Abstract
Background
The NK-92/5.28.z cell line (also referred to as HER2.taNK) represents a stable, lentiviral-transduced clone of ErbB2 (HER2)-specific, second-generation CAR-expressing derivative of clinically applicable NK-92 cells. This study addresses manufacturing-related issues and aimed to develop a GMP-compliant protocol for the generation of NK-92/5.28.z therapeutic doses starting from a well-characterized GMP-compliant master cell bank.
Materials and methods
Commercially available GMP-grade culture media and supplements (fresh frozen plasma, platelet lysate) were evaluated for their ability to support expansion of NK-92/5.28.z. Irradiation sensitivity and cytokine release were also investigated.
Results
NK-92/5.28.z cells can be grown to clinically applicable cell doses of 5 × 108 cells/L in a 5-day batch culture without loss of viability and potency. X-Vivo 10 containing recombinant transferrin supplemented with 5% FFP and 500 IU/mL IL-2 in VueLife 750-C1 bags showed the best results. Platelet lysate was less suited to support NK-92/5.28.z proliferation. Irradiation with 10 Gy completely abrogated NK-92/5.28.z proliferation and preserved viability and potency for at least 24 h. NK-92/5.28.z showed higher baseline cytokine release compared to NK-92, which was significantly increased upon encountering ErbB2(+) targets [GZMB (twofold), IFN-γ (fourfold), IL-8 (24-fold) and IL-10 (fivefold)]. IL-6 was not released by NK cells, but was observed in some stimulated targets. Irradiation resulted in upregulation of IL-8 and downregulation of sFasL, while other cytokines were not impacted.
Conclusion
Our concept suggests NK-92/5.28.z maintenance culture from which therapeutic doses up to 5 × 109 cells can be expanded in 10 L within 5 days. This established process is feasible to analyze NK-92/5.28.z in phase I/II trials.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-017-2055-2) contains supplementary material, which is available to authorized users.
Keywords: Natural killer cells, NK-92, CAR, HER2, Cancer immunotherapy, Glioblastoma
Introduction
NK-92 is a well-known IL-2-dependent cell line that is currently being developed for clinical application in a variety of different cancers. It is the only natural killer cell line established hitherto, which entered clinical trials and was shown to be safe even at high intravenous doses of up to 1010 cells with some beneficial effect reported in melanoma and lung cancer patients [1–3]. To further improve the efficacy of NK-92 cells, they were engineered to express a variety of chimeric antigen receptors (CARs) specific against tumor-associated proteins [e.g., CD38, CD19, CD20, epithelial cell adhesion molecule (EPCAM), disialoganglioside (GD2), epidermal growth factor receptor variant III (EGFRvIII) and ErbB2 (HER2)], which proved to render NK-92 cells cytotoxic against otherwise resistant hematologic and solid malignancies [4–8].
Considering that ErbB2 tumor-associated antigen is an attractive therapeutic target overexpressed by a broad spectrum of epithelial cancers [9], we recently focused on generating a fully stable, GMP-compliant transduced CAR-expressing NK-92 cell line. Following GMP-compliant procedures, parental NK-92 cells from an FDA-licensed master cell bank were transduced with a lentiviral vector encoding a humanized, second-generation CAR construct, comprising an ErbB2-specific single chain variable fragment (scFv) derived from the FRP5 monoclonal antibody, CD28, as a co-stimulatory molecule and the CD3-ζ signaling domain (5.28.z). The generated NK-92/5.28.z cell line was obtained after extensive screening of individual cell clones. The chosen clone revealed two vector integration sites, one in an intergenic region on chromosome 2, and one in the TRAF2 gene on chromosome 9 and exhibited highly specific cytotoxicity toward ErbB2-expressing tumor targets which were resistant to parental NK-92 cells [10]. In vivo antitumor efficacy was extensively tested in several established mouse models of orthotopic human glioblastoma (GBM), renal cell carcinoma and breast carcinoma xenografts, showing specific homing of NK-92/5.28.z cells to tumor sites, significant reduction of metastases and, in some cases, complete tumor clearance [10, 11]. To prepare for clinical evaluation in patients with advanced ErbB2-positive cancers, a robust GMP-compliant process yielding clinical doses up to 5 × 109 NK-92/5.28.z cells had to be established. The concept of cellular therapy product manufacturing presented here encompasses the expansion of individual patient dose from a pre-established and qualified master cell bank, comprising 200 ampules of NK-92/5.28.z cells at a concentration of 2 × 107 cells/mL.
Since NK-92 cells, like primary NK cells, are affected by cryopreservation and thawing, with loss of function rescued only after extended culture with IL-2 [12], it is necessary to develop a process which will allow the expansion of therapeutic doses in a timely manner without need of long recovery after cryopreservation. Other issues are the cell culture media and supplements used for expansion. Parental NK-92 cells represent IL-2-dependent cells that were previously reported to be cultured in X-Vivo 10 cell culture medium supplemented with varying amounts of IL-2 (100–1000 /mL) [3, 13]. Commercially available serum-free media usually contain high amounts of human serum albumin, which should make serum supplementation redundant to comply with GMP standards. However, efficient expansion of NK-92 cells so far was reported only in the presence of serum/plasma [1, 12].
To enable clinical application of NK-92/5.28.z in patients with ErbB2-overexpressing malignancies, the present study aimed to establish GMP-compliant procedures for robust generation of therapeutic doses. Since the clinical application of NK-92/5.28.z cells will most probably include γ-irradiation to prevent in vivo expansion, different irradiation doses were analyzed to find a dose which will inhibit proliferation but retain cell potency. Since CAR-T cell therapies were associated with cytokine release syndrome [14], it was of importance to determine the profile of soluble factors secreted by target-stimulated NK-92/5.28.z cells.
Methods
Cell lines and culture conditions
Human MDA-MB-453 and MDA-MB-468 (DSMZ) were maintained in DMEM, high glucose containing GlutaMAX. NK-sensitive K562 cells were cultured in RPMI 1640 medium containing GlutaMAX. Both media were supplemented with 10% of heat-inactivated (HI) FBS, 100 U/mL penicillin and 100 μg/mL streptomycin. For routine maintenance, NK cells were cultured in X-Vivo 10 medium containing recombinant transferrin (X-Vivo 10 rTF) (Lonza, Basel, Switzerland) supplemented with 500 U/mL IL-2 (Novartis Pharma) and 5% of HI human plasma, unless otherwise specified in the respective experiment.
Culture media and media supplements for expansion of NK-92/5.28.z
Cells were seeded at an initial concentration of 5 × 104/mL in triplicates in vented T75 cell culture flasks in respective combination of media supplements. Cell number and viability were measured using either Trypan blue exclusion assay or NucleoCounter NC-100 (Chemometec) according to the manufacturer’s protocol.
Clinical-scale expansion of NK-92/5.28.z
Clinical-scale expansion was conducted in gas-permeable VueLife 750-C1 culture bags (CellGenix, Freiburg, Germany) in a batch culture. NK-92/5.28.z cells were inoculated into culture bags prefilled with 1 L of X-Vivo 10 rTF supplemented with 5% HI human plasma and 500 U/mL of IL-2 at a concentration of 5 × 104 cells/mL. After 5 days of culturing, cells were harvested at a concentration of 5–6 × 105/mL by centrifugation in 400-mL transfer bags (Fenwal) and γ-irradiated with 10 Gy. Cell concentration in the final product was adjusted to 5 × 107/mL in X-Vivo 10 rTF supplemented with 100 U/mL of IL-2. Three independent final products were generated.
FACS-based cytotoxicity assay
Cytotoxicity of NK-92/5.28.z and parental NK-92 cells against tumor targets was analyzed using FACS-based killing assay where target cells labeled with calcein violet AM (Molecular Probes, Invitrogen) were co-incubated with effector cells at a 10:1 E/T ratio for 2 h at 37 °C. To determine spontaneous lysis, target cells were incubated in assay medium without effector cells. Afterwards, cells were labeled with 7-AAD (BD Pharmingen) viability dye at 0.5 µg/test and anti-human CD56-APC (BD Biosciences) for 15 min on ice. Samples were measured with a FACSCanto II flow cytometer and data were analyzed using FACSDiva software version 6.1.3. The percentage of 7-AAD and calcein violet AM double-positive cells measured in the test tube was considered as sample lysis and used for calculation of specific cytotoxicity according to the standard equation.
Europium TDA (EuTDA) cytotoxicity assay
Target cells loaded with acetoxymethyl ester of the fluorescence-enhancing ligand (BATDA) (Perkin Elmer) were co-incubated in triplicates at 5000 cells per well with effector cells at a 10:1 ratio. Supernatants were collected after a 2-h co-incubation for measuring of fluorescent signal reflecting target cell lysis with time-resolved fluorometry (TRF). Specific lysis was calculated according to the standard formula.
Control of cell identity and chimeric antigen receptor expression
Identity and CAR expression were monitored using two-step staining. First, cells were incubated with rhErbB2/Fc fusion protein (R&D Systems) for 30 min on ice and then stained with goat anti-human IgG F(ab′)2-APC (Jackson ImmunoResearch) forming FACS-detectable complexes. To confirm immunophenotype of the NK-92 cell line (CD16neg, CD56bright) and estimate cell viability, additional staining with anti-human CD56-PE, CD16-FITC (both BD Biosciences) and 7-AAD was performed. Parental NK-92 cells were included as a control.
PCR analysis of vector integration sites in single cell clone NK-92/5.28.z
Based on the vector integration site, DNA sequences established in the LAM-PCR analysis of a single cell clone NK-92/5.28.z [10], forward (F) and reverse (R) oligonucleotide primers, were designed for PCR amplification of the 5′ end of the vector integration in the TRAF2 gene on chromosome 9 (reaction ‘TRAF2-CAR’; primers TRAF2-F1 hybridizing to the TRAF2 sequence 5′ of the integrated CAR vector sequence and CAR-R1 hybridizing to the 5′ part of the integrated CAR vector sequence; expected amplification product: 587 bp) and the 3′ end of the vector integration in the TRAF2 gene on chromosome 9 (reaction ‘CAR-TRAF2′; primers CAR-F1 hybridizing to the 3′ part of the integrated CAR vector sequence and TRAF2-R1 hybridizing to the TRAF2 sequence 3′ of the integrated CAR vector sequence; expected amplification product: 503 bp). Likewise, oligonucleotide primers were designed for PCR amplification of the 5′ end of the vector integration in the intergenic region on chromosome 2 (reaction ‘IGCHR2-CAR’; primers IGCHR2-F1 hybridizing to the intergenic region 5′ of the integrated CAR vector sequence on chromosome 2 and CAR-R2 hybridizing to the 5′ part of the integrated CAR vector sequence; expected amplification product: 679 bp) and the 3′ end of the vector integration in the intergenic region on chromosome 2 (reaction ‘CAR-IGCHR2′; primers CARF2 hybridizing to the 3′ part of the integrated CAR vector sequence and IGCHR2-CAR-R1 hybridizing to a sequence overlapping the 3′ end of the integrated CAR vector sequence and the adjacent intergenic region 3′ of the integrated CAR vector sequence on chromosome 2; expected amplification product: 376 bp).
Flow cytometric analysis of natural cytotoxicity receptors
Staining of NCR was performed in triplicates 24 h post-irradiation with four sets of samples using the following antibodies: anti-CD56-APC, anti-CD16-FITC, anti-NKp44-PE, 7AAD (all obtained from BD Biosciences) anti-NKG2D-PE, anti-NKp46-PE and anti-NKp30-PE (all from BioLegend). Isotype control antibody was IgG1 k-PE (BD Biosciences). Non-irradiated cells were included for comparison.
Gamma irradiation
Cells were exposed to various irradiation doses ranging from 0 to 30 Gy using a cesium source with a dose rate of 3.75 Gy/min (IBL 437C blood irradiator), washed and seeded in fresh, complete culture medium.
Cytometric bead array
Release of soluble factors by NK-92/5.28.z and parental NK-92 upon stimulation with target cells was analyzed using FACS-based Cytometric Bead Array (BD Biosciences). 5 × 105 effector cells per sample were co-incubated with tumor cells at a 10:1 E/T ratio for 2 h at 37 °C. Unstimulated effector and target cells, as well as cells stimulated with PMA (50 ng/mL)/Ionomycin (500 ng/mL), were included for determination of basic and maximum release, respectively. After incubation, the plate was centrifuged and 50 µL of supernatant was collected from each well and further analyzed according to the manufacturer’s protocol. Data were collected using a BD LSRFortessa flow cytometer and analyzed with FCAP Array software version 3.0.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software version 5.02. Results are presented as Mean ± SEM as indicated in the figure legends. Two-tailed unpaired Student’s t test was used to analyze the results. Statistical differences were considered significant when P < 0.05.
Results
GMP-compliant, serum-free cell culture media and serum substitutes
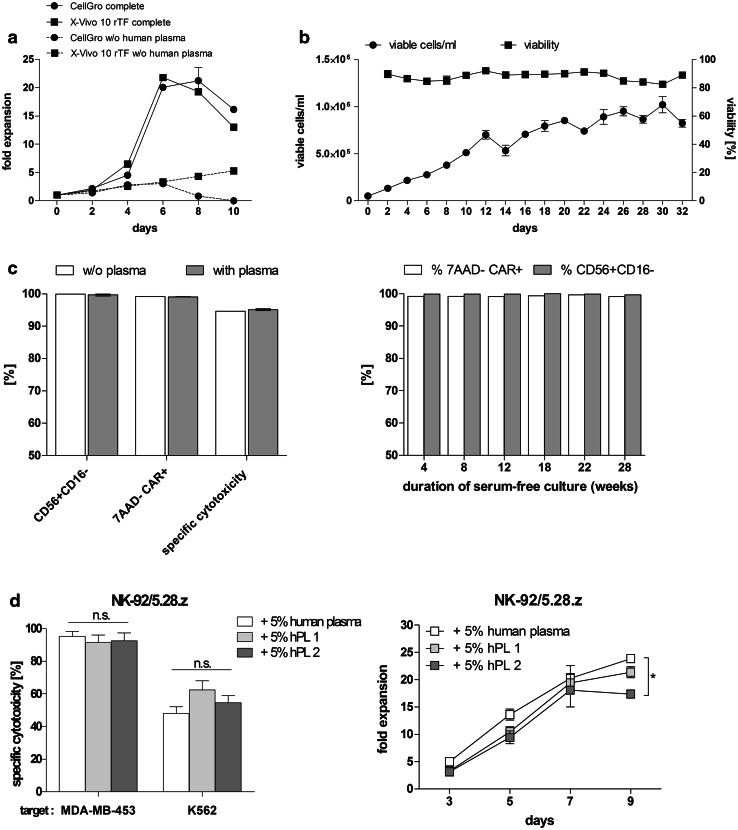
The NK-92 cell line was adapted to GMP-grade X-Vivo 10 culture medium to make it suitable for clinical application [1]. Due to its high human serum albumin content, X-Vivo 10 medium is intended to promote cell growth without the need for further human plasma substitution. Nevertheless, NK-92 cells cultured in X-Vivo 10 still require human plasma supplementation for efficient cell expansion. Therefore, commercially available GMP-grade cell culture media, 2 variants of X-Vivo 10 and CellGro, were compared with regard to their ability to promote growth of NK-92/5.28.z cells as a plasma-free and plasma-supplemented formulation. The first assessment was made between X-Vivo 10 containing human holo-transferrin and X-Vivo 10 containing recombinant transferrin (X-Vivo 10 rTF). Both media were supplemented with 500 U/mL of IL-2 and 5% of heat-inactivated human plasma. Although a difference in cell proliferation was not detected until day 6, the presence of recombinant transferrin seemed to improve cell growth in long-term culture. The most pronounced difference was observed at day 7 with fold expansion of 20.73 ± 0.33 and 15 ± 0.6 for X-Vivo 10 rTF and X-Vivo 10 containing human holo-transferrin, respectively (Supplementary Fig. 1). Subsequently, X-Vivo 10 rTF was compared with CellGro (SCGM) giving equivalent results in terms of supporting cell proliferation as plasma-supplemented composition. However, CellGro was not able to promote cell expansion in plasma-free conditions (max. conc./mL in batch culture 1.52 × 105 ± 0.1 × 105), whereas X-Vivo 10 rTF maintained gradual cell growth (max. conc./mL in batch culture 2.65 x 105 ± 0.09 x 105) (Fig. 1a), thus facilitating acclimation to plasma-free culture conditions.
Fig. 1.
Impact of culture medium supplements on proliferation and functionality of NK-92/5.28.z. a Cells were seeded in either X-Vivo 10 rTF or CellGro medium, both supplemented with 500 U/mL of IL-2 in the absence (w/o human plasma) or presence of 5% HI human plasma (complete). b Cells were seeded at an initial concentration of 5 × 104/mL in X-Vivo 10 rTF supplemented with 500 U/mL of IL-2 without addition of plasma/serum. Every second day, half the culture supernatant was removed and culture was replenished with fresh, plasma-free medium. Subsequently, cells were re-suspended and cell number and viability were determined. Cells were kept in batch culture for 32 days until reaching confluency defined as 1 × 106/mL. c At the end of the batch culture, identity (CD56+ CD16−), CAR expression (7AAD−CAR+) and specific cytotoxicity against ErbB2(+) targets (MDA-MB-453) were tested and compared with the results obtained with cells cultured in plasma-supplemented medium (with plasma) (left panel). CAR expression and identity were further tested up to 28 weeks (right panel). d Comparison of functionality (left panel) and proliferation (right panel) of NK-92/5.28.z cells cultured in human plasma or hPL supplemented medium. Experiments were performed in triplicates. Data are presented as Mean ± SEM, *P < 0.05
To eliminate undefined, donor-derived supplements, NK-92/5.28.z cells were subjected to the 32-day process of acclimation to serum/plasma-free conditions. An applied feeding regimen enabled maintenance of cell viability ≥80% during the whole acclimation course and maximum concentration in batch culture of 10.2 × 105 ± 0.85 × 105 cells/mL (Mean ± SEM) at day 30 (Fig. 1b). At the end of the acclimation procedure, the quality of the cells was evaluated with the following results for tested parameters: CAR expression >99%, identity (CD56+ CD16−) > 99% and specific cytotoxicity against ErbB2(+) targets with the Mean value of 94.4%. NK-92/5.28.z cells cultured in plasma-supplemented medium were included for comparison (Fig. 1c left panel). Established plasma-free culture was further maintained and analyzed providing favorable results in terms of cell proliferation when compared to non-acclimated control cells cultured in plasma-free medium with a doubling time of 47.37 h ± 2.75 h and 83.11 h ± 3.08 h (P < 0.001), respectively. CAR expression and phenotype of NK-92/5.28.z were further investigated and remained stable over the 28-week analysis period with values >99% (Fig. 1c right panel). However, plasma-free culture might not be efficient in a clinical setting providing significantly longer cell doubling time when compared to plasma-supplemented culture (47.37 ± 2.75 h and 28.84 ± 0.5 h, respectively) (Supplementary Table 1). Promising data on expansion of other cell types cultured in media supplemented with platelet lysate encouraged us to test the proliferation of NK-92/5.28.z in a similar setting. Platelet lysate from two companies in comparison with human plasma was assessed for its ability to promote cell expansion and cytotoxicity. There was no advantageous influence of cultivation in medium supplemented with 5% of human platelet lysate (hPL) in terms of cell functionality (Fig. 1d left panel); what is more, cells cultured in medium supplemented with 5% of human plasma proliferated significantly better (P < 0.05) when compared to hPL2 with fold expansion of 23.87 ± 0.22 and 17.37 ± 0.67, respectively, at day 9 (Fig. 1d right panel).
IL-2 supplementation
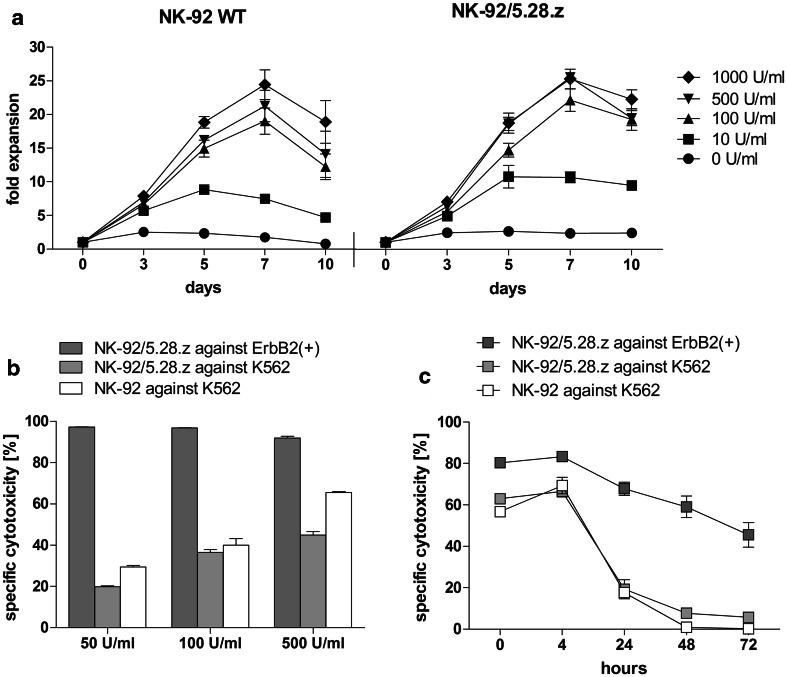
Since the growth and functionality of NK-92 cells is IL-2 dependent, we aimed to optimize the concentration of this cytokine for ex vivo cell expansion. Cell proliferation of NK-92 and NK-92/5.28.z cells was tested in five different concentrations of IL-2 ranging from 0 to 1000 U/mL, indicating 500 U/mL as an optimum. Doubling of this concentration did not promote further cell expansion (Fig. 2a). CAR-mediated specific cytotoxicity was not strictly dependent on IL-2 concentration in culture media. All three concentrations tested (50, 100, 500 U/mL) resulted in specific cytotoxicity of NK-92/5.28.z against ErbB2-positive targets of more than 90%. As expected, increasing levels of IL-2 supported natural killing tested against K562 cells, of both genetically modified and parental NK-92 cells (Fig. 2b). Furthermore, stability of cell potency in IL-2-free culture was tested up to 72 h. An instantaneous, significant decrease in natural killing of both NK-92 and NK-92/5.28.z was observed. Conversely, re-targeted killing of NK-92/5.28.z toward ErbB2-positive targets was decreasing gradually with cytotoxicity values of more than 50% up to 48 h (67.77% ± 3.16% at 24 h; 59.02% ± 5.16% at 48 h) and 45.50% ± 5.99% after 72 h (Fig. 2c).
Fig. 2.
Impact of IL-2 concentration on proliferation and functionality of NK-92 and NK-92/5.28.z. a Proliferation of parental (NK-92 WT) and genetically modified NK-92 cells (NK-92/5.28.z) in X-Vivo 10 rTF supplemented with 5% of human plasma and various concentrations of IL-2 up to 10 days. b Natural killing of NK-92 (white bars), NK-92/5.28.z (light grey bars) and retargeted killing of NK-92/5.28.z (dark grey bars) cultured for 5 days in X-Vivo 10 rTF supplemented with 5% of human plasma and 50, 100, 500 U/mL of IL-2. c Stability of natural killing of NK-92 (white symbol), NK-92/5.28.z (light grey symbol) and retargeted killing of NK-92/5.28.z (dark grey symbol) in IL-2-free culture was tested up to 72 h
Impact of gamma irradiation on proliferation and potency of NK-92/5.28.z
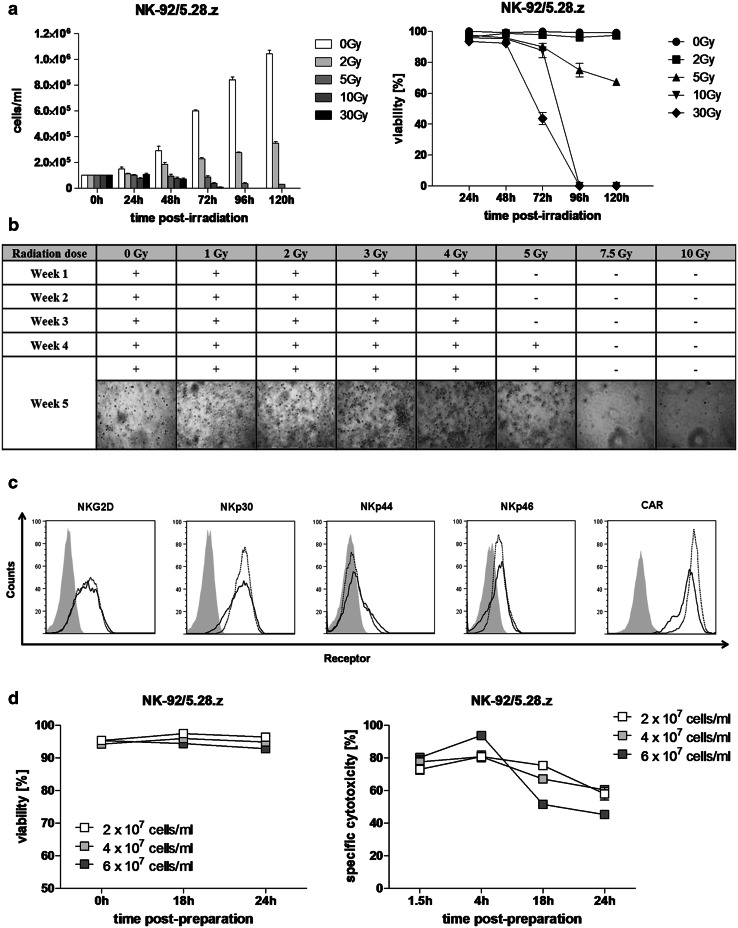
In all clinical trials performed hitherto, NK-92 cells were subjected to γ-irradiation exposure to prevent permanent engraftment in patients. In this study, we analyzed proliferation and viability of NK-92/5.28.z following γ-irradiation with 5 doses (range: from 0 Gy to 30 Gy) every 24 h up to 5 days. Figure 3a shows that 30 Gy, 10 Gy and 5 Gy, but not 2 Gy, caused the complete inhibition of cell proliferation when compared to the non-irradiated control, which resulted in a significant decline in viability first observed 72 h post-irradiation with 30 Gy with viability values of 43.59 ± 3.96% versus 99.86 ± 0.14% for non-irradiated cells. Treatment with 2 Gy significantly retarded cell growth but did not affect cell viability, which exceeded 90% over the entire analysis period. To determine the lowest irradiation dose efficiently inhibiting cell proliferation, we investigated long-term effect of exposure to various irradiation doses on cell growth. Our data show that irradiation doses ranging from 1 to 4 Gy were not able to inhibit cell proliferation. Cells irradiated with 5 Gy showed significant inhibition of cell growth up to 3 weeks post-exposure; however, further cultivation led to outgrowth of the cells first observed 4 weeks post-irradiation. Doses of 7.5 Gy and 10 Gy caused complete inhibition of cell proliferation, where after 5 weeks no viable cells were detected (Fig. 3b).
Fig. 3.
Radiation sensitivity of NK-92/5.28.z. a Cell concentration (left panel) and viability (right panel) of NK-92/5.28.z cells exposed to γ-irradiation were measured every 24 h up to 5 days. b Long-term analysis of NK-92/5.28.z cell proliferation exposed to γ-irradiation. c Receptor expression on irradiated (solid lines) and non-irradiated NK-92/5.28.z cells (dotted lines) 24 h post-exposure. Grey histograms represent isotype matched controls or, in the case of CAR analysis, parental NK-92 cells. d Viability (left panel) and specific cytotoxicity (right panel) of NK-92/5.28.z cells in high-density suspensions were analyzed at the indicated time points over storage under ambient conditions in X-Vivo 10 supplemented with 100 U/mL of IL-2 following γ-irradiation with 10 Gy
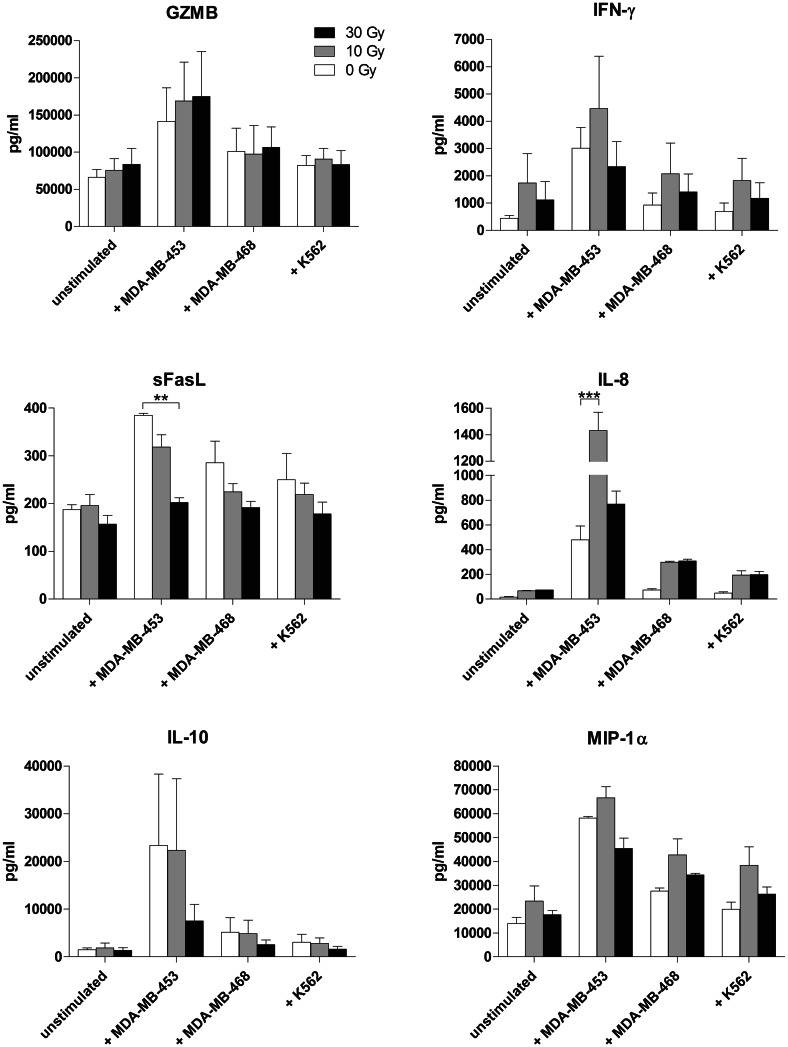
We investigated differences in the secretion of soluble factors between non-irradiated and irradiated NK-92/5.28.z upon stimulation with three cell lines routinely used as targets in our experiments. Granzyme B (GZMB), IFN-γ, IL-10 and MIP-1α were produced at similar levels by irradiated and non-irradiated NK-92/5.28.z cells, while secretion of IL-6 and TNF by effector cells was not detected. We observed a significant drop in sFasL production upon stimulation with MDA-MB-453 following irradiation with 30 Gy (385.02 pg/mL ± 4.04 pg/mL to 202.69 pg/mL ± 9.95 pg/mL) and significant increase in IL-8 secretion following irradiation with 10 Gy (479.38 ± 112.77 pg/mL to 1433.01 ± 135.12 pg/mL) but not with 30 Gy, when compared to the non-irradiated control (Fig. 4). FACS-based phenotype analysis performed 24 h post-irradiation showed slight upregulation of NKG2D (MFI: 0 Gy 1123 ± 12.9 vs. 10 Gy 1315.33 ± 18.7), NKp30 (MFI: 0 Gy 3588.33 ± 97.43, 10 Gy 3628.67 ± 15.72) and NKp44 (MFI: 0 Gy 384 ± 7, 10 Gy 573.33 ± 3.84) and downregulation of NKp46 (MFI: 0 Gy 511.67 ± 7.88, 10 Gy 474.67 ± 5.24) and CAR (MFI: 0 Gy 44841.33 ± 997.45, 10 Gy 27815.33 ± 1304.98) (Fig. 3c). Simultaneously performed functionality assays showed that observed changes did not attenuate the specific cytotoxicity against ErbB2(+) targets (0 Gy: 83.64% ± 0.39%, 10 Gy: 87.17% ± 1.07%; Mean ± SEM) (data not shown).
Fig. 4.
Influence of irradiation dose on soluble factors released by stimulated NK-92/5.28.z. NK-92/5.28.z were irradiated with 10 Gy (grey bars) and 30 Gy (black bars) and co-cultured with target cells at an E/T ratio of 10:1. NK-92/5.28.z cells cultured in absence of target cells, as well as non-irradiated NK-92/5.28.z (0 Gy, white bars), were included as a control. Concentrations of soluble proteins in test supernatants were measured using a cytometric bead array. Mean values ± SEM are shown; n = 3. **P < 0.01, ***P < 0.001
To analyze the shelf-life of a patient dose, we tested viability and potency of irradiated (10 Gy) NK-92/5.28.z in high-density cell suspensions stored under ambient conditions. Our data demonstrate high stability of the irradiated cells with a viability of >90% for 24 h and specific cytotoxicity against ErbB2(+) targets of >50% up to 18 h for all three tested concentrations (2 × 107/mL; 4 × 107/mL; 6 × 107/mL). However, cytotoxicity of NK-92/5.28.z at the density of 6 × 107/mL after 18 h was significantly lower (51.48% ± 1.5%) when compared to 4 × 107/mL (67.01% ± 1.89%, P < 0.0001) and 2 × 107/mL (75.36% ± 2.44%, P < 0.0001) (Fig. 3d), implying cell concentration rather than irradiation as a factor potentially limiting the stability of patient doses.
Taken together, the above results suggest that 10 Gy is an optimum irradiation dose for NK-92/5.28.z, inhibiting cell proliferation with satisfactory viability and functionality and a convenient safety margin for clinical application.
Generation of patient dose of NK-92/5.28.z
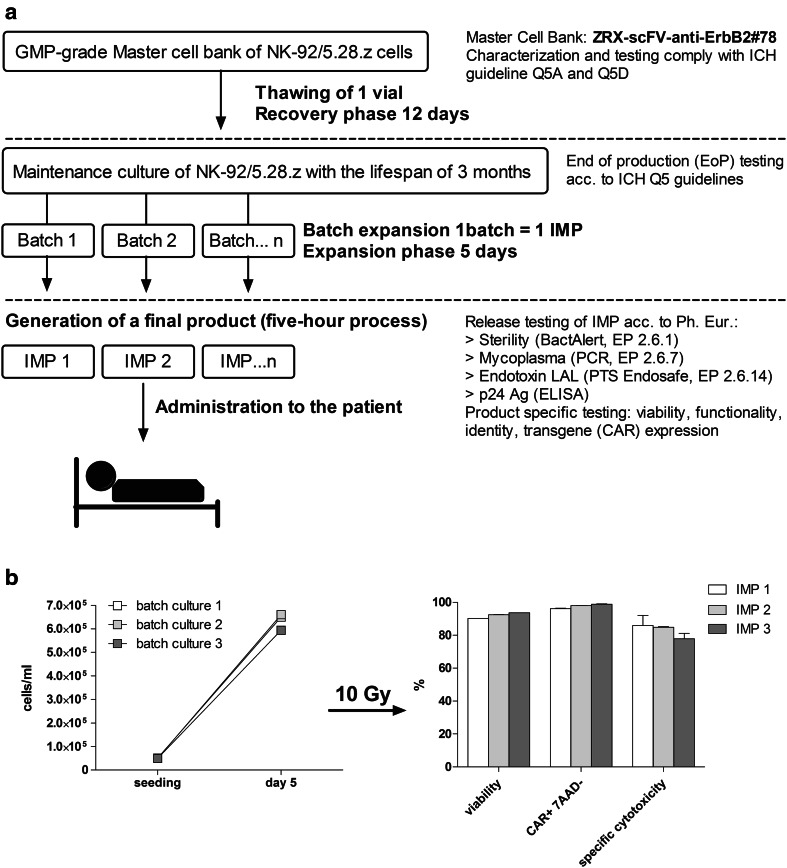
To avoid the post-thaw recovery phase, the investigational medicinal product (IMP) will be expanded on demand from large-scale maintenance culture with the lifespan of 3 months (Fig. 5a) during which time the cells preserve their phenotypic and functional features. We showed that CAR expression is stable over time and we did not observe a loss of any of the two integrated transgenes (Supplementary Fig. 2). To prove the feasibility of an established clinical-scale expansion protocol, three independent validation runs of patient dose generation were performed under class A conditions with subsequent testing of final product. NK-92/5.28.z cells showed stable and reproducible growth in VueLife 750-C1 culture bags with the average doubling time of 32.76 ± 0.32 h over a 5-day culturing period for three independent batches. All three generated patient doses fulfilled pre-defined specification in terms of cell viability (specification >80%; result: 92.16 ± 0.74%; Mean ± SEM; n = 3), CAR expression (specification >95%; result: 97.67 ± 0.4%; Mean ± SEM; n = 3) and specific cytotoxicity (specification >50%; result: 83.43 ± 2.48%; Mean ± SEM; n = 3) post-preparation (Fig. 5b). The generated cellular products were negative for culturable microbes (BacT/Alert) and free of mycoplasma and endotoxin.
Fig. 5.
Expansion and testing of individual patient dose of NK-92/5.28.z. a According to the established manufacturing process, cryopreserved cells of the ZRX-scFV-anti-ErbB2#78 Master Cell Bank are thawed and continuously cultured for up to 3 months (maintenance culture). Based on an individual prescription of the clinical trial center, NK-92/5.28.z cells are expanded in a VueLife 750-C1 bag until a defined cell density is reached. Cells are harvested, irradiated (10 Gy), washed and transferred in the final bag (CryoMACS 50). Release criteria are checked at this point. NK-92/5.28.z cells are characterized for identity/purity by flow cytometry for the expression of the surface markers CD56+, CD16− and the transgene CAR (≥95%), viability (≥80%), potency (cytolytic activity against an ErbB2(+) target) (≥50%), as well as on the presence of replication-competent lentiviral gene transfer vector. Microbial contamination is analyzed using the BacT/Alert system and the IMP will also be tested for the presence of endotoxins (LAL test) and mycoplasma (PCR). b Three independent batches of NK-92/5.28.z cells were expanded in VueLife 750-C1 culture bags prefilled with 1L of culture medium (left panel). After 5 days, cells were harvested, irradiated with 10 Gy and concentrated to desired density. Final products were tested in terms of viability, CAR expression and specific cytotoxicity (right panel)
Analysis of soluble factors secreted by stimulated NK-92/5.28.z
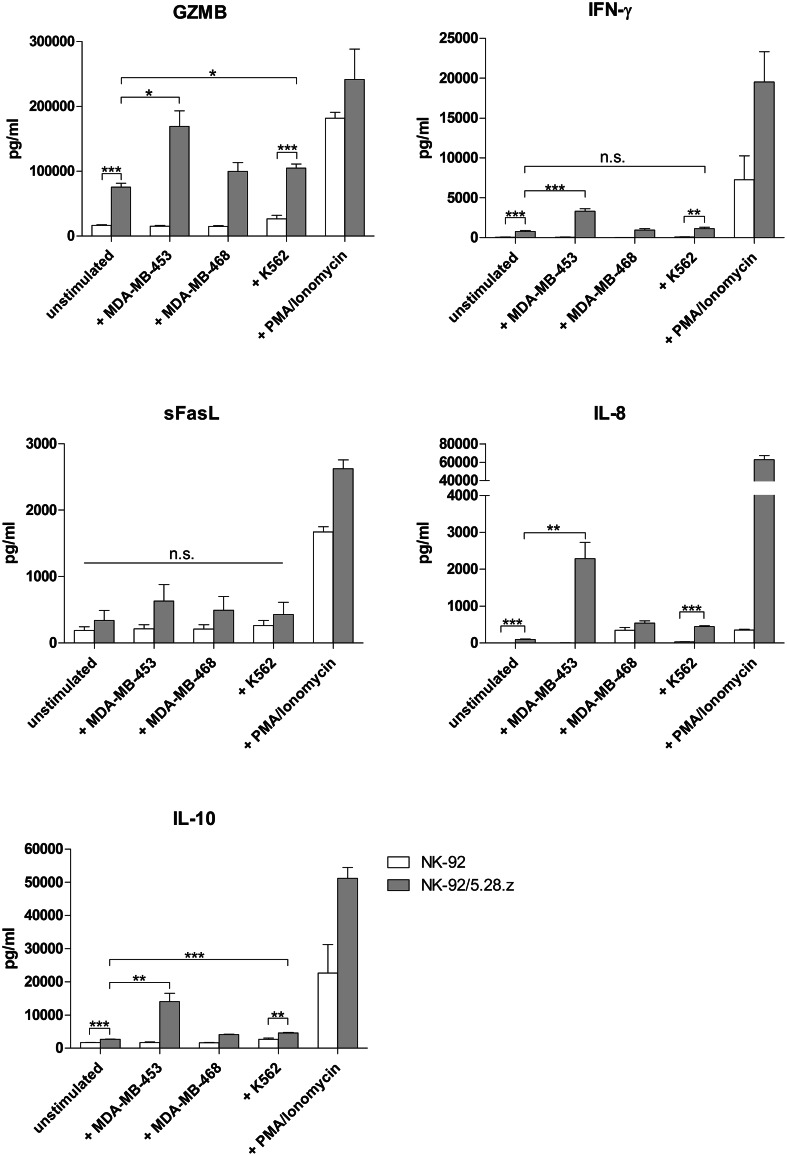
The profile of soluble factors, including effector molecules, secreted upon short-term stimulation was determined to test cell potency as well as to predict potential (systemic) side effects of the cell therapy. Release of GZMB, IFN-γ, sFasL, TNF, IL-2, IL-6, IL-8, IL-10, G-CSF and GM-CSF by parental NK-92 and NK-92/5.28.z was measured. Adequate controls of unstimulated and PMA/Ionomycin stimulated effector cells, as well as PMA/Ionomycin stimulated target cells, were included (Supplementary Table 2). Secretion of GZMB, IFN-γ, sFasL, IL-8 and IL-10 by NK-92/5.28.z was distinctly higher compared to parental cells, not only when stimulated with K562 but also in unstimulated controls; however, in the case of sFasL the differences did not reach the statistical significance (P = 0.1692 and P = 0.2110 for unstimulated and K562 stimulated cells, respectively). We observed a specific and significant increase in GZMB (twofold; P < 0.05), IFN-γ (fourfold; P < 0.001), IL-8 (24-fold; P < 0.01) and IL-10 (fivefold; P < 0.01) production by NK-92/5.28.z co-cultured with ErbB2-overexpressing targets (MDA-MB-453) when compared to baseline secretion, not observed in the case of parental NK-92 (Fig. 6). TNF was detected only in the PMA/Ionomycin stimulated control, whereas secretion of IL-6, GM-CSF, G-CSF and IL-2 by effector cells was below detection limit (data not shown).
Fig. 6.
Influence of second-generation ErbB2-CAR expression on the release of soluble factors by stimulated NK-92 cells. NK-92 (white bars) or NK-92/5.28.z (grey bars) at a concentration of 5 × 106 cells/mL were incubated for 2 h with ErbB2(+) MDA-MB-453, ErbB2(−) MDA-MB-468 or K562 cells at an 10:1 E/T ratio. Effector cells incubated without target cells (unstimulated), as well as stimulated with PMA/Ionomycin, served as a negative and positive control, respectively. Concentrations of GZMB, IFN-γ, sFasL, IL-8 and IL-10 were measured in test supernatants. TNF was detected only in the positive control, whereas secretion of IL-2, IL-6, G-CSF and GM-CSF by effector cells was not found (data not shown). Mean values ± SEM are shown; n = 3–4. *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
To meet the requirements of cell expansion for clinical use, culture conditions have to be well-defined to enable manufacturing of cellular product in a reproducible way. We assessed three GMP-grade culture media for their ability to support cell proliferation in plasma-free and plasma-supplemented cultures. X-Vivo 10 containing recombinant transferrin became our standard for cultivation of parental and re-targeted NK-92 cells, even as a plasma-free formulation, maintaining cell growth, phenotype and functionality at satisfactory levels. However, the comparison of doubling times of the cells cultivated in plasma-free and plasma-supplemented conditions stresses the need of human plasma usage for reaching the required cell numbers in a time-effective manner. Transferrin is a universal factor promoting the cell growth by providing the optimal iron delivery which is particularly important for DNA synthesis and thus, cell proliferation [15]. All culture media tested in this study contain transferrin; however, the protein differs in source (human-derived transferrin versus recombinant transferrin) and form (apo-transferrin versus holo-transferrin) which might be the reason for differences in cell proliferation. Our data demonstrate that X-Vivo 10 containing recombinant transferrin derived from Oryza sativa L. was the most efficient in promoting cell proliferation (when compared to X-Vivo 10 containing human holo-transferrin) and enabling the acclimation to serum-free culture which was not the case for CellGro medium. In addition rTF is a safe alternative to human-derived protein [16].
NK-92/5.28.z, same as the parental cell line, is dependent on exogenous IL-2 for cell survival and potency. Although other cytokines such as IL-7, IL-12 and IL-18 were tested as alternatives, only IL-2 was able to efficiently maintain long-term growth and functionality of NK-92 cells [17, 18]. In a systematic analysis, we found 500U/mL as an optimum concentration of IL-2 efficiently supporting cell proliferation and cytotoxicity in a batch culture.
Due to the immortalized nature of NK-92/5.28.z [17], additional safety measures are undertaken to prevent potential permanent engraftment in the patient. In previous clinical trials employing parental NK-92 cells treated with 10 Gy of γ-irradiation, lack of evidence of prolonged persistence was reported. Therefore, we investigated the radiation sensitivity of NK-92/5.28.z and confirmed irradiation dose of 10 Gy to prevent proliferation (Fig. 3a) of NK-92/5.28.z, as shown also in the late outgrowth experiment (Fig. 3b). The potency of the cells irradiated with 10 Gy was shown to be preserved for 24 h in terms of shelf-life of a final product at high cell densities (up to 5 × 107/mL). It will allow high clinical doses of up to 1.5 × 1010 in a typical transfusion volume of 300 mL. It was shown that under these conditions the cell density has an impact on viability and cytotoxicity. These results, however, do not reflect the overall cytotoxicity of NK-92/5.28.z cells after irradiation, which, as shown for parental cells [13], may exceed 72 h and was not analyzed here.
Additionally, we tested the release of soluble factors by effector cells as a stress response to γ-irradiation. As described for the other cell types we expected elevated levels of IL-8 and IL-6 after exposure [19]. However, we observed a significant increase only in IL-8 production, whereas release of IL-6 was not induced upon irradiation.
Formulation of the cellular product depends on the clinical application, where local injection into the vital body organs seems the most challenging. A good example of such an approach is an imminent clinical trial employing NK-92/5.28.z cells for the treatment of patients with ErbB2-overexpressing GBM, where intracranial injection of cell suspension will be performed into a resection cavity. This route of administration is associated with few limitations, where restricted injection volume and high cell density in a final product are the main issues. In this study, maximum, stable cell density in patient dose (5 × 107 cells/mL) has been established. Performed experiments showed that too high of a concentration of cells may limit efficacy of NK-92 cells. Moreover, prolonged storage in a closed system leads to nutrient and cytokine exhaustion and accumulation of metabolic products which may additionally impair cell quality. We addressed the issues of reproducible and efficient cell expansion, optimum irradiation dose and maximum cell concentration in the final product, which resulted in the establishment of a clinical grade manufacturing procedure enabling us to obtain sufficient numbers of functional cells forming stable patient dose. Successful generation of three independent cellular therapy products of NK-92/5.28.z cells proved the relevance of established conditions.
Cytokine release syndrome (CRS) is the most common and severe complication observed in patients subjected to immune-based therapies, e.g., CAR-engineered T cells or therapeutic antibodies. In most cases, CRS is fully reversible and manageable with combination of supportive care and IL-6 receptor-blocking antibody, tocilizumab, indicating IL-6 as a major player at least in the early, acute phase of the syndrome. Moreover, IL-6 and TNF-α were the first inflammatory cytokines highly elevated in the serum of pediatric ALL patients suffering from grade 3 CRS after CD19-CAR T cell infusion [20, 21]. In our experiments, IL-6 and TNF were not produced by target-stimulated effector cells suggesting the superiority of engineered NK cells over T cells in this pivotal safety aspect. On the other hand, intensified secretion of pro-inflammatory cytokines (IFN-γ, MIP-1α) by NK-92/5.28.z cells shown in our experiments may contribute to enhancement of endogenous antitumor immunity by stimulation of the host immune system as recently explored by Zhang et al. in mouse models of GMB [11].
In this study, we successfully established a GMP-compliant procedure enabling production of therapeutic doses of NK-92/5.28.z. We also discussed crucial issues concerning safety and efficacy in the context of imminent clinical trials. In summary, we showed that NK-92/5.28.z. cells retain main features of the parental line (phenotype, doubling time, the same optimum culture and cryopreservation conditions), while exhibiting superior, selective potency against ErbB2-positive targets normally resistant to NK-cell cytotoxicity. Results presented in this study describe a robust process to expand therapeutic doses of CAR-expressing NK-92 cells from a master cell bank under GMP-compliant conditions with no need for further GMP-processing at the clinical site. At very high densities, the current protocol is limited to a shelf-life of 24 h, which should allow the providing of cell products for immediate use in the clinics that can be reached over night. Our data also indicate that it may be feasible to further prolong the shelf-life under optimized conditions, which should include stabilizing transfusion solutions providing the necessary nutrients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported in part by grants from the German Federal Ministry of Education and Research (BMBF) (Cluster für individualisierte Immunintervention, Ci3; FKZ 131A009A, 131A009B, 131A009C) and the LOEWE Center for Cell and Gene Therapy Frankfurt (CGT). The LOEWE Center for Cell and Gene Therapy Frankfurt is funded by Hessisches Ministerium für Wissenschaft und Kunst, reference number: III L 5–518/17.004 (2013). We thank Barry J. Simon, Nantkwest Inc., for providing NK-92 cells from Nantkwest´s proprietary master cell bank. We thank Andrea Jochheim Richter, Kurt Schoenfeld and Hans Klingemann for helpful discussions and suggestions throughout the project.
Abbreviations
- APC
Allophycocyanin
- CRS
Cytokine release syndrome
- EGFRvIII
Epidermal growth factor receptor variant III
- EPCAM
Epithelial cell adhesion molecule
- Eu
Europium
- FDA
Food and Drug Administration
- FFP
Fresh frozen plasma
- GBM
Glioblastoma
- GD2
Disialoganglioside
- GZMB
Granzyme B
- HI
Heat inactivated
- hPL
Human platelet lysate
- HSA
Human serum albumin
- IMP
Investigational medicinal product
- NCR
Natural cytotoxicity receptors
- r
Recombinant
- SCGM
Stem cell growth medium
- TF
Transferrin
- TRF
Time-resolved fluorometry
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Tonn T, Becker S, Esser R, Schwabe D, Seifried E. Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J Hematother Stem Cell Res. 2001;10(4):535–544. doi: 10.1089/15258160152509145. [DOI] [PubMed] [Google Scholar]
- 2.Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, Klingemann H. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy. 2008;10:625–632. doi: 10.1080/14653240802301872. [DOI] [PubMed] [Google Scholar]
- 3.Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U, et al. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy. 2013;15:1563–1570. doi: 10.1016/j.jcyt.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Esser R, Muller T, Stefes D, Kloess S, Seidel D, Gillies SD, et al. NK cells engineered to express a GD2 -specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J Cell Mol Med. 2012;16:569–581. doi: 10.1111/j.1582-4934.2011.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genssler S, Burger MC, Zhang C, Oelsner S, Mildenberger I, Wagner M, et al. Dual targeting of glioblastoma with chimeric antigen receptor-engineered natural killer cells overcomes heterogeneity of target antigen expression and enhances antitumor activity and survival. Oncoimmunology. 2016;5:e1119354. doi: 10.1080/2162402X.2015.1119354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller T, Uherek C, Maki G, Chow KU, Schimpf A, Klingemann HG, et al. Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells. Cancer Immunol Immunother. 2008;57:411–423. doi: 10.1007/s00262-007-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romanski A, Uherek C, Bug G, Seifried E, Klingemann H, Wels WS, et al. CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies. J Cell Mol Med. 2016;20:1287–1294. doi: 10.1111/jcmm.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uherek C, Tonn T, Uherek B, Becker S, Schnierle B, Klingemann HG, et al. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood. 2002;100:1265–1273. [PubMed] [Google Scholar]
- 9.Santin AD, Bellone S, Roman JJ, McKenney JK, Pecorelli S. Trastuzumab treatment in patients with advanced or recurrent endometrial carcinoma overexpressing HER2/neu. Int J Gynaecol Obstet. 2008;102:128–131. doi: 10.1016/j.ijgo.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Schonfeld K, Sahm C, Zhang C, Naundorf S, Brendel C, Odendahl M, et al. Selective inhibition of tumor growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor. Mol Ther. 2015;23:330–338. doi: 10.1038/mt.2014.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang C, Burger MC, Jennewein L, Genßler S, Schönfeld K, Zeiner P, et al. ErbB2/HER2-specific NK cells for targeted therapy of glioblastoma. J Natl Cancer Inst. 2016 doi: 10.1093/jnci/djv375. [DOI] [PubMed] [Google Scholar]
- 12.Lapteva N, Durett AG, Sun J, Rollins LA, Huye LL, Fang J, Dandekar V, Mei Z, Jackson K, Vera J, Ando J, Ngo MC, Coustan-Smith E, Campana D, Szmania S, Garg T, Moreno-Bost A, Vanrhee F, Gee AP, Rooney CM. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy. 2012;14:1131–1143. doi: 10.3109/14653249.2012.700767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam YK, Martinson JA, Doligosa K, Klingemann HG. Ex vivo expansion of the highly cytotoxic human natural killer-92 cell-line under current good manufacturing practice conditions for clinical adoptive cellular immunotherapy. Cytotherapy. 2003;5:259–272. doi: 10.1080/14653240310001523. [DOI] [PubMed] [Google Scholar]
- 14.June CH, Maus MV, Plesa G, Johnson LA, Zhao Y, Levine BL, Grupp SA, Porter DL. Engineered T cells for cancer therapy. Cancer Immunol Immunother. 2014;63:969–975. doi: 10.1007/s00262-014-1568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laskey J, Webb I, Schulman HM, Ponka P. Evidence that transferrin supports cell proliferation by supplying iron for DNA synthesis. Exp Cell Res. 1988;176:87–95. doi: 10.1016/0014-4827(88)90123-1. [DOI] [PubMed] [Google Scholar]
- 16.Zhang D, Nandi S, Bryan P, Pettit S, Nguyen D, Santos MA, Huang N. Expression, purification, and characterization of recombinant human transferrin from rice (Oryza sativa L.) Protein Expr Purif. 2010;74:69–79. doi: 10.1016/j.pep.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8:652–658. [PubMed] [Google Scholar]
- 18.Kalina U, Kauschat D, Koyama N, Nuernberger H, Ballas K, Koschmieder S, et al. IL-18 activates STAT3 in the natural killer cell line 92, augments cytotoxic activity, and mediates IFN-gamma production by the stress kinase p38 and by the extracellular regulated kinases p44erk-1 and p42erk-21. J Immunol. 2000;165:1307–1313. doi: 10.4049/jimmunol.165.3.1307. [DOI] [PubMed] [Google Scholar]
- 19.Pasi F, Facoetti A, Nano R. IL-8 and IL-6 bystander signalling in human glioblastoma cells exposed to gamma radiation. Anticancer Res. 2010;30:2769–2772. [PubMed] [Google Scholar]
- 20.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.