Abstract
Objectives
Advanced age and female sex have been associated with worse outcomes in patients undergoing radical cystectomy for muscle-invasive bladder cancer. A reduced immune response has been implicated as a mechanism. The objective of our study was to analyze the expression patterns of various cellular proteins active in bladder cancer immune pathways, and assess the correlation between age, sex, and the expression of these immune markers.
Methods
We obtained surgical tissue samples from equally distributed male/female patients with/without lymph node metastasis who had undergone radical cystectomy for urothelial carcinoma (UC) of the bladder (n = 50). Immunohistochemistry (IHC) for CD3 (cluster of differentiation), CD4, CD8, CD56, LAG-3 (lymphocyte-activation gene), TIM-3 (T-cell immunoglobulin and mucin-domain), PD-1 (programmed death) and PD-L1 molecules was performed and scored by a single pathologist (high versus low). Spearman’s correlation and Chi square tests investigated the association between age, sex, and IHC results.
Results
Mean age at surgery was 67 years (range 50–78 years); all patients were Caucasians. The following percent of patients scored high for a stain: 18% CD3, 10% CD4, 0% CD8, 0% CD56, 20% LAG-3, 4% TIM-3, 0% PD-1 and 0% PD-L1. There was no association between patients’ age, sex, and the expression of any of the immune markers (p > 0.05 for all).
Conclusions
The association between advanced age, female sex, and worse outcomes in bladder cancer may be independent of the immune pathways active in the disease that we examined in this study.
Keywords: Bladder, Cancer, Immunohistochemistry, Age, Sex
Introduction
In the United States, bladder cancer is the 5th most common malignancy, 7th highest cause of death from cancer in males and 13th in females [1]. Multiple researchers have investigated factors that lead to poor outcomes in patients with muscle-invasive bladder cancer undergoing radical cystectomy. Such factors, among others, include older age and female sex [2–7]. A multi-institutional study by Messer et al. noted that women had 27% higher hazard of cancer-specific mortality as compared to men after controlling for standard clinical and pathological parameters [2]. Similarly, various study groups have shown that individuals aged ≥ 65 years have 15-times greater cancer-specific mortality rate than individuals aged < 65 years [7]. These authors hypothesized that such differences in outcomes related to age and sex are, at least in part, due to social and economic factors. However, translational studies have hinted that there may be an underlying immune mechanism as well—female and older adults may have a weaker immune response and thus succumb to the disease easier. In fact, there is evidence that physiological levels of estrogen are likely to promote immune tolerance [8], and that aging is associated with the reduced output of T-cells from lymphoid organs and skewing of T-cell response toward helper T-cell differentiation.
With the use of immune and targeted therapy to combat various cancers, an increasing drive for investigation of mechanism of action underlying immune cell response has begun. Both the immune cell checkpoints molecules and specific immune cellular proteins have been a target of various clinical trials with inconsistent efficacy. Monoclonal antibodies against these targets have shown effectiveness in many types of cancer, and a few of these agents, such as pembrolizumab and atezolizumab, are approved for treatment of bladder cancer [9, 10]. Our objective was to assess the relationship between age, sex, and the expression patterns of various cellular proteins active in immune pathways in patients undergoing radical cystectomy for muscle-invasive bladder cancer.
Materials and methods
Study population
Surgical tissue samples from patients who had undergone radical cystectomy between 2009 and 2011 for muscle-invasive urothelial carcinoma (n = 50) were obtained. The population was equally distributed between male and female patients. Tissue samples and all patient data were handled as stated in the study protocol approved by the Institutional Review Board.
Antibodies
Rabbit anti-human CD3 (cat# ab5690), anti-human CD4 (cat# ab133611), anti-human LAG-3 (cat# ab180187; lymphocyte-activation gene), anti-human programmed cell death (PD)-1 (cat# ab137132), and anti-human PD-L1 (cat# ab205921) antibodies were purchased from Abcam. Rabbit anti-human CD8 (cat# sc-7188) and anti-human CD56 (cat# sc-106) were purchased from Santa Cruz. Rabbit anti-TIM-3 (cat# 45208S; T-cell immunoglobulin and mucin-domain) was from Cell Signaling. Secondary anti-rabbit IgG conjugated to horseradish peroxidase (HRP) was from Jackson ImmunoResearch.
Immunohistochemical staining
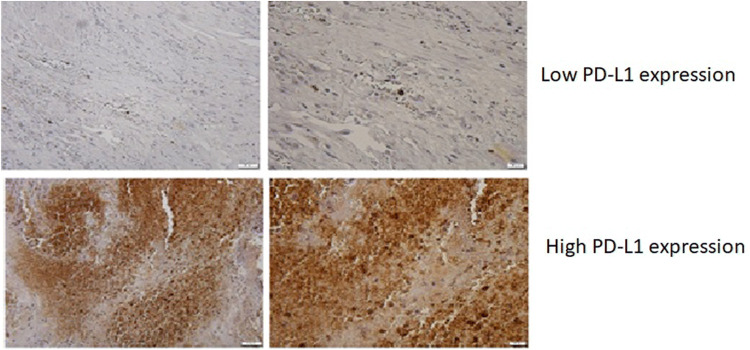
Immunohistochemical detection of PD-1, PD-L1, CD3, CD4, CD8, CD56, LAG-3, and TIM-3 proteins was performed at Southern Illinois University on formalin-fixed, paraffin-embedded sections of cystectomy tissues from all subjects. Slides were deparaffinized by passing 3 times through 100% of xylene, twice through 100% of ethanol, once through 95% ethanol and once 70% ethanol. This was followed by quenching endogenous peroxidase with 3% of H2O2 followed by three washes in PBS. All incubations were 5 min long. Deparaffinized slides were boiled for 20 min in antigen-retrieval solution consisted of Tris–HCl (10 mM, pH 9.0), 1 mM EDTA and 0.05% Tween-20. Slides were cooled to room temperature and washed three times in PBS before staining. Then, slides were incubated with primary antibodies and diluted 1:100 at 37 °C for 60 min followed by incubation with 10 µg/ml of anti-rabbit HRP-conjugated secondary antibody for 1 h at 37 °C. Slides were washed for 10 min in PBST and developed using ImmPACT DAB Eqv Development Kit (Vector Labs, cat# SK-4103). Slides were countered stained with hematoxylin and mounted using VectaMount permanent mounting medium (Vector Labs, cat# H-5000). Images were acquired using an Olympus BX41 upright microscope equipped with a DP70 digital camera and DP Controller software (Olympus, Center Valley, PA). All antibodies were validated on sections of human normal lymph nodes (a positive control). Omission of the primary antibody was used as a negative control. Stained slides were reviewed and scored by a single pathologist (Natalie Freed). To quantify staining-positive cells, the number of stain-positive cells was counted at the highest numbered five high-power fields (HPF). A uniform staining regimen has not been established and is greatly debated for these stains, specifically PD-L1 [11, 12]. Our regimen is as follows: less than 20% of infiltrated immune cells positive for all stains: CD3, CD4, CD56, CD8, LAG-3, TIM-3, PD-L1, and PD-1, and, was considered to be low, whereas ≥ 20% of stained immune cells as high infiltration (Figure 1: representative staining for PD-L1). For all stains, a score of < 1% was graded as negative. The cutoff scores for immunostaining were determined by the area under the curve of the receiver operating characteristic curve at the highest positive likelihood ratio point for overall survival in the study population. The association between OS and immunostaining results for the different biomarkers is not presented in this paper. Because the receiver operating characteristic curve is a plot of the true positive rate (sensitivity) versus the false positive rate (1 − specificity) for the determination of the death of patients, the cutoff level for the ideal test is presented as the sensitivity 1 and specificity 1 (Area under the curve 1.000). If antigens were expressed in tumor cells and infiltrating immune cells, both proteins were subjected to analysis and the total positive percentage was used for the score. The pathologist used internal positive and negative controls to assess a pattern and appropriateness of immuno-detected signal.
Fig. 1.
Representative slides for the staining levels for PD-L1 immune marker in tissue from patients treated with radical cystectomy for bladder cancer
Covariates
The following information was available about the patients: age at diagnosis, sex, race/ethnicity, cellular tumor percentage and percentage necrosis of surgical specimen, clinical tumor, node, metastasis (TNM) stage.
Statistical analysis
Descriptive statistics were computed for all study variables. Continuous variables were described with measures of central tendency and dispersion; categorical variables were summarized as frequencies and percentages. Associations of categorical variables were assessed with Chi square or Fisher’s exact test. Spearman’s correlation was used to test association between the different tumor markers and with age. Differences were considered statistically significant when the two-sided p value was < 0.05.
Results
Baseline characteristics
There were a total of 50 patient tissue samples, equally distributed between males and females. The mean age was 67 + 7 years, and all patients were of Caucasian ethnicity. Half (50%) of the patients had lymph node positive disease. All patients had a cellular tumor percentage of > 70% and a necrosis percentage of < 10%. The TNM stages at the time of surgery were divided as follows: 100% T2, 50% N0 and 50% N1, and 100% M0. Further baseline characteristics are reported in Table 1.
Table 1.
Baseline patient and disease characteristics in 50 patients that underwent radical cystectomy for muscle-invasive bladder cancer
| Median | Range | |
|---|---|---|
| Age (years) | 67 | 50–78 |
| Frequency | Percent | |
|---|---|---|
| Sex | ||
| Female | 25 | 50 |
| Male | 25 | 50 |
| Race | ||
| White | 50 | 100 |
| Pathological stage | ||
| pT Stage | ||
| 2 | 50 | 100 |
| pN Stage | ||
| 0 | 25 | 50 |
| 1 | 25 | 50 |
| pM Stage | ||
| 0 | 50 | 100 |
| Stage | ||
| 2 | 25 | 50 |
| 4 | 25 | 50 |
TNM and disease stage determined by American Joint Committee on Cancer staging system; LN positivity: 0 = negative, 1 = positive; Cellular tumor and necrosis percent of total specimen
Immunohistochemical results
The percentages of each stain scored as high, low or negative are presented in Table 2. Overall, a majority of the stains scored as low: 82% of CD3, 88% for CD4, 76% of CD8, 90% of CD56, 78% of LAG-3, 90% of TIM-3, 62% of PD-1, and 86% of PD-L1. PD-1 had the highest number of slides scored as negative at 38%, followed by CD56 at 24%. The two markers with the highest scoring were LAG-3 at 20% and CD3 at 18% of slides scored as high infiltration, with over 20% infiltration. No significant associations were found between any of the immune markers and sex or age (Tables 3, 4).
Table 2.
IHC stains applied to bladder cancer slides. Frequency and percentages per marker positivity scored as high, low, or negative
| Stain | Score | Frequency | Percent |
|---|---|---|---|
| CD3 score | High | 9 | 18 |
| Low | 41 | 82 | |
| Neg | 0 | 0 | |
| CD4 score | High | 5 | 10 |
| Low | 44 | 88 | |
| Neg | 1 | 2 | |
| CD8 score | High | 0 | 0 |
| Low | 38 | 76 | |
| Neg | 12 | 24 | |
| CD56 score | High | 0 | 0 |
| Low | 45 | 90 | |
| Neg | 5 | 10 | |
| LAG3 score | High | 10 | 20 |
| Low | 39 | 78 | |
| Neg | 1 | 2 | |
| TIM3 score | High | 2 | 4 |
| Low | 45 | 90 | |
| Neg | 3 | 6 | |
| PD-1 score | High | 0 | 0 |
| Low | 31 | 62 | |
| Neg | 19 | 38 | |
| PD-L1 score | High | 0 | 0 |
| Low | 43 | 86 | |
| Neg | 6 | 12 |
Table 3.
Percentage of immunohistochemical marker scoring associated with baseline patient characteristics demonstrating marker scoring correlation to gender; no value reveals any statistical significance
| Gender | Percent | p values | |||
|---|---|---|---|---|---|
| High (%) | Low (%) | Negative (%) | |||
| CD3 | Female | 16 | 84 | 0 | 1 |
| Male | 20 | 80 | 0 | ||
| CD4 | Female | 12 | 84 | 4 | 0.524 |
| Male | 8 | 92 | 0 | ||
| CD8 | Female | 0 | 68 | 32 | 0.321 |
| Male | 0 | 84 | 16 | ||
| CD56 | Female | 0 | 84 | 16 | 0.349 |
| Male | 0 | 96 | 4 | ||
| LAG3 | Female | 20 | 76 | 4 | 0.599 |
| Male | 20 | 80 | 0 | ||
| TIM3 | Female | 0 | 92 | 8 | 0.308 |
| Male | 8 | 88 | 4 | ||
| PD-1 | Female | 0 | 52 | 48 | 0.244 |
| Male | 0 | 72 | 28 | ||
| PDL1 | Female | 0 | 84 | 16 | 0.667 |
| Male | 0 | 92 | 8 | ||
Again, no statistical significance is seen. p values comparing different positivity of stain are given
Table 4.
Percentage of immunohistochemical marker scoring associated with baseline patient characteristics showing marker scoring based on nodal stage and cancer pathologic stage
| N stage | Stage | LN positive | Percent | p values | |||
|---|---|---|---|---|---|---|---|
| High (%) | Low (%) | Negative (%) | |||||
| CD3 | 0 | 2 | No | 20 | 80 | 0 | 1 |
| 1 | 4 | Yes | 16 | 84 | 0 | ||
| CD4 | 0 | 2 | No | 8 | 92 | 0 | 0.524 |
| 1 | 4 | Yes | 12 | 84 | 4 | ||
| CD8 | 0 | 2 | No | 0 | 80 | 20 | 0.742 |
| 1 | 4 | Yes | 0 | 72 | 28 | ||
| CD56 | 0 | 2 | No | 0 | 88 | 12 | 1 |
| 1 | 4 | Yes | 0 | 92 | 8 | ||
| LAG3 | 0 | 2 | No | 16 | 84 | 0 | 0.442 |
| 1 | 4 | Yes | 24 | 72 | 4 | ||
| TIM3 | 0 | 2 | No | 0 | 100 | 0 | 0.062 |
| 1 | 4 | Yes | 8 | 80 | 12 | ||
| PD-1 | 0 | 2 | No | 0 | 68 | 32 | 0.561 |
| 1 | 4 | Yes | 0 | 56 | 44 | ||
| PDL1 | 0 | 2 | No | 0 | 88 | 12 | 1 |
| 1 | 4 | Yes | 0 | 88 | 13 | ||
Again, no statistical significance is seen. p values comparing different positivity of stain are given
The relationship expression of different markers
Correlations between the immunohistochemical biomarkers are presented in Table 5. All significant correlations were positive. The strongest relationship was shown to be between CD8 and PD-1 (0.428, p = 0.002), followed by CD8 and PD-L1 (0.366, p = 0.010). Also, noteworthy, CD3 with CD4 (0.355, p = 0.011) and TIM-3 (0.357, p = 0.011).
Table 5.
Correlation of immunohistochemical biomarkers of bladder cancer
| Markers | Spearman’s rho | p values | |
|---|---|---|---|
| CD3 | CD4 | 0.355* | 0.011 |
| CD8 | 0.263 | 0.065 | |
| CD56 | 0.156 | 0.279 | |
| LAG3 | − 0.080 | 0.580 | |
| TIM3 | 0.357* | 0.011 | |
| PD-1 | 0.152 | 0.291 | |
| PDL1 | 0.177 | 0.223 | |
| CD4 | CD8 | 0.268 | 0.060 |
| CD56 | 0.082 | 0.571 | |
| LAG3 | 0.284* | 0.045 | |
| TIM3 | 0.001 | 0.996 | |
| PD-1 | 0.058 | 0.688 | |
| PDL1 | − 0.093 | 0.525 | |
| CD8 | CD56 | − 0.031 | 0.830 |
| LAG3 | − 0.108 | 0.454 | |
| TIM3 | 0.112 | 0.438 | |
| PD-1 | 0.428* | 0.002 | |
| PDL1 | 0.366* | 0.010 | |
| CD56 | LAG3 | 0.144 | 0.317 |
| TIM3 | − 0.022 | 0.878 | |
| PD-1 | 0.288* | 0.042 | |
| PDL1 | 0.285* | 0.047 | |
| LAG3 | TIM3 | 0.152 | 0.292 |
| PD-1 | 0.030 | 0.837 | |
| PDL1 | − 0.128 | 0.382 | |
| TIM3 | PD-1 | 0.211 | 0.141 |
| PDL1 | 0.218 | 0.133 | |
| PD-1 | PDL1 | 0.232 | 0.109 |
Spearman rho to determine correlation and p < 0.5 used to determine significance. *Significant results. All significant correlations were positive correlations
Discussion
This study of 50 men and women with bladder cancer showed no association between female sex or old age and positivity of immune biomarker staining. Various studies have shown the association between sex and poor outcomes in bladder cancer, but not many researchers examined this association from an immunologic perspective. Population-based studies have shown that female sex is associated with increased mortality after cystectomy even when adjusting for age, time to diagnosis, and stage [13]. Database studies have agreed with these findings showing an increased hazard ratio for recurrence for female bladder cancer patients [5]. There has been an association with interstitial cystitis and bladder pain syndrome suggesting that chronic inflammation could play a role [13, 14]. While this is one of the many proposed mechanisms making females at risk for more aggressive bladder cancer, the immune infiltration and biomarkers have not been extensively studied. We evaluated both cell checkpoint proteins involved in T-cell proliferation and the cellular biomarkers of immune cells; none of these were associated with female sex. Our findings suggest that the effect of sex on the immune response to bladder cancer could be independent of the expression of the immune pathway proteins we measured in our tissue samples.
We also showed no difference in percentage IHC staining compared to patient age. The Surveillance, Epidemiology, and End Results program (SEER) database studies have identified a decreased detection but increased cancer-specific mortality for patients aged over 70 [6]. Similar to the sex discrepancy, our results show no difference in IHC staining, suggesting that this increased mortality is likely not mediated by the immune cell components tested in this study. Many other immune system changes have been investigated to be the cause for the varying cancer prognosis in age and sex. Age has been shown to have an association with neutrophil to lymphocyte ratio, an immune system factor indicative of cancer-specific survival for multiple types of cancer [15, 16]. Innate immune changes including decreased presentation of antigens and eventual antibody production have shown decreased ability in advancing age and between sex [17]. These changes could be the potential source of varying cancer prognosis based on age and sex, as the immune factors examined in our study showed no significant difference.
Our study found overall low detection of TIM-3 and LAG-3 in bladder cancer, 90%, and 78% scoring low, respectively, with 6% and 2% scoring negative. Interestingly, TIM-3 had 20% of slides scored as high infiltration, which was higher than any other marker. The development of new agents targeted at TIM-3 and LAG-3 has increased the importance of these molecules in the treatment of cancer. Our findings do bring into question whether these expensive therapies would be useful in patients with bladder cancer, and should they be offered to them, or not. The activity of TIM-3 and LAG-3 in bladder cancer is significant to consider, from a clinical as well as a healthcare-economics point of view, as recently several phase-I trials opened for LAG-3 (NCT03250832, NCT01968109, NCT03005782) and TIM-3 (NCT02817633) molecules. Although it is such trials that will ultimately inform us about the efficacy of these drugs in various cancers, however, the zeal to test must be balanced against the rationale, and the costs and the risks to the patient.
We found multiple immunohistochemical stains that had positive correlations with other stains. CD3 was positively associated with CD4 and TIM-3. TIM-3 has previously been shown to have high levels in CD3 + cells of B-cell lymphomas, and the higher scoring of TIM-3 was correlated with higher disease stage [18]. Similarly, lung cancer research has seen an increased disease progression in patients with high TIM-3 staining on CD3 T-cells [19], although this correlation also came with CD56 NK cells. Our study showed bladder cancer does not exhibit the same correlation between CD56 and TIM-3, and no stain was associated with worse prognosis indicators. The strongest correlation of stains came from CD8 and PD-1 and PD-L1, Spearman’s rho of 0.428 and 0.366, respectively. This indicates a correlation of cytotoxic T cells with programmed cell death ligand and receptor. In colorectal cancer, a similar association has been seen in cancer draining lymph nodes, where a PD-1 expression on CD8 T-cells was upregulated compared to serum levels [20]. Hepatocellular carcinoma has seen similar results with increased PD-1 and CD8 markers in tumor compared to healthy controls; they also found poor prognosis in the patients with high PD-1 expression [21]. This correlation has not been elucidated in bladder cancer prior to this study.
Our study has several limitations. The first limitation is the relatively small sample size and the retrospective nature of the study design and analysis. The access to tissue of sufficient quality and quantity to perform these tests, and the cost to obtain these, has to be taken into consideration. Speaking further of the study population, a cohort of equal males and females was chosen to elucidate any possible association between sex and the association of immune markers in our tissue samples. The second drawback is the lack of utilization of more sensitive methods of detection of protein expression in bladder cancer specimens such as flow cytometry. We were also unable to evaluate differences in ethnicity as all patients were Caucasian. Finally, with urothelial carcinoma being sensitive to immunotherapy, a lower cutoff point for immune staining could have been more appropriate to use. We think that the small size of the population is probably what made this cutoff point higher. We also think that a higher number of study subjects would have let us use a lower cutoff score. Nonetheless, limitations notwithstanding, our study represents the first study to examine the expression profiles of various targetable immune checkpoint molecules in bladder cancer and demonstrate that there does not exist a relationship between advanced age, sex, and expression of these immune molecules.
Conclusion
We did not find a correlation between age, sex, or lymph node positivity and the expression level of many of the essential proteins active in immune pathways.
Abbreviations
- CD
Cluster of differentiation
- IHC
Immunohistochemistry
- LAG-3
Lymphocyte-activation gene 3
- PD-1
Programmed death receptor 1
- PD-L1
Programmed death receptor ligand 1
- SEER
Surveillance, Epidemiology and End Results program
- TIM-3
T-cell immunoglobulin and mucin-domain 3
- TNM
Tumor, node, metastasis
- UC
Urothelial carcinoma of the bladder
Author contributions
BCH: data collection, manuscript writing. AS: manuscript writing. KD: statistical analysis, manuscript writing. DID: regulatory assistance. SR: immune staining, manuscript writing. NF: immune staining, manuscript writing. SA: study concept, manuscript writing, supervision.
Funding
Southern Illinois University School of Medicine research fund.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the institutional review board of Southern Illinois University School of Medicine (5/13/2015). No human subjects or animals were involved in the study as it was done on stored tissue.
Informed consent
No informed consent was required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bradley C. Holland and Akshay Sood equally contributed.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Messer JC, Shariat SF, Dinney CP, et al. Female gender is associated with a worse survival after radical cystectomy for urothelial carcinoma of the bladder: a competing risk analysis. Urology. 2014;83:863. doi: 10.1016/j.urology.2013.10.060. [DOI] [PubMed] [Google Scholar]
- 3.Zeegers MP, Tan FE, Dorant E, et al. The impact of characteristics of cigarette smoking on urinary tract cancer risk: a meta-analysis of epidemiologic studies. Cancer. 2000;89:630. doi: 10.1002/1097-0142(20000801)89:3<630::AID-CNCR19>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 4.Lucca I, Fajkovic H, Klatte T. Sex steroids and gender differences in nonmuscle invasive bladder cancer. Curr Opin Urol. 2014;24:500. doi: 10.1097/MOU.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 5.Chamie K, Litwin MS, Bassett JC, et al. Recurrence of high-risk bladder cancer: a population-based analysis. Cancer. 2013;119:3219. doi: 10.1002/cncr.28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan ES, Yip SK, Hou SM, et al. Age, tumour stage, and preoperative serum albumin level are independent predictors of mortality after radical cystectomy for treatment of bladder cancer in Hong Kong Chinese. Hong Kong Med J. 2013;19:400. doi: 10.12809/hkmj134122. [DOI] [PubMed] [Google Scholar]
- 7.Shariat SF, Sfakianos JP, Droller MJ, et al. The effect of age and gender on bladder cancer: a critical review of the literature. BJU Int. 2010;105:300. doi: 10.1111/j.1464-410X.2009.09076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paiola M, Knigge T, Duflot A, et al. Oestrogen, an evolutionary conserved regulator of T cell differentiation and immune tolerance in jawed vertebrates? Dev Comp Immunol. 2018;84:48. doi: 10.1016/j.dci.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Sundahl N, Rottey S, De Maeseneer D, et al. Pembrolizumab for the treatment of bladder cancer. Expert Rev Anticancer Ther. 2018;18:107. doi: 10.1080/14737140.2018.1421461. [DOI] [PubMed] [Google Scholar]
- 10.Massari F, Di Nunno V, Cubelli M, et al. Immune checkpoint inhibitors for metastatic bladder cancer. Cancer Treat Rev. 2018;64:11. doi: 10.1016/j.ctrv.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Udall M, Rizzo M, Kenny J, et al. PD-L1 diagnostic tests: a systematic literature review of scoring algorithms and test-validation metrics. Diagn Pathol. 2018;13:12. doi: 10.1186/s13000-018-0689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Troncone G, Gridelli C. The reproducibility of PD-L1 scoring in lung cancer: can the pathologists do better? Transl Lung Cancer Res. 2017;6:S74. doi: 10.21037/tlcr.2017.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tracey E, Watt H, Currow D, et al. Investigation of poorer bladder cancer survival in women in NSW, Australia: a data linkage study. BJU Int. 2014;113:437. doi: 10.1111/bju.12496. [DOI] [PubMed] [Google Scholar]
- 14.Keller J, Chiou HY, Lin HC. Increased risk of bladder cancer following diagnosis with bladder pain syndrome/interstitial cystitis. Neurourol Urodyn. 2013;32:58. doi: 10.1002/nau.22283. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi Y, Horio H, Hato T, et al. Prognostic significance of preoperative neutrophil-lymphocyte ratios in patients with stage I non-small cell lung cancer after complete resection. Ann Surg Oncol. 2015;22(Suppl 3):S1324. doi: 10.1245/s10434-015-4735-5. [DOI] [PubMed] [Google Scholar]
- 16.Proctor MJ, McMillan DC, Morrison DS, et al. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer. 2012;107:695. doi: 10.1038/bjc.2012.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giefing-Kroll C, Berger P, Lepperdinger G, et al. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14:309. doi: 10.1111/acel.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Du H, Xiao TW, et al. Prognostic value of PD-1 and TIM-3 on CD3 + T cells from diffuse large B-cell lymphoma. Biomed Pharmacother. 2015;75:83. doi: 10.1016/j.biopha.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 19.Xu LY, Chen DD, He JY, et al. Tim-3 expression by peripheral natural killer cells and natural killer T cells increases in patients with lung cancer–reduction after surgical resection. Asian Pac J Cancer Prev. 2014;15:9945. doi: 10.7314/APJCP.2014.15.22.9945. [DOI] [PubMed] [Google Scholar]
- 20.Wu X, Zhang H, Xing Q, et al. PD-1(+) CD8(+) T cells are exhausted in tumours and functional in draining lymph nodes of colorectal cancer patients. Br J Cancer. 2014;111:1391. doi: 10.1038/bjc.2014.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi F, Shi M, Zeng Z, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128:887. doi: 10.1002/ijc.25397. [DOI] [PubMed] [Google Scholar]