Abstract
Objectives
To clarify comprehensive immunological signature patterns of tumour tissue-infiltrating lymphocytes in patients with renal cell carcinoma and show its clinical significance.
Materials and methods
We investigated the surface marker expressions of tumour tissue-infiltrating lymphocytes quantitatively and classified them based on their functional populations. We extracted 109 sets of tumour tissue-infiltrating lymphocytes from 80 patients who underwent surgical resection of renal cell carcinoma, of which 44 tumour tissue-infiltrating lymphocytes were multiply extracted from 15 patients. Each tumour tissue-infiltrating lymphocyte was characterised on the basis of functional T-cell populations using ten surface marker expressions measured by flow cytometry.
Results
All sets of the tumour tissue-infiltrating lymphocytes were classified into three groups, which correlated significantly with Fuhrman grade (OR 0.253, 95% CI 0.094–0.678, P = 0.006). Importantly, both overall metastasis-free survival (HR 0.449, 95% CI 0.243–0.832, P = 0.011) and recurrence-free survival (HR 0.475, 95% CI 0.238–0.948, P = 0.035) of the patients with the higher marker expressions were significantly inferior to those of the patients with the lower marker expressions by multivariate analysis. Six specific genes for this classification identified by microarray analysis verified our results using the TCGA KIRC data set. In addition, we discovered the presence of intra-tumoural diversity in the classification of 3 (20%) of the 15 patients.
Conclusions
This study showed that the presence of classable diversity in the immunological signature of tumour tissue-infiltrating lymphocytes correlated with prognosis and tumour aggressiveness that was observed even within individual tumours in some patients with renal cell carcinoma.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-017-2060-5) contains supplementary material, which is available to authorized users.
Keywords: Immunological classification, Intra-tumour diversity, Tim-3, PD-1, Heterogeneity, Hot cluster
Introduction
Recently, novel immunotherapies that can target the surface molecules on cytotoxic T lymphocytes and regulatory T cells (Tregs) have shown magnificent clinical responses for many kinds of cancer types including renal cell carcinoma (RCC) [1]. However, there is still a large population of non-responders. To circumvent this issue and develop combination therapy for them, a better immunological understanding based on phenotypic analysis of not only peripheral blood but also tumour tissues and normal tissues is needed [2].
In terms of the nature of tumours, RCC is well known as an immunogenic tumour [1], and several studies have already reported on the relationship between the tumour microenvironment, including the immunogenic condition, and tumour staging [3], prognosis [4], clonality [5], and functionality [6]. However, each report was mainly based on each functional immune cell type, and no reports evaluated or revealed the immunological condition comprehensively and quantitatively using multiple immune cell types, which were reported to have important interactions with each other in many experimental studies [7, 8]. Omics analysis of tumour immunity using comprehensive genetic data across various tumour types was recently reported [9], but there have been no studies to clarify the clinical importance of the comprehensive immune-related evaluation quantitatively in the tumour microenvironment of RCC by multivariate analysis, although some studies were just recently reported in both non-small cell lung cancer and RCC patients [10, 11].
Here, we evaluated the immunological signature of patients with surgically resected RCC based on T-cell subsets of circulating lymphocytes (CLs), adjacent normal tissue-infiltrating lymphocytes (NILs), and tumour tissue-infiltrating lymphocytes (TILs) measured by FACS. As a result, we successfully found that immunological classification significantly correlated with both tumour aggressiveness and prognosis using multivariate analysis and also revealed the presence of intra-tumour diversity of surface marker expressions in some patients.
Patients and methods
Patients
Eighty RCC patients surgically treated from October 2014 to July 2016 were enrolled in this study (Fig. 1a; Table 1). The initially diagnosed tumours were staged according to the 7th American Joint Committee on Cancer staging classification [12]. The patient characteristics including laboratory findings were evaluated at the time of surgical resection. Analysis of clinical laboratory data included the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), C-reactive protein (CRP) [13], serum sodium concentration [14], and modified Glasgow Prognostic Score (mGPS) [15].
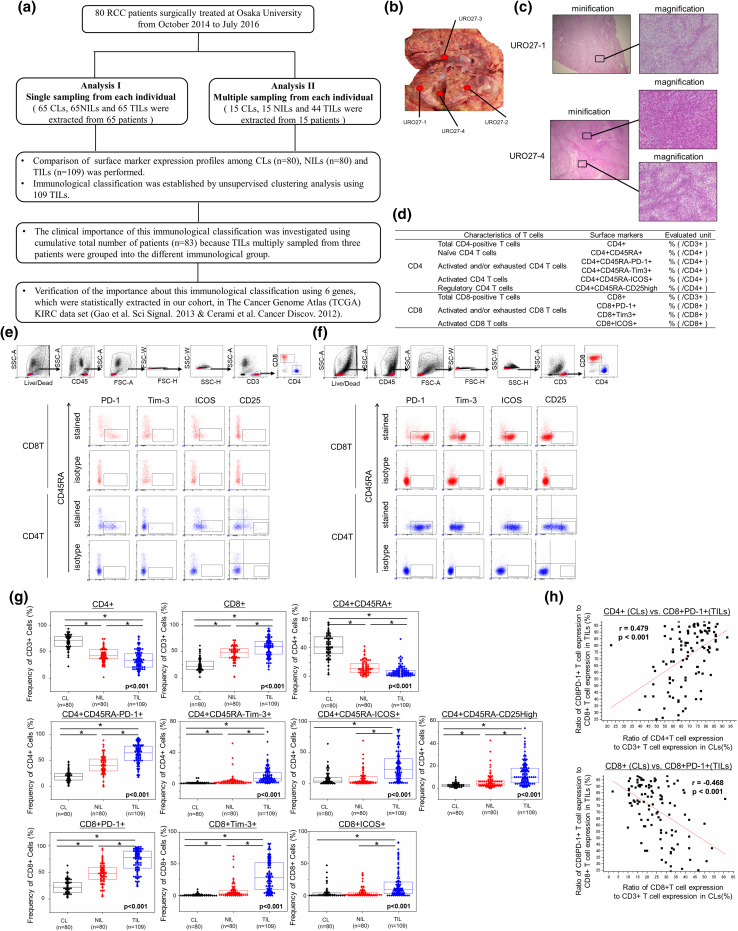
Fig. 1.
Flowchart illustrating the samples that were used for analysis (a). Tumour tissues in 12 patients were multiply sampled as shown in (b). Staining of each sample with hematoxylin and eosin staining is shown in (c). Populations of tissue-infiltrating lymphocytes based on T-cell characteristics analyzed in this study (d). The method of gating of FACS analysis for NILs (e) and TILs (f). Comparison of surface markers on CLs, NILs, and TILs (g). Expression ratios of ten surface markers were compared among CLs, NILs, and TILs. First, comparison of three sample types was performed by Kruskal–Wallis analysis, and then, a comparison of each sample type was performed by Bonferroni-corrected Mann–Whitney U test. The central tendency of the box plot shows the median of each group, and the upper and lower ranges of the box plot show the 25th and 75th percentiles of each data set, respectively. *P < 0.01, **P < 0.05. Correlation of the expression rate of surface markers between CLs and TILs (h). The correlation coefficient graphs show the relationship between the CD8+PD-1+ expression ratio in TILs (Y-axis) and the CD4+ expression ratio in CLs (X-axis) (upper panel), and the relationship between the CD8+PD-1+ expression ratio in TILs (X axis) and the CD8+ expression ratio in CLs (Y-axis) (lower panel). CLs circulating lymphocytes, KIRC ccRCC samples and matched normal kidney tissues, NILs adjacent normal tissue-infiltrating lymphocytes, TILs tumour tissue-infiltrating lymphocytes, RCC renal cell carcinoma
Table 1.
Clinical characteristics of the patients (n = 80)
| Age (median) (years) | 27–82 (66) |
| Gender | |
| Male | 58 |
| Female | 22 |
| Histological type | |
| Clear cell | 71 |
| Clear cell with sarcomatoid change | 1 |
| Sarcomatoid | 1 |
| Papillary | 3 |
| Chromophobe | 2 |
| Mucinous tubular and spindle | 1 |
| Unclassified | 1 |
| pT stage | |
| 1a/1b | 38/9 |
| 2 | 4 |
| 3a/3b/3c | 17/9/3 |
| Fuhrman grade (highest) | |
| 1/2/3/4 | 4/52/15/9 |
| INF | |
| Α | 60 |
| Β | 20 |
| pN stage | |
| 0 | 75 |
| I | 5 |
| M stage | |
| 0 | 72 |
| I | 8 |
| Lymphovascular invasion | |
| Yes | 28 |
| No | 52 |
| NLR (median) | 0.82–9.68 (2.32) |
| PLR (median) | 0.26–4.65 (0.84) |
| Serum sodium concentration (median) (mEq/L) | 134–145 (140) |
| CRP (median) (mg/dl) | 0.0–16.33 (0.06) |
| Modified Glasgow Prognostic Score | |
| 0 (CRP <1 mg/dl) | 61 |
| 1 (CRP ≥1 mg/dl and Alb ≥3.5 g/dl) | 7 |
| 2 (CRP ≥1 mg/dl and Alb <3.5 g/dl) | 12 |
| Follow-up time (median) (months) | 1.2–22.3 (7.9) |
INF infiltration, NLR neutrophil-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio, CRP C-reactive protein, Alb albumin
This study was approved by the local ethics committee of the Graduate School of Medicine, Osaka University (#13397-2, #14069-3), and written informed consent was obtained from all patients.
Lymphocyte isolation
CLs were extracted from 5 ml peripheral blood of RCC patients using BD Pharm Lyse lysing buffer (BD Biosciences, Tokyo, Japan). Both cancer and adjacent normal tissues macroscopically distinguished were sampled just after resection of the tumour (Fig. 1b, c). After measuring the weights of each tissue every time, TILs and NILs were extracted using gentleMACS Dissociator (Miltenyi Biotec, Tokyo, Japan) and dissociated by a Tumour Dissociation Kit for humans (Miltenyi Biotec) according to the manufacturer’s protocol. Subsequently, the cell suspension was applied to a 40-μm nylon cell strainer (BD Biosciences), and the strainer was repeatedly rinsed with wash buffer (Hank’s balanced salt solution supplemented with 2% foetal bovine serum and 10 mM HEPES). The filtered single-cell suspension was centrifuged, and the cell pellet was resuspended in wash buffer after decanting the supernatant.
Extracellular antibody staining and data acquisition by multicolour flow cytometry
Cells were stained by fluorophore-conjugated antibodies for 30 min after FcR block (Human TruStain FcX Fc Receptor blocking solution). These cells were then subjected to LSRFortessa (BD Biosciences). Live cells discriminated by Zombie NIR (BioLegend, San Diego, CA, USA) were drilled down, and singlet and CD45-positive cells were focussed on for further cell surface marker assessment. FOXP3/Transcription Factor Staining Buffer Set (Affymetrix Japan K.K., Tokyo, Japan) was used for intracellular staining according to the manufacturer’s protocol. Data collection was performed using the DiVA software (BD Biosciences).
Monoclonal antibodies, anti-CD45-BV785 [HI30], anti-CD3-Alexafluore 700 [UCTH1], anti-CD4-BV711 [RPA-T4], anti-CD8-BV510 [RPA-T8], anti-CD45RA-FITC [H100], anti-ICOS-PerCP/Cy5.5 [ISA-3], anti-Tim3-APC [F38-2E2], and anti-CD25-PE [BC96] were purchased from BioLegend. Anti-PD-1-PE-Cy7 [EH12.1] was obtained from BD Biosciences. Anti-Foxp3-PE [PCH101] antibody was bought from eBioscience. Human TruStain FcX Fc Receptor Blocking Solution was purchased from BioLegend.
Definition of subpopulation of extracted lymphocytes
To investigate the comprehensive immune condition based on the expression of functional T cells, we chose ten sets of subpopulations, as defined in Fig. 1d. Although consensus in defining the phenotype and function of human T-lymphocyte subsets was already reported [16], we mainly used immune check-point markers closer to the clinical situations that are treated in general practice. In the population of CD8+ T cells, activated and/or exhausted CD8+ T cells were defined as CD8+PD-1+ and CD8+Tim3+, because these phenotypes were reported to have hetero-functionality in a murine model [17, 18]. In the population of CD4+ T cells, effector CD4+ T cells were defined as both CD4+CD45RA-PD-1+ and CD4+CD45RA-Tim3+, whereas naïve CD4+ T cells were defined as CD4+CD45RA+ [9]. In both CD4+ and CD8+ T cells, ICOS was used as an activation surface marker as previously reported [19]. Tregs were defined as CD4+CD45RA-CD25high in CD4+ T cells as previously reported [20–22]. Beforehand, we confirmed that the CD4+CD45RA-CD25high cells correspondingly expressed FOXP3, because the higher expression of FOXP3 is reported to be essential to identify effector Tregs as previously reported (Supplemental Fig. S1) [20, 22]. The methods of tumour sampling and gating of each surface marker of NILs and TILs are shown in Fig. 1e, f, respectively. The positively stained cells were defined above the negative region for isotype control.
Extraction of total RNA and microarray analysis for mRNA
Total RNA from 11 fresh tissue samples was isolated using an RNeasy Mini Kit (Qiagen, Tokyo, Japan) according to the manufacturer’s protocol. Affymetrix HTA 2.0 DNA microarrays (Affymetrix, Santa Clara, CA, USA) were processed according to the manufacturer’s protocol after confirming RNA integrity using a NanoDrop 2000 spectrophotometer (Labtech International, Uckfield, UK). These arrays were scanned using a GeneChip Scanner 3000 7G (Affymetrix), and the scanned data were processed with GeneChip Command Console Software 4.0 (Affymetrix). Specific genes for this immunological classification were extracted using selection criterion levels (p < 0.05 in comparison with each sample type and fold change >).
Verification of the results of microarray analysis in The Cancer Genome Atlas (TCGA) study
Expression data for 538 primary clear cell RCC (ccRCC) samples were downloaded from the TCGA ccRCC samples and matched normal kidney tissues (KIRC) sample set through cBioportal for Cancer Genomics [23, 24]. In addition, the corresponding clinical data were downloaded from the same site. After 22 patients with neoadjuvant therapy (n = 19) and no mRNA expression data (n = 3) were excluded, 516 patients were analyzed to verify the results of our previous analysis. Altered mRNA expression was defined as a Z-score > in the TCGA KIRC cohort, which indicated significant upregulation or downregulation in that cohort.
Statistical analysis
Comparisons between the surface marker expressions and clinical features were evaluated by Kruskal–Wallis test, Bonferroni-corrected Mann–Whitney U test, Fisher exact test, and Cramer’s coefficient test as appropriate. The correlations between each surface marker expression among CLs, NILs, and TILs were evaluated by Spearman’s test, and a correlation coefficient of greater than 0.4 indicated the presence of a mild relationship. Overall metastasis-free survival (MFS) of both M0 and M1 patients was measured from the date of surgical resection until radiological or histological confirmation of overall metastasis. For the M0 patients only at the time of surgery, recurrence-free survival (RFS) was measured from the date of surgical resection until radiological or histological confirmation of local recurrence or distant metastasis. Distributions of MFS and RFS times were estimated with the Kaplan–Meier method, and associations between MFS, RFS, and the immunological classification were assessed with the log-rank test. As a multivariate analysis, Cox regression analysis using a stepwise forward selection with P < 0.1 as the criterion for model entry or stay was used. Unsupervised clustering analysis of TILs based on the surface marker expressions and the other statistical analyses described above were also performed using the SPSS software, version 20.0 (IBM SPSS Statistics, Tokyo, Japan) and R version 3.3.0 with Heatmap 3 package (ver. 1.1.1) (The R Foundation for Statistical Computing). A value of P < 0.05 was considered statistically significant.
Results
Patient characteristics
The clinical characteristics of the 80 patients are listed in Table 1. Median patient age was 66 (range 27–82) years, and the histology of 71 patients (88.8%) was clear cell carcinoma. At diagnosis, eight patients had distant metastases. Twenty-four patients (30%) had a higher Fuhrman grade (Grade 3 or 4) tumour. As shown in Fig. 1a, 44 tumour sites were multiply sampled from 15 patients. Totally, 109 sets of TILs, 80 sets pf NILs, and 80 sets of CLs were included in this study. During follow-up time, distant metastasis had newly occurred in 11 patients (lung: 6, bone: 4, and liver: 1).
Median NLR and PLR were 2.32 (range 0.82–9.68) and 0.84 (0.26–4.65), respectively. The mGPS of 61 patients was 0, 7 patients had a score of 1, and 12 patients had a score of 2. Median follow-up time was 7.9 (1.2–22.3) months.
Differences in immune signatures between CLs, NILs, and TILs
The numbers of tissue-infiltrating CD3+, CD4+, and CD8+ T cells were significantly higher in tumour tissues than in adjacent normal tissues (Supplemental Fig. S2). All surface markers except for ICOS on both CD4+ and CD8+ T cells were significantly different among CLs, NILs, and TILs. Indeed, total CD4+ and naïve CD4+ T cells (CD45RA+) uniformly decreased in the order of CLs, NILs, and TILs, whereas total CD8+ T cells, activated and/or exhausted markers (PD-1+ and Tim-3+) of both CD4+ and CD8+ T cells, and Tregs (CD4+CD45RA-CD25high) uniformly increased in the order of CLs, NILs, and TILs. The ratios of activation marker ICOS+ of both CD4+ and CD8+ T cells were significantly upregulated compared to those in NILs and CLs, although there were also significant differences between those in NILs and CLs (Fig. 1g).
Correlation of expressions of surface markers within each sample type
Using the expression ratio mentioned above, we analysed the correlation of each marker expression ratio within CLs, NILs, and TILs, respectively. In CLs, the CD4+PD-1+ was conversely correlated with the CD4+CD45RA+ (correlation coefficient (r) = −0.536, P < 0.001) and was positively correlated with the CD4+Tim-3+ (r = 0.417, P < 0.001), and ICOS on CD4+ and CD8+ T cells were also positively correlated (r = 0.773, P < 0.001) (Supplemental Fig. S3). In NILs, both PD-1 (r = 0.579, P < 0.001) and ICOS (r = 0.623, P < 0.001) of CD4+ and CD8+ T cells were positively correlated with each other. Moreover, CD8+PD-1+ was positively correlated with CD8+Tim-3+ (r = 0.457, P < 0.001) (Supplemental Fig. S4). In TILs, 14 kinds of marker combinations were significantly correlated. Representatively, the CD4+CD45RA-CD25high was positively correlated with the CD4+CD45RA-Tim-3+ (r = 0.672, P < 0.001) and CD4+CD45RA-ICOS+ (r = 0.587, P < 0.001). In addition, the CD4+CD45RA-PD-1+ was significantly correlated with the CD4+CD45RA-Tim-3+ (r = 0.527, P < 0.001), as was the CD8+PD-1+ with the CD8+ (r = 0.548, P < 0.001) and CD8+Tim3+ (r = 0.703, P < 0.001). Both Tim3 (r = 0.493, P < 0.001) and ICOS (r = 0.647, P < 0.001) on CD4+ and CD8+ T cells were positively correlated with each other (Supplemental Fig. S5).
Correlation of CD4+/CD3+ ratio in CLs and CD8+PD1+/CD8+ ratio in TILs
Next, we investigated whether immunological phenotypes in TILs were predicted by peripheral blood. There were two combinations that had significant correlations between CLs and TILs. The CD4+ ratio in CLs was positively correlated with the CD8+PD-1+ ratio in TILs (r = 0.479, P < 0.001) (Fig. 1h), whereas the CD8+ ratio in CLs was inversely correlated with the CD8+PD1+ ratio in TILs (r = −0.468, P < 0.001) (Fig. 1h). There were no other markers with significant correlation between CLs and TILs.
Immunological classification based on the surface marker expressions
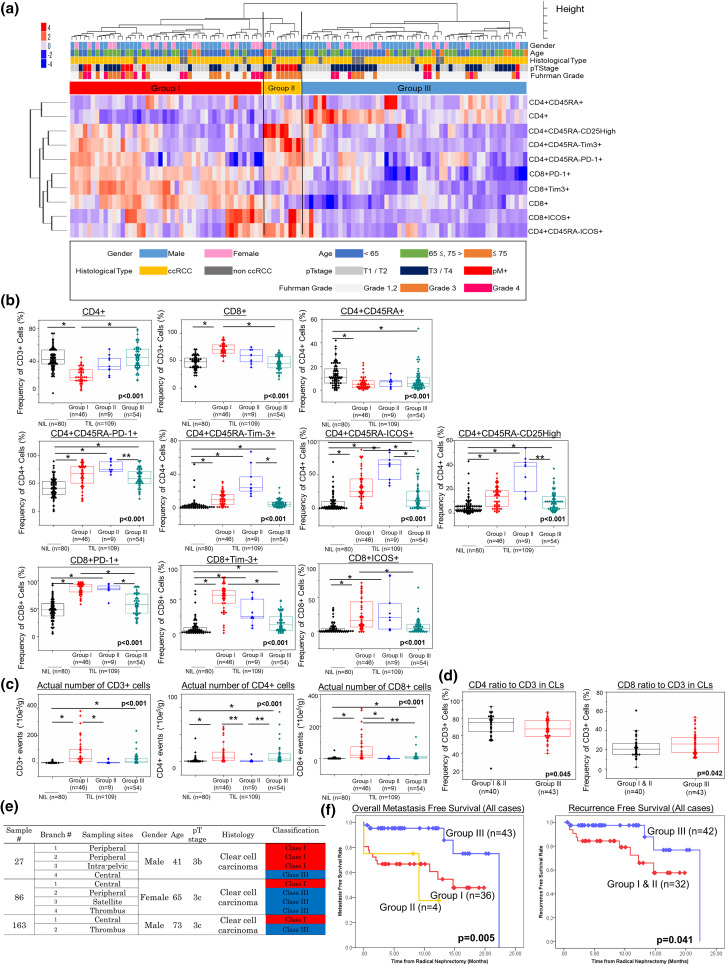
Then, 109 sets of TILs were classified based on the expression profiles of ten kinds of surface markers using unsupervised clustering analysis (Fig. 2a). These TILs could be classified into three groups (Group I: activated CD8 T cell-dominant group, Group II: regulatory CD4 T cell-dominant group, and Group III: immunologically silent group). In Group III (54 samples; 49.5%), all of the surface markers except for total CD4+ T cells were downregulated compared to the other two groups. Compared to Group III, the surface marker expressions related to CD8+ T cells and CD4+ activated marker (ICOS) in Group I (46 samples; 43.1%) increased significantly and CD4+ T cells decreased significantly, whereas all of the surface marker expressions related to CD4+ T cells and CD8+PD-1+T cells in Group II (nine samples; 8.3%) increased significantly (Fig. 2b). In terms of the number of TILs, the numbers of CD3+ T cells in Group I were significantly higher than those in Group II (Fig. 2c). Among the three groups, the numbers of CD8+ T cells were significantly highest in Group I, whereas those of CD4+ T cells were significantly lowest in Group II (Fig. 2c). From the above analysis, this classification based on the functional T-cell populations could indicate the immunological status in topical cancer tissues.
Fig. 2.
Clustering analysis and clinical information of 109 sets of TILs. Clinical information shows gender, age, tumour histology, pathological T stage, and Fuhrman grade (a). Expression ratios of ten surface markers are compared among the three groups of TILs and NILs (b). The actual numbers of CD3-, CD4-, and CD8-positive cells per extracted tissue weight (g) are compared between the three groups of TILs and NILs (c). The graphs show the relationships between two markers of CLs and the immunological classification (d). First, comparison of three sample types was performed by Kruskal–Wallis analysis, and then, a comparison of each sample type was performed by Bonferroni-corrected Mann–Whitney U test. The central tendency of the box plot indicates the median of each group, and the upper and lower ranges of the box plot show the 25th and 75th percentiles of each data set, respectively. Clinical features, tumour sites, and immunological classification of each sample within the multiply sampled patients with intra-tumour diversity of surface marker expressions (e). Probability estimates of overall metastasis-free survival time of all patients (n = 83) stratified by immunological classification. Also shown are the probability estimates of the recurrence-free survival time of the patients without metastasis at the time of surgery (n = 74) stratified by immunological classification (f). The patients having surface marker diversity within each individual are counted as one patient for each classification. Statistical analysis was performed by log-rank test. *P < 0.01, **P < 0.05. CLs circulating lymphocytes, NILs adjacent normal tissue-infiltrating lymphocytes, TILs tumour tissue-infiltrating lymphocytes
The signature of the above TILs found here prompted us to investigate the relationship between the classification of TILs and CLs. In CD3+ cells of CLs, the presence of CD4+ T cells was higher in Groups I and II than in Group III, whereas that of CD8+ T cells was lower in Groups I and II than in Group III (Fig. 2d).
Intra-tumour diversity of surface marker expressions in RCC patients
Forty-four samples multiply extracted from 15 patients were the focus of the next analysis (Supplemental Table S1, Fig. 2e). Thirty-two samples were sampled from primary tumour sites. Moreover, nine and three samples were sampled from inferior vena cava (IVC) tumour thrombus and lymph node metastasis, respectively. Most of them (34 samples from 12 patients) were classified into the same group within each individual (Supplemental Table S1). Intriguingly, intra-tumour diversity of surface marker expressions was present within ten samples from three patients (20.0%) (Fig. 2e). Two (22.2%) of the nine patients with IVC tumour thrombus had diversity of surface marker expressions between the IVC tumour thrombus and the primary sites. Interestingly, there was no diversity of surface marker expression between lymph node metastases and the primary sites; however, only three patients were analysed (Supplemental Table S1).
Immunological classification relates only to Fuhrman grade
Thirty-five patients (81.4%) within Group III had tumours of lower Fuhrman grade (G1, 2), whereas 19 patients (47.5%) within Groups I and II had tumours of higher Fuhrman grade (G3, 4) (P = 0.028) (Supplemental Table S2). There was no significant correlation between this immunological classification and the other clinical items including pathological T stage and blood inflammation markers (NLR, PLR, and CRP) (Supplemental Table S2). In univariate and multivariate analyses, Fuhrman grade was also significantly correlated with this immunological classification [odds ratio 0.253, 95% confidence interval (CI) 0.094–0.678, P = 0.006] (Table 2).
Table 2.
Univariate and multivariate analyses with logistic regression of clinical characteristics correlated with the present immunological classification (classes I, II vs. III)
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| P | OR | 95% CI | P | |
| Age (<65 vs. ≥65 years) | 0.240 | |||
| Gender (male vs. female) | 0.055 | 0.076 | ||
| Histological type (ccRCC vs. non-ccRCC) | 0.524 | |||
| pT stage (T1/2 vs. T3/4) | 0.476 | |||
| Fuhrman grade (1, 2 vs. 3, 4) | 0.005 | 0.253 | 0.094–0.678 | 0.006 |
| INF (α vs. β) | 0.055 | 0.880 | ||
| Lymphovascular invasion (no vs. yes) | 0.978 | |||
| NLR (<2.4 vs. ≥2.4) | 0.099 | 0.845 | ||
| PLR (<1.8 vs. ≥1.8) | 0.219 | |||
| Hyponatremia (≥138 vs. <138 mEq/L) | 0.892 | |||
| Modified Glasgow Prognostic Score (0 vs. 1, 2) | 0.146 | |||
Bold values were defined as p <0.05
OR odds ratio, CI confidence interval, ccRCC clear cell renal cell carcinoma, INF infiltration, NLR neutrophil-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio
Immunological classification was significantly correlated with overall MFS and RFS
First, our immunological classification was significantly correlated with the overall MFS rate (Fig. 2f). Especially, all of the higher stage patients of Groups I and II had metastatic disease within 15 months after surgical resection (Supplemental Fig. S6a). In multivariate analysis, our immunological classification was significantly correlated with overall MFS rate [hazard ratio (HR) 0.449, 95% CI 0.243–0.832, P = 0.011] along with pT stage (P = 0.016) and modified GPS score (P = 0.037) (Table 3a). Even in ccRCC patients only, our immunological classification was significantly correlated with overall MFS rate (Supplemental Fig. S7a, b). In multivariate analysis, our immunological classification in only ccRCC patients was significantly correlated with overall MFS rate (HR 0.302, 95% CI 0.146–0.625, P = 0.001) along with pT stage (P = 0.001) and mGPS score (P = 0.005) (Supplemental Table S3a).
Table 3.
Univariate and multivariate analyses of prognostic factors for metastasis-free survival (n = 83) (a) and recurrence-free survival (n = 74) (b) in all histological cases
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| P | HR | 95% CI | P | |
| (a) Metastasis-free survival (n = 83) | ||||
| Age (<65 vs. ≥65 years) | 0.718 | |||
| Gender (male vs. female) | 0.453 | |||
| Histological type (ccRCC vs. non-ccRCC) | 0.466 | |||
| pT stage (T1/2 vs. T3/4) | <0.001 | 5.814 | 1.391–24.305 | 0.016 |
| Fuhrman grade (1, 2 vs. 3, 4) | <0.001 | 0.119 | ||
| INF (α vs. β) | <0.001 | |||
| Lymphovascular invasion (no vs. yes) | <0.001 | |||
| Immunological classification (Group I, II vs. Group III) | 0.001 | 0.449 | 0.243–0.832 | 0.011 |
| NLR (<2.4 vs. ≥2.4) | <0.001 | |||
| PLR (<1.8 vs. ≥1.8) | <0.001 | |||
| Hyponatremia (≥138 vs. <138 mEq/L) | <0.001 | |||
| Modified Glasgow Prognostic Score (0 vs. 1, 2) | <0.001 | 3.247 | 1.071–9.843 | 0.037 |
| (b) Recurrence-free survival (n = 74) | ||||
| Age (< 65 vs. ≥65 years) | 0.267 | |||
| Gender (male vs. female) | 0.982 | |||
| Histological type (ccRCC vs. non-ccRCC) | 0.414 | |||
| pT stage (T1/2 vs. T3/4) | <0.001 | 0.915 | ||
| Fuhrman grade (1, 2 vs. 3,4) | <0.001 | 0.912 | ||
| INF (α vs. β) | <0.001 | |||
| Lymphovascular invasion (no vs. yes) | <0.001 | |||
| Immunological classification (Group I, II vs. Group III) | 0.041 | 0.475 | 0.238–0.948 | 0.035 |
| NLR (<2.4 vs. ≥2.4) | 0.019 | |||
| PLR (<1.8 vs. ≥1.8) | 0.009 | 0.922 | ||
| Hyponatremia (≥138 vs. <138 mEq/L) | <0.001 | |||
| Modified Glasgow Prognostic Score (0 vs. 1, 2) | 0.001 | |||
Bold values were defined as p <0.05
HR hazard ratio, CI confidence interval, ccRCC clear cell renal cell carcinoma, INF infiltration, NLR neutrophil-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio
Second, this immunological classification was significantly correlated with RFS rate in all patients (Fig. 2f and Supplemental Fig. S6b) and in only the ccRCC patients (Supplemental Fig. S7c, d). Multivariate analysis showed only this immunological classification to be significantly correlated with RFS rate both in all patients (HR 0.475, 95% CI 0.238–0.948, P = 0.035) (Table 3b) and in only ccRCC patients (HR 0.370, 95% CI 0.151–0.904, P = 0.029) (Supplemental Table S3b). Moreover, pT stage was also significantly correlated with RFS rate only in the ccRCC patients (P = 0.016) (Supplemental Table S3b).
Six genes were identified to classify our immunological classification and were verified using TCGA data
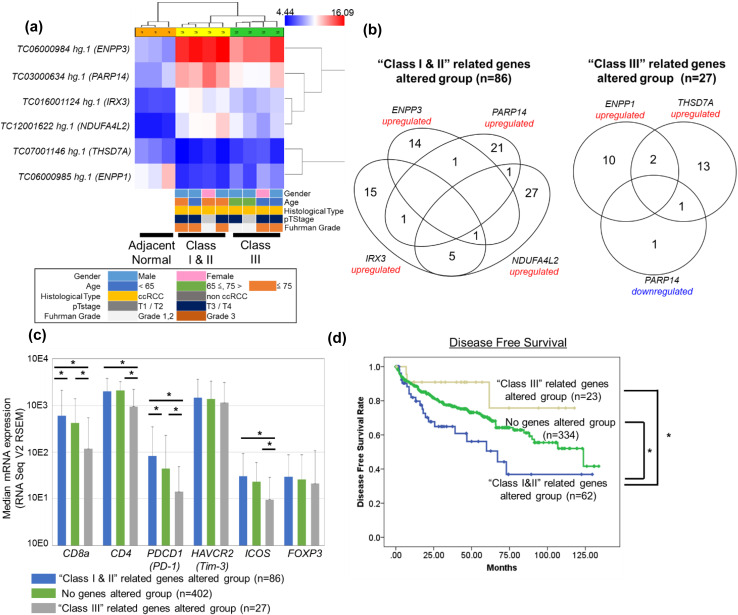
To verify our immunological classification using another cohort study, we tried to identify the specific genes from microarray analysis. Comparing among adjacent normal samples (n = 3), Group III samples [pT1a (n = 1), pT3a (n = 1), and pT3b (n = 2)] and Group I and II samples [pT1a (n = 1), pT3a (n = 1), and pT3b (n = 2)], six genes were identified with which to classify these groups (four genes were upregulated in Groups I and II samples: ENPP3 (encoding ectonucleotide pyrophosphatase/phosphodiesterase family member 3), PARP14 [encoding poly (ADP-ribose) polymerase 14], IRX3 (encoding iroquois-class homeodomain protein IRX-3), and NDUFA4L2 [encoding NADH dehydrogenase (ubiquinone) 1 alpha subcomplex subunit 4-like 2], and 2 genes were downregulated in Group III samples: THSD7A (encoding thrombospondin type-1 domain-containing protein 7A) and ENPP1 (ectonucleotide pyrophosphatase/phosphodiesterase family member 1) (Fig. 3a). In the TCGA KIRC cohort, ENPP3, PARP14, IRX3, and NDUFA4L2 were significantly upregulated in 16, 25, 22, and 34 patients, respectively. Cumulative total number of 86 patients had 4 significantly altered genes specific to Groups I and II (“Groups I and II”-related genes altered group). Whereas, THSD7A and ENPP1 were significantly upregulated in 12 and 16 patients, respectively, and PARP14 was significantly downregulated in two patients. Cumulative total number of 27 patients had three significantly altered genes specific to Group III (“Group III”-related genes altered group) (Fig. 3b). There were no significant correlations between these groups and clinical items including tumour grade and pathological T stage. However, four (CD8, CD4, PD-1, and ICOS) of the six mRNA expressions of the surface markers analysed in our study were significantly upregulated in the patients of the “Groups I and II”-related genes altered group compared to those of the “Group III”-related genes altered group (Fig. 3c). In addition, disease-free survival in the “Groups I and II”-related genes altered group was significantly poorest compared to that of the “Group III”-related genes altered group and the “no genes” altered group (Fig. 3d).
Fig. 3.
Identification of specific gene sets of a previous immunological classification compared between adjacent normal samples (n = 3), Group I and II samples (n = 4) and Group III samples (n = 4) using selection criterion levels (P < 0.05 in comparison with each sample type and fold change >) (a). The Venn diagram shows the total number of patients with altered four genes [upregulated; ENPP3 (n = 16), PARP14 (n = 25), IRX3 (n = 22), NDUFA4L2 (n = 34)] specific to Groups I and II (“Groups I and II”-related genes altered group, n = 86) and altered three genes [upregulated; ENPP1 (n = 12), THSD7A (n = 16), downregulated; PARP14 (n = 2)] specific to Group III (“Group III”-related genes altered group, n = 27) in TCGA KIRC cohort. Altered mRNA expression was defined as a Z-score > (b). Median mRNA expressions of six surface markers analysed in our FACS study stratified by three groups of the TCGA KIRC cohort (RNA Seq V2 RSEM). First, comparison of three sample types was performed by Kruskal–Wallis analysis, and then, a comparison of each sample type was performed by Bonferroni-corrected Mann–Whitney U test. Error bars showed the standard error of each data set, respectively. *P < 0.01, **P < 0.05 (c). Probability estimates of disease-free survival time of the patients stratified by the three groups in the patients of the TCGA KIRC cohort (d). TCGA The Cancer Genome Atlas, KIRC ccRCC samples and matched normal kidney tissues
Discussion
Novel cancer immunotherapies targeting the PD-1/PD-L1 axis have shown magnificent clinical benefits for many cancer types [25]. However, established biomarkers to predict the clinical response and the mechanism of acquiring drug resistance are still unknown [26]. Therefore, it is important to understand the immune microenvironment of tumours phenotypically and quantitatively to develop tailored immunotherapies and discover more ideal agents and types of combination therapy.
To our knowledge, this is the first study based on phenotypic analysis using FACS to show that the immunological condition of TILs could be classified into three groups, which were significantly correlated with tumour aggressiveness and prognosis using multivariate analysis. In addition, our results could show the presence of intra-tumour diversity in surface marker expressions of TILs in some, but not all, of the patients.
Recently, the phenotypic immunological condition of RCC patients was reported to be classified into three groups: (1) immune-regulated; (2) immune-activated; and (3) immune-silent, and they were significantly correlated with tumour grade and prognosis in univariate analysis in the same way as ours [11]. Although the number and kinds of analysed surface markers were different between the two studies, the definition of the immune-silent group in their analysis was similar to “Group III” in our cohort. However, the definitions of immune-regulated and immune-activated groups were different from our “Group I” and “Group II” in terms of immunological conditions and clinical importance. These studies including ours showed that the immunological condition in RCC patients could be first divided into two groups (immune-silent or immune-non-silent), and the other patients could be classified into immune-regulated and immune-activated groups depending on the kinds of surface markers present and the patient characteristics.
The immunological classification established in our study was correlated with the expression profile of CLs. In the relationship between the circulating lymphocytes and our classification, several items were significantly correlated similar to the single surface marker. This result implied that the circulating lymphocytes possibly reflected the immune condition in the microenvironment of the tumour, although this was always believed to be difficult to prove [27]. To define the importance of our immune classification for general use, further analysis is needed.
We successfully discovered that our immunological classification was significantly correlated with only tumour aggressiveness not histological type nor pathological T stage in multivariate analysis. Immunological classification of phenotypic analysis in lung cancer was recently reported [10], and the results showed that the immunological condition of lung cancer was divided into two groups (“hot cluster”, which had the higher expression of surface markers, and “cold cluster”, which had the lower expression of surface markers). Our results showed that the Groups I and II patients with higher levels of most surface markers had higher Fuhrman grade and an inferior prognosis. Although several surface markers evaluated in our study were independently reported to be correlated with prognosis in RCC patients [28–30], our results suggested the importance of comprehensive and quantitative analysis to understand tumour immunity in RCC as recently reported [11].
Actually, we could extract six genes that were relevant to our immunological classification and validate our results using them in the TCGA KIRC cohort [23, 24]. Most of the genes were included in the pathway of metabolism (ENPP3, PARP14, NDUFA4L2, and ENPP1) and glycolysis (THSD7A). Actually, the relationship between NDUFA4L2 and prognosis of RCC patients was recently reported [31]. Hanahan et al. advocated the complexity of cancer and the hallmarks of cancer comprising ten biological capabilities including immunity and metabolism [32]. Our study showed that immunological classification was significantly correlated with the other hallmark of cancer and further study is needed to clarify the relationship between RCC aggressiveness and tumour microenvironment across the several hallmarks of cancer. In addition, these six genes might become promising predictive and prognostic markers for new cancer immunotherapy for immunocheckpoint and further study is needed.
The presence of both inter- and intra-tumour heterogeneity in RCC patients and that of intra-tumour heterogeneity of T-cell clones is already well known [5, 33]. However, no phenotypic analyses have reported the diversity of PD-1 expression within one individual, and we revealed the presence of intra-individual diversity in some patients. These results might support the presence of radiologic heterogeneity in response to anti-PD-1 treatment as previously reported [34]. However, the diversity of immunological condition existed just 20% in our study and was much lower than we expected. Although most of the TILs from the same individual were grouped in the same group, and the expression pattern of each functional T cell might be similar within one individual, as previously reported, high functional heterogeneity was reported to exist even in phenotypically similar T cells [35]. Thus, a more sophisticated and comprehensive immune definition based on both phenotype and functional analysis should be required from now on.
This study has several limitations. First, the number of analysed surface markers was limited. In addition, we could not include the expression of cytokines or other immune cells, which are reported to play important roles in the microenvironment of RCC [36]. Second, the sample types were mostly extracted from primary lesions. The lymphocyte subsets between primary and metastatic RCC were reported to be slightly different [37], and we need to perform an expanded study that includes both primary and metastatic lesions.
In conclusion, this study showed that the presence of classable diversity in the immunological signature of TILs correlated with prognosis and tumour aggressiveness that was observed even within individual tumours in some patients with RCC. A comprehensive understanding of the immune condition is needed for the upcoming era of novel cancer immunotherapy using not only genetic but also phenotypic and functional classifications.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by Shionogi & Co., Ltd. (Osaka, Japan), the Project for Development of Innovative Research on Cancer Therapeutics (P-DIRECT), the Practice Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development, AMED (H. Wada), Osaka Kidney Bank (Osaka, Japan) (A. Kawashima), and the Public Trust Surgery Research Fund (Tokyo, Japan) (A. Kawashima).
Abbreviations
- ccRCC
Clear cell RCC
- CI
Confidence interval
- CLs
Circulating lymphocytes
- CRP
C-reactive protein
- HR
Hazard ratio
- IVC
Inferior vena cava
- KIRC
ccRCC samples and matched normal kidney tissues
- MFS
Metastasis-free survival
- mGPS
Modified Glasgow Prognostic Score
- NILs
Adjacent normal tissue-infiltrating lymphocytes
- NLR
Neutrophil-to-lymphocyte ratio
- PLR
Platelet-to-lymphocyte ratio
- RCC
Renal cell carcinoma
- RFS
Recurrence-free survival
- TCGA
The Cancer Genome Atlas
- TILs
Tumour tissue-infiltrating lymphocytes
- Tregs
Regulatory T cells
Compliance with ethical standards
Conflict of interest
H. Wada has received research funding from Shionogi & Co., Ltd. T. Kanazawa, K. Goto, and M. Matsumoto are employees of Shionogi & Co., Ltd. The other authors declare that they have no conflict of interest.
Footnotes
Atsunari Kawashima and Takayuki Kanazawa contributed equally to this work.
Contributor Information
Atsunari Kawashima, Phone: +81-6-6879-3531, Email: kawashima@uro.med.osaka-u.ac.jp.
Takayuki Kanazawa, Phone: +81-6-6210-8413, Email: takayuki.kanazawa@shionogi.co.jp.
References
- 1.Massari F, Santoni M, Ciccarese C, Santini D. The immunocheckpoints in modern oncology: the next 15 years. Expert Opin Biol Ther. 2015;15:917–921. doi: 10.1517/14712598.2015.1035251. [DOI] [PubMed] [Google Scholar]
- 2.Park HJ, Park JS, Jeong YH, et al. PD-1 upregulated on regulatory T cells during chronic virus infection enhances the suppression of CD8+ T cell immune response via the interaction with PD-L1 expressed on CD8+ T cells. J Immunol. 2015;194:5801–5811. doi: 10.4049/jimmunol.1401936. [DOI] [PubMed] [Google Scholar]
- 3.Bromwich EJ, McArdle PA, Canna K, McMillan DC, McNicol AM, Brown M, Aitchison M. The relationship between T-lymphocyte infiltration, stage, tumour grade and survival in patients undergoing curative surgery for renal cell cancer. Br J Cancer. 2003;89:1906–1908. doi: 10.1038/sj.bjc.6601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giraldo NA, Becht E, Pages F, et al. Orchestration and prognostic significance of immune checkpoints in the microenvironment of primary and metastatic renal cell cancer. Clin Cancer Res. 2015;21:3031–3040. doi: 10.1158/1078-0432.CCR-14-2926. [DOI] [PubMed] [Google Scholar]
- 5.Gerlinger M, Quezada SA, Peggs KS, et al. Ultra-deep T cell receptor sequencing reveals the complexity and intratumour heterogeneity of T cell clones in renal cell carcinomas. J Pathol. 2013;231:424–432. doi: 10.1002/path.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attig S, Hennenlotter J, Pawelec G, et al. Simultaneous infiltration of polyfunctional effector and suppressor T cells into renal cell carcinomas. Cancer Res. 2009;69:8412–8419. doi: 10.1158/0008-5472.CAN-09-0852. [DOI] [PubMed] [Google Scholar]
- 7.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 8.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 9.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lizotte PH, Ivanova EV, Awad MM, et al. Multiparametric profiling of non-small-cell lung cancers reveals distinct immunophenotypes. JCI Insight. 2016;1:e89014. doi: 10.1172/jci.insight.89014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giraldo NA, Becht E, Vano Y, et al. Tumor-Infiltrating and peripheral blood T-cell immunophenotypes predict early relapse in localized clear cell renal cell carcinoma. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-16-2848. [DOI] [PubMed] [Google Scholar]
- 12.Edge SB, American Joint Committee on Cancer . AJCC cancer staging manual. 7. London: Springer, New York; 2010. [DOI] [PubMed] [Google Scholar]
- 13.Hu H, Yao X, Xie X, Wu X, Zheng C, Xia W, Ma S. Prognostic value of preoperative NLR, dNLR, PLR and CRP in surgical renal cell carcinoma patients. World J Urol. 2017;35:261–270. doi: 10.1007/s00345-016-1864-9. [DOI] [PubMed] [Google Scholar]
- 14.Kawashima A, Tsujimura A, Takayama H, et al. Impact of hyponatremia on survival of patients with metastatic renal cell carcinoma treated with molecular targeted therapy. Int J Urol. 2012;19:1050–1057. doi: 10.1111/j.1442-2042.2012.03115.x. [DOI] [PubMed] [Google Scholar]
- 15.Proctor MJ, Talwar D, Balmar SM, O’Reilly DS, Foulis AK, Horgan PG, Morrison DS, McMillan DC. The relationship between the presence and site of cancer, an inflammation-based prognostic score and biochemical parameters. Initial results of the Glasgow Inflammation Outcome Study. Br J Cancer. 2010;103:870–876. doi: 10.1038/sj.bjc.6605855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 17.Paley MA, Kroy DC, Odorizzi PM, et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338:1220–1225. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singer M, Wang C, Cong L, et al. A distinct gene module for dysfunction uncoupled from activation in tumor-infiltrating T cells. Cell. 2016;166(1500–11):e9. doi: 10.1016/j.cell.2016.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okazaki T, Iwai Y, Honjo T. New regulatory co-receptors: inducible co-stimulator and PD-1. Curr Opin Immunol. 2002;14:779–782. doi: 10.1016/S0952-7915(02)00398-9. [DOI] [PubMed] [Google Scholar]
- 20.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol. 2014;27:1–7. doi: 10.1016/j.coi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Asma G, Amal G, Raja M, Amine D, Mohammed C, Amel BA. Comparison of circulating and intratumoral regulatory T cells in patients with renal cell carcinoma. Tumour Biol. 2015;36:3727–3734. doi: 10.1007/s13277-014-3012-8. [DOI] [PubMed] [Google Scholar]
- 22.Saito T, Nishikawa H, Wada H, et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22:679–684. doi: 10.1038/nm.4086. [DOI] [PubMed] [Google Scholar]
- 23.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carbognin L, Pilotto S, Milella M, et al. Differential activity of nivolumab, pembrolizumab and MPDL3280A according to the tumor expression of programmed death-ligand-1 (PD-L1): sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS One. 2015;10:e0130142. doi: 10.1371/journal.pone.0130142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blum KS, Pabst R. Lymphocyte numbers and subsets in the human blood. Do they mirror the situation in all organs? Immunol Lett. 2007;108:45–51. doi: 10.1016/j.imlet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 29.Minarik I, Lastovicka J, Budinsky V, Kayserova J, Spisek R, Jarolim L, Fialova A, Babjuk M, Bartunkova J. Regulatory T cells, dendritic cells and neutrophils in patients with renal cell carcinoma. Immunol Lett. 2013;152:144–150. doi: 10.1016/j.imlet.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Granier C, Dariane C, Combe P, et al. Tim-3 Expression on tumor-infiltrating PD-1+CD8+T cells correlates with poor clinical outcome in renal cell carcinoma. Cancer Res. 2017;77:1075–1082. doi: 10.1158/0008-5472.CAN-16-0274. [DOI] [PubMed] [Google Scholar]
- 31.Minton DR, Fu L, Mongan NP, Shevchuk MM, Nanus DM, Gudas LJ. Role of NADH dehydrogenase (ubiquinone) 1 alpha subcomplex 4-like 2 in clear cell renal cell carcinoma. Clin Cancer Res. 2016;22:2791–2801. doi: 10.1158/1078-0432.CCR-15-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Velasco G, Krajewski KM, Albiges L, Awad MM, Bellmunt J, Hodi FS, Choueiri TK. Radiologic heterogeneity in responses to anti-PD-1/PD-L1 therapy in metastatic renal cell carcinoma. Cancer Immunol Res. 2016;4:12–17. doi: 10.1158/2326-6066.CIR-15-0197. [DOI] [PubMed] [Google Scholar]
- 35.Ma C, Fan R, Ahmad H, et al. A clinical microchip for evaluation of single immune cells reveals high functional heterogeneity in phenotypically similar T cells. Nat Med. 2011;17:738–743. doi: 10.1038/nm.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gustafson MP, Lin Y, Bleeker JS, et al. Intratumoral CD14+ cells and circulating CD14+ HLA-DRlo/neg monocytes correlate with decreased survival in patients with clear cell renal cell carcinoma. Clin Cancer Res. 2015;21:4224–4233. doi: 10.1158/1078-0432.CCR-15-0260. [DOI] [PubMed] [Google Scholar]
- 37.Giraldo NA, Becht E, Remark R, Damotte D, Sautes-Fridman C, Fridman WH. The immune contexture of primary and metastatic human tumours. Curr Opin Immunol. 2014;27:8–15. doi: 10.1016/j.coi.2014.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.