Abstract
Cysteine cathepsins are lysosomal peptidases involved in the regulation of innate and adaptive immune responses. Among the diverse processes, regulation of granule-dependent cytotoxicity of cytotoxic T-lymphocytes (CTLs) and natural killer (NK) cells during cancer progression has recently gained significant attention. The function of cysteine cathepsins is regulated by endogenous cysteine protease inhibitors—cystatins. Whereas other cystatins are generally cytosolic or extracellular proteins, cystatin F is present in endosomes and lysosomes and is thus able to regulate the activity of its target directly. It is delivered to endosomal/lysosomal vesicles as an inactive, disulphide-linked dimer. Proteolytic cleavage of its N-terminal part leads to the monomer, the only form that is a potent inhibitor of cathepsins C, H and L, involved in the activation of granzymes and perforin. In NK cells and CTLs the levels of active cathepsin C and of granzyme B are dependent on the concentration of monomeric, active cystatin F. In tumour microenvironment, inactive dimeric cystatin F can be secreted from tumour cells or immune cells and further taken up by the cytotoxic cells. Subsequent monomerization and inhibition of cysteine cathepsins within the endosomal/lysosomal vesicles impairs granzyme and perforin activation, and provokes cell anergy. Further, the glycosylation pattern has been shown to be important in controlling secretion of cystatin F from target cells, as well as internalization by cytotoxic cells and trafficking to endosomal/lysosomal vesicles. Cystatin F is therefore an important mediator used by bystander cells to reduce NK and T-cell cytotoxicity.
Keywords: Cystatin F, Cathepsins, Natural killer cells, T cells, Cell cytotoxicity, CITIM 2017
Introduction
Peptidases, enzymes that mediate the hydrolysis of peptide bonds, control a variety of physiological processes, such as food digestion, protein recycling, apoptosis, the cell cycle and the immune response. Depending on their catalytic mechanism they are classified as aspartic, cysteine, serine, metallo or threonine peptidases [1, 2]. While peptidases from different classes are involved in the immune response [3], in this review we focus primarily on cysteine peptidases, i.e. the cysteine cathepsins, due to their important roles in diverse immune processes, such as antigen presentation, T-cell activation and migration, cytokine activation and regulation of cytotoxicity of cytotoxic lymphocytes [3, 4]. In addition, we present the regulation of their activity by cystatins, a group of protein inhibitors that can regulate the immune response by inhibiting enzyme activities and, also, independently from their inhibitory function [5].
Cysteine cathepsins comprise a group of 11 lysosomal peptidases, cathepsins B, C, F, H, K, L, O, S, V, X and W, all related to papain and classified as clan CA [6]. Cathepsins B, C, H and L are expressed ubiquitously, while that of others is tissue and cell specific. In addition to their different expression profiles they differ from each other in their expression levels, cell localization and substrate specificities, resulting in their various physiological roles [6, 7].
In the immune response the cysteine cathepsins are probably best known for their role in antigen processing and presentation. They are involved in the degradation of both endocytosed and endogenous antigens, and in the generation of antigenic peptides that can then be presented to T cells by MHC class II molecules located on antigen-presenting cells. Furthermore, the action of cysteine cathepsins is essential for the degradation of invariant chain (Ii) chaperone and for its removal from major histocompatibility complex (MHC) molecules, a prerequisite step prior to peptide binding [3, 8].
In innate immunity, cysteine cathepsins are responsible for activating Toll-like receptors (TLR). TLRs recognize pathogen-derived material, and their activation leads to inflammation, so their activity needs to be tightly regulated, again by proteolytic activation by cysteine cathepsins [9, 10]. In addition, cysteine cathepsins can activate/inhibit cytokine signalling or, conversely, their activity can be regulated by cytokines [11, 12].
Moreover, cathepsin X has been shown to interact with integrin receptors and, thus, modulate cell migration and adhesion and other events contingent on adhesion. It can either cleave integrin receptors at the C-terminal and/or can, independently of its enzymatic activity, bind to integrins via the arginyl-glycyl-aspartic acid (RGD) motif in its pro-region [13, 14]. Modulation of integrin activation by cathepsin X is important in several types of immune cells. In dendritic cells, proteolytic cleavage of β2-integrin receptor Mac-1 (CD11b/CD18) regulates their adhesion and, consequently, their activation [15]. Similarly, cathepsin X regulates the adhesion of monocytes and macrophages and, thus, phagocytosis and T-cell activation [16]. Finally, cathepsin X has been shown to be important for the migration, invasiveness and homotypic aggregation of T-lymphocytes [17].
The effector functions of cytotoxic T-lymphocytes (CTLs), natural killer (NK) cells, mast cells and neutrophils depend on the activation of their granule-localized serine peptidases by cysteine cathepsins [3]. The first cathepsin shown to activate serine peptidases was cathepsin C, which removes two amino acid residues at the N-terminus. This activation is crucial in neutrophils and mast cells while, in cytotoxic lymphocytes (CTLs and NK cells), other cathepsins can substitute for this cathepsin C function. This was first demonstrated in cathepsin C null mice, in which granzyme B activity is reduced, but not absent [18]. Additionally, Papillon–Lefévre syndrome, a human genetic disorder caused by a mutation in the cathepsin C gene, is characterized by skin infections and periodontitis but not by general T-cell immunodeficiency [19]. It was discovered, at least for granzyme B, that the additional convertase is cathepsin H [20]. However, even in mice lacking both cathepsins C and H, the granzyme B activity was still not absent, suggesting that other convertases might be involved [20]. Granules of cytotoxic cells contain, in addition to granzymes, perforin, a potent pore-forming protein crucial for killing target cells. Similarly to the granzymes, perforin is also synthesized as an inactive form that needs to be processed proteolytically at the C-terminus for its activation [21]. In this process several peptidases are involved, among the cysteine cathepsins a role in perforin processing was demonstrated for cathepsin L [22].
The role of cystatins in the immune response
Cystatins constitute a protein superfamily with members present in all living organisms. The human cystatins can be grouped into three types, according to their structure. Type I cystatins contain approximately 100 amino-acids, with no disulphide bridges or glycosylation. They also lack signal peptides and are mainly cytosolic, but can be secreted. This group includes stefins A and B. Type II cystatins are synthesized containing signal peptides and are mainly secreted in the extracellular space. They comprise 120 amino-acids, contain at least two disulphide bridges and can be glycosylated. The members of this group are cystatins C, D, E/M, F, S, SA, SN, cystatins 11, 12, 13, 14, cystatin-related epididymal spermatogenic protein (CRES) and testatin. The most complex of the cystatins are type III cystatins, the kininogens. They are multifunctional proteins, containing three tandemly repeated type II-like cystatin domains and are present in blood and other body fluids, where they provide systemic protection from endogenous and exogenous cysteine peptidases [23]. Besides other functions they have been shown to act as prototypic danger signals inducing full-fledged maturation of dendritic cells [24].
Irrespective of their type, cystatins act mainly as reversible, tight-binding inhibitors of cysteine peptidases. They are predominantly inhibitors of cysteine peptidases from the C1 family, although some cystatins can inhibit peptidases from the C13 family, utilizing a second reactive site [25]. Cystatins are involved in the immune response by regulating several processes in which cysteine cathepsins are involved. Furthermore, they can inhibit pathogen-derived cysteine peptidases, thus strengthening the immune response against microbes [23]. In addition, cystatins can act independently of their inhibitory function [26].
Since cysteine cathepsins are well known for their involvement in antigen presentation, it is not surprising that the majority of studies have focused on the role of cystatins in the modulation of antigen presentation, especially in dendritic cells. The first cystatin shown to be implicated in antigen presentation was cystatin C. During maturation of dendritic cells, cystatin C expression and protein levels were found to decrease [27, 28]. In addition, different populations of mouse dendritic cells have different levels of cystatin C [29]. However, using dendritic cells from cystatin C-deficient mice, cystatin C was found not to be essential for regulating antigen presentation [29]. Another candidate proposed as being implicated in antigen presentation is cystatin F. Compared to other type II cystatins, it is present in significantly higher levels inside cells [30] and is targeted to the endosomal/lysosomal vesicles through the mannose-6-phosphate pathway [31], making it a likely candidate for direct regulation of cathepsin activity. Indeed, it was shown that cystatin F inhibits, at least in vitro, peptidases involved in antigen presentation, such as cathepsins S and V and legumain [25, 32, 33]. Accordingly, the localization of cystatin F was found to change during maturation of primary human dendritic cells; in immature dendritic cells it is strongly co-localized with cathepsin S whereas, during maturation, it is translocated to endosomes and lysosomes, remaining only partially co-localized with cathepsin S in mature dendritic cells. Conversely, cystatin F is not co-localized with cathepsin L in immature dendritic cells but only in mature, adherent dendritic cells [34]. By inhibiting cathepsin L, cystatin F can regulate the adhesion and maturation of dendritic cells through the processing of procathepsin X, as described above. Furthermore, in monocyte-derived dendritic cells stimulated with ligands for TLR3 (poly I:C) or TLR4 (LPS), cystatin F is strongly upregulated [27, 35] while, in the U937 cell line differentiated towards the granulocytic pathway by trans-retinoic acid or towards macrophages by phorbol ester cystatin F is downregulated [30]. Similarly to cystatins C and F, cystatin D was also found in antigen-presenting cells and, because of its preferential inhibition of cathepsin S, it was proposed to play a role in antigen presentation [36]. Another prominent role of cysteine cathepsins in the immune response is the activation of granzymes by cathepsins C and H, which is important in the cytotoxicity of NK and T cells. This process is regulated by cystatin F and will be explained below.
In addition, the importance of cystatins in infectious diseases has been underlined in several studies. Although the precise mechanism is yet to be elucidated, it has been shown that cystatins can modulate the activity of exogenous peptidases derived from pathogens and the pro-apoptotic reactions initiated by endogenous cysteine peptidases [8]. Stefin A is expressed selectively in cells associated with the first line immune response, such as skin epithelial cells [37] and polymorphonuclear granulocytes [38] and, probably, inhibits pathogens invading the skin. Stefin B has been suggested as playing a role in the response to bacterial infections, since it is upregulated in LPS-stimulated human monocytes [39]. The cystatins D, SN and SA, found in saliva and tears [40], inhibit cysteine peptidases from parasites such as cruzipain from Trypanosoma cruzi [41]. In viral infections, salivary cystatins were shown to suppress the infectivity of influenza A virus [42] and of herpes simplex virus 1 [43], while cystatin C was shown to inhibit the replication of herpes simplex virus [44]. Furthermore, cystatin F was demonstrated to be important for successful clearance of the nematode parasite Brugia malayi, since cystatin F null mice were unable to eliminate the infection [45]. In the same study, in a model of allergic lung inflammation, cystatin F was shown to be involved in eosinophil-mediated allergic pathology. Cystatin F was demonstrated to be a critical factor for the survival of eosinophils, for normal granule biogenesis and for eosinophil development [45].
Finally, cystatins are also associated with inflammation in several diseases. For example, in systemic lupus erythematosus, cystatin C was found to be associated with inflammation [46] and, in chronic obstructive pulmonary disease, it was shown to be an acute-phase reactant [47]. Biochemical markers of inflammatory processes accompanying lung disorders are associated with increased cystatin F levels in pleural effusions [48].
Regulation of cell cytotoxicity by cystatin F
Cytotoxic lymphocytes, namely CTLs and NK cells, are major cell effectors of anti-microbial and anti-tumour immune responses. Although these cells are activated by different signals, the mechanisms by which they kill target cells are the same. The key one is the perforin/granzyme pathway, where cytotoxic lymphocytes first recognize a virus-infected or a transformed cell as a target cell and release the content of their cytotoxic granules, also termed “secretory lysosomes” [49], into the lytic synapse between the killer cell and target cell. The most important cargo of the cytotoxic granules comprises perforin and granzymes. Perforin is a pore-forming protein, crucial for the penetration of granzymes into the target cells. Granzymes are serine peptidases that, once in the target cell, trigger apoptosis through activation of caspases, but can also trigger cell death in the absence of activated caspases [50–52]. In addition to perforin and granzymes, there are several other important granule constituents, among them cysteine cathepsins C, H and L [4].
The most important role of cysteine cathepsins in cytotoxic cells is to activate granzymes. As noted above, the main cathepsin in this process is cathepsin C, although cathepsin H is an additional convertase, at least for granzyme B. However, even in mice lacking both cathepsins C and H, the active granzyme B is present, implying considerable redundancy among the peptidases involved in this process [20]. Similarly to granzymes, perforin is also synthesized in an inactive form with a C-terminal propeptide that prevents oligomerization of perforin monomers [21]. In cytotoxic granules, perforin is activated by proteolytic removal of the C-terminal peptide [53]. Among the cysteine cathepsins, cathepsin L was shown to be involved in perforin activation [22]. However, even inhibition of the entire family of cysteine cathepsins with inhibitor E64 does not completely abolish perforin activation, suggesting again that multiple peptidases are involved in perforin processing [21, 53].
Cystatin F has been proposed to be the most important candidate for regulating the activity of cysteine cathepsins in cytotoxic lymphocytes. It is expressed specifically in immune cells such as neutrophils, eosinophils, mast cells, macrophages, dendritic cells, CTLs and NK cells [33, 54, 55]. It is synthesized as an inactive dimer and has to be converted to the monomeric form to inhibit cathepsins [32]. Furthermore, to gain the inhibitory function, cystatin F is cleaved at the N-terminus, supposedly by cathepsin V [56]. Cleavage facilitates conversion of the dimeric form into a monomeric one and also changes the inhibitory profile of the monomeric form. In fact, full-length monomeric cystatin F does not inhibit cathepsin C, while the N-terminally truncated monomeric form does [35]. Moreover, different forms of cystatin F are found at different cell localizations—the inactive dimeric form is localized in the Golgi apparatus, endoplasmic reticulum and extracellular fluids, while the monomeric form is localized in lysosomes and endosomes [31, 35]. Interestingly, cystatin F can also function in trans, meaning that the secreted inactive dimeric form can be internalized and activated in bystander cells [31]. Preferential expression in immune cells, existence of different forms with different inhibitory profiles, endosomal/lysosomal localization and the ability to function in trans are features that suggest an important regulatory role of cystatin F in the immune response. Indeed, in mouse CTLs, overexpression of cystatin F attenuates the activity of cathepsin C, and, moreover, it has been shown that cystatin F null CTLs can take up cystatin F secreted by wild-type CTLs, confirming its ability to function in trans. In addition, the internalized cystatin F decreased the activity of cathepsin C [31]. In human CD8 + T cell blasts, cystatin F was shown to co-localize with granzyme A, perforin and LAMP-1 [35]. Co-localization of cystatin F with cathepsin C in cytotoxic granules of CTLs has been demonstrated by our group (Fig. 1).
Fig. 1.
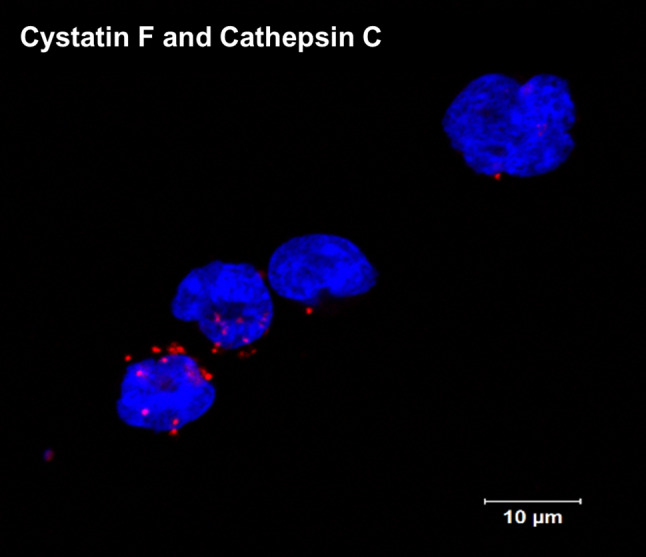
Co-localization/interaction of cystatin F and cathepsin C in TALL-104 cells determined by proximity ligation assay. Blue: DAPI stained nuclei; red: proximity ligation assay signals. The method was carried out as described in Soderberg and collaborators [57]. Images were taken on a Carl Zeiss LSM 710 confocal microscope. DAPI 4′,6′-Diamidino-2-phenylindole, LSM laser scanning microscope
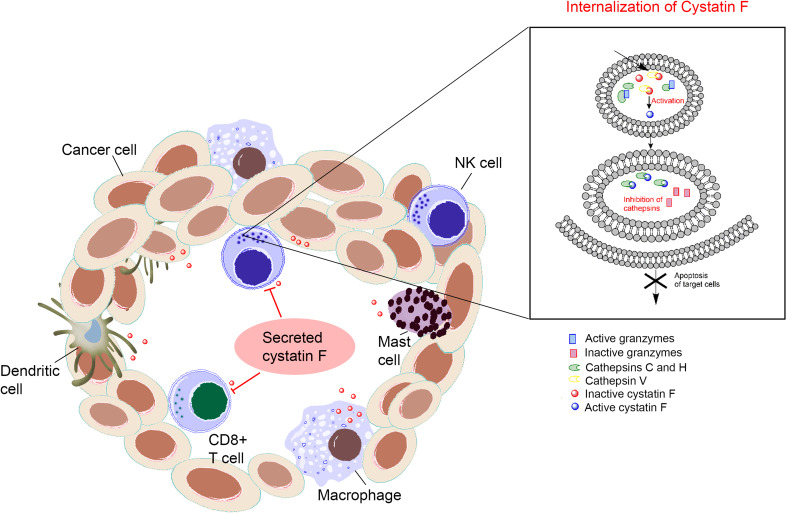
In NK cells, cystatin F expression is even higher than in CTLs [55]. In NK-92 cells, cystatin F co-localized with cathepsins C and H [58] and is N-terminally processed in both NK-92 cells and primary human NK cells [58]. It is thus not unexpected that increased levels of cystatin F in NK cells were associated with lower cytotoxicity in anergic NK cells [58]. NK cells become anergic following interaction with NK-sensitive targets and undergo functional and phenotypic changes that lead to lower cytotoxicity and increased cytokine secretion—the status called split anergy. These cells also display different functions: non-anergized NK cells are implicated in the selection of stem cells, while anergized NK cells are important for resolution of inflammation and for differentiation and regeneration of tissues [59]. As demonstrated by our group, anergized NK cells exhibit lower levels of cathepsin C, decreased processing of cathepsin C from the pro-form to the mature form, and lower levels of cathepsin H and granzyme B. In contrast, levels of the truncated monomeric form of cystatin F are significantly increased and consequently able to reduce the convertase activity of cathepsins C and H [58]. To test the hypothesis that cystatin F is a key regulator of granzyme activation, different mutants of cystatin F were prepared. They were analysed for cell uptake, inhibition of intracellular peptidases and changes in subcellular trafficking. Furthermore, their impact on the cytotoxicity of NK-92 cells and primary NK cells was evaluated [60]. In HeLa and Hek293 cells, the N-glycosylation pattern was found to direct the secretion, uptake and subcellular sorting of cystatin F. Besides dimeric cystatin F, active, N-terminally truncated monomeric form can also be internalized by recipient cells and targeted to endosomes/lysosomes, and may affect granzyme activation and cell cytotoxicity in cells with low contents of endogenous cystatin F or those lacking the cystatin F activating peptidase. The cystatin F mutants capable of entering the cells and the endosomal/lysosomal pathway, either by transfection or in trans by using conditioned media, significantly decreased cathepsin C and H activities in HeLa and Hek293 cells. Further, these mutants suppressed granule-mediated cytotoxicity in IL-2 stimulated NK-92 and primary NK cells. These results designate cystatin F as an important mediator that is used by tumour cells or bystander immune cells to impair NK cell cytotoxicity and to escape the anti-tumour immune response [61] (Fig. 2).
Fig. 2.
Increasing extracellular concentration of cystatin F negatively affects the granule-mediated cytotoxicity of NK cells and CTLs. Cystatin F originating from macrophages, mast cells, dendritic cells and/or tumour cells can be taken up by NK cells and CTLs and transported to secretory lysosomes where it is activated. In secretory lysosomes, cystatin F inhibits the cathepsins C and H needed for proteolytic activation of granzymes—the major cytotoxic mediators of NK cells and CTLs
Analysis of the promoter region of the cystatin F gene, CST7, showed that it is devoid of the elements typical of a house-keeping gene such as multiple Sp1 and GC-box elements. However, it has been shown that CST7 contains a unique C/EBP α binding site that might account for restricted expression in immune cells [30]. C/EBP α is a member of a family of transcription factors that promote the expression of certain genes by interaction with a specific cytosine–cytosine–adenosine–adenosine–thymidine (CCAAT) box motif, CCAAT enhancer-binding proteins (C/EBP) [62]. In unstimulated promonocytic U937 and promyeloblastic HL-60 cells, this transcription factor was shown to bind to the promotor region of cystatin F (Dautović et al., unpublished results), which strengthens the role of C/EBP α as a possible regulator of cystatin F expression.
Finally, high levels of cystatin F could, in addition, decrease the activity of other cathepsins such as cathepsin L and of legumain. It has been reported that NK cells from legumain null mice display lower cytotoxicity, implying an important role for legumain in cell cytotoxicity [63]. Cathepsin L has been shown to be involved in perforin processing and lower levels of active perforin could further impair the cytotoxic function of NK cells [22].
Conclusions
Cysteine cathepsins are involved in several immune processes, among them activation of granule serine peptidases, a mechanism essential for the activation of the cytotoxicity of NK and T cells. The mechanism is regulated by cystatin F, the only cystatin that is localized in endosomal/lysosomal vesicles and capable of direct regulation of the granzyme convertases cathepsins C and H. Cystatin F inhibitory activity depends on the rate of monomerization from the inactive dimeric form that is enhanced by proteolytic cleavage of the N-terminal peptide by the activating peptidase. As an inactive dimer, cystatin F can be secreted by bystander cells and internalized by cytotoxic lymphocytes, targeting cathepsins in endosomes/lysosomes and impairing their cytotoxic function. In NK cells we showed that increased levels of cystatin F correlate well with split anergy, a status of NK cells characterized by loss of cytotoxicity and associated with failure of the anti-tumour immune response. Moreover, it was demonstrated that the level of cystatin F is higher in tumour tissue of patients with colorectal cancer and that these levels correlate with liver metastasis and a poorer prognosis [64]. Likewise, in patients with glioma, the association between cystatin F expression and worse prognosis has also been reported (Cancer Genome Atlas of Glioblastoma Multiforme and Low Grade Glioma Data Collection, http://gliovis.bioinfo.cnio.es).
The function of cystatin F could be regulated in several ways. Modification of its glycosylation profile could alter its cell trafficking and/or prevent its internalization. Additionally, regulation of transcription factors could decrease its expression. Finally, its activation and monomerization could be prevented by targeting the activating peptidase. The opportunity for precise regulation of cystatin F activity makes it a promising target for improving cancer immunotherapy.
Acknowledgements
We are grateful to Professor Roger H. Pain for critical reading of the manuscript.
Abbreviations
- C/EBP
CCAAT/enhancer-binding protein
- CCAAT box motif
Cytosine–cytosine–adenosine–adenosine–thymidine box motif
- CRES
Cystatin-related epididymal spermatogenic protein
- CTLs
Cytotoxic T-lymphocytes
- E64
N-[N-(l-3-trans-Carboxyirane-2-carbonyl)-l-leucyl]-agmatine
- LAMP-1
Lysosomal-associated membrane protein 1
- LPS
Lipopolysaccharide
- Mac-1
Macrophage-1 antigen
- MHC
Major histocompatibility complex
- NK cells
Natural killer cells
- poly I:C
Polyinosinic:polycytidylic acid
- RGD motif
Arginyl-glycyl-aspartic acid motif
- TLR
Toll-like receptors
Author contributions
MP, MPN and ED drafted the manuscript. MPN and MP designed the figures. JK finalized the manuscript in consultation with JS and AJ.
Funding
This work was supported by the Grants P4-0127 and J4 6811 from the Research Agency of the Republic of Slovenia (to Janko Kos).
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Barrett AJ, Rawlings, Woessner JF, editors. Handbook of proteolytic enzymes. 3. San Diego: Academic; 2012. [Google Scholar]
- 2.Turk B, Turk D, Turk V. Protease signalling: the cutting edge. EMBO J. 2012;31(7):1630–1643. doi: 10.1038/emboj.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colbert JD, Matthews SP, Miller G, Watts C. Diverse regulatory roles for lysosomal proteases in the immune response. Eur J Immunol. 2009;39(11):2955–2965. doi: 10.1002/eji.200939650. [DOI] [PubMed] [Google Scholar]
- 4.Perisic Nanut M, Sabotic J, Jewett A, Kos J. Cysteine cathepsins as regulators of the cytotoxicity of NK and T cells. Front Immunol. 2014;5:616. doi: 10.3389/fimmu.2014.00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prunk M, Nanut MP, Sabotic J, Kos J. Cystatins, cysteine peptidase inhibitors, as regulators of immune cell cytotoxicity. Period Biol. 2016;118:353–362. doi: 10.18054/pb.v118i4.4504. [DOI] [Google Scholar]
- 6.Rawlings ND, Waller M, Barrett AJ, Bateman A. MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2014;42(Database issue):D503-509. doi: 10.1093/nar/gkt953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turk V, Turk B, Turk D. Lysosomal cysteine proteases: facts and opportunities. EMBO J. 2001;20(17):4629–4633. doi: 10.1093/emboj/20.17.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bird PI, Trapani JA, Villadangos JA. Endolysosomal proteases and their inhibitors in immunity. Nat Rev Immunol. 2009;9(12):871–882. doi: 10.1038/nri2671. [DOI] [PubMed] [Google Scholar]
- 9.Asagiri M, Hirai T, Kunigami T, Kamano S, Gober HJ, Okamoto K, Nishikawa K, Latz E, Golenbock DT, Aoki K, Ohya K, Imai Y, Morishita Y, Miyazono K, Kato S, Saftig P, Takayanagi H. Cathepsin K-dependent toll-like receptor 9 signaling revealed in experimental arthritis. Science. 2008;319(5863):624–627. doi: 10.1126/science.1150110. [DOI] [PubMed] [Google Scholar]
- 10.Ewald SE, Engel A, Lee J, Wang M, Bogyo M, Barton GM. Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. J Exp Med. 2011;208(4):643–651. doi: 10.1084/jem.20100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohashi K, Naruto M, Nakaki T, Sano E. Identification of interleukin-8 converting enzyme as cathepsin L. Biochim Biophys Acta. 2003;1649(1):30–39. doi: 10.1016/S1570-9639(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 12.Fiebiger E, Meraner P, Weber E, Fang IF, Stingl G, Ploegh H, Maurer D. Cytokines regulate proteolysis in major histocompatibility complex class II-dependent antigen presentation by dendritic cells. J Exp Med. 2001;193(8):881–892. doi: 10.1084/jem.193.8.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kos J, Vizin T, Fonovic UP, Pislar A. Intracellular signaling by cathepsin X: molecular mechanisms and diagnostic and therapeutic opportunities in cancer. Semin Cancer Biol. 2015;31:76–83. doi: 10.1016/j.semcancer.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Lechner AM, Assfalg-Machleidt I, Zahler S, Stoeckelhuber M, Machleidt W, Jochum M, Nagler DK. RGD-dependent binding of procathepsin X to integrin alphavbeta3 mediates cell-adhesive properties. J Biol Chem. 2006;281(51):39588–39597. doi: 10.1074/jbc.M513439200. [DOI] [PubMed] [Google Scholar]
- 15.Obermajer N, Svajger U, Bogyo M, Jeras M, Kos J. Maturation of dendritic cells depends on proteolytic cleavage by cathepsin X. J Leukoc Biol. 2008;84(5):1306–1315. doi: 10.1189/jlb.0508285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obermajer N, Premzl A, Zavasnik Bergant T, Turk B, Kos J. Carboxypeptidase cathepsin X mediates beta2-integrin-dependent adhesion of differentiated U-937 cells. Exp Cell Res. 2006;312(13):2515–2527. doi: 10.1016/j.yexcr.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Jevnikar Z, Obermajer N, Bogyo M, Kos J. The role of cathepsin X in the migration and invasiveness of T lymphocytes. J Cell Sci. 2008;121(Pt 16):2652–2661. doi: 10.1242/jcs.023721. [DOI] [PubMed] [Google Scholar]
- 18.Sutton VR, Waterhouse NJ, Browne KA, Sedelies K, Ciccone A, Anthony D, Koskinen A, Mullbacher A, Trapani JA. Residual active granzyme B in cathepsin C-null lymphocytes is sufficient for perforin-dependent target cell apoptosis. J Cell Biol. 2007;176(4):425–433. doi: 10.1083/jcb.200609077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pham CT, Ivanovich JL, Raptis SZ, Zehnbauer B, Ley TJ. Papillon-Lefevre syndrome: correlating the molecular, cellular, and clinical consequences of cathepsin C/dipeptidyl peptidase I deficiency in humans. J Immunol. 2004;173(12):7277–7281. doi: 10.4049/jimmunol.173.12.7277. [DOI] [PubMed] [Google Scholar]
- 20.D’Angelo ME, Bird PI, Peters C, Reinheckel T, Trapani JA, Sutton VR. Cathepsin H is an additional convertase of pro-granzyme B. J Biol Chem. 2010;285(27):20514–20519. doi: 10.1074/jbc.M109.094573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.House IG, House CM, Brennan AJ, Gilan O, Dawson MA, Whisstock JC, Law RH, Trapani JA, Voskoboinik I. Regulation of perforin activation and pre-synaptic toxicity through C-terminal glycosylation. EMBO Rep. 2017;18(10):1775–1785. doi: 10.15252/embr.201744351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konjar S, Sutton VR, Hoves S, Repnik U, Yagita H, Reinheckel T, Peters C, Turk V, Turk B, Trapani JA, Kopitar-Jerala N. Human and mouse perforin are processed in part through cleavage by the lysosomal cysteine proteinase cathepsin L. Immunology. 2010;131(2):257–267. doi: 10.1111/j.1365-2567.2010.03299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zavasnik-Bergant T. Cystatin protease inhibitors and immune functions. Front Biosci. 2008;13:4625–4637. doi: 10.2741/3028. [DOI] [PubMed] [Google Scholar]
- 24.Scharfstein J, Schmitz V, Svensjo E, Granato A, Monteiro AC. Kininogens coordinate adaptive immunity through the proteolytic release of bradykinin, an endogenous danger signal driving dendritic cell maturation. Scand J Immunol. 2007;66(2–3):128–136. doi: 10.1111/j.1365-3083.2007.01983.x. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez-Fernandez M, Barrett AJ, Gerhartz B, Dando PM, Ni J, Abrahamson M. Inhibition of mammalian legumain by some cystatins is due to a novel second reactive site. J Biol Chem. 1999;274(27):19195–19203. doi: 10.1074/jbc.274.27.19195. [DOI] [PubMed] [Google Scholar]
- 26.Sokol JP, Schiemann WP. Cystatin C antagonizes transforming growth factor beta signaling in normal and cancer cells. Mol Cancer Res. 2004;2(3):183–195. [PubMed] [Google Scholar]
- 27.Hashimoto SI, Suzuki T, Nagai S, Yamashita T, Toyoda N, Matsushima K. Identification of genes specifically expressed in human activated and mature dendritic cells through serial analysis of gene expression. Blood. 2000;96(6):2206–2214. [PubMed] [Google Scholar]
- 28.Zavasnik-Bergant T, Repnik U, Schweiger A, Romih R, Jeras M, Turk V, Kos J. Differentiation- and maturation-dependent content, localization, and secretion of cystatin C in human dendritic cells. J Leukoc Biol. 2005;78(1):122–134. doi: 10.1189/jlb.0804451. [DOI] [PubMed] [Google Scholar]
- 29.El-Sukkari D, Wilson NS, Hakansson K, Steptoe RJ, Grubb A, Shortman K, Villadangos JA. The protease inhibitor cystatin C is differentially expressed among dendritic cell populations, but does not control antigen presentation. J Immunol. 2003;171:5003–5011. doi: 10.4049/jimmunol.171.10.5003. [DOI] [PubMed] [Google Scholar]
- 30.Nathanson CM, Wasselius J, Wallin H, Abrahamson M. Regulated expression and intracellular localization of cystatin F in human U937 cells. Eur J Biochem. 2002;269(22):5502–5511. doi: 10.1046/j.1432-1033.2002.03252.x. [DOI] [PubMed] [Google Scholar]
- 31.Colbert JD, Plechanovova A, Watts C. Glycosylation directs targeting and activation of cystatin f from intracellular and extracellular sources. Traffic. 2009;10(4):425–437. doi: 10.1111/j.1600-0854.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langerholc T, Zavasnik-Bergant V, Turk B, Turk V, Abrahamson M, Kos J. Inhibitory properties of cystatin F and its localization in U937 promonocyte cells. FEBS J. 2005;272(6):1535–1545. doi: 10.1111/j.1742-4658.2005.04594.x. [DOI] [PubMed] [Google Scholar]
- 33.Ni J, Fernandez MA, Danielsson L, Chillakuru RA, Zhang J, Grubb A, Su J, Gentz R, Abrahamson M. Cystatin F is a glycosylated human low molecular weight cysteine proteinase inhibitor. J Biol Chem. 1998;273(38):24797–24804. doi: 10.1074/jbc.273.38.24797. [DOI] [PubMed] [Google Scholar]
- 34.Magister S, Obermajer N, Mirkovic B, Svajger U, Renko M, Softic A, Romih R, Colbert JD, Watts C, Kos J. Regulation of cathepsins S and L by cystatin F during maturation of dendritic cells. Eur J Cell Biol. 2012;91(5):391–401. doi: 10.1016/j.ejcb.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton G, Colbert JD, Schuettelkopf AW, Watts C. Cystatin F is a cathepsin C-directed protease inhibitor regulated by proteolysis. EMBO J. 2008;27(3):499–508. doi: 10.1038/sj.emboj.7601979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvarez-Fernandez M, Liang YH, Abrahamson M, Su XD. Crystal structure of human cystatin D, a cysteine peptidase inhibitor with restricted inhibition profile. J Biol Chem. 2005;280(18):18221–18228. doi: 10.1074/jbc.M411914200. [DOI] [PubMed] [Google Scholar]
- 37.Rasanen O, Jarvinen M, Rinne A. Localization of the human SH-protease inhibitor in the epidermis. Immunofluorescent studies. Acta Histochem. 1978;63(2):193–196. doi: 10.1016/S0065-1281(78)80025-7. [DOI] [PubMed] [Google Scholar]
- 38.Brzin J, Kopitar M, Turk V, Machleidt W. Protein inhibitors of cysteine proteinases.1. Isolation and characterization of stefin, a cytosolic protein inhibitor of cysteine proteinases from human polymorphonuclear granulocytes. Hoppe Seylers Z Physiol Chem. 1983;364:1475–1480. doi: 10.1515/bchm2.1983.364.2.1475. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki T, Hashimoto S-i, Toyoda N, Nagai S, Yamazaki N, Dong H-Y, Sakai J, Yamashita T, Nukiwa T, Matsushima K. Comprehensive gene expression profile of LPS-stimulated human monocytes by SAGE. Blood. 2000;96:2584–2591. [PubMed] [Google Scholar]
- 40.Freije JP, Balbin M, Abrahamson M, Velasco G, Dalboge H, Grubb A, Lopez-Otin C. Human cystatin D. cDNA cloning, characterization of the Escherichia coli expressed inhibitor, and identification of the native protein in saliva. J Biol Chem. 1993;268(21):15737–15744. [PubMed] [Google Scholar]
- 41.Stoka V, Nycander M, Lenarcic B, Labriola C, Cazzulo JJ, Bjork I, Turk V. Inhibition of cruzipain, the major cysteine proteinase of the protozoan parasite, Trypanosoma cruzi, by proteinase inhibitors of the cystatin superfamily. FEBS Lett. 1995;370(1–2):101–104. doi: 10.1016/0014-5793(95)00798-E. [DOI] [PubMed] [Google Scholar]
- 42.Teran LM, Ruggeberg S, Santiago J, Fuentes-Arenas F, Hernandez JL, Montes-Vizuet AR, Xinping L, Franz T. Immune response to seasonal influenza A virus infection: a proteomic approach. Arch Med Res. 2012;43(6):464–469. doi: 10.1016/j.arcmed.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Gu M, Haraszthy GG, Collins AR, Bergey EJ. Identification of salivary proteins inhibiting herpes simplex virus 1 replication. Oral Microbiol Immun. 1995;10:54–59. doi: 10.1111/j.1399-302X.1995.tb00118.x. [DOI] [PubMed] [Google Scholar]
- 44.Bjorck L, Grubb A, Kjellen L. Cystatin C, a human proteinase inhibitor, blocks replication of herpes simplex virus. J Virol. 1990;64(2):941–943. doi: 10.1128/jvi.64.2.941-943.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matthews SP, McMillan SJ, Colbert JD, Lawrence RA, Watts C. Cystatin F ensures eosinophil survival by regulating granule biogenesis. Immunity. 2016;44(4):795–806. doi: 10.1016/j.immuni.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lertnawapan R, Bian A, Rho YH, Raggi P, Oeser A, Solus JF, Gebretsadik T, Shintani A, Stein CM. Cystatin C is associated with inflammation but not atherosclerosis in systemic lupus erythematosus. Lupus. 2012;21(3):279–287. doi: 10.1177/0961203311425527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang M, Li Y, Yang X, Shan H, Zhang Q, Ming Z, Xie Y, Chen H, Liu Y, Zhang J. Serum cystatin C as an inflammatory marker in exacerbated and convalescent COPD patients. Inflammation. 2016;39(2):625–631. doi: 10.1007/s10753-015-0287-x. [DOI] [PubMed] [Google Scholar]
- 48.Werle B, Sauckel K, Nathanson CM, Bjarnadottir M, Spiess E, Ebert W, Abrahamson M. Cystatins C, E/M and F in human pleural fluids of patients with neoplastic and inflammatory lung disorders. Biol Chem. 2003;384(2):281–287. doi: 10.1515/BC.2003.031. [DOI] [PubMed] [Google Scholar]
- 49.Page LJ, Darmon AJ, Uellner R, Griffiths GM. L is for lytic granules: lysosomes that kill. Biochim Biophys Acta. 1998;1401(2):146–156. doi: 10.1016/S0167-4889(97)00138-9. [DOI] [PubMed] [Google Scholar]
- 50.Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2(10):735–747. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- 51.Andersen MH, Schrama D, Thor Straten P, Becker JC. Cytotoxic T cells. J Invest Dermatol. 2006;126(1):32–41. doi: 10.1038/sj.jid.5700001. [DOI] [PubMed] [Google Scholar]
- 52.Chowdhury D, Lieberman J. Death by a thousand cuts: granzyme pathways of programmed cell death. Annu Rev Immunol. 2008;26:389–420. doi: 10.1146/annurev.immunol.26.021607.090404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uellner R, Zvelebil MF, Hopkins J, Jones J, MacDougall LK, Griffiths GM, Morgan BP, Podack E, Waterfield MD. Perforin is activated by a proteolytic cleavage during biosynthesis which reveals a phospholipid-binding C2 domain. EMBO J. 1997;16(24):7287–7296. doi: 10.1093/emboj/16.24.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halfon S, Ford J, Foster J, Dowling L, Lucian L, Sterling M, Xu Y, Weiss M, Ikeda M, Liggett D, Helms A, Caux C, Lebecque S, Hannum C, Menon S, McClanahan T, Gorman D, Zurawski G. Leukocystatin, a new Class II cystatin expressed selectively by hematopoietic cells. J Biol Chem. 1998;273(26):16400–16408. doi: 10.1074/jbc.273.26.16400. [DOI] [PubMed] [Google Scholar]
- 55.Obata-Onai A, Hashimoto S, Onai N, Kurachi M, Nagai S, Shizuno K, Nagahata T, Mathushima K. Comprehensive gene expression analysis of human NK cells and CD8(+) T lymphocytes. Int Immunol. 2002;14:1085–1098. doi: 10.1093/intimm/dxf086. [DOI] [PubMed] [Google Scholar]
- 56.Maher K, Konjar S, Watts C, Turk B, Kopitar-Jerala N. Cystatin F regulates proteinase activity in IL-2-activated natural killer cells. Protein Pept Lett. 2014;21(9):957–965. doi: 10.2174/0929866521666140403124146. [DOI] [PubMed] [Google Scholar]
- 57.Soderberg O, Leuchowius KJ, Gullberg M, Jarvius M, Weibrecht I, Larsson LG, Landegren U. Characterizing proteins and their interactions in cells and tissues using the in situ proximity ligation assay. Methods. 2008;45(3):227–232. doi: 10.1016/j.ymeth.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 58.Magister S, Tseng HC, Bui VT, Kos J, Jewett A. Regulation of split anergy in natural killer cells by inhibition of cathepsins C and H and cystatin F. Oncotarget. 2015;6(26):22310–22327. doi: 10.18632/oncotarget.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jewett A, Man YG, Cacalano N, Kos J, Tseng HC. Natural killer cells as effectors of selection and differentiation of stem cells: role in resolution of inflammation. J Immunotoxicol. 2014;11(4):297–307. doi: 10.3109/1547691X.2013.877104. [DOI] [PubMed] [Google Scholar]
- 60.Perisic Nanut M, Sabotic J, Svajger U, Jewett A, Kos J. Cystatin F affects natural killer cell cytotoxicity. Front Immunol. 2017;8:1459. doi: 10.3389/fimmu.2017.01459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kos J, Perisic Nanut M, Prunk M, Sabotic J, Jakoš T, Jewett A. Tumor cell derived cystatin F as mediator of NK and T cell cytotoxicity. Presented at 42nd Congress of the Federation of European Biochemical Societies (FEBS), “From Molecules to Cells and Back” Jerusalem, Israel; September 10–14, 2017. FEBS J. 2017;284:4.1–051. doi: 10.1111/febs.14174. [DOI] [Google Scholar]
- 62.Poli V, Mancini FP, Cortese R. IL-6DBP, a nuclear protein involved in interleukin-6 signal transduction, defines a new family of leucine zipper proteins related to C/EBP. Cell. 1990;63(3):643–653. doi: 10.1016/0092-8674(90)90459-R. [DOI] [PubMed] [Google Scholar]
- 63.Chan CB, Abe M, Hashimoto N, Hao C, Williams IR, Liu X, Nakao S, Yamamoto A, Zheng C, Henter JI, Meeths M, Nordenskjold M, Li SY, Hara-Nishimura I, Asano M, Ye K. Mice lacking asparaginyl endopeptidase develop disorders resembling hemophagocytic syndrome. Proc Natl Acad Sci USA. 2009;106(2):468–473. doi: 10.1073/pnas.0809824105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Utsunomiya T, Hara Y, Kataoka A, Morita M, Arakawa H, Mori M, Nishimura S. Cystatin-like metastasis-associated protein mRNA expression in human colorectal cancer is associated with both liver metastasis and patient survival. Clin Cancer Res. 2002;8(8):2591–2594. [PubMed] [Google Scholar]