Abstract
Background
PD-1 antibodies (PD1ab) are increasingly used in metastatic melanoma and other malignancies. Arthralgia is an underestimated side effect of PD-1 antibody treatment with unknown cause. Our aim was to characterize PD1ab-induced arthralgia.
Patients and methods
We retrospectively included patients with metastatic cutaneous malignancies treated with pembrolizumab or nivolumab ± ipilimumab at the National Center for Tumor Diseases (Heidelberg) between 01/2013 and 09/2016. Arthralgia was characterized by laboratory diagnostics, imaging, and if indicated, rheumatologic consultation.
Results
26 of 195 patients (13.3%) developed arthralgia. The median onset of symptoms was 100 days (7–780 days). Most frequently, arthralgia involved large joints (shoulders, knees) in a predominantly symmetrical pattern. Only two patients were seropositive for rheumatoid factor and/or anti-citrullinated protein antibodies. Ten patients developed the clinical picture of arthritis, with seven of them showing synovitis in MRI or PET/CT. Five patients showed inflammation in joints pre-damaged by osteoarthritis. In 11 patients arthralgia could not be specified. The majority of patients was satisfactorily treated with non-steroidal anti-inflammatory drugs (NSAIDs), 23.1% required additional low-dose corticosteroids and only 7.6% of our patients received further immunosuppressive treatment. Patients with arthralgia showed a better treatment response and improved PFS and OS.
Conclusion
Arthralgia is frequent during PD1ab treatment. The clinical picture varies between synovitis of predominantly large joints, progressive osteoarthritis and arthralgia without evident joint damage. Vast majority of cases can be satisfactorily managed by NSAID and/or low-dose corticosteroids.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-017-2069-9) contains supplementary material, which is available to authorized users.
Keywords: PD-1 antibody, Pembrolizumab, Nivolumab, Arthralgia, Arthritis, Melanoma
Introduction
PD-1 antibodies (PD1ab) are increasingly used to treat advanced malignancies. Even though well tolerated in general immune related adverse events (irAE) such as exanthema, colitis and hepatitis are frequent. Arthralgia is reported with a frequency of 5–12% under treatment with pembrolizumab or nivolumab in metastatic melanoma [1, 2]. In contrast, only 5–6% of patients reported arthralgia under ipilimumab monotherapy [1, 3]. To date, the pathomechanisms of arthralgia under PD1ab is not understood and this symptom was not listed among the irAEs in previous studies [4]. Moreover, arthralgia might be reported under different terms such as “arthralgia“, “arthritis”, “back pain”, “bone pain”, “joint effusion”, “joint—range of motion decreased”, “pain in extremity” and “pain” according to common terminology criteria for adverse events (CTC-AE) v4.03 [5]. Hence, the CTC-AE grading system possibly underestimates this symptom and is not adapted to classify rheumatologic side effects [6]. So far, rheumatologic side effects have been described only in small number of PD1ab treated patients [7, 8] but not yet in larger cohorts. Therefore, we aimed to characterize arthralgia in patients on PD1ab treatment.
Materials and methods
Patients
This retrospective analysis systematically included patients treated at the National Center for Tumor Diseases (NCT) Heidelberg between 01/2013 and 09/2016 for metastatic melanoma or other advanced cutaneous malignancies with the PD1abs pembrolizumab or nivolumab ± ipilimumab in the approved doses. At each visit, patients underwent systematic pain assessment by an institutional questionnaire (including pain intensity by visual analogue scale, pain character, and pain localization by illustration, pain medication, and other symptomatic treatment). In case of new onset of “joint pain”, the treating physician proceeded with further work-up including personal and family history for rheumatic diseases, clinical examination of joints, and laboratory tests including rheumatoid factor (RF), anti-citrullinated peptide antibodies (ACPA), antinuclear antibodies (ANA), and, if applicable, HLA-B27. Routine tumour assessments were performed every 12 weeks using CT of the neck/chest/abdomen or whole body FDG-PET/CT scan plus MRI of the brain. Depending on the nature of joint symptoms, patients received additional joint imaging by X-ray or MRI. In case of severe joint symptoms rheumatologic or orthopaedic consultations were arranged.
Upon retrospective review of imaging data, the presence of synovitis was defined radiologically if MRI showed synovial thickening, synovial edema or synovial hyperenhancement and/or FDG-PET/CT displayed increased glucose uptake. Osteoarthritic pre-damage was diagnosed if the following criteria were present in imaging (X-ray, CT or MRI): joint space narrowing, subchondral sclerosis, and osteophytosis.
Data collection
Patients with new onset of arthralgia during PD1ab therapy were included in our analysis. Patients were eligible if they had received at least one infusion of PD1ab before July 9, 2016. From a total of 220 patients, patients with concurrent BRAF inhibitor therapy were excluded from our analysis (n = 7) as well as patients who were not evaluable for side effects due to rapid deterioration of general status (n = 18). Furthermore, we excluded patients with joint pain due to metastatic disease (n = 4), and patients with a known medical history of rheumatic disease (n = 2). The detailed patient selection process is displayed in Supplementary Figure 1. Final follow-up was completed on October 20, 2016. Best response to treatment was defined according to RECIST criteria version 1.1 [9] and indicated as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). All available joint imaging was reviewed by a radiologist (T. F. Weber), nuclear medicine physicians (A. Dimitrakopoulou-Strauss, H. Anwar) and by rheumatologists (K. Benesova, H.-M. Lorenz). All cases with medical history, clinical findings, laboratory results and imaging were reviewed by rheumatologists (K. Benesova, H.-M. Lorenz), and dermatooncologists (K. Buder-Bakhaya, J. C. Hassel).
Statistical analyses
Statistical analysis was conducted using SPSS version 22 (IBM, Ehningen, Germany). Progression-free survival (PFS) and overall survival (OS) were calculated as time from onset of PD1ab treatment until progression or death from any cause, respectively. In patients with no events of progression or death at time of final data analysis, the date of last contact was used for censored calculation. Survival was estimated by the Kaplan–Meier method. Delayed onset of joint symptoms bears the risk of a guarantee-time bias wherefore a landmark analysis was performed to assess the effect of arthralgia on PFS and OS. Univariate comparisons of Kaplan–Meier estimators were done with the log-rank test. Comparisons among groups with categorical variables were assessed by two-sided Fisher’s exact and Chi-square test. p values were considered significant with values of p < 0.05.
Results
Patient characteristics
26 of 195 patients (13.3%) reported arthralgia as a new symptom. Detailed patient characteristics of “total cohort” and “arthralgia cohort” are displayed in Table 1. Patients characteristics did not differ significantly with the exception of longer median treatment duration in the arthralgia cohort (314 versus 104 days, p < 0.001). Of note, irAEs other than arthralgia occurred with similar frequency in patients with arthralgia and in the total cohort.
Table 1.
Patient characteristics of the total cohort and the arthralgia cohort.*
| Parameter | Number of patients in total cohort (%) | Number of patients in arthralgia cohort (%) | ||
|---|---|---|---|---|
| Total number of patients | 195 | (100%) | 26 | (13.3%) |
| Age (in years), median [range] | 63.9 | [17–91] | 65.6 | [46–82] |
| Gender | ||||
| Male | 106 | (54.4) | 15 | (57.7) |
| Female | 89 | (45.6) | 11 | (42.3) |
| Disease entity | ||||
| Cutaneous melanoma | 164 | (84.1) | 22 | (84.6) |
| Mucosal melanoma | 12 | (6.2) | 2 | (7.7) |
| Uveal melanoma | 9 | (4.6) | 1 | (3.8) |
| Merkel cell carcinoma | 4 | (2.1) | 1 | (3.8) |
| Squamous cell carcinoma | 3 | (1.5) | 0 | |
| Basal cell carcinoma | 3 | (1.5) | 0 | |
| Type of PD-1 antibody | ||||
| Pembrolizumab | 111 | (56.9) | 17 | (65.4) |
| Nivolumab | 48 | (24.6) | 6 | (23.1) |
| Nivolumab + ipilimumab | 36 | (18.5) | 3 | (11.5) |
| Duration of treatment (in days), median [range] | 104 | [1–1168] | 314 | [75–1168] |
| Number of prior treatments | ||||
| 0 | 59 | (30.3) | 13 | (50.0) |
| 1 | 77 | (39.5) | 4 | (15.4) |
| 2 | 28 | (14.4) | 4 | (15.4) |
| ≥3 | 36 | (16.9) | 5 | (19.2) |
| Types of previous treatment | ||||
| Ipilimumab | 103 | (52.8) | 12 | (46.2) |
| PD-1 antibody | 27 | (13.8) | 1 | (3.9) |
| Targeted therapy | 44 | (22.6) | 5 | (19.2) |
| Chemotherapy | 38 | (19.5) | 7 | (26.9) |
| irAEs other than arthralgia | 51 | (26.2) | 7 | (26.9) |
| Treatment discontinued due to irAEs | 20 | (10.3) | 2 | (7.7) |
| Follow-up (in days), median [range] | 306 | [25–1194] | 374 | [129–1057] |
irAE immune-related adverse events
* There were no significant between-group differences with exception of treatment duration (p < 0.001) and follow-up (p < 0.01)
Characterization of arthralgia
17 out of 26 patients (65.4%) reported CTC-AE grade 1, 9 patients (34.6%) grade 2 arthralgia; nothing more severe occurred [5]. Median onset of symptoms was 100 days (range 7–780 days) with an earlier onset in patients with simultaneous nivolumab plus ipilimumab therapy (n = 3, onset after 7, 14 and 244 days which was after 1 cycle in 2 patients and after 4 cycles of combination followed by 10 cycles of nivolumab monotherapy in 1 patient) compared to a later onset of arthralgia in PD1ab monotherapy (median onset 116 days [range 11–780]) with a median onset after 5 cycles of pembrolizumab, and 8 cycles of nivolumab, respectively.
Most frequently involved joints were shoulders (61.5%), knees (50%), foot joints (42.3%), and wrists (38.5%). Less frequently affected were finger joints (26.9%), spine (19.2%), elbows (15.4%), and hips (11.5%). 19 patients showed symptoms in large joints only (73.1%) whereas 7 patients (26.9%) had both large and small joints (MCP, PIP, DIP) involved. No patient had an isolated involvement of small joints. In the majority of patients arthralgia occurred symmetrically (16 of 26 patients, 62%). Median number of involved joints was 4 (range 1–10), joint swelling was found in 10 of 26 patients (38.5%). RF was positive in 2 patients (7.7%) with one simultaneously being positive for ACPA. 6 patients underwent rheumatologic or orthopaedic consultation because of severity of symptoms. In two patients a synovial fluid analysis was performed because of severe knee effusions showing a clear, sterile effusion with lymphocytes and neutrophils without crystals.
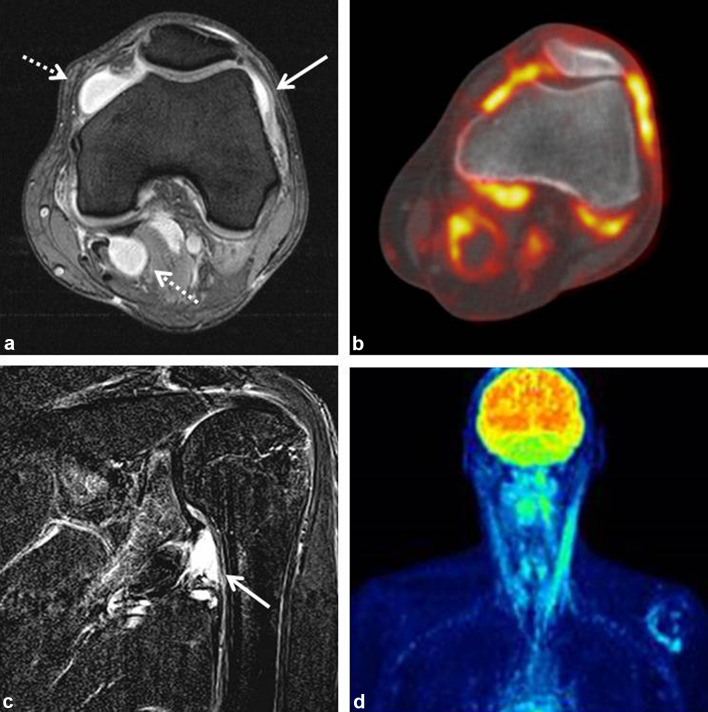
After review of clinical and imaging data, two different clinical patterns could be delineated: (1) patients with imaging signs of synovitis in joints pre-damaged by OA named “activated OA” group (n = 5) and (2) patients with signs of synovitis (without signs of OA) and/or clinical signs of arthritis (joint swelling, decreased range of motion) named “arthritis” group (n = 10). Patients who could not be classified into these two groups because clinical and/or imaging data were not typical for one of the groups or imaging was not done were subsumed as “arthralgia, not specified” (n = 11). One major difference between the groups was that patients with activated OA revealed symptoms in only a few joints whereas arthritis patients more often had involvement of 5 or more joints (p < 0.05). In addition, arthritis patients were younger than OA patients (p < 0.05), had more often a positive family history for rheumatologic diseases, and a higher incidence of other irAEs. Detailed features of these patient groups are displayed in Table 2. 70% of the arthritis patients showed radiological disease manifestations in MRI including thickening, edema, or hyperenhancement of synovial tissue and joint effusion (Fig. 1a, c) and/or PET-CT displaying increased FDG-uptake by synovial tissue (Fig. 1b, d).
Table 2.
Clinical, serological and imaging characteristics of the different arthralgia patterns in our cohort of 26 patients
| Arthritis | Activated osteoarthritis | Arthralgia (not specified) | Total number of patients with available data | |
|---|---|---|---|---|
| Total number of patients | 10 (38.5%) | 5 (19.2%) | 11 (42.3%) | 26 |
| Median age, male/female (% female patients) | 56 years, 6/4 (40%) | 75 years, 1/4 (80%) | 68 years, 8/3 (27%) | 26 |
| Number of patients with < 5 involved joints | 5 (50%) | 5 (100%) | 6 (54.6%) | 26 |
| Number of patients with ≥ 5 involved joints | 5 (50%) | 0 | 5 (45.4%) | 26 |
| Only large joints involved | 6 (60%) | 3 (60%) | 10 (90.9%) | 26 |
| Large and small joints involved | 4 (40%) | 2 (40%) | 1 (9.1%) | 26 |
| Positive family history of joint- or joint related diseases |
3/7 (42.9%) 1 RA, 2 psoriasis |
1/4 (25%) 1 OA |
3/6 (50%) 2 RA, 1 psoriasis |
17 |
| Rheumatoid factor positive | 1/7 (14.3%) | 0/3 (0%) | 1/8 (12.5%) | 18 |
| ACPA positive | 1/7 (14.3%) | 0/3 (0%) | 0/7 (0%) | 17 |
| Elevated ANA titer with specific pattern | 0/6 (0%) | 1/3 (33.3%)* | 0/7 (0%) | 16 |
| HLA-B27 positive | 2/7 (28.6%) | 1/4 (25%) | 0/7 (0%) | 18 |
| MRI showed synovitis | 2/3 (66.7%) | 2/2 (100%) | 0/2 (0%) | 7 |
| PET showed FDG-uptake in joints | 5/5 (100%) | NA | 0/1 (0%) | 6 |
| Other irAE | 4 (40%) | 1 (20%) | 2 (18.2%) | 26 |
| Treatment | ||||
| NSAIDs only | 6/10 (60%) | 4/5 (80%) | 9/11 (81.8%) | 26 |
| Low-dose prednisolone | 4/10 (40%) | 0/5 (0%) | 1/11 (9.1%) | 26 |
| Additional immuno-suppression | 2/10 (20%) | 0/5 (0%) | 0/11 (0%) | 26 |
ACPA anti-citrullinated protein antibodies, ANA antinuclear antibodies, irAE immune-related adverse events, NSAIDs non-steroidal anti-inflammatory drugs, OA osteoarthritis, RA rheumatoid arthritis
* This patient subsequently developed autoimmune hepatitis
Fig. 1.
a MRI of the left knee of a patient with oligoarthritis showing synovialitis (arrow) and joint effusion (dashed arrows). b PET-CT in the same patient with markedly increased FDG-uptake by synovial tissue. c MRI of the left shoulder in another patient with oligoarthritis showing focal synovitis of the inferior glenoid (arrow). d PET-CT of the same patient showing increased FDG-uptake by synovial tissue of the left shoulder
One patient with polyarthritis was found to be seropositive for RF and ACPA and was subsequently diagnosed with classical RA. Retrospectively, RF and ACPA were already detectable in cryo-preserved blood samples collected prior to PD1ab-start.
In the majority of patients (73.1%) symptoms were satisfactorily managed by only non-steroidal anti-inflammatory drugs (NSAID; e.g. ibuprofen). 5 patients (19.2%) further needed low-dose prednisolone (5–10 mg per day). Interestingly, 50% of patients in the arthritis group required low-dose prednisolone whereas none of the activated OA patients and only 9.1% of patients with arthralgia, not specified (Table 2). Of the arthritis patients, one who was diagnosed with seronegative polyarthritis subsequently required high-dose corticosteroids and another patient with seropositive RA additionally received sulfasalazine and hydroxychloroquine.
During the further course, four patients stopped the PD-1 antibody treatment because of CR/PR which was followed by complete clearing of arthralgia. 9 patients stopped PD-1 antibody therapy due to toxicity or progression. Only one of these nine patients suffers from ongoing arthralgia requiring NSAIDs intake. Of 13 patients who are still under treatment, 5 patients were able to stop NSAIDs ± low dose prednisolone completely, 6 patients constantly require NSAIDs ± low dose steroids, in 1 patient immunosuppression was deescalated to low dose prednisolone, and 1 patient refused to take further medication despite ongoing arthralgia.
Treatment efficacy
Efficacy and survival analyses were performed for patients with cutaneous or mucosal melanoma (n = 176). Objective tumour response data were available for 173 patients. Response rates (CR + PR) of patients with arthralgia (n = 24) were significantly better than in non-arthralgia patients (n = 149) (Chi-square test: p < 0.0001, Table 3). Notably, no patient in the arthralgia group progressed at the first staging -having in mind that the median time to onset of symptoms was 100 days and hence about the time of first staging.
Table 3.
Comparison of best responses to treatment in cutaneous and mucosal melanoma patients with and without arthralgia
| Total number of patients | Number of patients in the non-arthralgia cohort (%) | Number of patients with new onset arthralgia (%)* | ||
|---|---|---|---|---|
| 149 | 24 | |||
| Complete response | 6 | (4.0%) | 4 | (16.7%) |
| Partial response | 32 | (21.5%) | 15 | (62.5%) |
| Stable disease | 34 | (22.8%) | 5 | (20.8%) |
| Progressive disease | 77 | (51.7%) | 0 | |
* The difference in response rate (CR + PR) versus no response (SD + PD) between arthralgia- and non-arthralgia patients was statistically significant (Chi-square test: p < 0.0001)
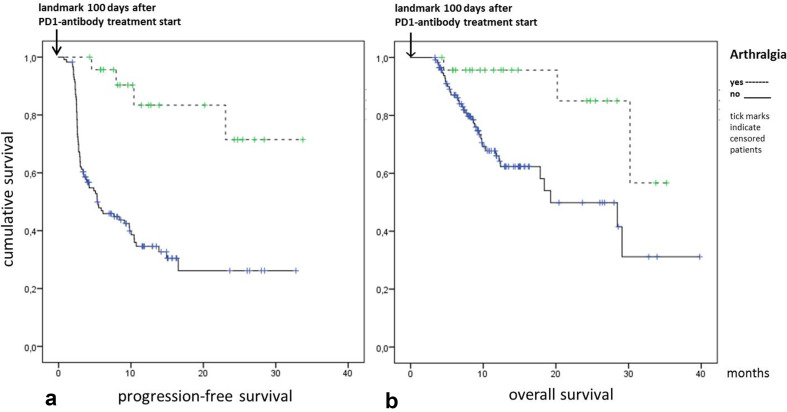
Median follow-up time for all patients was 294 days [range 25–1194], 280 days [25–1194] in the non-arthralgia cohort and 374 days [129–1057] in the arthralgia cohort (p < 0.01). The median PFS in non-arthralgia patients was 4.2 months (95% CI 2.5–6.0) with a median OS of 17.8 months (95% CI 11.2–24.5). In the arthralgia cohort neither median PFS nor median OS were reached. To control for the guarantee-time bias we performed a landmark analysis for PFS and OS at 100 days after start of the treatment as this was equal to the median onset of arthralgia. Patients with arthralgia had an improved estimated PFS as compared to patients without arthralgia (log rank test, p < 0.001), Fig. 2a. The Kaplan–Meier estimated OS was significantly longer for arthralgia patients as opposed to non-arthralgia patients (p < 0.01), Fig. 2b.
Fig. 2.
Landmark analysis of Kaplan–Meier estimated PFS (a) and OS (b) at 100 days after PD1i treatment start showing improved PFS and OS in patients with arthralgia (log rank test, p < 0.001 for PFS and p < 0.01 for OS)
Discussion
Arthralgia has not been interpreted as an irAE under immune-checkpoint inhibition until recently [4, 10]. However, arthralgia under PD1ab treatment was reported in phase III trials in 5–12% of patients with melanoma or non-small cell lung cancer [1–3, 11]. In our patient cohort arthralgia occurred with a slightly higher frequency of 13.3%. Joint symptoms will certainly gain importance in daily clinical practice with increasing use of PD1abs.
Herein, we characterize PD1ab-induced arthralgia in detail for the first time leading to two specific clinical patterns of joint involvement: typical arthritis and inflammatory OA, whereas in a proportion of patients arthralgia remained unclassifiable partly due to incomplete imaging. All patients showed involvement of large joints or combination of large and small joints. The median onset of arthralgia in our cohort was about 3 months which is comparable to previous case series [7] and less than reported for ipilimumab induced arthralgia (9 months) [12]. Combined checkpoint blockade might induce more severe, erosive arthritis as reported by Capelli et al. [7]. However, in our cohort only 3 of 40 patients (7.5%) on combination treatment developed inflammatory arthritis or inflammatory OA. Hence, incidence seems to be not increased. A higher incidence of arthralgia on PD1ab treatment as compared to ipilimumab monotherapy [1, 3] might be explained by different roles of the molecules in T cell activity regulation. Whereas CTLA-4 is important during early immune response, PD-1 limits T cell activity in peripheral tissues.
Three mechanisms of PD1ab induced arthralgia have been postulated [8]: First, PD1ab activate previously existing dormant arthritogenic clones. This hypothesis is supported by the course of our patient who developed classical RA and was asymptomatic before the start of PD1ab despite in retrospect already detectable RF and ACPA. Second, PD1ab treatment leads to a lack of suppression of newly presented autoantigens. These autoantigens could be presented in the circumstance of minimal traumata or infections that might not have been recognized by the patients. In addition, this could explain arthritis in pre-damaged joints as seen in OA. Underlining this, the majority of patients were seronegative for autoantibodies similar to previously published cases [7, 8]. Third, it is discussed that a direct PD1ab drug effect on synovial tissue might lead to metabolic changes and inflammation, e.g. by synovial expression of PD-L1.
Increased FDG-uptake of joints has been described for patients with arthritis under anti-CTLA-4 antibody treatment [12]. Synovitis was objectified in the majority of our patients who received MRI or PET/CT. Despite significant radiographic findings most arthritis patients had mild symptoms manageable by NSAIDs or low-dose steroids. This contradicts previous data about regularly required high-dose corticosteroid treatment, and even methotrexate or anti-TNF-alpha inhibitors for arthritis under PD1abs [7]. This discrepancy might be explained by the fact that the data were generated in a rheumatologic department where only severe cases will be referred to.
The role of arthralgia or other irAEs as predictors for improved response and survival is a challenging question. In a case series, three of four patients who developed arthritis under ipilimumab showed disease control [12]. Correlation of response and irAEs under PD1ab treatment tend towards improved response and longer PFS in patients with occurrence of irAEs [13, 14]. Our data show improved response, estimated PFS and OS for patients with arthralgia. However, side effects occur in a time-dependent manner and patients with a fast disease progression will not be able to receive PD1ab long enough to develop irAEs. Hence, it is important to control the results for the guarantee time bias. Concerning the better response rates, first staging of patients was done after 3 months which is about the time of median onset of arthralgia. For survival data we used the conditional landmark analysis starting at the median onset of arthralgia [15].
One limitation of our analysis is the retrospective character. A higher incidence of arthralgia could potentially be found in a prospective setting. That would also open up the possibility to perform imaging and laboratory examinations early in all affected patients as well as additional work-up with MRI and/or ultrasonography where arthralgia was not classifiable in retrospect.
Conclusion
Arthralgia is frequently induced by PD1abs and mainly affects large joints. Clinical manifestation of arthritis requires further diagnostic work-up including inflammation parameters, autoantibodies as well as imaging to identify patients who likely need more intensive treatment. All other patients are typically seronegative and symptoms are manageable by NSAIDs or low-dose steroids. In our cohort patients with arthralgia revealed a better clinical outcome in the landmark analysis. However, a prospective setting is needed for better characterization and the correlation with treatment efficacy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank our pain nurses for systematic pain assessment. We thank all patients and their families for supporting our clinical research.
Abbreviations
- ACPA
Anti-citrullinated protein antibodies
- ANA
Antinuclear antibodies
- CR
Complete response
- CT
Computed tomography
- CTC-AE
Common terminology criteria for adverse events
- FDG-PET
Fludeoxyglucose positron emission tomography
- irAE
Immune-related adverse event
- MRI
Magnetic resonance imaging
- NCT
National Center for Tumor Diseases, Heidelberg
- NSAID
Non-steroidal antirheumatic drug
- OA
Osteoarthritis
- PD
Progressive disease
- PD1ab
PD-1 antibody
- PFS
Progression-free survival
- PR
Partial response
- RA
Rheumatoid arthritis
- RF
Rheumatoid factor
- SD
Stable disease
Author contributions
Study conception and design was done by Kristina Buder-Bakhaya, Karolina Benesova, Hanns-Martin Lorenz, and Jessica C. Hassel. Kristina Buder-Bakhaya, Karolina Benesova, Carsten Schulz, Hoda Anwar, Antonia Dimitrakopoulou-Strauss, Tim F. Weber, Hanns-Martin Lorenz, and Jessica C. Hassel are responsible for the integrity of acquired data. Statistical analysis was performed by Kristina Buder-Bakhaya and Jessica C. Hassel. Kristina Buder-Bakhaya and Karolina Benesova prepared the manuscript. All authors made substantial contributions to data analysis and interpretation, manuscript editing, review and approval.
Compliance with ethical standards
Ethical approval
Retrospective analyses of clinical data were approved by the institutional review board of the Medical Faculty of the University Hospital Heidelberg (no. S-069/2010). The ethical committee had agreed to the retrospective analysis of routinely collected clinical data without prior informed consent of patients.
Informed consent
Informed consent of patients for publication of imaging data was obtained.
Funding
No relevant funding.
Conflict of interest
K Buder-Bakhaya received honoraria and travel reimbursements from TEVA Pharmaceutical Industries GmbH, MSD Sharp & Dome GmbH Oncology (MSD), and Roche Pharma AG (Roche). K. Benesova received payment for lectures from Roche and Abbvie Germany GmbH & Co, KG (Abbvie), travel expenses and/or conference fees from Abbvie, Pfizer Pharma GmbH (Pfizer) and Bristol-Myers Squibb (BMS). H.-M. Lorenz received consultancy fees, honoraria for lectures, support for scientific projects or travel reimbursements from Abbvie, MSD, BMS, Pfizer, Roche, Celgene GmbH, Baxter Germany GmbH, Swedish Orphan Biovitrum GmbH, Biogen GmbH, Medac GmbH, GlaxoSmithKline GmbH & Co. KG (GSK), Chugai Pharma Europe Ltd., Novartis Pharma GmbH (Novartis), UCB Pharma GmbH, Janssen-Cilag GmbH, AstraZeneca GmbH, Lilly Germany GmbH, Actelion Pharmaceuticals Germany GmbH, Bayer Vital GmbH, Shire Germany GmbH, and Octapharm GmbH. A. Enk received consultancy fees and honoraria for lectures from Biotest AG, Galderma Laboratorium GmbH, Janssen-Cilag GmbH, AbbVie, BMS, MSD, and Roche. J.C. Hassel received consultancy fees from Amgen GmbH, and MSD, payment for lectures from BMS, MSD, Roche, GSK, Novartis, and Pfizer and travel reimbursements from BMS, MSD, Amgen, and GSK. All other authors declare that they have no conflicts of interest.
References
- 1.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A, Investigators K Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 2.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Jr, Lao CD, Linette GP, Thomas L, Lorigan P, Grossmann KF, Hassel JC, Maio M, Sznol M, Ascierto PA, Mohr P, Chmielowski B, Bryce A, Svane IM, Grob JJ, Krackhardt AM, Horak C, Lambert A, Yang AS, Larkin J. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 3.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institutes of Health (2010) Common terminology criteria for adverse events (CTCAE), Version 4.03. U.S. Department of Health and Human Services, Bethasda
- 6.Woodworth T, Furst DE, Alten R, Bingham CO, 3rd, Yocum D, Sloan V, Tsuji W, Stevens R, Fries J, Witter J, Johnson K, Lassere M, Brooks P. Standardizing assessment and reporting of adverse effects in rheumatology clinical trials II: the Rheumatology Common Toxicity Criteria v. 2.0. J Rheumatol. 2007;34(6):1401–1414. [PubMed] [Google Scholar]
- 7.Cappelli LC, Gutierrez AK, Baer AN, Albayda J, Manno RL, Haque U, Lipson EJ, Bleich KB, Shah AA, Naidoo J, Brahmer JR, Le D, Bingham CO., 3rd Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis. 2017;76(1):43–50. doi: 10.1136/annrheumdis-2016-209595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan MM, Kefford RF, Carlino M, Clements A, Manolios N. Arthritis and tenosynovitis associated with the anti-PD1 antibody pembrolizumab in metastatic melanoma. J Immunother. 2015;38(1):37–39. doi: 10.1097/CJI.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Melero I, Grimaldi AM, Perez-Gracia JL, Ascierto PA. Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res. 2013;19(5):997–1008. doi: 10.1158/1078-0432.CCR-12-2214. [DOI] [PubMed] [Google Scholar]
- 11.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L, Investigators K- Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 12.Bronstein Y, Ng CS, Hwu P, Hwu WJ. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. AJR Am J Roentgenol. 2011;197(6):W992–W1000. doi: 10.2214/AJR.10.6198. [DOI] [PubMed] [Google Scholar]
- 13.Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, Viollet R, Thomas M, Roy S, Benannoune N, Tomasic G, Soria JC, Champiat S, Texier M, Lanoy E, Robert C. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152(1):45–51. doi: 10.1001/jamadermatol.2015.2707. [DOI] [PubMed] [Google Scholar]
- 14.Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, Sznol M, Long GV, Li H, Waxman IM, Jiang J, Robert C. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35(7):785–792. doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 15.Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol. 2013;31(23):2963–2969. doi: 10.1200/JCO.2013.49.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.