Abstract
Background
Due to the strong tumoricidal activities of activated natural killer T (NKT) cells, invariant NKT cell-based immunotherapy has shown promising clinical efficacy. However, suppressive factors, such as regulatory T cells (Tregs), may be obstacles in the use of NKT cell-based cancer immunotherapy for advanced cancer patients. Here, we investigated the suppressive effects of Tregs on NKT cells and the underlying mechanisms with the aim to improve the antitumor activities of NKT cells.
Methods
Peripheral blood samples were obtained from healthy donors, patients with benign tumors, and patients with head and neck squamous cell carcinoma (HNSCC). NKT cells, induced with α-galactosylceramide (α-GalCer), and monocyte-derived dendritic cells (DCs) were co-cultured with naïve CD4+ T cell-derived Tregs to investigate the mechanism of the Treg suppressive effect on NKT cell cytotoxic function. The functions and phenotypes of NKT cells were evaluated with flow cytometry and cytometric bead array.
Results
Treg suppression on NKT cell function required cell-to-cell contact and was mediated via impaired DC maturation. NKT cells cultured under Treg-enriched conditions showed a decrease in CD4− NKT cell frequency, which exert strong tumoricidal responsiveness upon α-GalCer stimulation. The same results were observed in HNSCC patients with significantly increased effector Tregs.
Conclusion
Tregs exert suppressive effects on NKT cell tumoricidal function by inducing more CD4− NKT cell anergy and less CD4+ NKT cell anergy. Both Treg depletion and NKT cell recovery from the anergy state may be important for improving the clinical efficacy of NKT cell-based immunotherapy in patients with advanced cancers.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02417-6) contains supplementary material, which is available to authorized users.
Keywords: Head and neck cancer, Regulatory T cell, Immune suppression, NKT cell, Anergy
Introduction
Invariant natural killer T (NKT) cells form a unique small lymphocyte subpopulation characterized by the expression of the invariant T cell receptor, which is composed of the Vα24-Jα18 chain and Vβ11 chain in humans. NKT cells are activated by a glycolipid ligand, α-galactosylceramide (α-GalCer), that is presented by CD1d on antigen-presenting cells (APCs) [1–3]. Upon stimulation by α-GalCer, NKT cells exhibit not only direct tumoricidal activity against various cancers but also indirect antitumor activity via cytotoxic T cells and natural killer cells [4–7].
Our research efforts have focused on the dramatic tumoricidal activity induced by NKT cells, and we previously developed a NKT cell-based immunotherapy for patients with advanced head and neck squamous cell carcinoma (HNSCC) and non-small cell lung cancer (NSCLC) [8, 9]. Our clinical trials showed that the administration of α-GalCer-pulsed APCs induced the proliferation and activation of endogenous NKT cells. Furthermore, the increase in interferon (IFN)-γ-producing NKT cells in the peripheral blood was associated with the prolonged survival of NSCLC patients [10, 11]. Although significant tumor size reductions were detected in some advanced HNSCC patients after NKT cell-based immunotherapy, no case achieved a complete response.
Recent studies have reported that immunosuppressive factors, such as regulatory T cells (Tregs) and myeloid-derived suppressor cells, are higher in patients with advanced cancers [12]. Tregs play a critical role in the maintenance of peripheral immune tolerance [13]. However, in the tumor microenvironment, Tregs suppress the antitumor activity of effector T cells and promote the immune evasion of tumors [14, 15]. Recent studies found that higher Treg levels in the peripheral blood of HNSCC patients were associated with tumor progression and poor prognosis [16, 17]. These findings suggest that the antitumor activities of NKT cells and T cells in cancer patients are both negatively affected by Tregs, and high Treg populations could be a major obstacle for the efficacy of various treatments, including NKT cell-based immunotherapy. However, the suppressive effects of Tregs on NKT cells in humans and the potential mechanisms for these processes are not clear.
Our study investigated the suppressive effect of Tregs on NKT cells with the aim of improving the efficacy of NKT cell-based immunotherapy.
Materials and methods
Blood samples
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized venous blood samples by density gradient centrifugation. Blood samples were obtained from healthy donors, five HNSCC patients (three males and two females with a mean age of 76.6 years, range 65–85 years), and five patients with benign tumors (three males and two females with a mean age of 57.8 years, range 52–64 years), who were diagnosed and received treatment in Chiba University Hospital. The blood samples were diluted 1:1 with saline before being layered onto Ficoll-Paque PLUS (GE Healthcare Bio-Sciences, Chicago, Illinois, USA). After centrifugation, PBMCs were collected from the plasma–Ficoll interphase.
Antibodies and flow cytometry
PBMCs were stained with the following anti-human antibodies for surface staining: anti-CD3-APC (BD Bioscience, San Diego, CA, USA), anti-CD4-Pacific blue (BD Bioscience), anti-CD8-PE-Cy5 (BioLegend, San Diego, CA, USA), anti-CD45RA-FITC (BD Bioscience), anti-CD25-APC-Cy7 (BioLegend), anti-CD39-PE (eBioscience, Santa Clara, CA, USA), anti-CCR4-PE (BD Bioscience), anti-CD11c-Pacific Blue (BioLegend), anti-CD80-PE (BioLegend), anti-CD86-FITC (eBioscience), anti-CD1d-APC (eBioscience), CD14-APC-Cy7 (BioLegend), anti-CD40-APC (BD Bioscience), anti-HLA-DR-PE-Cy5 (BioLegend), anti-Vα24-FITC (Beckman Coulter, Brea, CA, USA), anti-Vβ11-PE (Beckman Coulter), and anti-CTLA-4-PE (BioLegend). Additionally, anti-Foxp3-eflour660 (eBioscience), anti-IL-4-PE (BD Bioscience), anti-IL-10-APC (BD Bioscience), anti-IFN-γ-PE (BioLegend), anti-TNF-α-Pacific Blue (BioLegend), anti-IL-12-FITC (eBioscience) were used for intracellular staining. Cell surface and intracellular staining were each performed in accordance with the manufacturer’s protocols. The expressions of surface molecules and cytokine production levels were evaluated by multicolor flow cytometry using a FACSCanto II (BD Biosciences). Data were analyzed with FlowJo software (FlowJo, LLC, Ashland, OR, USA).
Induction of Tregs from PBMCs
Naïve CD4+ T cells were isolated from the PBMCs of healthy donors using the Naïve CD4+ T cell isolation kit II (Miltenyi Biotec, Germany) in accordance with the manufacturer’s protocol. Isolated CD4+ T cells were cultured with IL-2 (100 JRU/ml, Shionogi, Japan), TGF-β (5 ng/ml, PeproTech, Rocky Hill, NJ, USA), and anti-CD3/28-coated beads (Life Technologies, Carlsbad, CA, USA) in RPMI1640 medium supplemented with 10% fetal bovine serum and penicillin–streptomycin (Gibco, USA) (RPMI1640 complete medium) for 7 days.
Induction of activated NKT cells and preparation of dendritic cells (DCs)
PBMCs obtained from healthy donors were cultured with α-GalCer (100 ng/ml) and IL-2 (100 JRU/ml) in RPMI1640 complete medium. After 7 days, the NKT cells were isolated via positive selection of Vα24-FITC-stained cells. Monocyte-derived DCs were prepared as follows: CD14+ cells were isolated from PBMCs by autoMACS using CD14+ MicroBeads (Miltenyi Biotec) and then cultured with IL-4 (50 ng/ml, PeproTech) and GM-CSF (75 µg/ml, GeneTech, China) for 7 days.
T cell proliferation assay
CD4+ cells and CD8+ cells were isolated from peripheral blood obtained from healthy donors using the RosetteSep Human CD4+ T cell Enrichment Cocktail and Human CD8+ T cell Enrichment Cocktail (STEMCELL, Vancouver, BC, Canada). T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) using the CellTrace CFSE Cell Proliferation Kit (Molecular Probes, USA) in accordance with the manufacturer’s instructions. CFSE-labeled T cells (5 × 105/well) were stimulated with anti-CD3/28-coated beads in the presence of IL-2 (100 JRU/ml) and cultured with induced Tregs (2.5 × 105/well) or as a counterpart to Tregs, cultured with CD4+ T cells (2.5 × 105/well) and anti-CD3/28-coated beads and IL-2 (100 JRU/ml) for 7 days following isolation from peripheral blood, as mentioned above, for 72 h. The CFSE dilutions in these T cells were then analyzed using a FACSCanto II. The proliferation index was calculated using FlowJo software as the number of divisions divided by the number of cells that went into the division.
Suppression assays with NKT cells
Purified NKT cells (5 × 105) were co-cultured with Tregs at different ratios in the presence of IL-2 (100 JRU/ml) and α-GalCer-pulsed monocyte-derived DCs (5 × 105) for 7 days, and the proliferation and cell number of NKT cells were analyzed. The cytokine productions of NKT cells (2 × 105) were evaluated using FACSCanto II after a 4-h co-culture with α-GalCer-pulsed monocyte-derived DCs (2 × 105) and Tregs (2 × 105).
For experiments in Transwell plates (Corning, USA), NKT cells (5 × 105) and α-GalCer-pulsed DCs (5 × 105) were placed in the lower wells of Transwell chambers, and Tregs (5 × 105) were placed in the upper wells in the presence of IL-2 (100 JRU/ml). To block CD39, cytotoxic T-lymphocyte-associated antigen (CTLA)-4, or programmed cell death ligand 1 (PD-L1) on the surface of Tregs, the Tregs were incubated with anti-CD39 antibody (10 µg/ml, BioLegend), anti-CTLA-4 antibody (20 µg/ml, BioLegend), anti-PD-L1 antibody (20 µg/ml, BioLegend), respectively, or with an isotype control antibody (20 µg/ml, BioLegend) for 1 h, after which the cells were washed to remove unbound antibodies. The blocked Tregs were added to the culture medium of NKT cells in the presence of IL-2 and α-GalCer. On day 7, the NKT cell proliferation was analyzed. To examine the direct suppressive effect of Tregs on NKT cells, purified NKT cells were re-stimulated with plate-bounded CD1d dimer in the presence or absence of Tregs. After 4 h of stimulation, NKT cells were collected, and intracellular staining was performed to determine cytokine production.
Suppression assays with DCs
Monocytes were isolated from PBMCs using autoMACS with CD14+ MicroBeads (Miltenyi Biotec). The isolated CD14+ cells were cultured with IL-4 (50 ng/ml) and GM-CSF (75 µg/ml) for 7 days. After 7 days of culture, monocyte-derived DCs were stimulated with 0.1 KE/ml of OK-432 (Chugai Pharmaceuticals, Japan) to induce maturation, in the presence (Treg-DC) or absence (control DC) of Tregs. The surface molecules on DCs and the levels of IL-10 and IL-12 production were analyzed using a FACSCanto II.
Subsequently, CD4+ cells were depleted from both DC fractions (Treg-DC and control DC) using autoMACS, and α-GalCer was loaded on these isolated DC fractions. Then, purified NKT cells were restimulated with the α-GalCer-loaded DCs.
Induction assay of anergic NKT cells
NKT cells were cultivated from PBMCs with different concentration of Tregs; Tregs were added into the culture medium of PBMCs at a PBMC/Treg ratio of 4:1 (Treg-enriched condition). For the control condition, Tregs were not added into the culture medium. Vα24+Vβ11+ cells were isolated on day 7 using a FACSAria (BD Biosciences). Purified NKT cells were re-stimulated with plate-bounded CD1d dimer (BD Biosciences). The supernatants of these cells were collected at 24 h after stimulation. A cytometric bead array (BD Biosciences) was performed in accordance with the manufacturer’s protocols to evaluate the cytokine production.
Functional assay of CD4+ NKT cells, CD4−CD8− NKT cells, and CD8+ NKT cells
NKT cells were cultured as described above. On day 7 of culture, the Vα24+ cells were isolated using autoMACS. On day 8, the CD4+ NKT cells, CD4−CD8− (double negative; DN) NKT cells, and CD8+ NKT cells were sorted by FACSAria based on the expression of CD4 and CD8 on the NKT cells. On day 9, each isolated subset of NKT cells was re-stimulated with plate-bounded CD1d dimer. After a 24-h incubation, the supernatant of these cells was collected and used in a cytometric bead array to evaluate the cytokine production. To evaluate the cytotoxic activities of each subset of NKT cells, a cytotoxicity assay was also performed on day 9. NKT cells were cocultured with K562 cells at effector/target (E/T) ratios ranging from 2.5:1 to 20:1 in 96-well plates. Cancer cell lines were labeled using a CellTrace Violet Cell Proliferation Kit (CTV) (Life Technologies, USA), in accordance with the manufacturer’s protocol. After 4 h of cancer and NKT cell coculture, the cells were stained using a Dead Cell Apoptosis Kit (Invitrogen), in accordance with the manufacturer’s protocol, to detect apoptotic cells. Cytotoxicity was assessed based on the amount of Annexin V+ cells.
Surface phenotype and proliferative responses of NKT cells in patients with benign tumors or HNSCC
PBMCs were collected from HNSCC patients and from patients with benign tumors. Following α-GalCer stimulation, the PBMCs were cultured for 7 days. The NKT cell expansion and the NKT cell CD4 and CD8 expressions were evaluated using a FACSCanto II.
Statistical analyses
Statistical analyses of the means and correlations were conducted using a parametric unpaired t test and a two-way ANOVA, respectively. All p values were two-sided, and p values < 0.05 were considered statistically significant.
Results
Characteristics of induced Tregs and their suppressive effect on T cells
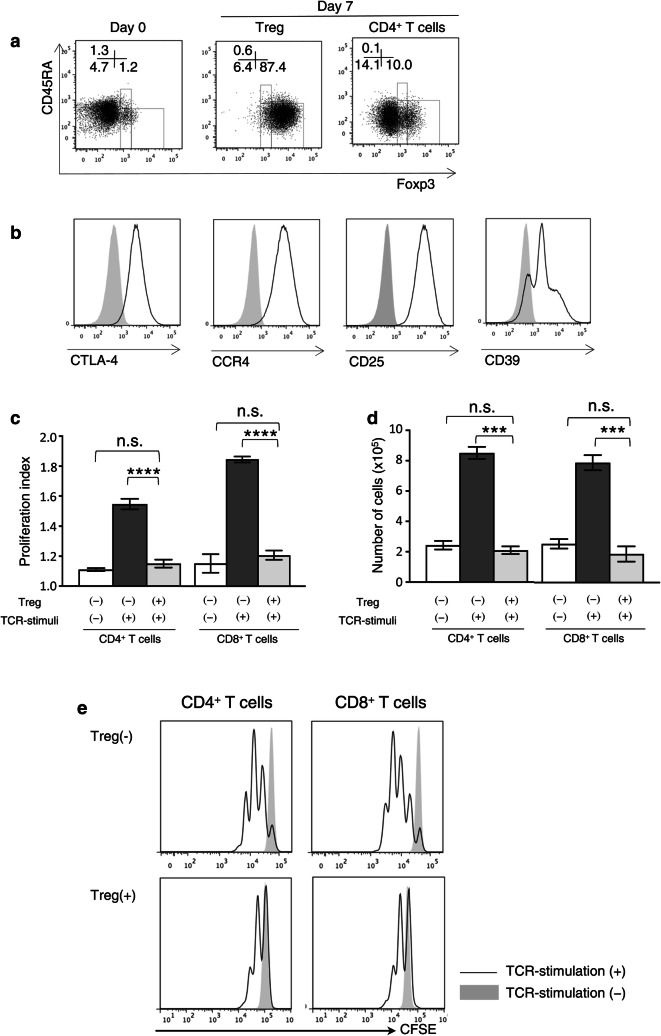
The majority of Tregs derived from the PBMCs of healthy donors were CD45RA−Foxp3high Tregs (effector Tregs, eTregs). In contrast, cultured CD4+ T cells included a low frequency of eTregs. Representative staining profiles of the CD45RA and Foxp3 expression on these Tregs and cultured CD4+ T cells are shown in Fig. 1a. The expression levels of CTLA-4, CCR4, CD25, and CD39 in the Tregs were high (Fig. 1b). We next examined the suppressive effects of Tregs against T cells. CFSE-labeled isolated CD4+ T cells and CD8+ T cells were stimulated with anti-CD3/28-coated beads for 72 h in the presence of Tregs or cultured CD4+ T cells. The proliferation levels of both CD4+ and CD8+ T cells were significantly suppressed in the presence of Tregs (Fig. 1c–e).
Fig. 1.
Characteristics and suppressive effects of Tregs. a Representative profiles of Tregs before and after 7 days of cultivation and of cultured CD4+ T cells. b Expression of surface molecules on Tregs. The gray-shaded histogram: the isotype control, the unshaded histograms: each molecule. c The proliferation index, d numbers and e representative proliferation profiles of CD4+ cells and CD8+ cells co-cultured with Tregs (Treg (+)) or control CD4+ T cells (Treg (−)) in the presence of anti-CD3/28-coated beads for 72 h. Data are shown as mean ± SD. Statistical comparisons were performed using unpaired t tests. ***p < 0.001, ****p < 0.0001, ns not significant. Results are representative of three experiments. CTLA-4 cytotoxic T-lymphocyte-associated antigen, CFSE carboxyfluorescein succinimidyl ester
Suppressive effect of Tregs on NKT cells
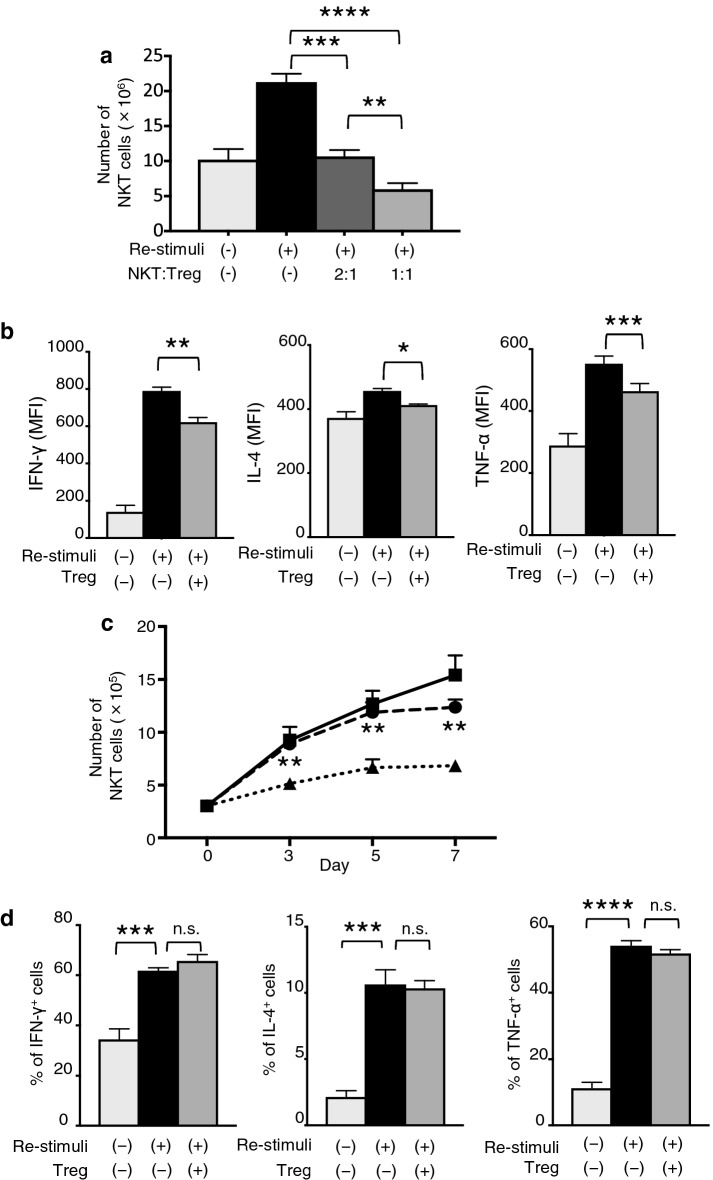
We next examined the suppressive effects of Tregs on NKT cells. The purity of the cultivated and isolated NKT cells was greater than 95% (Fig. S1A). Purified NKT cells were co-cultured with Tregs at various ratios. The NKT cell proliferation levels were significantly suppressed in a dose-dependent manner following the addition of Tregs (Fig. 2a). The IFN-γ and TNF-α production levels of NKT cells were also significantly suppressed in the presence of Tregs. In contrast, there was a significant but minor increase in the IL-4 production level of NKT cells cultured without Tregs in comparison with that of NKT cells cultured with Tregs (Fig. 2b and S1B).
Fig. 2.
Treg-mediated NKT cell suppression via cell-to-cell contact. a Numbers of NKT cells co-cultured with Tregs at 7 days after re-stimulation with α-GalCer-pulsed DCs. b Cytokine secretions of NKT cells upon α-GalCer-pulsed DC stimulation with or without Tregs by the intracellular flow cytometry staining. c NKT cells and α-GalCer-pulsed DCs were seeded with Tregs in Transwell chambers as indicated (NKT cells:DCs:Tregs = 1:1:1). The NKT cells were co-cultured with DCs and Tregs in the same chamber (dotted line), or NKT cells and DCs were seeded in the lower chambers and Tregs were seeded in the upper chambers (dash line), or NKT cells and DCs were cultured without Tregs (solid line). Statistical analyses were performed between the NKT cells and DCs cultured with Tregs in the same chamber and those cultured in separate chambers. d Cytokine secretion of NKT cells upon plate-bound α-GalCer-loaded CD1d dimer stimulation with or without Tregs was evaluated by intracellular flow cytometry staining. Data are shown as mean ± SD. Statistical comparisons were performed using unpaired t tests. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns not significant. Results are representative of at least three experiments. MFI mean fluorescence intensities
Treg-mediated NKT cell suppression via cell-to-cell contact
To investigate the suppressive mechanism of Tregs on NKT cells, Tregs and NKT cells were co-cultured using Transwell plates. The level of proliferation by NKT cells cultured separately from Tregs in the Transwell system was equivalent to that of NKT cells cultured without Tregs (Fig. 2c). The suppressed proliferation of NKT cells co-cultured with Tregs was not recovered by prior blockades of CD39, CTLA-4, or PD-L1 on the surface of Tregs (data not shown). We next examined the Treg suppressive effects on NKT cell activation by plate-bound α-GalCer stimulation in the absence of DCs. The presence of Tregs did not suppress the cytokine production by NKT cells (Fig. 2d and S1C).
Suppressive effect of Tregs on DC maturation
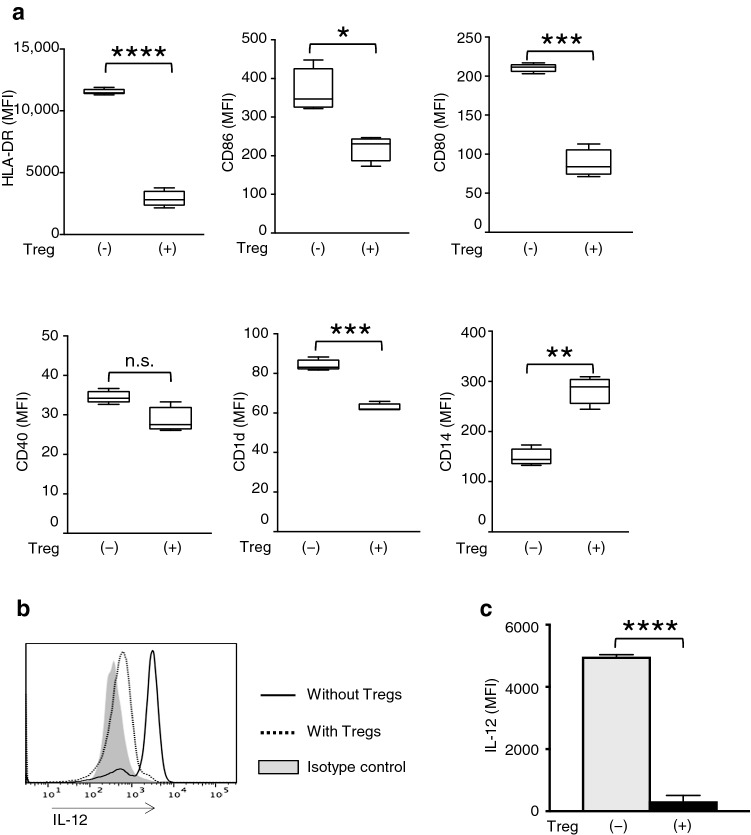
We next examined the suppressive effects of Tregs on DCs. The expression levels of HLA-DR, CD86, CD80, and CD1d on monocyte-derived DCs were significantly lower in the Treg-DC than the Control-DC; in contrast, the level of the monocyte marker CD14 was significantly higher, and no significant difference between these groups was found for the expression of CD40 (Fig. 3a, S2C). Furthermore, a significantly lower level of IL-12 production by DCs was observed in the presence of Tregs (Fig. 3b, c). Additionally, Treg-DC activated NKT cells equivalent to Control-DC if Tregs were depleted from the DC fraction prior to the co-culture with NKT cells (Fig. S2A and D). Of note, the majority of CD4+ cells were depleted from the mixture of Tregs and DCs (Fig. S2B). These results were confirmed in another two experiments.
Fig. 3.
Suppressive effects of Tregs on DCs. a The expression levels of the surface molecules HLA-DR, CD86, CD80, CD40, CD1d, and CD14 on monocyte-derived DCs co-cultured with or without Tregs. b A representative FACS profile and c MFI of IL-12 expression on DCs. Data are shown as mean ± SD. Statistical comparisons were performed using unpaired t tests. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns not significant. Results are representative of at least three experiments. MFI mean fluorescence intensities
Change in the NKT subset ratio and anergy induction of NKT cells by Tregs
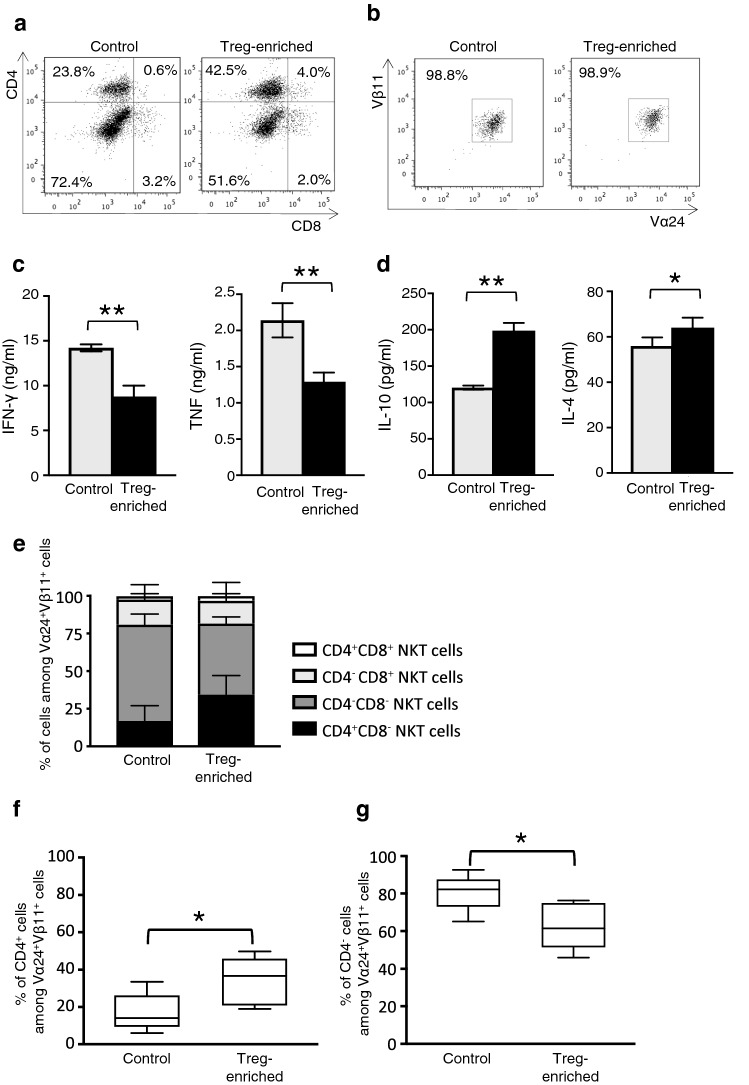
The effects of Tregs on the surface phenotype of NKT cells were examined. Representative CD4 and CD8 expression profiles of NKT cells cultured for 7 days under the Treg-enriched condition or control condition are shown in Fig. 4a. The purity of the isolated NKT cells in these two groups was equal (Fig. 4b). The levels of IFN-γ and TNF production by NKT cells cultivated under the Treg-enriched condition were significantly lower compared with those of NKT cells cultivated under the control condition, even though Tregs were depleted at the time of re-stimulation (Fig. 4c). In contrast, NKT cells cultured under the Treg-enriched condition secreted higher amounts of IL-10 and IL-4 compared with NKT cells cultured under the control condition (Fig. 4d). The frequency of the CD4+ phenotype in Vα24+Vβ11+ NKT cells (CD4+ NKT cells) was higher under the Treg-enriched condition compared with the control condition (Fig. 4e, f). In contrast, the frequency of the CD4− phenotype (CD4− NKT cells) was significantly lower in Vα24+Vβ11+ NKT cells cultured under the Treg-enriched condition compared with the control condition (Fig. 4e, g).
Fig. 4.
Change in the NKT cell subset ratio and cytokine production by Tregs. a Representative FACS profiles of the CD4/CD8 expressions on NKT cells cultured under control conditions and Treg-enriched conditions for 7 days, and b the purity of sorted NKT cells. c IFN-γ and TNF, d IL-4 and IL-10 production levels were evaluated using a cytometric bead array stimulation with α-GalCer-loaded CD1d dimers. e Quantification of each fraction and f the frequencies of CD4+ NKT cells and g CD4− NKT cells after 7 days of cultivation under Treg-enriched or control conditions. Data are shown as mean ± SD. Statistical comparisons were performed using unpaired t tests. *p < 0.05, **p < 0.01. Results are representative of assays performed using cells from four healthy donors. Control control conditions, Treg-enriched Treg-enriched conditions
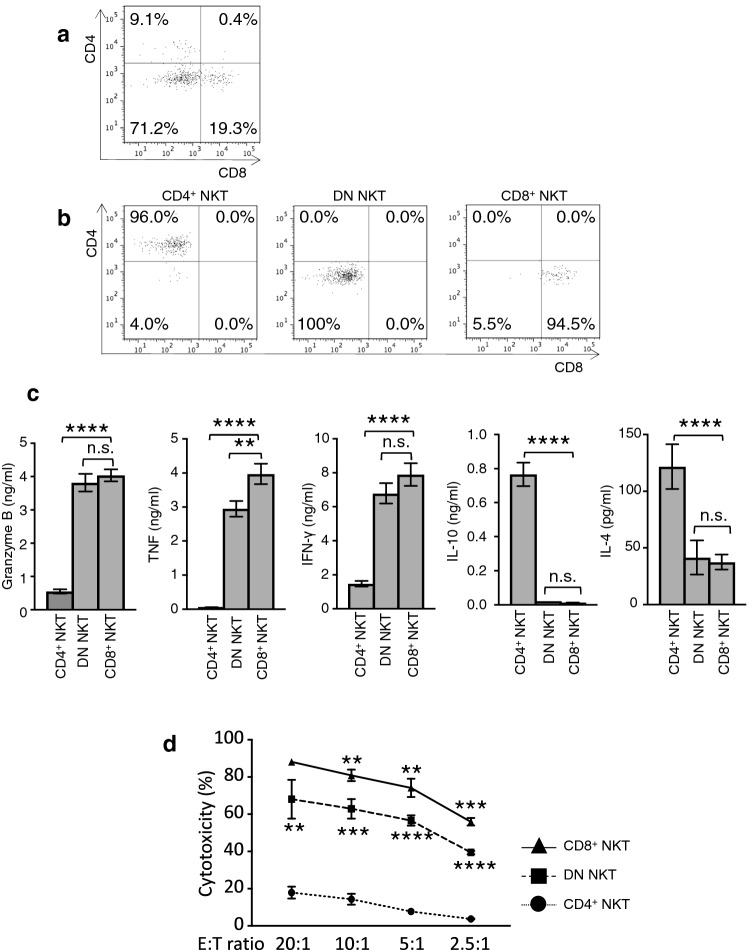
Functional characteristics of CD4+ NKT cells, DN NKT cells, and CD8+ NKT cells
Cultured NKT cells were sorted by a FACSAria into subsets of CD4+ NKT cells, DN NKT cells, and CD8+ NKT cells. Representative profiles of the pre-sorted NKT cells and of each sorted subset of NKT cells are shown in Fig. 5a, b. The amounts of Granzyme B, TNF, and IFN-γ secreted from CD4+ NKT cells were significantly lower than those secreted from DN NKT cells or CD8+ NKT cells. In contrast with the production levels of those cytokines, the IL-10 and IL-4 secretion levels from CD4+ NKT cells were significantly higher compared with levels from the other two groups (Fig. 5c). The cytotoxic activity of CD4+ NKT cells was dramatically weaker than those of DN NKT cells and CD8+ NKT cells (Fig. 5d). A representative Annexin V-PI staining pattern for each subset at an E/T ratio of 10:1 is shown in Figure S3A.
Fig. 5.
Functional characteristics of NKT cell subsets. Representative FACS profiles of cultured NKT cells are shown for a unsorted NKT cells and b each sorted subset of NKT cells. c Cytokine production from CD4+ NKT cells, DN NKT cells, and CD8+ NKT cells. d The cytotoxicity of CD8+ NKT cells (solid line), DN NKT cell (dashed line) and CD4+ NKT cells (dotted line) targeted to K562 cells. Statistical analyses were performed comparing the cytotoxic activity levels between the CD4+ NKT cells and DN NKT cells and those between the DN NKT cells and CD8+ NKT cells at every E/T ratio. Data are shown as mean ± SD. Statistical comparisons were performed using unpaired t tests. **p < 0.01, ***p < 0.001, ****p < 0.0001. ns not significant. Results are representative of assays performed using cells from three healthy donors. CD4+ NKT: CD4+ NKT cells, DN NKT: CD4−CD8− NKT cells, and CD8+ NKT: CD8+ NKT cells
Surface phenotype and proliferative responses of NKT cells in patients with benign tumors or HNSCC
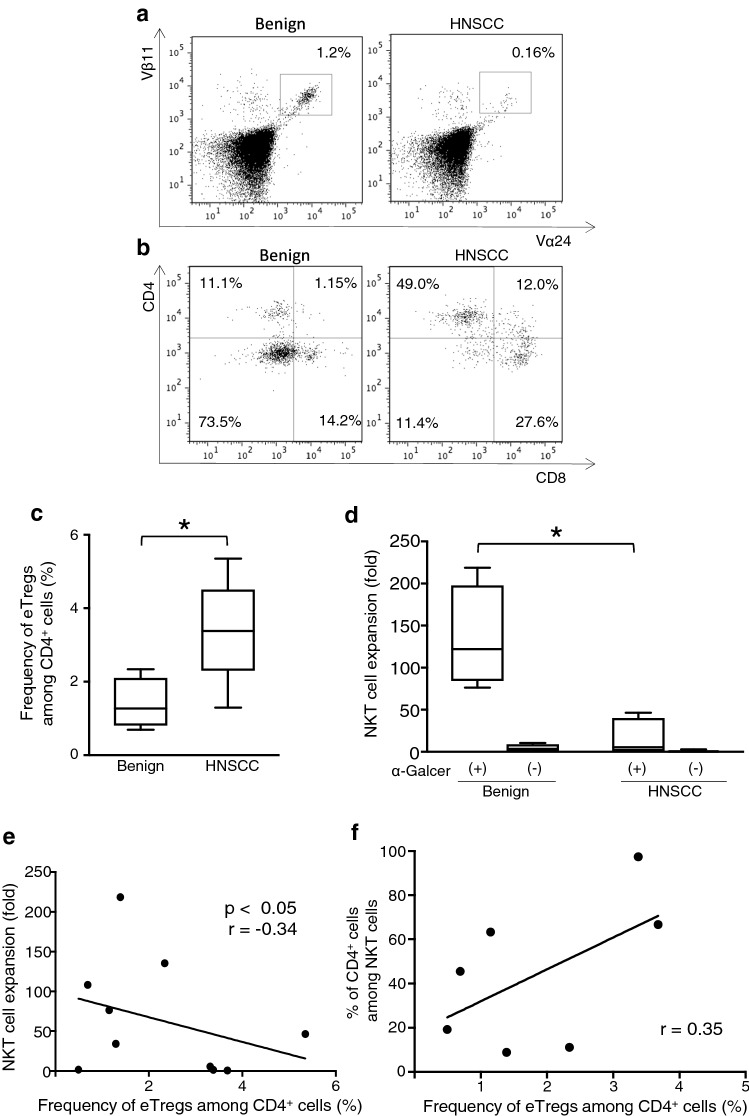
PBMCs were obtained from five patients with HNSCC and five patients with benign tumors, and then cultured with α-GalCer for 7 days. The frequencies of Vα24+Vβ11+ NKT cells among the PBMCs from HNSCC patients were comparable to those from benign tumor patients (data not shown). However, the expansion of NKT cells from HNSCC patients was lower compared with that from benign tumor patients (Fig. 6a, d). The percentage of Vα24+Vβ11+ NKT cells with a CD4+ phenotype was higher in HNSCC patients than in benign tumor patients, whereas the percentage of Vα24+Vβ11+ NKT cells with a CD4− phenotype, especially a CD4−CD8− phenotype, was lower. The results of representative cases are shown in Fig. 6b, and the remaining participant data are shown in Fig. S4. Data obtained from a benign patient (Benign 05) and two HNSCC patients (HNSCC 04 and 05) showed extremely low NKT cell expansion and undetectable CD4/CD8 expression. The percentage of eTregs among CD4+ cells in HNSCC patients was significantly higher, more than twofold, compared with that in benign tumor patients (Fig. 6c). Additionally, the levels of NKT cell expansion and the frequency of eTregs among CD4+ cells exhibited a significant negative correlation (Fig. 6e). Although two patients with a high frequency of eTregs among CD4+ cells on day 0 had a higher frequency of CD4+ NKT cells among NKT cells in the seven analyzable patients, a significant correlation was not detected (Fig. 6f).
Fig. 6.
Characteristics of HNSCC patient-derived NKT cell subsets. a Representative profile of cultured NKT cells and b the CD4/CD8 expressions from a benign tumor patient and head and neck squamous cell carcinoma (HNSCC) patient on day 7. c Frequency of effector Tregs (eTreg) among CD4+ cells on day 0, and d proliferation of NKT cells after 7 days of cultivation with or without α-GalCer from benign and HNSCC patients. e Correlation between the eTreg frequency among CD4+ cells on day 0 and the level of NKT cell expansion after 7 days of cultivation. f Correlation between the eTreg frequency among CD4+ cells on day 0 and CD4+ NKT cells. Data are shown as mean ± SD. Statistical comparisons were performed using unpaired t tests. *p < 0.05. eTregs effector Tregs, HNSCC head and neck squamous cell carcinoma
Discussion
Tregs are divided into functional subsets based on the expression of CD45RA and Foxp3 [18]. CD45RA−Foxp3high Tregs (eTregs) exert more vigorous suppressive effects compared with CD45RA+Foxp3low Tregs (naïve Tregs). A previous report demonstrated a correlation between an increased frequency of peripheral eTregs before treatment and the poor prognosis of cancer patients [16]. Here, Tregs derived from naïve CD4+ T cells showed a CD45RA−Foxp3high and CTLA4+ effector phenotype and suppressed the proliferative activity of NKT cells as well as those of CD4+ T cells and CD8+ T cells in a dose-dependent manner.
The results of our transwell experiments revealed that the suppressive effects of Tregs on NKT cells required cell-to-cell contact, indicating that secreted factors, such as immunosuppressive cytokines IL-10 and TGF-β, were not involved in this effect. CTLA-4 and CD39 expressed on Tregs have been shown to play an important role in the regulation of effector T cells by cell-to-cell contact [17, 19, 20]. However, the blockade of these suppressive molecules on Tregs by specific antibodies did not restore the proliferation of NKT cells. Additionally, Tregs did not exert this suppressive effect on the cytokine secretions from NKT cells in the absence of DCs.
Because the above results suggest that Tregs cannot exert a suppressive effect on NKT cells directly, we focused on the impact of Tregs on DCs. In the tumor microenvironment, DC function has been found to be inclined toward tolerance [21]. It has also been reported that Tregs suppress the maturation of DCs [22, 23]. Immature DCs are not only poorly immunogenic, they also convert naïve T cells into Tregs [23, 24]. Here, a downregulation of HLA-DR, CD86, CD80, and CD1d on the surface of DCs and a lower level of IL-12 production by DCs was observed in the presence of Tregs. However, no significant difference in the IL-10 production of DCs was observed with or without Tregs (data not shown), and the depletion of Tregs restored DC activating function toward NKT cells. These results indicate that Tregs did not induce tolerogenic DCs but rather suppressed the differentiation of immature DCs into mature DCs. Furthermore, the co-existence of Tregs and DCs at the phase of antigen presentation was considered the pivotal mechanism involved in the Treg suppression of NKT cell function. Immature DCs, which express low levels of co-stimulatory molecules, induce T cell anergy [25, 26]. The optimal responses of NKT cells also require CD28/B7 interactions [27].
The levels of IFN-γ and TNF secretion by NKT cells cultured under the Treg-enriched condition were lower compared with cells cultured under control conditions, despite a depletion of Tregs from the culture. These results indicate that activation stimuli by immature DCs induced NKT cells into an anergic state. However, the levels of IL-10 and IL-4 production by NKT cells cultured under a Treg-enriched condition were higher compared with culture under the control condition. Thus, all subsets of NKT cells may not be equally induced into anergic state by Tregs. Given the increase in the ratio of CD4+ NKT cells to CD4− NKT cells under the Treg-enriched culture condition, CD4− NKT cells are more likely to be induced into the anergic state.
Recent studies showed that peripheral NKT cells are heterogeneous and can be divided into functional subsets based on the CD4 and CD8 expression on their cell surfaces [28], specifically CD4+, CD4−CD8− (DN), and CD8+ NKT cells [29–32]. As shown in our results, the DN NKT cells and CD8+ NKT cells secrete inflammatory cytokines and exert strong tumoricidal activities, which play a pivotal role in the anti-tumor activity. In contrast, the CD4+CD8− NKT cells have only weak tumoricidal activity. Therefore, the increased percentage of the CD4+ NKT cell subset caused by Tregs may also contribute to diminishing the NKT cell cytotoxic function. Furthermore, CD4+ NKT cells promote Treg differentiation as well as the M2 differentiation of tumor-associated macrophages via a high level of IL-10 production, resulting in tumor microenvironments being inclined toward immune suppression [33]. NKT cells obtained from HNSCC patients who had increased eTreg levels were also skewed toward a CD4+ NKT cell-dominant population. The high sensitivity of CD4− NKT cells to anergy induced by Tregs might contribute to tumor progression in HNSCC patients [32].
Only a few studies have focused on the mechanisms of the Treg suppressive function on NKT cells [34, 35]. Our studies indicate that Tregs suppress the NKT cell cytotoxic function through the induction of anergy targeting mainly CD4− NKT cells. These results also demonstrate that Tregs are one of the major obstacles to the tumoricidal activities of both NKT cells and T cells in the treatment of cancer patients. Therefore, novel cancer therapies aimed at the elimination of Tregs and the improvement of DC function are desired for the recovery of NKT cells from their anergic state, especially for use in advanced cancer patients with a high population of Tregs. In our previous clinical trials for NSCLC and HNSCC patients, an increased frequency peripheral NKT cells and IFN-γ production by these cells were observed as a result of the administration of ex vivo-activated NKT cells [9, 11]. The percentage of CD4− NKT cells was also increased after this treatment in some cancer patients. Other studies showed that a PD-L1 blockade on DCs enhanced the NKT cell function of NSCLC patients and that the NKT cell anergy can be reversed by an exogenous addition of abundant IL-2 [36, 37]. These findings indicate that NKT cells could recover their functions under conducive conditions, even in advanced cancer patients for whom Treg suppression has been already established [36, 38].
Further investigations of NKT anergy are required. However, we propose that not only Treg depletion but also the recovery of NKT cells from anergy may be required to improve the efficacy of NKT cell-based immunotherapy for patients with increased levels of Tregs.
Conclusions
Our study demonstrates that Tregs suppressed NKT cell function in a cell-to-cell contact-dependent manner via preventing DC maturation. The higher levels of immature DCs induce more anergy in CD4− NKT cells and less anergy in CD4+ NKT cells. The corresponding decrease in the CD4− NKT cell subset may participate in the suppression of NKT cell tumoricidal activity. Therefore, the development of a Treg-targeted therapy could be a novel immunotherapy strategy for reinforcing the tumoricidal function of NKT cells.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Saori Tagi for her excellent technical assistance and thank Katie Oakley, PhD, from Edanz Group (http://www.edanzediting.com/ac) for editing a draft of this paper.
Abbreviations
- α-GalCer
α-Galactosylceramide
- APCs
Antigen-presenting cells
- CFSE
Carboxyfluorescein succinimidyl ester
- CTLA
Cytotoxic T-lymphocyte-associated antigen
- DCs
Dendritic cells
- E/T
Effector/target
- HNSCC
Head and neck squamous cell carcinoma
- IFN
Interferon
- NKT cells
Invariant natural killer T cells
- MFI
Mean fluorescence intensity
- NSCLC
Non-small cell lung cancer
- PBMCs
Peripheral blood mononuclear cells
- PD-L1
Programmed cell death ligand 1
- Tregs
Regulatory T cells
Author contributions
FI, DS, SM and YO designed the study. FI, DS, MT, TK performed experiments. FI, DS, MK, TK, NK, KY, TI, TN, SM and YO discussed the experimental design. MT and TK contributed to analysis and interpretation. NK, KY and TI contributed to collecting the samples and analyzing the data. FI and DS analyzed the data and wrote the manuscript. TN, SM and YO critically revised the manuscript. All authors reviewed the manuscript.
Funding
This study was funded by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) KAKENHI (Grant numbers 15K10799 and 17K16892).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
Blood samples from all healthy donors and patients were collected only after obtaining written informed consent. All procedures involving human participants were approved on August 2, 2013 by the institutional review board of Chiba University Hospital and were conducted in accordance with the Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study. All participants consented to providing their peripheral blood and the use of their specimens and data for research and for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fumie Ihara and Daiju Sakurai contributed equally to this study.
References
- 1.Vicari AP, Zlotnik A. Mouse NK1.1+ T cells: a new family of T cells. Immunol Today. 1996;17:71–76. doi: 10.1016/0167-5699(96)80582-2. [DOI] [PubMed] [Google Scholar]
- 2.Taniguchi M, Koseki H, Tokuhisa T, Masuda K, Sato H, Kondo E, Kawano T, Cui J, Perkes A, Koyasu S, Makino Y. Essential requirement of an invariant Vα 14 T cell antigen receptor expression in the development of natural killer T cells. Proc Natl Acad Sci USA. 1996;93:11025–11028. doi: 10.1073/pnas.93.20.11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of Vα 14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 4.Kawano T, Nakayama T, Kamada N, Kaneko Y, Harada M, Ogura N, Akutsu Y, Motohashi S, Iizasa T, Endo H, Fujisawa T, Shinkai H, Taniguchi M. Antitumor cytotoxicity mediated by ligand-activated human Vα 24 NKT cells. Cancer Res. 1999;59:5102–5105. [PubMed] [Google Scholar]
- 5.Motohashi S, Nakayama T. Clinical applications of natural killer T cell-based immunotherapy for cancer. Cancer Sci. 2008;99:638–645. doi: 10.1111/j.1349-7006.2008.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nieda M, Nicol A, Koezuka Y, Kikuchi A, Lapteva N, Tanaka Y, Tokunaga K, Suzuki K, Kayagaki N, Yagita H, Hirai H, Juji T. TRAIL expression by activated human CD4+Vα 24NKT cells induces in vitro and in vivo apoptosis of human acute myeloid leukemia cells. Blood. 2001;97:2067–2074. doi: 10.1182/blood.V97.7.2067. [DOI] [PubMed] [Google Scholar]
- 7.Toura I, Kawano T, Akutsu Y, Nakayama T, Ochiai T, Taniguchi M. Cutting edge: inhibition of experimental tumor metastasis by dendritic cells pulsed with α-galactosylceramide. J Immunol. 1999;163:2387–2391. [PubMed] [Google Scholar]
- 8.Uchida T, Horiguchi S, Tanaka Y, Yamamoto H, Kunii N, Motohashi S, Taniguchi M, Nakayama T, Okamoto Y. Phase I study of α-galactosylceramide-pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol Immunother. 2008;57:337–345. doi: 10.1007/s00262-007-0373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motohashi S, Ishikawa A, Ishikawa E, Otsuji M, Iizasa T, Hanaoka H, Shimizu N, Horiguchi S, Okamoto Y, Fujii S, Taniguchi M, Fujisawa T, Nakayama T. A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2006;12:6079–6086. doi: 10.1158/1078-0432.CCR-06-0114. [DOI] [PubMed] [Google Scholar]
- 10.Kunii N, Horiguchi S, Motohashi S, Yamamoto H, Ueno N, Yamamoto S, Sakurai D, Taniguchi M, Nakayama T, Okamoto Y. Combination therapy of in vitro-expanded natural killer T cells and α-galactosylceramide-pulsed antigen-presenting cells in patients with recurrent head and neck carcinoma. Cancer Sci. 2009;100:1092–1098. doi: 10.1111/j.1349-7006.2009.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamasaki K, Horiguchi S, Kurosaki M, Kunii N, Nagato K, Hanaoka H, Shimizu N, Ueno N, Yamamoto S, Taniguchi M, Motohashi S, Nakayama T, Okamoto Y. Induction of NKT cell-specific immune responses in cancer tissues after NKT cell-targeted adoptive immunotherapy. Clin Immunol. 2011;138:255–265. doi: 10.1016/j.clim.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Frydrychowicz M, Boruczkowski M, Kolecka-Bednarczyk A, Dworacki G. The dual role of Treg in cancer. Scand J Immunol. 2017;86:436–443. doi: 10.1111/sji.12615. [DOI] [PubMed] [Google Scholar]
- 13.Wing JB, Sakaguchi S. Foxp3+ Treg cells in humoral immunity. Int Immunol. 2014;26:61–69. doi: 10.1093/intimm/dxt060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138:105–115. doi: 10.1111/imm.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alizadeh D, Larmonier N. Chemotherapeutic targeting of cancer-induced immunosuppressive cells. Cancer Res. 2014;74:2663–2668. doi: 10.1158/0008-5472.CAN-14-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ihara F, Sakurai D, Horinaka A, Makita Y, Fujikawa A, Sakurai T, Yamasaki K, Kunii N, Motohashi S, Nakayama T, Okamoto Y. CD45RA−Foxp3high regulatory T cells have a negative impact on the clinical outcome of head and neck squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1275–1285. doi: 10.1007/s00262-017-2021-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng C, Cao LJ, Song HF, Xu P, Chen H, Xu JC, Zhu XY, Zhang XG, Wang XF. Expression of PD-L1 on CD4+CD25+Foxp3+ regulatory T cells of patients with chronic HBV infection and its correlation with clinical parameters. Viral Immunol. 2015;28:418–424. doi: 10.1089/vim.2015.0062. [DOI] [PubMed] [Google Scholar]
- 18.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt A, Oberle N, Krammer PH. Molecular mechanisms of treg-mediated T cell suppression. Front Immunol. 2012;3:51. doi: 10.3389/fimmu.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amodio G, Gregori S. Human tolerogenic DC-10: perspectives for clinical applications. Transplant Res. 2012;1:14. doi: 10.1186/2047-1440-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 23.Mahnke K, Ring S, Johnson TS, Schallenberg S, Schonfeld K, Storn V, Bedke T, Enk AH. Induction of immunosuppressive functions of dendritic cells in vivo by CD4+CD25+ regulatory T cells: role of B7-H3 expression and antigen presentation. Eur J Immunol. 2007;37:2117–2126. doi: 10.1002/eji.200636841. [DOI] [PubMed] [Google Scholar]
- 24.Raker VK, Domogalla MP, Steinbrink K. Tolerogenic dendritic cells for regulatory T cell induction in man. Front Immunol. 2015;6:569. doi: 10.3389/fimmu.2015.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz RH, Mueller DL, Jenkins MK, Quill H. T-cell clonal anergy. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 2):605–610. doi: 10.1101/SQB.1989.054.01.072. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 27.van den Heuvel MJ, Garg N, Van Kaer L, Haeryfar SM. NKT cell costimulation: experimental progress and therapeutic promise. Trends Mol Med. 2011;17:65–77. doi: 10.1016/j.molmed.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson FC, Berzofsky JA, Terabe M. NKT cell networks in the regulation of tumor immunity. Front Immunol. 2014;5:543. doi: 10.3389/fimmu.2014.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi T, Chiba S, Nieda M, Azuma T, Ishihara S, Shibata Y, Juji T, Hirai H. Cutting edge: analysis of human Vα 24+CD8+ NK T cells activated by α-galactosylceramide-pulsed monocyte-derived dendritic cells. J Immunol. 2002;168:3140–3144. doi: 10.4049/jimmunol.168.7.3140. [DOI] [PubMed] [Google Scholar]
- 31.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 32.Singh AK, Gaur P, Shukla NK, Das SN. Differential dendritic cell-mediated activation and functions of invariant NKT-cell subsets in oral cancer. Oral Dis. 2015;21:e105–e113. doi: 10.1111/odi.12238. [DOI] [PubMed] [Google Scholar]
- 33.Krijgsman D, Hokland M, Kuppen PJK. The role of natural killer T cells in cancer-a phenotypical and functional approach. Front Immunol. 2018;9:367. doi: 10.3389/fimmu.2018.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hua J, Liang S, Ma X, Webb TJ, Potter JP, Li Z. The interaction between regulatory T cells and NKT cells in the liver: a CD1d bridge links innate and adaptive immunity. PLoS ONE. 2011;6:e27038. doi: 10.1371/journal.pone.0027038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh KH, Lee C, Lee SW, Jeon SH, Park SH, Seong RH, Hong S. Activation of natural killer T cells inhibits the development of induced regulatory T cells via IFNγ. Biochem Biophys Res Commun. 2011;411:599–606. doi: 10.1016/j.bbrc.2011.06.193. [DOI] [PubMed] [Google Scholar]
- 36.Kamata T, Suzuki A, Mise N, Ihara F, Takami M, Makita Y, Horinaka A, Harada K, Kunii N, Yoshida S, Yoshino I, Nakayama T, Motohashi S. Blockade of programmed death-1/programmed death ligand pathway enhances the antitumor immunity of human invariant natural killer T cells. Cancer Immunol Immunother. 2016;65:1477–1489. doi: 10.1007/s00262-016-1901-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okita R, Saeki T, Takashima S, Yamaguchi Y, Toge T. CD4+CD25+ regulatory T cells in the peripheral blood of patients with breast cancer and non-small cell lung cancer. Oncol Rep. 2005;14:1269–1273. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.