Abstract
Background
PD-1 inhibition (PD-1i) is the standard of care in melanoma and other malignancies. In patients with bone metastases of solid tumors, the monoclonal antibody denosumab directed against RANKL is approved for the prevention of skeletal-related events. However, RANKL is not only relevant in osteoclastogenesis, but also has immunological effects. Hence, we aimed at investigating, whether the combination of PD-1i and denosumab produces synergistic effects in metastatic melanoma treatment.
Methods
We retrospectively collected and analyzed clinical data of metastatic melanoma patients with bone metastases, who received PD-1i and denosumab therapy.
Results
29 patients were identified with a median age of 60.7 years: 20 were male and 9 were female. 20 patients (69%) were in stage IV M1c and 9 (31%) in stage IV M1d; 52% had an increased serum LDH. 24 patients (83%) received PD-1i as first-line therapy and five patients (17%) as second- or third-line therapy. 13 patients received the triple combination nivolumab, ipilimumab and denosumab (N + I+D), 16 patients received PD-1i and denosumab (PD-1i + D). Within a median follow-up time of 19.8 months, 17 patients progressed with a median time to progression of 6 months. The objective response rate was 54% in the N + I + D group and 50% in the PD-1i + D group. Recalcification of bone metastases was radiologically observed in 18 (62%) patients. No unexpected treatment-related adverse events emerged.
Conclusions
The combination therapy of metastatic melanoma with PD-1i and denosumab was feasible without unexpected safety issues and showed a promising efficacy signal. Further investigation in prospective studies is needed.
Keywords: Melanoma, Immunotherapy, RANK/RANKL, Bone metastasis, Adverse events
Introduction
Immune checkpoint blockade (ICB) targeting the checkpoints CTLA-4 and PD-1 became the standard treatment of advanced melanoma since it has been shown to prolong PFS and OS [1–3].
Bone was found to be the fourth most common site of melanoma visceral metastasis after lung, liver and brain in 11–18% of patients [4]. These bone metastases can lead to skeletal-related events (SREs), such as pathological fracture, spinal cord compression, and severe bone pain [5].
Denosumab, a recombinant fully monoclonal human IgG2 antibody directed against the receptor activator of nuclear factor kappa-B ligand (RANKL), represents a bone-modifying agent and is approved for the prevention of SREs in advanced solid tumors with metastasis to the bone. Denosumab blocks the binding between RANKL and its receptor RANK on osteoclasts resulting in the inhibition of osteoclastogenesis. However, the RANK/RANKL pathway also plays a role in many other processes [6, 7], e.g., immunological processes as it is a member of the tumor necrosis factor (TNF) family starting at thymic development of T cells to regulation of T cell responses in the periphery [8].
Therefore, the combined blockade of immune checkpoints as well as RANKL could have synergistic effects. This has been suggested by a case report of a patient with metastatic melanoma and widespread bone metastases, who showed a remarkable response to the CTLA-4 inhibitor ipilimumab in combination with denosumab [9]. Furthermore, murine tumor models suggest synergistic effects of the combined administration of CTLA-4 inhibitors and denosumab [10] or PD-1 inhibition (PD-1i) and denosumab [11].
To obtain further evidence for the clinical tolerability and efficacy of checkpoint inhibition, we retrospectively collected data from patients treated at four German skin cancer centers with denosumab and PD-1i for metastatic melanoma and bone metastases.
Methods
Study design
This is a retrospective multicenter study initiated by the German Dermatologic Cooperative Oncology Group (DeCOG). Patients meeting the following criteria were eligible: diagnosis of unresectable metastatic melanoma including bone metastases, therapy with denosumab and PD-1i with nivolumab, pembrolizumab or nivolumab plus ipilimumab combination therapy with a minimum concurrent administration of 4 weeks. These treatments were performed as the standard of care according to the summary of product characteristics (SmPC), e.g., before denosumab treatment a dental check was performed and calcium was substituted during therapy.
Data collection
Participating centers retrospectively searched their electronic databases for eligible patients. Data were provided on a standardized form including demographics (age, sex), melanoma characteristics (preceding therapies, stage of disease according to the AJCC classification [12], ECOG performance status), the PD-1i under investigation (type of inhibitor, date of therapy start, best response according to RECIST 1.1 criteria [13] [graded into complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD)], change of bone lesions such as sclerosis and details on the denosumab therapy (onset, duration). Imaging for restaging was performed every 2–3 months according to German melanoma guidelines [14]. Adverse events were graded according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 [15].
Statistical analysis
The Kaplan–Meier method was used to calculate estimates of PFS und OS using the software XLSTAT 2018. OS and PFS were defined as the time between the first administration of the immune checkpoint inhibitor and death or occurrence of disease progression, respectively. The results of OS and PFS were both presented in months.
Results
Patients
29 eligible patients were identified from four clinical centers. 20 patients were male, 9 were female, with a mean age of 60.7 years (range 35–82 years, Table 1). 13 of the patients received a combination therapy with nivolumab, ipilimumab and denosumab (N + I+D), while 16 others received a monotherapy with a PD-1-inhibitor plus denosumab (PD-1i + D). Eight patients received nivolumab and eight patients received pembrolizumab, respectively. The minimum of concomitant ICB and denosumab exposure was 1 month, the median 7 months and the maximum 44 months.
Table 1.
Baseline characteristics of patients receiving checkpoint inhibitors plus denosumab treatment
| Nivolumab + ipilimumab + denosumab (N = 13) |
PD-1-inhibitor + denosumab (N = 16) | Total (N = 29) | |
|---|---|---|---|
| Age (years) | |||
| Mean | 59.9 | 61.4 | 60.7 |
| Range | 39–82 | 35–82 | 35–82 |
| Sex: no. (%) | |||
| Male | 10 (77%) | 10 (63%) | 20 (69%) |
| Female | 3 (23%) | 6 (37%) | 9 (31%) |
| Metastasis stage IV: no. (%) | |||
| M1c | 8 (62%) | 12 (75%) | 20 (69%) |
| M1d | 5 (38%) | 4 (25%) | 9 (31%) |
| Lactate dehydrogenase: no. (%) | |||
| Normal | 7 (54%) | 7 (44%) | 14 (48%) |
| Elevated | 6 (46%) | 9 (56%) | 15 (52%) |
| BRAF-status: no. (%) | |||
| Mutated | 10 (77%) | 7 (44%) | 17 (59%) |
| Wild type | 3 (23%) | 9 (56%) | 12 (41%) |
| Therapy line: no. (%) | |||
| 1st | 12 (92%) | 12 (75%) | 24 (83%) |
| 2nd | 1 (8%) | 1 (6%) | 2 (7%) |
| 3rd | 0 (0%) | 3 (19%) | 3 (10%) |
A total of 31% of the patients had stage IV M1d and 69% had stage IV M1c metastatic melanoma, respectively. 52% of the patients had an elevated lactate dehydrogenase (LDH) serum level, and 59% had a BRAF V600 mutation. Four patients had received a BRAF-directed targeted therapy before ICB and denosumab. 24 patients had received PD-1i as the first-line treatment, two as the second-line and three others as the third-line treatment, respectively (Table 1).
Efficacy
In the N + I + D group, a CR was observed in two patients (15%), a PR in five patients (39%), three patients (23%) had a SD and three patients (23%) had a PD. In the PD-1i + D group, three patients (19%) experienced a CR, five patients (31%) a PR, four patients (25%) had a SD and four patients (25%) had a PD (Table 2).
Table 2.
Efficacy of checkpoint inhibitors plus denosumab in 29 patients
| Nivolumab + ipilimumab + denosumab (N = 13) | PD-1-inhibitor + denosumab (N = 16) | Total (N = 29) | |
|---|---|---|---|
| Best overall response: no. (%) | |||
| Complete response | 2 (15%) | 3 (19%) | 5 (17%) |
| Partial response | 5 (39%) | 5 (31%) | 10 (35%) |
| Stable disease | 3 (23%) | 4 (25%) | 7 (24%) |
| Progressive disease | 3 (23%) | 4 (25%) | 7 (24%) |
| Objective response: no. (%) | 7 (54%) | 8 (50%) | |
| Median duration of response (months) | 10.2 | 12.5 | |
| Median (range) PFS (months) | 6 (0–24) | 6 (1–44) | |
| Median (range) OS (months) | NR | NR | |
| Sclerosis of bone metastases: no. (%) | 6 (46%) | 12 (75%) | 18 (62%) |
At the time of analysis, progressive disease was observed in 16 patients (8/13 from N + I + D group and 8/16 from PD-1i + D group). Five (17%) patients had died due to tumor progression (1/13 from N + I+D group and 4/16 from PD-1i + D group). The median values of OS are not reached (Fig. 1).
Fig. 1.
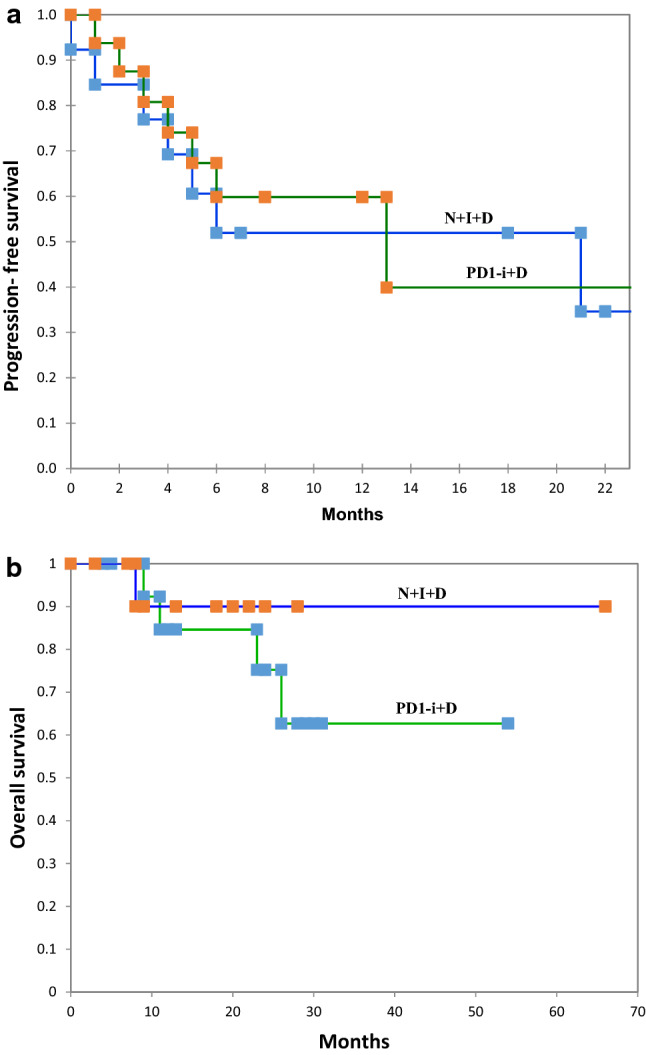
Kaplan–Meier curves of PFS (a) and OS (b), separated for patients receiving N + I+D and patients receiving PD-1i + D
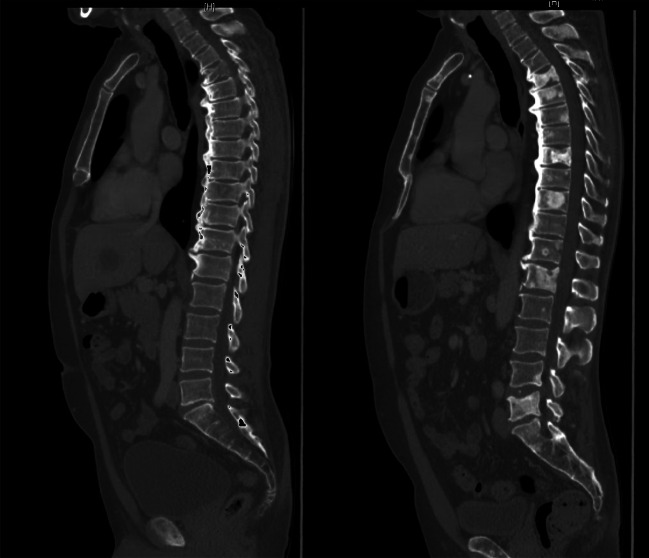
Bone metastases responding to treatment as assessed radiographically by sclerotic changes within a metastatic deposit were seen in 18 patients (6/13 patients in the N + I + D group; 12/16 patients in the PD-1i + D group) (Fig. 2). All of these 18 patients with sclerotic changes in the bone metastases responded. Of the 11 other patients without sclerotic changes of bone metastases, two patients have reached PR, two patients had a SD and seven patients had a PD.
Fig. 2.
Sclerotic changes within bone metastases as response to treatment. The left CT scan shows the metastases before treatment, the right CT scan with sclerotic changes of the bone metastases after 12 months of treatment with N + I+D. The patient achieved a partial response
The overall response rate in the subgroup of patients with BRAF V600 mutation melanoma (47%; 1 CR and 7 PR) was similar compared to patients with BRAF V600 wild type (58%; 4 CR and 3 PR) (Table 3). Two (13%) patients with elevated LDH had a CR, seven (47%) patients had a PR, two (13%) had a SD and four (27%) patients had a PD. In the group of patients with normal LDH, CR was observed in three patients (21%), PR in three patients (21%), three patients (21%) had a SD and five patients (36%) had a PD. Overall, the patients with an elevated LDH level had a slightly higher response rate (60%) compared to patients with normal levels of LDH (43%). Four patients (20%) without brain metastases (stage IV M1c) achieved a CR, seven patients (35%) had a PR, seven patients (35%) had a SD and three patients (15%) had a PD. In the group of patients in stage IV M1d, a CR was observed in one patient (11%), a PR in three patients (33%), a PD in five patients (56%) and no patients in this group had a SD. Thus, response rates of patients with stage IV M1d were 44% and those with stage IV M1c were 55% (Table 3).
Table 3.
Efficacy of checkpoint-inhibitor plus denosumab treatment in certain subgroups
| BRAF V600E-mutation (N = 17) | BRAF V600E wild type (N = 12) | Total (N = 29) | |
|---|---|---|---|
| Best overall response: no. (%) | |||
| Complete response | 1 (6%) | 4 (33%) | 5 (17%) |
| Partial response | 7 (41%) | 3 (25%) | 10 (34%) |
| Stable disease | 5 (29%) | 2 (17%) | 7 (24%) |
| Progressive disease | 4 (24%) | 3 (25%) | 7 (24%) |
| Objective response: no. (%) | 8 (47%) | 7 (58%) | |
| Elevated LDH level (N = 15) | Normal LDH level (N = 14) | Total (N = 29) | |
| Best overall response: no. (%) | |||
| Complete response | 2 (13%) | 3 (21%) | 5 (17%) |
| Partial response | 7 (47%) | 3 (21%) | 10 (34%) |
| Stable disease | 2 (13%) | 5 (36%) | 7 (24%) |
| Progressive disease | 4 (27%) | 3 (21%) | 7 (24%) |
| Objective response: no. (%) | 9 (60%) | 6 (43%) | |
| M1c (N = 20) | M1d (N = 9) | Total (N = 29) | |
| Best overall response: no. (%) | |||
| Complete response | 4 (20%) | 1(11%) | 5 (17%) |
| Partial response | 7 (35%) | 3 (33%) | 10 (34%) |
| Stable disease | 7 (35%) | 0 (0%) | 7 (24%) |
| Progressive disease | 2 (10%) | 5 (56%) | 7 (24%) |
| Objective response: no. (%) | 11 (55%) | 4 (44%) | |
Adverse events
Treatment-related adverse events of any grade occurred in 85% of the patients in the N + I + D group and 50% of those in the PD-1i + D group (Table 4). The most frequently reported treatment-related adverse events in N + I+D group were colitis of grades 3–4 (in 31% of the patients), followed by hypothyroidism of grade 1 or 2 (in 23%). Fatigue, rash and dyspnea were observed in two patients each (15%). On the other hand, fatigue and rash of grade 1 or 2 (in 25% of patients) were the most common adverse events in the PD-1i + D group, and no grade 3 or 4 treatment-related adverse events were reported in this group. Two patients (15%) had to discontinue the treatment due to adverse events in the N + I + D group, but none in the PD-1i + D group. The discontinuations were due to colitis (grade 4) and hepatitis (grade 4), respectively. Adverse events after injection of denosumab were reported in two patients. Myalgia occurred as AE in one patient, whereas the other had pyrexia. The AEs occurred repetitively within 1–2 days after denosumab injection but were not observed after ICB-application and were, therefore, considered as denosumab-related AEs. No other adverse events under denosumab therapy including osteonecrosis of the jaw were reported in our patients.
Table 4.
Adverse events in patients receiving checkpoint inhibitors plus denosumab treatment
| Nivolumab + ipilimumab + denosumab (N = 13) | PD-1-ihibitor + denosumab (N = 16) | |||
|---|---|---|---|---|
| Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | |
| Number of patients (%) | ||||
| Treatment-related adverse events | 11 (85%) | 8 (50%) | ||
| Fatigue | 2 (15%) | 0 | 4 (25. %) | 0 |
| Pruritus | 1 (8%) | 0 | 1 (6%) | 0 |
| Vitiligo | 0 | 0 | 2 (12%) | 0 |
| Rash | 2 (15%) | 0 | 4 (25%) | 0 |
| Nausea/other gastrointestinal symptoms | 1 (8%) | 2 (15%) | 2 (12%) | 0 |
| Decreased appetite | 0 | 1 (8%) | 1 (6%) | 0 |
| Increased aspartate or alanine transaminase | 0 | 1 (8%) | 0 | 0 |
| Increased lipase and amylase | 1 (8%) | 0 | 0 | 0 |
| Hypothyroidism | 3 (23%) | 0 | 1 (6%) | 0 |
| Colitis | 0 | 4 (31%) | 0 | 0 |
| Arthalgia | 1 (8%) | 0 | 2 (12%) | 0 |
| Dyspnea | 2 (15%) | 0 | 3 (19%) | 0 |
| Pyrexia | 0 | 1 (8%) | 0 | 0 |
| Myasthenic syndrome | 0 | 1 (8%) | 0 | 0 |
| Treatment-related adverse events leading to discontinuation | 2 (15%) | 0 | ||
The severity of adverse events was graded according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 (for download see link http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf)
Discussion
This study aimed to provide clinical evidence that the use of denosumab as a targeted therapy against RANKL in combination with immune checkpoint blockade is feasible and may enhance the antitumor effect in metastatic melanoma patients with bone metastases.
We identified 29 patients, who received PD-1-directed checkpoint inhibition plus denosumab for metastatic melanoma with bone metastases.
With regard to AEs, the overall incidence of treatment-related AEs was significantly higher in the N + D+I group (85%) than in the PD-1i + D group (50%). The incidence of treatment-related AEs of grade 3 or 4 was only reported in N + I + D, notably for colitis. Overall, the safety profile of the combination therapy remained consistent with previous experiences within the combination therapy with nivolumab and ipilimumab and with PD-1i monotherapy [16]. For denosumab, a well-known serious adverse event is osteonecrosis of the jaw which occurs in 1–2% of patients [17]. In our case series, we observed no osteonecrosis of the jaw. Thus, our case series did not show any unexpected safety issues with regard to type, frequency or severity of AEs.
With regard to efficacy, response rates of the patients in the N + I + D group (54%) were slightly higher compared to the response rates of the patients in the PD-1i + D group (50%). These response rates are in line with previous studies investigating ICB in melanoma without concomitant denosumab treatment. The Checkmate 67 study revealed a response rate of 45% for nivolumab and 58% for nivolumab plus ipilimumab [18]. Furthermore, the Keynote 6 study showed a response rate of 42% for pembrolizumab [16]. To our knowledge, there are no data focusing on the response of melanoma bone metastases on ICB or denosumab alone. In our experience, bone metastases also respond to the treatment if extraosseous metastases respond. This is also reflected in our study, where all 18 patients with sclerotic changes of bone metastases had an overall response. Out of the 11 patients without sclerotic changes, there were only two overall responders.
Recently, there were two case series reporting the combination of ICB and denosumab. The study from Liede et al. [19] retrieved melanoma and lung cancer patients from the Flatiron Health electronic health records, who started both ICB (CTLA-4 inhibitor ipilimumab or PD-1 inhibitor nivolumab or pembrolizumab) and denosumab within a period of 30 days. This analysis reported a 47% response rate for PD1i + D treatment in 30 patients, which is similar to the response rate of 50% observed in our patients. However, they did not report on the combination of N + I+D or adverse events. Furthermore, Afzal and Shirai [20] performed a retrospective chart review of melanoma patients from two cancer centers who received ICB in combination with denosumab. Of the 11 patients they identified, 8 received pembrolizumab, 1 nivolumab, 1 ipilimumab, and 1 nivolumab + ipilimumab, respectively. They reported a response in 6/11 patients (55%). Hypocalcemia (2/11 patient grade 2) and myalgia/back pain (5/11 patients grade 1 or 2) were more common in the denosumab group as compared to a matched control group with ICB alone (26 patients with metastatic melanoma without bone metastases).
The mechanism as to how RANKL blockade with denosumab might affect immunotherapy is a matter of speculation. One option is a direct effect on tumor cells. RANK can be expressed by melanoma cells, but RANK expressing melanomas and RANK-negative melanomas have similar rates of bone metastases; thus, the functional role of RANK on melanoma cells is unclear [21–24]. Another option is an effect of the RANKL/RANK pathway in immune reactions. RANKL expression has been described on various immune cells, such as regulatory T cells, myeloid suppressor cells and CD8 + as well as CD4 + T cells [25–29]. Thus, immunological effects of denosumab are possible, which is also suggested by murine models, where the concomitant administration of anti-CTLA-4 and RANKL-Inhibitor resulted in an increased number of tumor-infiltrating CD8 + T cells [10, 30].
Of course, our case series implies the usual limitations of all retrospective studies, such as possible bias by patient selection and incomplete reporting of data. However, it provides further clinical evidence for the tolerability and efficacy of both N + I+D as well as PD-1i + D. These findings encourage the conduct of prospective clinical trials investigating the combination of PD-1i plus denosumab.
Appropriate prospective clinical trials are currently ongoing. The CHARLI-trial (NCT 03161756) is a two-arm study performed in Australia in patients with metastatic melanoma. Arm A investigates denosumab plus nivolumab, arm B denosumab plus nivolumab + ipilimumab. The primary endpoints are median PFS and the occurrence of grade 3–4 selected immune-related adverse events of interest. The BONEMET trial (EudraCT-Nr. 2016-001925-15) is performed in Germany and includes patients who receive treatment with PD-1i plus denosumab for metastatic melanoma with bone metastases.
The results of these trials are expected earliest in 2021. Since the combination of denosumab plus PD-1-directed checkpoint inhibition is currently frequently used in the clinical care of metastatic melanoma patients, our retrospectively collected evidence is important as a basis for the potential use of this combination.
Conclusion
We show in a retrospective analysis of 29 patients that the combination of PD-1i or nivolumab plus ipilimumab with denosumab is feasible with no unexpected safety concerns and promising efficacy. Thus, prospective trials are encouraged to further investigate this combination.
Abbreviations
- AEs
Adverse events
- AJCC
American Joint Committee on Cancer
- CR
Complete response
- CTCAE
Common Terminology Criteria for Adverse Events
- CTLA4
Cytotoxic T-lymphocyte-associated protein 4
- EMA
European Medicines Agency
- FDA
Food and Drug Administration
- ICB
Immune checkpoint blockade
- LDH
Lactate dehydrogenase
- N
Number
- N + I+D
Nivolumab plus ipilimumab plus denosumab
- NR
Not reached
- ORR
Objective rate response
- PD
Progressive disease
- PD-1i
Programmed cell death protein1 inhibitor/inhibitors
- PD-1i + D
PD1 inhibitor plus denosumab
- PR
Partial response
- RANK
Receptor activator of NF-kB
- RANKL
Receptor activator of NF-kB ligand
- RECIST
Response evaluation criteria in solid tumors
- SD
Stable disease
- SmPC
Summary of product characteristics
- SREs
Skeletal-related events
Author contributions
Authors whose names appear on this paper have contributed sufficiently to the scientific work and, therefore, share collective responsibility and accountability for the results. YA and RG planned the project. All authors contributed to the data collection. YA and RG contributed to the interpretation of the results. YA took the lead in writing the manuscript. All authors provided critical feedback and helped to shape the manuscript.
Funding
No specific funding was received for this study.
Compliance with ethical standards
Ethics approval
This is a retrospective multicenter study initiated by the German Dermatologic Cooperative Oncology Group (DeCOG). Human participants (melanoma patients) were involved but only by the retrospective chart review. Ethics approval for the retrospective data collection and anonymous analysis without informed consent of the patients was obtained from the Ethics committee of Hannover Medical School (vote number 1612). This vote states that there are no ethical concerns with regard to the anonymous retrospective analysis of routine clinical care data from own patients and that there is no general need to notify the ethics committee on such studies. The patient who is shown in Fig. 2 provided written informed consent for the use of his CT scans within this publication.
Conflict of interest
Ralf Gutzmer served as speaker to Roche, Bristol-Myers Squibb (BMS), Novartis, Merck Serono, Merck Sharp & Dohme (MSD), Almirall-Hermal, Amgen, Merck Serono, Pierre Fabre, SUN Pharma and received research grants from Novartis, Pfizer, Johnson & Johnson. He served as consultant to Roche Pharma, BMS, Novartis, MSD, Almirall-Hermal, LEO-Pharma, Amgen, Pfizer, Pierre Fabre, Roche Posay, Merck Serono, Regeneron, SUN Pharma and Incyte. Selma Ugurel received research support from BMS, medac, and Merck Serono and serves as speaker/consultant to BMS, MSD, Merck Serono, and Roche, and received travel grants from BMS, Medac, and MSD. Jürgen C. Becker received speaker fees from Amgen, MerckSerono, and Pfizer, advisory board honoraria from Amgen, CureVac, eTheRNA, Lytix, MerckSerono, Novartis, Rigontec, and Takeda as well as research grants from Alcedis, Boehringer Ingelheim, BMS and Merck Serono. He also received travel fees from 4SC and Incyte. Carsten Weishaupt served as speaker to Amgen, BMS, Curevac, La Roche Posay, Leo Pharma, MSD, Novartis, Roche, Takeda, TEVA. All other authors declared that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Petrella TM, Robert C, Richtig E, Miller WH, Masucci GV, Lebbe C, Steven N, Middleton MR, Hille D, Zhou W, Ibrahim N, Cebon J. Patient-reported outcomes in KEYNOTE-006, a randomised study of pembrolizumab versus ipilimumab in patients with advanced melanoma. Eur J Cancer. 2017;86:115–124. doi: 10.1016/j.ejca.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 2.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbé C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 3.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbé C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, Larkin J. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tas F. Metastatic behavior in melanoma: timing, pattern, survival, and influencing factors. J Oncol. 2012;2012:647684. doi: 10.1155/2012/647684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saad F, Lipton A, Cook R, Chen YM, Smith M, Coleman R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110:1860–1867. doi: 10.1002/cncr.22991. [DOI] [PubMed] [Google Scholar]
- 6.de Groot AF, Appelman-Dijkstra NM, van der Burg SH, Kroep JR. The anti-tumor effect of RANKL inhibition in malignant solid tumors—a systematic review. Cancer Treat Rev. 2018;62:18–28. doi: 10.1016/j.ctrv.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, Morony S, Rubin E, Sarao R, Hojilla CV, Komnenovic V, Kong YY, Schreiber M, Dixon SJ, Sims SM, Khokha R, Wada T, Penninger JM. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440:692–696. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 8.Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, Karin M. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470:548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smyth MJ, Yagita H, McArthur GA. Combination Anti-CTLA-4 and Anti-RANKL in metastatic melanoma. J Clin Oncol. 2016;34:e104–e106. doi: 10.1200/JCO.2013.51.3572. [DOI] [PubMed] [Google Scholar]
- 10.Ahern E, Harjunpää H, Barkauskas D, Allen S, Takeda K, Yagita H, Wyld D, Dougall WC, Teng MWL, Smyth MJ. Co-administration of RANKL and CTLA4 antibodies enhances lymphocyte-mediated antitumor immunity in mice. Clin Cancer Res. 2017;23:5789–5801. doi: 10.1158/1078-0432.CCR-17-0606. [DOI] [PubMed] [Google Scholar]
- 11.Ahern E, Harjunpää H, O’Donnell JS, Allen S, Dougall WC, Teng MWL, Smyth MJ. RANKL blockade improves efficacy of PD1-PD-L1 blockade or dual PD1-PD-L1 and CTLA4 blockade in mouse models of cancer. Oncoimmunology. 2018;7:e1431088. doi: 10.1080/2162402X.2018.1431088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, Lazar AJ, Faries MB, Kirkwood JM, McArthur GA, Haydu LE, Eggermont A, Flaherty KT, Balch CM, Thompson JF, for members of the American Joint Committee on Cancer Melanoma Expert Panel and the International Melanoma Database and Discovery Platform Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:472–492. doi: 10.3322/caac.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2008;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Pflugfelder A, Kochs C, Blum A, Capellaro M, Czeschik C, Dettenborn T, Dill D, Dippel E, Eigentler T, Feyer P, Follmann M, Frerich B, Ganten MK, Gärtner J, Gutzmer R, Hassel J, Hauschild A, Hohenberger P, Hübner J, Kaatz M, Kleeberg UR, Kölbl O, Kortmann RD, Krause-Bergmann A, Kurschat P, Leiter U, Link H, Loquai C, Löser C, Mackensen A, Meier F, Mohr P, Möhrle M, Nashan D, Reske S, Rose C, Sander C, Satzger I, Schiller M, Schlemmer HP, Strittmatter G, Sunderkötter C, Swoboda L, Trefzer U, Voltz R, Vordermark D, Weichenthal M, Werner A, Wesselmann S, Weyergraf AJ, Wick W, Garbe C, Schadendorf D, German Dermatological Society; DermatologicCooperative Oncology Group Malignant melanoma S3-guideline “diagnosis, therapy and follow-up of melanoma”. J Dtsch Dermatol Ges. 2013;6:1–116. doi: 10.1111/ddg.12113_suppl. [DOI] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services; National Institutes of Health; National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. Available at: https://evs.nci.nihgov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Accessed June 27, 2017
- 16.Long GV, Schachter J, Ribas A, Arance AM, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil CM, Lotem M, Larkin JMG, Lorigan P, Neyns B, Blank CU, Petrella TM, Hamid O, Anderson J, Krepler C, Ibrahim N, Robert C. 4-year survival and outcomes after cessation of pembrolizumab (pembro) after 2-years in patients (pts) with ipilimumab (ipi)-naive advanced melanoma in KEYNOTE-006. J Clin Oncol. 2018;36:9503. doi: 10.1200/JCO.2018.36.15_suppl.9503. [DOI] [Google Scholar]
- 17.Khan AA, Morrison A, Hanley DA, Felsenberg D, McCauley LK, O’Ryan F, Reid IR, Ruggiero SL, Taguchi A, Tetradis S, Watts NB, Brandi ML, Peters E, Guise T, Eastell R, Cheung AM, Morin SN, Masri B, Cooper C, Morgan SL, Obermayer-Pietsch B, Langdahl BL, Al Dabagh R, Davison KS, Kendler DL, Sándor GK, Josse RG, Bhandari M, El Rabbany M, Pierroz DD, Sulimani R, Saunders DP, Brown JP, Compston J. International task force on osteonecrosis of the Jaw. J Bone Miner Res. 2015;30:3–23. doi: 10.1002/jbmr.2405. [DOI] [PubMed] [Google Scholar]
- 18.Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, Lao CD, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hill A, Hogg D, Marquez-Rodas I, Jiang J, Rizzo J, Larkin J, Wolchok JD. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480–1492. doi: 10.1016/S1470-2045(18)30700-9. [DOI] [PubMed] [Google Scholar]
- 19.Liede A, Hernandez RK, Wade SW, Bo R, Nussbaum NC, Ahern E, Dougall WC, Smyth MJ. An observational study of concomitant immunotherapies and denosumab in patients with advanced melanoma or lung cancer. Oncoimmunology. 2018;7:e1480301. doi: 10.1080/2162402X.2018.1480301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afzal MZ, Shirai K. Immune checkpoint inhibitor (anti-CTLA-4, anti-PD-1) therapy alone versus immune checkpoint inhibitor (anti-CTLA-4, anti-PD-1) therapy in combination with anti-RANKL denosumab in malignant melanoma: a retrospective analysis at a tertiary care center. Melanoma Res. 2018;28:341–347. doi: 10.1097/CMR.0000000000000459. [DOI] [PubMed] [Google Scholar]
- 21.Kupas V, Weishaupt C, Siepmann D, Kaserer ML, Eickelmann M, Metze D, Luger TA, Beissert S, Loser K. RANK is expressed in metastatic melanoma and highly upregulated on melanoma-initiating cells. J Invest Dermatol. 2011;131:944–955. doi: 10.1038/jid.2010.377. [DOI] [PubMed] [Google Scholar]
- 22.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and tregs in the melanoma tumor microenvironment is driven by CD8 + T Cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, Ezoe S, Kanakura Y, Sato E, Fukumori Y, Karbach J, Jäger E, Sakaguchi S. Anti-CCR25 mAb selectively depletes effector-type FoxP3 + CD4 + regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci USA. 2013;110:17945–17950. doi: 10.1073/pnas.1316796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker LS. Treg and CTLA-4: two intertwining pathways to immune tolerance. J Autoimmun. 2013;45:49–57. doi: 10.1016/j.jaut.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahern E, Smyth MJ, Dougall WC, Teng MWL. Roles of the RANKL-RANK axis in antitumour immunity—implications for therapy. Nat Rev Clin Oncol. 2018;15:676–693. doi: 10.1038/s41571-018-0095-y. [DOI] [PubMed] [Google Scholar]
- 26.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 27.Santini D, Perrone G, Roato I, Godio L, Pantano F, Grasso D, Russo A, Vincenzi B, Fratto ME, Sabbatini R, et al. Expression pattern of receptor activator of NFkB (RANK) in a series of primary solid tumors and related metastases. J Cell Physiol. 2011;226:780–784. doi: 10.1002/jcp.22402. [DOI] [PubMed] [Google Scholar]
- 28.Standal T, Seidel C, Hjertner Ø, Plesner T, Sanderson RD, Waage A, Borset M, Sundan A. Osteoprotegerin is bound, internalized, and degraded by multiple myeloma cells. Blood. 2002;100:3002–3007. doi: 10.1182/blood-2002-04-1190. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs JF, Nierkens S, Figdor CG, de Vries IJ, Adema GJ. Regulatory T cells in melanoma: the final hurdle to-wards effective immunotherapy? Lancet Oncol. 2012;13:e32–e42. doi: 10.1016/S1470-2045(11)70155-3. [DOI] [PubMed] [Google Scholar]
- 30.Bakhru P, Zhu ML, Wang HH, Hong LK, Khan I, Mouchess M, Gulati AS, Starmer J, Hou Y, Sailer D, Lee S, Zhao F, Kirkwood JM, Moschos S, Fong L, Anderson MS, Su MA. Combination central tolerance and peripheral checkpoint blockade unleashes antimelanoma immunity. JCI Insight. 2017;2(18):93265. doi: 10.1172/jci.insight.93265. [DOI] [PMC free article] [PubMed] [Google Scholar]