Abstract
Background
Checkpoint inhibitors are first-line therapies in melanoma, but safety in older adults has not yet been assessed. Ipilimumab improves survival, but immunologic-related adverse events (AEs) can be threatening, and its use in elderly people raises questions.
Aim
To assess safety in a cohort of very elderly patients treated with ipilimumab.
Methods
All patients over 80 years treated with ipilimumab for melanoma were retrospectively included. AE occurrence, management, and outcome, as well as response rate at week 16 and overall survival were recorded, and compared to data for a group of younger patients treated in our institution during the same period.
Results
In the elderly group, 23 patients were included with a median age of 82 years [80–90]. AEs amounting to 23 occurred in 15 patients (65%) with 5 grade 3 (22%) and 1 grade 5 (opportunistic infection) AEs. Corticosteroids were required for five (22%) patients, additive immunosuppressive therapy for two, hospitalization for four, and definitive interruption of ipilimumab for three. Median overall survival was 14 months. In the younger group, 29 patients were included with a median age of 58 years. AEs occurred in 15/29 (52%) with 4 grade 3 (19%) and 1 grade 4 (7%). Median OS was 17 months.
Conclusion
Serious AEs occurred in 80 + adults at the same rate as observed in our younger patients and as previously reported in younger populations. Ipilimumab can be an option in elderly patients, as patients may benefit from therapy and safety seems to be manageable.
Keywords: Ipilimumab, Checkpoint inhibitors, Elderly, Older adults, Adverse events, Melanoma
Introduction
Ipilimumab, an anti-cytotoxic T-cell lymphocyte antigen-4 antibody, was the first molecule to improve overall survival (OS) in metastatic melanoma [2]. In a pooled analysis of 1861 patients enrolled in randomized clinical trials (RCT), median overall survival was 11.4 months, with a plateau at 21% in the survival curve beginning around 3 years, indicating a long-term benefit [3]. If programmed-death 1 (PD-1) checkpoint inhibitors are prescribed as first-line therapy for most patients with metastatic melanoma, ipilimumab remains useful and is prescribed frequently in cases of refractory or progressive disease, or in combination therapies. Checkpoint inhibitors can result in activation of immune responses in healthy tissues, leading to immunologic-related adverse events (irAEs). Severe irAEs (≥ grade 3) occur in about 30% of cases with ipilimumab [4]. Standard management is based on corticosteroids as first-line treatment, and additional immunosuppressive agents or anti-tumor-necrosis-factor alpha inhibitors (anti-TNFα) can be necessary [5]. Furthermore, invasive procedures may be required to investigate these irAEs. In elderly patients, ipilimumab safety has been reported from RCT subgroups and retrospective studies [4, 6], but often, a 65- or 70-year-old cutoff was chosen, though older patients are seen in practice. This prompted us to analyze the “real-life” safety of ipilimumab in patients over 80 years treated in our department. The primary endpoint was to evaluate the safety of ipilimumab and adverse event management; the secondary endpoint was its efficacy.
Materials and methods
Inclusion criteria
All patients over 80 years treated with ipilimumab (3 mg/kg) for advanced melanoma at the University Hospital of Bordeaux, France between June 2010 and March 2016 with a least 16 week follow-up were retrospectively systematically included from pharmacy databases. A second group of patients, aged less than 80 years, were randomly determined among all patients treated during the same period.
Data collection and statistics
The following data were collected at the time of first infusion: age, sex, general condition (comorbidities, Charlson’s score, body mass index, ECOG status, and social personal situation), biologic data (albumin and lymphocyte count), disease characteristics (TNM stage, BRAF status, LDH, and cerebral involvement), and prior therapies. Occurrence of AEs was recorded focusing on the grade, type, number, and duration of hospitalizations related to AE management, invasive medical procedures, treatments, and outcome. Response rates were evaluated with complete body CT scan at week 16, then every 12 weeks up to database lock (September 2017). Progression-free survival (PFS) and OS were calculated as time from onset of treatment until progression or death from any cause, respectively. In patients with no progression or death at the time of final data analysis, the date of last contact was used for censored calculation. Statistical analysis was performed using Graphpad Prism V.7.01.
Results
Population and treatment regimen
Twenty-three patients were included in the elderly group with a median age of 82 years. ECOG status was ≤ 2 for all patients. Out of 23, 14 (61%) received ipilimumab after one or more previous therapies. Baseline characteristics of the population study are detailed in Table 1. Twenty patients (87%) completed four ipilimumab infusions and four (17%) required four more. Twenty-nine patients were included in the younger group with a median age of 58 years, including five (17%) who received ipilimumab as a first-line therapy. Radiotherapy was used as a complementary treatment for one patient in the elderly group and for nine in the “young” group. In the elderly group, two patients received ipilimumab as a second-line therapy after the previous PD-1 inhibitor treatment, as did one in the younger group.
Table 1.
Baseline characteristics of the study group (n = 52)
| Baseline characteristics | No. (%) | ||
|---|---|---|---|
| Elderly patients (n = 23) | Younger patients (n = 29) | p value | |
| Age (years): median, range | 82 [80–90] | 58.3 [32–77] | |
| Sex: male/female | 14/9 | 16/13 | 0.68a |
| M Stage | |||
| M1a | 7 (31) | 4 (14) | |
| M1b | 4 (17) | 5 (18) | 0.30b |
| M1c | 12 (52) | 20 (69) | |
| Cerebral metastasis | 3 (13) | 7 (25) | |
| Active | 1 | 1 | |
| Inactive | 2 | 6 | |
| BRAF status | |||
| BRAF-mutated (V600) | 2 (9) | 8 (26) | 0.07b |
| BRAF-WT | 21 (91) | 17 (59) | |
| NA | 4 (14) | ||
| Lactate dehydrogenase level | |||
| < Upper limit of normal range | 5 (22) | 16 (55) | 1b |
| > Upper limit of normal range | 1 (4) | 5 (17) | |
| NA | 17 (74) | 8 (28) | |
| Previous systemic therapy | |||
| Ipilimumab as first-line therapy | 9 (39) | 5 (17) | |
| Ipilimumab as second-line therapy or more | |||
| After antiPD1 | 2 (9) | 1 (3) | 0.21b |
| After targeted therapy | 3 (13) | 8 (27) | |
| After chemotherapy | 9 (39) | 15 (51) | |
| Number of doses of ipilimumab | |||
| Median, range | 4 [2–8] | 3.8 [1–8] | |
| Geriatric assessment | |||
| ECOG status | |||
| 0 | 7 (31) | 9 (31) | |
| 1 | 15 (65) | 15 (51) | 0.55b |
| 2 | 1 (4) | 4 (14) | |
| ≥ 3 | 0 | 1 (3) | |
| Comorbidities | |||
| Charlson score: median, range | 0 [0–3] | 0 [0–3] | |
| Myocardial infarction | 1 | 0 | |
| Congestive heart failure | 2 | 2 | |
| Peripheral vascular disease | 1 | 1 | |
| Cerebrovascular disease | 1 | 0 | |
| Dementia | 0 | 0 | |
| Connective tissue disease | 0 | 0 | |
| Chronic pulmonary disease | 2 | 2 | |
| Peptic ulcer disease | 1 | 1 | |
| Liver disease | 0 | 0 | |
| Diabetes | 1 | 5 | |
| Renal disease | 0 | 0 | |
| Other tumor (non-metastatic) | 3 | 1 | |
| BMI | |||
| Median, range | 25.7 [16–41, 6] | 25.7 [15, 92–41, 23] | |
| Personal situation | |||
| With family at home | 14 (61) | 11 (38) | |
| Alone at home | 9 (39) | 17 (62) | |
| Institution | 0 | 0 | |
| NA | 0 | 1 | |
| Serum albumin (g/L) | |||
| Median, range | 39.7 [33–43, 3] | 44.5 [34–45, 7] | |
| > 30 | 16 (70) | 14 (48) | |
| ≤ 30 | 0 | 0 | |
| NA | 7 (30) | 15 (52) | |
| Lymphocyte count (g/L) | |||
| Median, range | 1.2 [0.49–4.12] | 1.4 [0.6–4.2] | |
BMI body mass index, NA not available, WT wild type
aIndependent t test
bFisher’s exact test
Adverse events
Detailed characteristics of AEs reported in the elderly group and younger group are provided in Table 2. AE occurrence and severity did not differ between the two groups (Fisher’s exact test).
Table 2.
Adverse events reported in the study and management
| AEs | Grades according to CTCAE v4.0 | Action required for the AE management | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elderly patients | younger patients | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Hospitalization | Invasive procedure | Systemic corticosteroids | Additive immunosuppressive therapy | |||||||||
| E | Y | E | Y | E | Y | E | Y | E | Y | E | Y | E | Y | E | Y | E | Y | ||
| Skin reaction | 5 | 7 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Asthenia | 4 | 2 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Nausea | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Hypereosinophilia | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Hypophysitis | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | |
| Colitis | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | |
| Hepatitis | 0 | 0 | 0 | 0 | 2a | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | |
| Pneumopathy | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Severe infection | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1a | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total n (%) | 11 (48) | 9 (43) | 6 (26) | 7 (33) | 5 (22) | 4 (19) | 0 | 1 (5) | 1 (4) | 0 | b5 (22) | 2 (9) | c4 (17) | 2 (9) | d5 (22) | 4 (19) | e2 (9) | 1 (4) | |
| p value (Fisher’s exact test) | 0.44 | 1.00 | 0.72 | 1.00 | 0.45 | 0.24 | 0.40 | 0.72 | 0.58 | ||||||||||
aAEs observed in patients previously treated with PD1 inhibitor in a sequential treatment
bHospitalization: in elderly group (median 32 days, [4; 64]) was required for 4 patients (including 1 patient for 2 successive AEs)/in younger group (median 17 days, [10; 24])
cInvasive procedures were: in elderly group: rectosigmoidoscopy (n = 1), liver biopsy (n = 2), bronchial fibroscopy (n = 1)/in younger group: rectosigmoidoscopy (n = 2)
dFive patients needed systemic corticosteroids (including 1 patient for 2 successive AEs)
eAdditive immunosuppressive therapy: in elderly group: mycophenolate mofetil (n = 1), infliximab (n = 1)/in younger group: infliximab (n = 1)
Among the elderly patients, 15 (65%) experienced at least one AE, mainly of grade 1 (n = 11). Six grade ≥ 3 AEs were reported in five patients (22%). Among them, two patients had been previously treated with PD-1 inhibitors as a sequential therapy. One patient with grade 3 hepatitis requiring corticosteroid pulses developed a Nocardia transvalensis infection leading to death (grade 5). The most common AEs were skin reactions (n = 10, 44%), grade 1 or 2. Severe AEs included colitis (1/23), hepatitis (2/23), hypophysitis (1/23) pneumonitis (1/23), and infection (nocardiosis) (1/23). Hospitalization and invasive procedures were required for four patients. Five patients (22%) received corticosteroids, three of them with high-dose infusions. Four patients developed corticosteroid-induced diabetes. Because of inadequate resolution of AEs (one hepatitis and one colitis), mycophenolate mofetil and infliximab, respectively, were added to corticosteroids. Ipilimumab was definitively stopped in three patients (13%) due to hepatitis or colitis.
In the younger patients, 15 (52%) experienced at least one AE. The most common (Table 2) AEs were grade 1 and 2 skin reactions (n = 7, 33%) and asthenia (n = 9, 43%). Four patients experienced a grade 3 AEs (19%) and one a grade 4 colitis (5%). None of them had been treated in a sequence after PD-1 inhibitor therapy. The patient who had grade 3 nausea and grade 4 colitis after three infusions required hospitalization and rectosigmoidoscopy. Intravenous methylprednisolone (1 mg/kg) was started, but three infusions of infliximab were then required due to corticosteroid dependence. Two patients had grade 3 hypophysitis. Finally, ipilimumab had to be definitively discontinued for three patients (10%) because of colitis or hypophysitis.
Evaluation of response, progression-free survival, and overall survival (Table 3; Fig. 1)
Table 3.
Response to treatment at week 16 and survival of elderly and young patients included in the study
| Elderly patients | Younger patients | |
|---|---|---|
| Number of patients | 23 | 29 |
| Response at week 16: n (%) | ||
| Complete response | 0 (0) | 1 (3) |
| Partial response | 3 (13) | 1 (3) |
| Stable disease | 9 (39) | 10 (34) |
| Progressive disease | 11 (48) | 17 (59) |
| Disease control rate at week 16 | 52% | 41% |
| Response rate at week 16 | 13% | 6% |
| Median progression-free survival (months) | 4 | 4 |
| Median overall survival (months) | 14 | 17 |
Fig. 1.
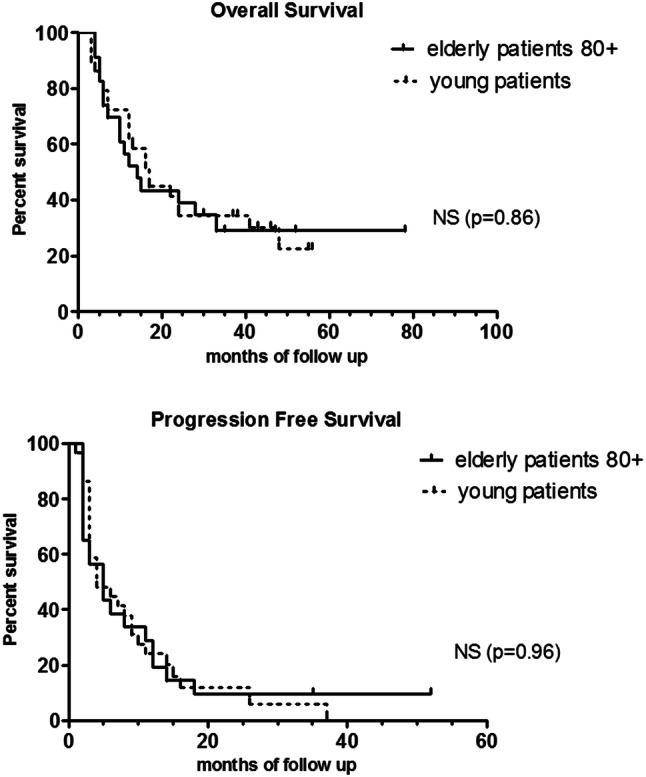
Overall survival and progression-free survival of the patients reported in the study
In the elderly group, disease control and response at week 16 were observed in 12/23 (52%) and 3/23 (13%) patients, respectively. At last follow-up, 7/23 patients were alive (three in complete remission, four with disease). Three patients aged 84, 90, and 93 years experienced long-term disease control without additional therapy and maintained independent physical and social function.
In the younger patients, disease control and response at week 16 were observed in 12/29 (41%) and 2/29 (6%) patients, respectively. A total of 72% (n = 21) of patients died of the disease. At the last follow-up, 8/29 patients were alive (four in complete remission, one with partial response, and three with stable disease).
Median progression-free survival was 4 months in both groups, and median overall survival was 14 months in the elderly group, and 17 months in the younger group, which is not significantly different (log rank test, p = 0.86).
Discussion
In this retrospective study focused on ipilimumab usage in patients over 80 years of age, we report AEs in 65% of patients, with 26% severe AEs (≥ grade 3), compared to 52% AE occurrence and 24% AE ≥ grade 3 in a group of younger patients treated during the same period at our institution. Due to the limited size of the series and the retrospective bias, the incidence can be under or overestimated. Interestingly, these results do not show any increase in high-grade AEs occurrence over the rate observed both in our younger group and in RCTs and retrospective studies of younger patients [7]. Our series of elderly patients is characterized by a low comorbidity Charlson’s score and a preserved general condition, as patients had been initially selected by physicians for ipilimumab therapy. However, irAE management can be challenging in elderly patients, as high-dose corticosteroids (22% of our patients), invasive procedures, and repeated hospitalizations may lead to degradation of general health status or death. Furthermore, the sequence of a PD-1 inhibitor followed by ipilimumab may increase irAE occurrence, so caution is needed in this population. Friedman et al. underlined special considerations for management of irAEs in older adults and the need for closer monitoring in this population [8]. They reported a grade 3–4 AE rate in patients over 80 years treated with ipilimumab of 29.7% [8]. Mian et al. reported a series of 858 patients over 65 years (mean age 74.8) with a 60% rate of irAEs and 20.7% considered as severe irAEs [9]. However, an age 65 cutoff is very different to that of 80 years; even if an 80-year-old patient starts ipilimumab with a good general status, each medical issue could destabilize a frail condition as well as social status functioning. Thus, assessment of the general condition and comorbidities of elderly patients should be recommended before any initiation of treatment [10], and Charlson’s score or the G-8 screening tool could also be useful.
Here, we report a median overall survival of 14 months and long-term disease control of 13% in the 80 + group. These efficacy results are not different to those observed in our younger group (Fig. 1), and are comparable to those reported in the literature, regarding patients with a median age of 65 years [7]. Although it has been hypothesized that older adults could benefit less from checkpoint inhibitors, we and others do not report lower survivals [8]. Immunosenescence is associated with a complex dysregulation of immune components involving antigen presenting cells (dendritic cells, macrophages, etc) and T-lymphocyte population percentages and action capacities. A decrease of naive T-cell populations and impaired function of CD8 T cells (cytokine production and cytotoxic functions) associated with an increase of immunosuppressive cells such as regulatory T cells and myeloid derived suppressive cells [11] may enhance tumor cells’ immune escape and impair immune checkpoint efficacy, providing a scientific rationale for thinking that prospective studies of checkpoint inhibitors in elderly patients are needed. In a recent meta-analysis focusing on efficacy of immune checkpoint inhibitors in elderly patients (age cutoff: 65–70 years) compared to younger patients, 4725 patients from eight studies were included, including three studies on ipilimumab [12]. There was no significant difference in overall survival between younger patient subgroups and older patient subgroups. Subgroup analysis according to type of checkpoint inhibitor showed a significant improvement in overall survival with ipilimumab in both younger and older patients (HR 0.82 95% CI [0.71–0.95]). These results are consistent with those observed by Sileni et al. in the Italian Expanded Access Programme cohort with an age cutoff 70 years [6].
Although retrospective and single center, this series in a real-life setting suggests that ipilimumab may be as safe and as efficient in very elderly patients as in younger ones, after adequate evaluation of their comorbidities and general condition. However, we have to keep in mind that older adults remain a more vulnerable population, above all when an AE occurs. Specific studies assessing ipilimumab and all new immunomodulatory drugs in very elderly patients are necessary, because immunosenescence could modify efficacy and/or safety, and because AE consequences may have deleterious effects on physical, and social function and quality of life.
Acknowledgements
We thank Dr Marie-Laure Jullie, Pathology Department, CHU Bordeaux, France, for language editing of the manuscript.
Abbreviations
- AE
Adverse events
- anti-TNFα
Anti-tumor-necrosis-factor alpha inhibitors
- BRAF
B-Raf proto-oncogene
- CI
Confidence interval
- CT scan
Computed tomography scan
- ECOG status
Eastern Cooperative Oncology Group status
- HR
Hazard ratio
- irAEs
Immunologic-related adverse events
- LDH
Lactate dehydrogenase
- OS
Overall survival
- PD-1
Programmed-death 1
- PFS
Progression-free survival
- RCT
Randomized clinical trials
- TNM
Tumor node metastasis
Author contributions
All the authors had full access to all the data in the study. Manuscript was prepared by Vaianu Leroy, Emilie Gerard and Anne Pham-Ledard. Clinical data were prepared and interpreted by Vaianu Leroy, Emilie Gerard, Caroline Dutriaux, Sorilla Prey, Aurelia Gey, Marie Beylot-Barry, Cecile Mertens, and Anne Pham-Ledard. Marie Beylot-Barry and Anne Pham-Ledard reviewed the paper and provided important advice.
Funding
No relevant funding.
Compliance with ethical standards
Conflict of interest
Consulting and advisory role for Bristol-Myers Squibb (Dutriaux, Beylot-Barry); speaker honorarium (Pham-Ledard, Dutriaux, Beylot-Barry) travelling, expenses and accommodation (Pham-Ledard, Dutriaux, Beylot-Barry, Prey). All other authors declare that they have no conflict of interest.
Informed consent
A written informed consent was obtained from all individual participants included in the study, allowing authors to exploit data anonymously.
Ethical approval and ethical standards
This study has been approved by the ethics committee of the University Hospital of Bordeaux, reference number GP-CE2018/11, and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Footnotes
Vaianu Leroy and Emilie Gerard contributed equally to this study.
References
- 1.Leroy V, Gerard E, Dutriaux C, Prey S, Gey A, Mertens C, et al. Tolérance et efficacité de l’ipilimumab chez les patients âgés. Ann Dermatol Venereol. 2016;143(Suppl 12):abstract:198. doi: 10.1016/j.annder.2016.09.242. [DOI] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horvat TZ, Adel NG, Dang T-O, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33:3193–3198. doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaehler KC, Piel S, Livingstone E, et al. Update on immunologic therapy with anti-CTLA-4 antibodies in melanoma: identification of clinical and biological response patterns, immune-related adverse events, and their management. Semin Oncol. 2010;37:485–498. doi: 10.1053/j.seminoncol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Chiarion Sileni V, Pigozzo J, Ascierto PA, et al. Efficacy and safety of ipilimumab in elderly patients with pretreated advanced melanoma treated at Italian centres through the expanded access programme. J Exp Clin Cancer Res. 2014;33:30. doi: 10.1186/1756-9966-33-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertrand A, Kostine M, Barnetche T, et al. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. doi: 10.1186/s12916-015-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman CF, Wolchok JD. Checkpoint inhibition and melanoma: considerations in treating the older adult. J Geriatr Oncol. 2017;8:237–241. doi: 10.1016/j.jgo.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mian I, Yang M, Hui Z, Mohsin S, Adi D, Shannon V, et al. Immune related adverse events and survival in elderly patients with melanoma treated with ipilimumab. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.34.15_suppl.3047. [DOI] [Google Scholar]
- 10.Du-Thanh A, Lesage C, Ferreira E, Dereure O, Guillot B. Innovative therapies for metastatic melanoma in elderly patients. Ann Dermatol Venereol. 2015;142:549–556. doi: 10.1016/j.annder.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Elias R, Karantanos T, Sira E, Hartshorn KL. Immunotherapy comes of age: immune aging and checkpoint inhibitors. J Geriatr Oncol. 2017;8(3):229–235. doi: 10.1016/j.jgo.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Nijhishima TF, Muss HB, Shachar SS, Moschos SJ. Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: a systematic review and meta-analysis. Cancer Treat Rev. 2016;45:30–37. doi: 10.1016/j.ctrv.2016.02.006. [DOI] [PubMed] [Google Scholar]


