antigen-specific T cells against MAGE-A3 antigen during the clinical study (Fig. 3b, c).
Fig. 3.
Tumour antigen-specific response during DCVAC/PCa treatment in the peripheral blood. a The frequency of PSA-specific T cells at both tested time points (DCVAC-4 and DCVAC-12) was significantly increased, *p < 0.05, as well as the maintenance of stable levels of T cells specific against MAGE-A1 (b) and MAGE-A3 (c), was detected. Concentrations of IgG antibodies against PSA (d) and MAGE-A3 (e) were measured in the patients’ sera. The cut-off value (red line) designating a positive reaction was calculated as the mean OD of the 15 healthy control human sera + 3SD. f The increase in the frequency of PSA-specific T cells in the subsequent DCVAC/PCa cycle, *p < 0.05, and maintenance of stable levels of T cells specific against MAGE-A1 (g) and MAGE-A3 (h)
Moreover, we evaluated the presence of tumour-specific IgG antibodies during the treatment period in patient sera. PSA and MAGE-A3 IgG positive antibodies were detected in 9 of 27 (33%) and in 9 of 27 (33%) patients, respectively (Fig. 3d, e). There was no correlation between IgG and the CTL (cytotoxic T lymphocytes) response against either PSA or MAGE-A3 (Supplemental Figure 5a, b). Moreover, in the subsequent DCVAC/PCa cycle, we observed a statistically significant increase in the frequency of PSA-specific T cells (Fig. 3f) and the maintenance of stable frequencies of T lymphocytes specific against MAGE-A1 and MAGE-A3 (Fig. 3g, h). Comparing the immune response characteristics with the clinical status, we observed no significant correlations between PSADT and specific humoural IgG responses and cellular CTL responses (data not shown).
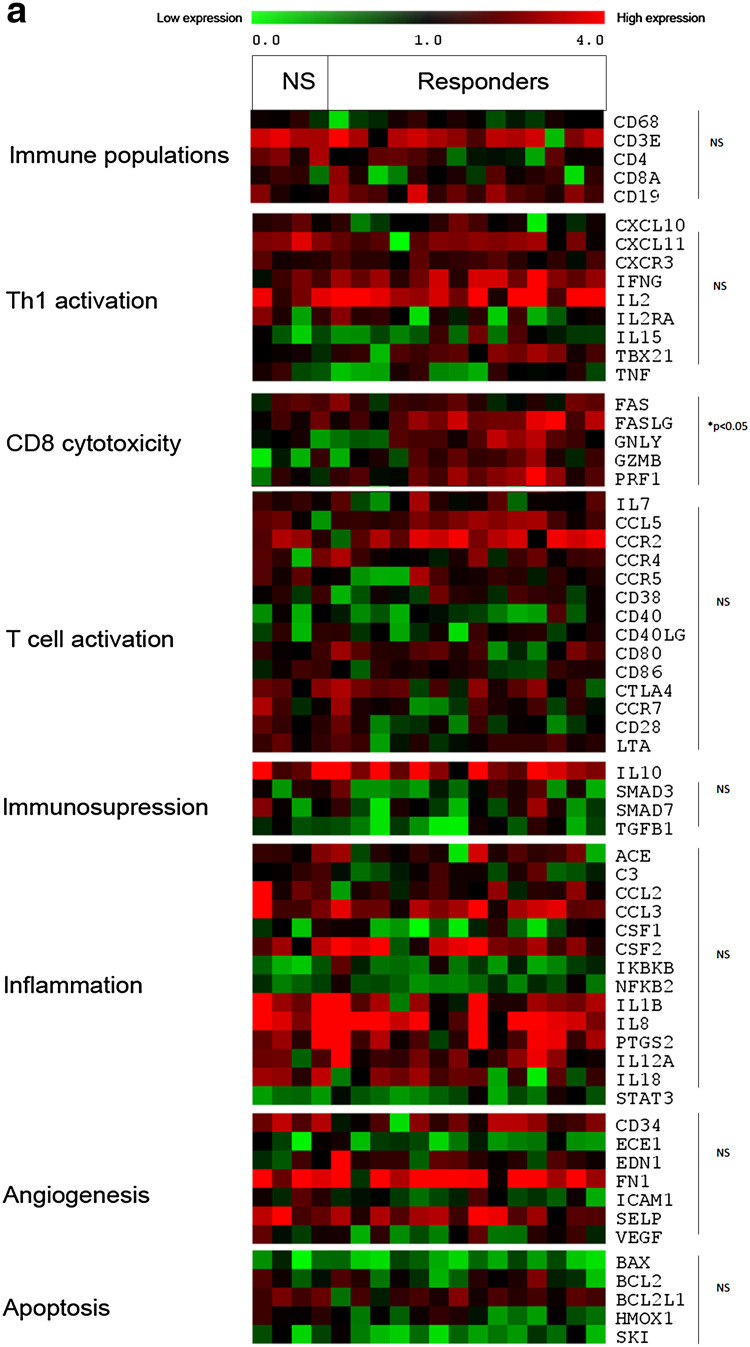
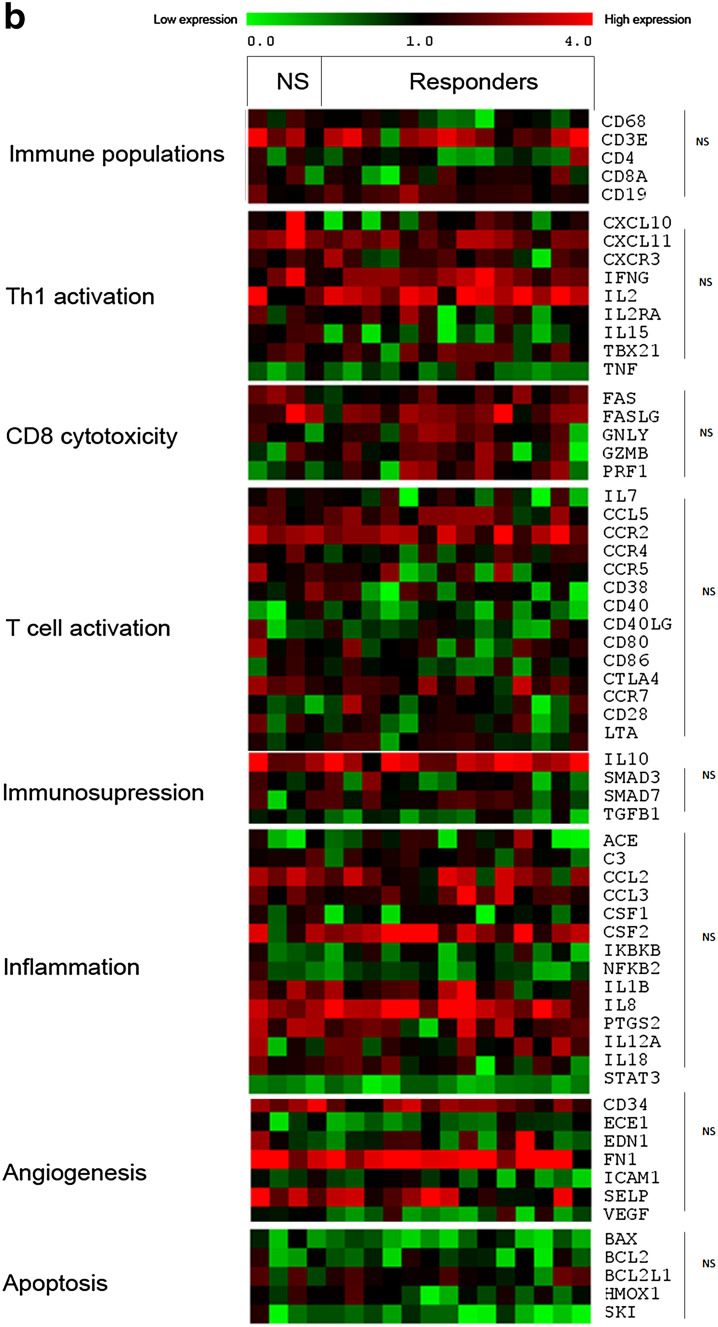
Because the T cell populations may play an important role in the patient response to DCVAC/PCa, we next evaluated the gene expression profile of PBMCs focussing on T lymphocytes. The expression levels of genes related to the main immune population, Th1, CD8 T cell cytotoxicity, T cell activation, immunosuppression, inflammation, and angiogenesis were assessed in the peripheral blood of 18 patients (4 non-responders and 14 responders) (Fig. 4). Several genes related to CD8/NK (natural killer) cell cytotoxicity (Fas, Fas-L, granzyme, granulysin, PRF1) were significantly overexpressed in the group of responders compared with non-responders (Fig. 4a). We observed no similar pattern after DCVAC/PCa treatment (Fig. 4b). By contrast, genes involved in Th1, immunosuppression, inflammation, angiogenesis, and T cell activation were not differentially expressed between these groups of patients both before and after DCVAC/PCa treatment. Moreover, significant differences between the timepoints, pre- and post-DCVAC treatment, were only detected in a few genes, namely, for the group of non-responders: BCL2L1 (p = 0.038); IL15 (p = 0.038) and for responders: NFKB2 (p = 0.016); CD19 (p = 0.022); ACE (p = 0.044). Altogether, these results suggest that the up-regulation of genes associated with immune cell cytotoxicity in peripheral blood before the initiation of treatment is higher in patients with a favourable PSA response to DCVAC/PCa treatment.
Fig. 4.
Gene expression levels related to Immune populations, Th-orientation, cytotoxicity, T cell activation, Immunosuppression, inflammation, and angiogenesis according to the patient clinical status. Gene expression levels were assessed by qRT-PCR and were determined using threshold cycle values normalized to the reference genes in 18 patients (4 were classified based on clinical data as non-responders, and 14 were classified as responders). The heat map representation of clusters of genes related to different immune populations, T cell activation, immunosuppression, inflammation, and angiogenesis before and after treatment with DCVAC/PCa is shown in a and b, respectively. The minimal level of expression (green) to the maximal level (red) is shown. Statistical analysis was performed using unpaired T-test
Discussion
Biochemical recurrence of PCa defined by rising PSA measured by ultrasensitive testing represents a clinical situation that corresponds to the minimal residual disease and might be well suited for immunotherapy approaches [11, 24]. However, it has been estimated that even the lowest detectable concentrations of PSA, such as 0.0035 ng/mL, still correspond to a tumour mass of approximately 105−6 tumour cells [25–27]. In this phase I/II trial, we evaluated the DC-based immunotherapy in such patients. Repeated, long-term administration of DCVAC/PCa was safe. Significant prolongation of PSADT was recorded in 22 of 25 evaluable patients after 1 year of treatment. While the median pre-treatment PSADT was approximately 6 months, in accordance with previously published data, it is more than tripled to a median of 18.85 months at the end of the one-year treatment course. In 16 patients, PSADT exceeded 15 months after the first cycle of immunotherapy, and, in four of them, the PSA curve even declined. Twelve patients underwent the second leukapheresis procedure and an additional cycle of immunotherapy, and the median PSADT remained stable. In comparison, a recent study published by DiPaola et al. in a similar patient population reported an increase in PSADT from 5.3 to 7.7 months after 6 months of immunotherapy by PROSTVAC, a viral vector-based PSA vaccine [28].
The prognosis and clinical outcome of patients with the biochemical recurrence of PCa is related to PSADT as reported by several studies [9]. In patients with a PSADT < 3 months, the median metastasis-free survival can be as short as 2 years; however, if PSADT is longer than 15 months, the median metastasis-free survival exceeds 10 years. As reported by Freedland et al., if PSADT after biochemical recurrence exceeds 15 months, the probability of death from the PCa in men is negligible [9]. Although the design and timeframe of this study did not allow for the evaluation of efficacy parameters, such as metastasis-free survival or overall survival, the prolongation of PSADT in most of the DCVAC/PCa-treated patients is intriguing and suggests a possible biological activity of the tested compound. This is further supported by the increase in PSA-specific T cells in the peripheral blood. However, as it was designed as a single-arm study, the possibility remains that the alteration of PSADT occurred by chance and does not correspond to the effect of the treatment. It is unlikely that DCVAC/PCa altered PSA secretion or elimination in any way because we found no correlation between the presence of anti-PSA antibodies and PSADT. The single-arm character of this study also limits the interpretation of the finding that responding patients have an up-regulated gene signature associated with T/NK cell cytotoxicity in the peripheral blood prior to the start of immunotherapy. If validated in the ongoing phase II trial with a control cohort, this relatively simple test could help to identify patients who are likely to respond to immunotherapy. However, this finding might also indicate that patients with up-regulated cytotoxic markers are more likely to spontaneously develop an effective antitumour immune response and control the outgrowth of residual tumour cells over long time periods, even in the absence of immunotherapy. Only subsequent clinical trials with an appropriate control cohort will provide answers to this question.
A significant increase in antigen-specific T cells against PSA already after the fourth dose supports the immunomodulating effect of DCVAC/PCa. Sustained levels of PSA-reacting T cells were detected through the study. Changes in other evaluated immune parameters were insignificant with the exception of a significant decrease in the frequency of Tregs in patients with long-term (second cycle) immunotherapy. The lack of correlation between PSA serum kinetics and induction of tumour-specific immune responses further highlights the urgent need for the identification of other biomarkers predictive of a clinical outcome in immune-based therapies. It is also likely that the peripheral blood, although easily accessible, might not represent the optimal compartment for the analysis of tumour-specific immune responses.
Despite the outlined limitations, this study supports the feasibility of immunotherapy with DCVAC/PCa in patients with low-volume disease and warrants further evaluation of this platform in larger clinical trials with an appropriate cohort of control patients included.
Conclusion
This study indicates that continuous cancer immunotherapy with DCVAC/PCa represents a feasible treatment modality for prostate cancer patients with early signs of disease recurrence. This study supports the use of immunotherapy early in the course of the disease provided that relevant surrogate endpoints predictive of improved prognosis of early stage patients will be identified. Long-term follow-up and additional supportive data from large clinical studies in this patient population are needed to understand how the modified kinetics of PSA affects the clinical outcome of patients with the biochemical recurrence of PCa.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The work of the Department of Immunology of Charles University is supported by the Ministry of Health, Czech Republic-Conceptual Development of Research Organization (University Hospital Motol, Prague, Czech Republic, 00064203) and Grant AZV ČR (agency for medical research, Czech Republic) 16-28135A.
List of abbreviations
- AEs
Adverse events
- BCR
Biochemical relapse
- CTL
Cytotoxic T lymphocytes
- DCs
Dendritic cells
- DCVAC/PCa
Dendritic cells pulsed with the killed prostate-cancer cell line LNCaP
- GMP
Good manufacturing practice
- LNCaP
Androgen-sensitive human prostate adenocarcinoma cells
- NKs
Natural killer cells
- PBMCs
Peripheral blood mononuclear cells
- PCa
Prostate cancer
- PSA
Prostate-specific antigen
- PSADT
PSA doubling time
- RP
Radical prostatectomy
- RT
Radiotherapy
- SAEs
Serious adverse events
- s.c.
Subcutaneously
- SRT
Salvage radiotherapy
- Tregs
Regulatory T cells
Compliance with ethical standards
Conflict of interest
Jitka Fucikova, Michal Podrazil, Pavla Bilkova, Michal Hensler, Anna Fialova, Klara Sochorova, Daniela Rozkova are part-time employees of Sotio; Jirina Bartunkova and Radek Spisek are minority shareholders of Sotio. The other authors declare that they have no conflict of interest.
Informed consent
Written informed consent was obtained from all patients before any of the study procedures was conducted.
Footnotes
Jitka Fucikova, Michal Podrazil, Radek Spisek and Jirina Bartunkova contributed equally to this work.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, Bray F. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61(6):1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 3.Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nat Rev Cancer. 2002;2(5):389–396. doi: 10.1038/nrc801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 5.Walsh PC, DeWeese TL, Eisenberger MA. Clinical practice. Localized prostate cancer. N Engl J Med. 2007;357(26):2696–2705. doi: 10.1056/NEJMcp0706784. [DOI] [PubMed] [Google Scholar]
- 6.D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA, Kaplan I, Beard CJ, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 7.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 8.Abdollah F, Schmitges J, Sun M, Jeldres C, Tian Z, Briganti A, Shariat SF, Perrotte P, Montorsi F, Karakiewicz PI: comparison of mortality outcomes after radical prostatectomy versus radiotherapy in patients with localized prostate cancer: a population-based analysis. Int J Urol. 2012;19(9):836–844. doi: 10.1111/j.1442-2042.2012.03052.x. [DOI] [PubMed] [Google Scholar]
- 9.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, Partin AW. Death in patients with recurrent prostate cancer after radical prostatectomy: prostate-specific antigen doubling time subgroups and their associated contributions to all-cause mortality. J Clin Oncol. 2007;25(13):1765–1771. doi: 10.1200/JCO.2006.08.0572. [DOI] [PubMed] [Google Scholar]
- 10.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6(10):715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 11.Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol. 2010;10(8):580–593. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 13.Sheikh NA, Petrylak D, Kantoff PW, Dela Rosa C, Stewart FP, Kuan LY, Whitmore JB, Trager JB, Poehlein CH, Frohlich MW, et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. 2013;62(1):137–147. doi: 10.1007/s00262-012-1317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vacchelli E, Vitale I, Eggermont A, Fridman WH, Fucikova J, Cremer I, Galon J, Tartour E, Zitvogel L, Kroemer G, et al. Trial watch: dendritic cell-based interventions for cancer therapy. Oncoimmunology. 2013;2(10):e25771. doi: 10.4161/onci.25771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Draube A, Klein-Gonzalez N, Mattheus S, Brillant C, Hellmich M, Engert A, von Bergwelt-Baildon M. Dendritic cell based tumor vaccination in prostate and renal cell cancer: a systematic review and meta-analysis. PLoS One. 2011;6(4):e18801. doi: 10.1371/journal.pone.0018801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geary SM, Salem AK. Prostate cancer vaccines: update on clinical development. Oncoimmunology. 2013;2(5):e24523. doi: 10.4161/onci.24523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lubaroff DM. Prostate cancer vaccines in clinical trials. Expert Rev Vaccines. 2012;11(7):857–868. doi: 10.1586/erv.12.54. [DOI] [PubMed] [Google Scholar]
- 18.Butterfield LH. Dendritic cells in cancer immunotherapy clinical trials: are we making progress? Front Immunol. 2013;4:454. doi: 10.3389/fimmu.2013.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galluzzi L, Vacchelli E, Bravo-San Pedro JM, Buque A, Senovilla L, Baracco EE, Bloy N, Castoldi F, Abastado JP, Agostinis P, et al. Classification of current anticancer immunotherapies. Oncotarget. 2014;5(24):12472–12508. doi: 10.18632/oncotarget.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Podrazil M, Horvath R, Becht E, Rozkova D, Bilkova P, Sochorova K, Hromadkova H, Kayserova J, Vavrova K, Lastovicka J, et al. Phase I/II clinical trial of dendritic-cell based immunotherapy (DCVAC/PCa) combined with chemotherapy in patients with metastatic, castration-resistant prostate cancer. Oncotarget. 2015;6(20):18192–18205. doi: 10.18632/oncotarget.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hermans IF, Chong TW, Palmowski MJ, Harris AL, Cerundolo V. Synergistic effect of metronomic dosing of cyclophosphamide combined with specific antitumor immunotherapy in a murine melanoma model. Cancer Res. 2003;63(23):8408–8413. [PubMed] [Google Scholar]
- 22.Fucikova J, Rozkova D, Ulcova H, Budinsky V, Sochorova K, Pokorna K, Bartunkova J, Spisek R. Poly I: c-activated dendritic cells that were generated in Cell Gro for use in cancer immunotherapy trials. J Transl Med. 2011;9:223. doi: 10.1186/1479-5876-9-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rozkova D, Tiserova H, Fucikova J, Last’ovicka J, Podrazil M, Ulcova H, Budinsky V, Prausova J, Linke Z, Minarik I, et al. FOCUS on FOCIS: combined chemo-immunotherapy for the treatment of hormone-refractory metastatic prostate cancer. Clin Immunol. 2009;131(1):1–10. doi: 10.1016/j.clim.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Schweizer MT, Drake CG. Immunotherapy for prostate cancer: recent developments and future challenges. Cancer Metastasis Rev. 2014;33(2–3):641–655. doi: 10.1007/s10555-013-9479-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimonte G. A cell kinetics model for prostate cancer and its application to clinical data and individual patients. J Theor Biol. 2010;264(2):420–442. doi: 10.1016/j.jtbi.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 26.Dimonte G, Bergstralh EJ, Bolander ME, Karnes RJ, Tindall DJ. Use of tumor dynamics to clarify the observed variability among biochemical recurrence nomograms for prostate cancer. Prostate. 2012;72(3):280–290. doi: 10.1002/pros.21429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anscher MS. Adjuvant radiotherapy following radical prostatectomy is more effective and less toxic than salvage radiotherapy for a rising prostate specific antigen. Int J Cancer. 2001;96(2):91–93. doi: 10.1002/ijc.1011. [DOI] [PubMed] [Google Scholar]
- 28.DiPaola RS, Chen YH, Bubley GJ, Stein MN, Hahn NM, Carducci MA, Lattime EC, Gulley JL, Arlen PM, Butterfield LH, et al. A National Multicenter Phase 2 Study of Prostate-specific Antigen (PSA) Pox Virus Vaccine with Sequential Androgen Ablation Therapy in Patients with PSA Progression: ECOG 9802. Eur Urol. 2015;3:365–371. doi: 10.1016/j.eururo.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.