Abstract
Anti-PD-1 and anti-CTLA-4 antibodies cause immune-related side effects such as autoimmune type 1 diabetes (T1D). It has also been suggested that by increasing TNF-α, IL-2 and IFN-γ production, anti-PD-1 and/or anti-CTLA-4 treatment could affect pancreatic beta cell function and insulin sensitivity. This study was based on a retrospective observational analysis from 2 July 2014 to 27 June 2016, which evaluated the occurrence of T1D and changes in glycemia and C-reactive protein (CRP) plasma concentrations in patients undergoing anti-PD-1 and/or anti-CTLA-4 treatment for melanoma at the Saint Louis Hospital. All cases of T1D that developed during immunotherapy registered in the French Pharmacovigilance Database (FPVD) were also considered. Among the 132 patients included, 3 cases of T1D occurred. For the remaining subjects, blood glucose was not significantly affected by anti-PD-1 treatment, but CRP levels (mg/l) significantly increased during anti-PD-1 treatment (p = 0.017). However, 1 case of type 2 diabetes (T2D) occurred (associated with a longer therapy duration). Moreover, glycemia of patients pretreated (n = 44) or concomitantly treated (n = 8) with anti-CTLA-4 tended to increase during anti-PD-1 therapy (p = 0.068). From the FPVD, we obtained 14 cases of T1D that occurred during immunotherapy and were primarily characterized by the rapidity and severity of onset. In conclusion, in addition to inducing this rare immune-related diabetes condition, anti-PD-1 treatment appears to increase CRP levels, a potential inflammatory trigger of insulin resistance, but without any short-term impact on blood glucose level.
Keywords: Melanoma, Anti-PD-1 antibody, Type 1 diabetes, Type 2 diabetes, Insulin resistance, Adverse events
Introduction
Programmed cell death-1 (PD-1) antibodies are immune-checkpoint inhibitors that selectively block the interaction of the PD-1 receptor with its ligand, disrupting the negative signal that regulates T-cell activation and proliferation against tumor cells. Inhibition of PD-1/PD-L1 signaling, either through antibody blockade or PD-1 deficiency, has been shown to improve the clinical outcome, restore a functional T-cell response in several cancers and increase TNF-α, IL-2 and IFN-γ production [1]. Approved by the Food and Drug Administration for the treatment of advanced melanoma since September 2014, anti-PD-1 therapy has been associated with significant improvements in overall survival and progression-free survival compared to dacarbazine and ipilimumab [2, 3]. However, anti-PD-1 treatment can cause immune-related side effects, and 25 cases of type 1 diabetes (T1D) induced during treatment have been described worldwide [4–14]. In addition, combination therapy with a cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor (disrupting the negative signal induced by the CTLA-4/CD80-86 interaction) has shown an increased occurrence of immune-related side effects [15].
Inflammation is now recognized as an important contributor to the pathophysiology of type 2 diabetes (T2D) [16]. Anti-inflammatory treatments such as anti-TNFα [17, 18], IL-1 inhibitors and salsalate [19] have been shown to reduce the risk of developing T2D or to moderately improve glucose control in T2D patients, suggesting that T2D may be an inflammatory disease initiated by the innate and adaptive immune systems. Consequently, anti-PD-1 treatment, by stimulating cellular immunity, may have an impact on T2D genesis in treated patients.
In this study, in a cohort of 132 consecutive patients with unresectable or metastatic melanoma treated with anti-PD-1 and/or anti-CTLA-4 drugs at our oncodermatology center, we retrospectively evaluated diabetes occurrence and blood glucose, insulin, C-peptide, and pancreatic beta-cell auto-antibody changes before and after diabetes onset. In the remaining subjects, we assessed blood glucose and HbA1c level changes over the 2 years of observation. We also considered all cases of T1D induced by immunotherapy reported to the French Pharmacovigilance database (FPVD) since the availability of these drugs in France.
Methods
All the patients attending the Oncodermatology Department of Saint Louis Hospital in France (Paris) from 2 July 2014 to 27 June 2016 and treated for unresectable or metastatic melanoma with anti-PD-1 agents were eligible (n = 148).
All patients underwent biological and clinical standardized follow-up as described below.
The study was conducted in accordance with the principles of the Declaration of Helsinki. All participants gave written informed consent (inclusion in the Melbase register or non-opposition note) to laboratory analyses, clinical examinations, sampling of biomaterial, and the use of their records for research purposes.
The drafting of the manuscript followed the STROBE guidelines for cohort studies.
Criteria (and characteristics) underpinning our clinical investigation
Clinical data were directly collected from a computerized database. All medical records were reviewed to ascertain the quality of coding: type of anti-PD-1 treatment (pembrolizumab or nivolumab), anti-cytotoxic T-lymphocyte associated protein 4 (CTLA-4) treatment before or in combination with anti-PD-1, duration of anti-PD-1 treatment, and treatment side effects.
T1D has been defined as follows [20]: symptomatic hyperglycemia (polyuria polydipsia, asthenia, polyphagia, body weight loss) associated with ketosis (with or without ketoacidosis) and undetectable C-peptide. Patients with no T1D-associated autoimmunity were considered T1D if the C-peptide concentration was undetectable.
T2D was defined as fasting glycemia ≥ 1.26 g/l (7.00 mmol/l) or random glycemia ≥2 g/l (11.1 mmol/l) plus symptoms of hyperglycemia or HbA1c ≥ 6.5% (47.5 mmol/mol) [20].
Prediabetes was defined as fasting glycemia between 1.00 and 1.25 g/l (5.60–6.99 mmol/l) or HbA1c between 5.7 (38.8 mmol/mol) and 6.4% (46.4 mmol/mol).
For the analysis of T2D occurrence, the exclusion criteria were as follows: patients with T2D or steroid-induced hyperglycemia prior to inclusion, patients who developed T1D after anti-PD-1 treatment initiation, patients receiving fewer than 2 anti-PD-1 infusions, and those with fewer than 2 glycemia results available.
To highlight possible bias in the analysis of T2D development, confounding variables such as age, gender, corticosteroid treatment, and cardiovascular risk factors including arterial hypertension, dyslipidemia, obesity and T2D prior to treatment were collected. Due to the probable impact of corticosteroids on glycemia and CRP level—especially in the group of patients who received prior or concomitant ipilimumab—glycemia and CRP variations were compared between patients who received corticosteroids and those who did not.
Overweight and obesity were defined as a BMI over 25 and 30 kg/m2, respectively.
Biological analyses
Biological data such as glycemia, CRP and HbA1c were retrospectively analyzed from collected blood samples.
For each subject, random (not necessarily fasting) glycemia and CRP measures were performed before starting treatment and then again every 2 weeks (nivolumab) or 3 weeks (pembrolizumab). The last glycemia and CRP measures were performed on the day of the last drug infusion, before the infusion. CRP was measured by immunoturbidimetry, insulin and C-peptide by electro-chemiluminescence, and glycemia using the glucose oxidase method (Modular, Roche, Meylan, F).
For half of the cohort, HbA1C% (IFCC) was measured at the end of treatment and 3 months before the end of treatment (due to changes in follow-up practice). HbA1c was determined by electrophoresis using a Capillary 3 Tera (Sebia, Evry, F).
Glycemia, HbA1c and CRP are presented as the median [q1–q3], unless otherwise stated. Comparisons between before and after anti-PD-1 treatment for the overall population and subgroups (anti-CTLA-4 previously used or associated) were performed using a Wilcoxon paired test. Differences between glycemia and CRP variations in the two subgroups were evaluated with a t test. Distributions of confounding variables such as corticosteroid treatment were evaluated with Fisher’s exact test. Correlations between CRP evolution and glycemia modifications were analyzed with linear regression. The level of significance was set at p < 0.05.
Islet cell autoantibodies, C-peptide, and insulin analysis were retrospectively performed on frozen samples for patients who presented with an extreme glycemia increase (T1D suspected).
GAD, tyrosine-phosphatase inhibitor (IA2), zinc transporter 8 (ZnT8) auto-antibodies (GADA, IA2A, ZnT8A) and anti-insulin antibodies (IAA) were measured using enzyme immunoassays (RSR, Cardiff, UK; Medipan, Berlin, Germany, respectively). Cut-off values for positive results for GADA, IA2A, ZnT8A and IAA were 5.0, 7.5, 15.0 and 2.7 U/ml, respectively.
FPVD investigation of immune-related diabetes cases
The FPVD was established in 1985 to record Adverse Drug Reactions (ADR) reported to a network of 31 French Regional Pharmacovigilance Centers. For each report, information about the patient, the ADR coded according to the Medical Dictionary for Drug Regulatory Activities (MedDRA), drug exposure and summary of the clinical description are recorded. If possible causality is found between the drug and the occurrence of the ADR, the drug is defined as “suspected” of having caused the ADR. A request of the FPVD was made on 16 December 2016 for all diabetes cases “suspected” to have been induced by ipilimumab, pembrolizumab or nivolumab or to have appeared/been aggravated by concomitant use of these immunotherapies and registered in FPVD since their availability. All the records were analyzed in this study.
Results
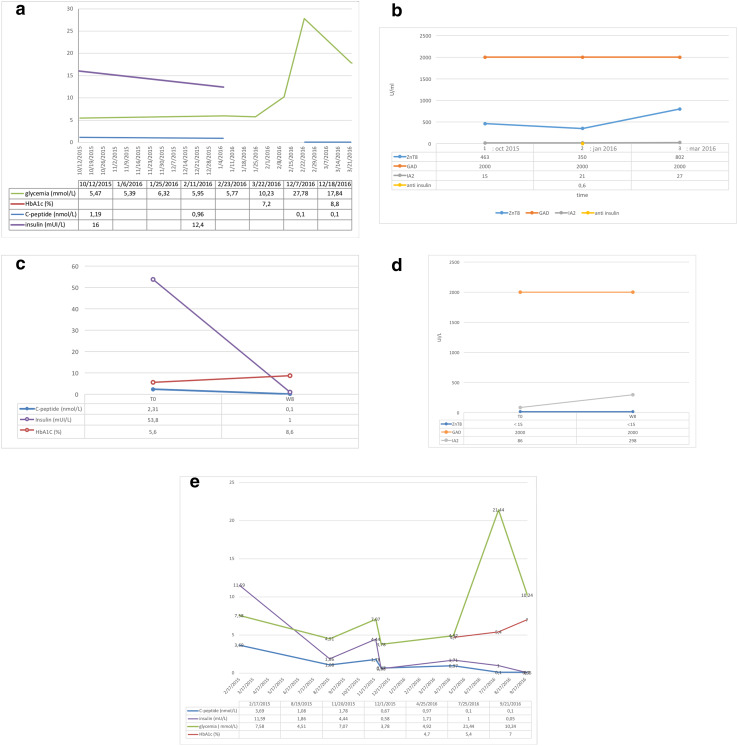
From 2 July 2014 to 27 June 2016, 148 subjects attending the department were eligible for anti-PD-1 treatment. The database revealed that data were missing (fewer than two glycemia tests reported) for 11 (7.4%), and 5 subjects (3.4%) did not receive anti-PD-1 treatment. Among the 132 remaining patients, three presented with T1D (2.3%) in the 2 years of observation and were excluded for glycemia evolution analysis. Descriptions of these cases are provided in Table 1 and Fig. 1a–e.
Table 1.
Characteristics of type 1 diabetic patients under anti-PD-1 ± anti CTLA-4 therapy in our center
| Characteristics | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Age (years) | 73 | 84 | 34 |
| Gender | Male | Female | Male |
| Time (weeks) between anti PD-1 treatment initiation and hyperglycemia | 6 | 6 | 11 |
| Melanoma characteristics | Breslow 2.65 mm, BRAF-mutated melanoma. He received adjuvant interferon treatment for 10 months, which was stopped because of Graves’ disease occurrence. He received first-line treatment with vemurafenib and cobimetinib combination because of lung, lymph node and hepatic metastatic progression (3 months). Second-line treatment consisted of anti PD-1 treatment (nivolumab) because of progression | A nasal cavity, BRAF wild-type but NRAS mutated melanoma, with secondary lung metastasis evolution after surgical treatment and adjuvant radiotherapy. Her first-line treatment was anti PD-1 (pembrolizumab) | He received third-line treatment with combination therapy ipilimumab and nivolumab after ipilimumab (with hypophysitis occurrence) then nivolumab alone |
| Symptoms at diabetes diagnosis | Abdominal pain and vomiting associated with a polyuria-polydipsia syndrome | Asthenia and somnolence, polyuria-polydypsia syndrome, and loss of 5 kg |
No symptoms No family history of T2D No personal history of hypertension, overweight or dyslipidemia |
| Glycemia (mmol/l) | 27.78 | 26.71 | 21.44 |
| Dipstick test | 3 crosses glucose and ketone | 3 crosses glucose and ketone | No ketonuria |
| Creatinine (µmol/l) | 177 (baseline: 90) | 156 (baseline: 60) | N/A |
| HCO3-/pH | 18 mmol/l/N/A | 6 mmol/l/7.128 | N/A |
| HbA1c% (mmol/mol) | 7.2 (55.2) | 8.6 (70.5) | 5.4 (35.5) |
| Auto antibodies: GADA/IA-2A/ZnT8A (UI/l) | > 2000/27/802.3 | > 2000/298/<10 | Negatives |
| C peptide/insulin | Undetectable | ||
| Management of immune-related adverse event | Anti PD-1 maintained 3.5 weeks, then resumed with insulin therapy | Pembrolizumab was stopped after the acute event and glycemia normalization with insulin therapy treatment despite tumor response. Follow-up and resumption will be discussed depending on evolution | Anti PD-1 continuation with insulin therapy |
Fig. 1.
Glycemia, C peptide and insulin concentration changes in patient 1 (P1) (a); Pancreatic beta-cell auto-antibody changes before, D0 and at the onset of diabetes in patient 1 (P1) (b); Glycemia, C peptide and insulin concentration changes at anti-PD-1 treatment initiation and at week 8 of treatment in patient 2 (P2) (c); Autoantibody changes before, D0 and at the initiation of diabetes symptoms in patient 2 (P2) (d); Glycemia, C peptide and insulin changes over time in patient 3 (P3) (e). D day, W week
Thirteen patients (8.8%) who already had diabetes at treatment initiation (T2D n = 12, steroids-induced diabetes n = 1) were also excluded. Thus, 116 were included in the final analysis. The mean follow-up was 7 months (± 6.68 SD), and the median follow-up was 5 months (range 0.5–39). The median age was 62.5 (range 26–93) years. There were 56 (48.3%) women and 60 (51.7%) men. The median BMI was 24.89 kg/m2 (range 15.57–46.87). Anti-PD-1 treatment was pembrolizumab for 55 subjects and nivolumab for 61. 52 (46%) patients received ipilimumab (anti-CTL-4) before or concomitant with anti-PD-1 therapy (n = 24 before nivolumab, n = 19 before pembrolizumab, n = 1 before both treatments, and n = 8 ipilimumab concomitant with nivolumab). 55 patients (47%) presented with at least one cardiovascular risk factor at treatment initiation. 16 of the 44 patients treated with ipilimumab before anti-PD1 presented at least one ipilimumab-related immune side effect. Immunotherapy-related side effects occurred in 25 patients on anti-PD-1 and in 3 patients on combination therapy (Table 2).
Table 2.
Characteristics of patient cohort
| Patients | 116 | |
| Age (years) | 62.5 (range 26–93) | |
| Gender | Female: n = 56 (48.3%); male: n = 60 (51.7%) | |
| Median BMI (kg/m2) | 24.89 (range 15.57–46.87) | |
| At least one cardiovascular risk factor | n = 55 (47%) | |
| Treatment | ||
| Ipilimumab before | Total: n = 44 | |
| Nivolumab: n = 24 | ||
| Pembrolizumab: n = 19 | ||
| Both: n = 1 | ||
| Ipilimumab concomitantly | n = 8 | |
| Ipilimumab after anti PD-1 | n = 4 | |
| Nivolumab/pembrolizumab | n = 55/n = 61 | |
| Median anti PD-1 therapy duration | 5 months (0.5;39) | |
| Median line number | 2 with 4 re-challenges | |
| Concomitant corticosteroid treatment | n = 32 (28%) | |
| Immunotherapy-related adverse events | ||
| Side effects | Ipilimumab: n = 16 | |
| Anti PD-1: n = 25 | ||
| Concomitant treatment n = 3 | ||
| Ipilimumab | Hypophysitis n = 10 | |
| Colitis n = 4 | ||
| Hepatitis n = 2 | ||
| Nivolumab/pembrolizumab | Meningitis n = 1 | |
| Hepatitis n = 4 | ||
| Thyroiditis n = 9 | ||
| Pneumonitis n = 5 | ||
| Hypophysitis n = 3 | ||
| Vitiligo n = 2 | ||
| Surrenal insufficiency n = 1 | ||
| Ipilimumab + nivolumab | Colitis n = 1 | |
| Hypophysitis n = 1 | ||
| Hepatitis n = 1 | ||
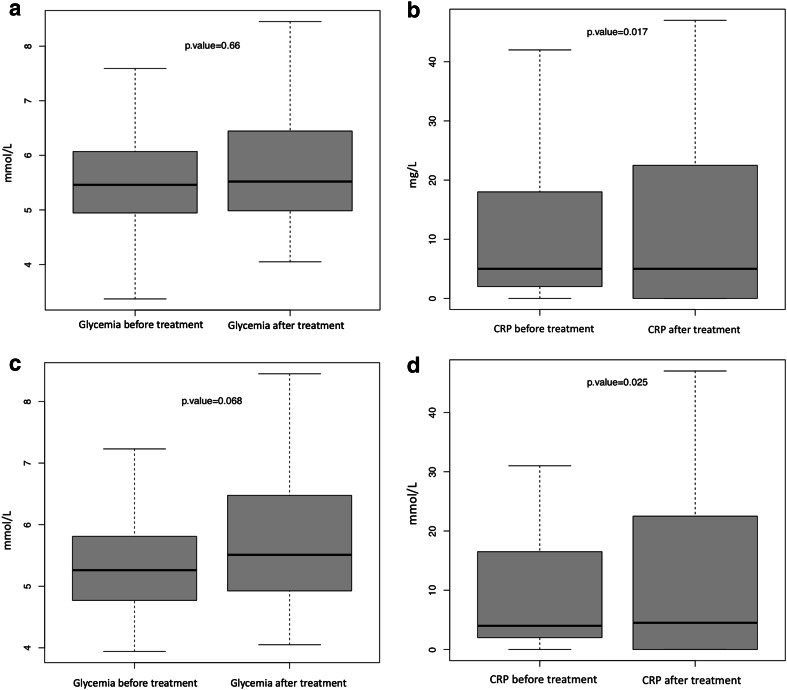
CRP concentration (mg/l) changes—before and after anti-PD-1 therapy—were analyzed to research a potential proinflammatory trigger induced by immunotherapy. It changed from a median of 5 [2–18] to 5 (0–21.75) with a 44% increase in the mean (21.54–31.08 mg/l; n = 114, p = 0.017) after anti-PD-1 treatment (Fig. 2a). Glycemia (mmol/l) was measured in a fasting state in 60 (52%) patients and in a non-fasting state in 41 (35%). The conditions of blood glucose sampling were not available for 15 patients (13%). No changes in glycemia (mmol/l) were observed: 5.46 (4.95–6.07) before versus 5.52 (4.98–6.44) after treatment (n = 116, p = 0.66) (Fig. 2b). Additionally, fasting glycemia was not affected by anti-PD-1: from 5.5 (4.9–5.59) to 5.4 (4.86–6.06) (n = 60, p = 0.85). Indeed, in case of patients for whom fasting glycemias were available and who did not receive corticosteroids, linear regression did not highlight any short-term correlation between CRP evolution and glycemia modification (n = 44; R2 = 0.07; p = 0.48).
Fig. 2.
Random glycemia (mmol/l) changes before and after anti-PD-1 treatment. Before treatment median 5.46 (q1:4.95-q3:6.07); after treatment median 5.52 (q1:4.98-q3:6.44) (a). Median CRP (mg/L) changes before and after anti-PD-1 treatment; before treatment median: 5 (q1:2-q3:18); after treatment median: 5 (q1:0-q3:21.75) (b). Glycemia (mmol/l) changes before and after anti-PD-1 treatment with concomitant or pretreatment with ipilimumab; before treatment median: 5.26 (q1:4.78-q3:5.78); after treatment median: 5.51 (q1:4.94-q3:6.46) (c). Median CRP (mg/l) changes before and after anti-PD-1 treatment in the subgroup that received prior or concomitant anti-CTLA-4; before treatment: 4 (2; 16.5); after treatment: 4.5 (0; 20.25); p = 0.025 (d)
However, 6 new cases of prediabetes occurred in the course of treatment follow-up among the 58 subjects who were tested for HbA1c. One patient was newly diagnosed with T2D and was treated with metformin. He was a 49-year-old obese man (BMI 34.64 kg/m2) and had hypertension. He did not receive ipilimumab before anti-PD-1 treatment and presented with immune asymptomatic and limited pneumonitis first, which did not require corticosteroid therapy. He was still responding after 39 months of anti-PD-1 treatment. Moreover, glycemia (mmol/l) of anti-CTLA-4 pretreated (n = 44) or concomitantly treated (n = 8) patients tended to increase between the beginning and the end of anti-PD-1 therapy: 5.26 (4.78; 5.78) to 5.51 (4.94; 6.46) (n = 52, p = 0.068) (Fig. 2c) and was associated with an increased CRP (mg/l) from 4 (2; 16.5) to 4.5 (0; 20.25) (p = 0.025) (Fig. 2d). It should be stated that glycemia decreased or was stable in some patients (n = 20), corresponding with those with a shorter therapy duration [median: 4.5 (n = 20) versus 5.25 (n = 32) months]. These data could contribute to this tendency. No additional increase in cases of fasting glycemia was found in this subgroup of patients [from 5.32 (4.82; 5.92) to 5.34 (4.87; 6.19); n = 35 p = 0.30]. Moreover, no difference was found in glycemia variation with (n = 16) versus without (n = 36) corticosteroids (p = 0.51), and there was non-significant glycemia modification in both subgroups [5.56 (4.81; 5.925) to 5.705 (4.83; 6.885) p = 0.56; n = 16 (corticosteroids) versus 5.25 (4.8; 5.89) to 5.375 (4.97; 6.15) p = 0.37; n = 36 (no corticosteroids)]. However, corticosteroids seemed to decrease CRP (mg/l) [7 (2; 13.5) to 4 (4.83; 6.69)], whereas CRP increased significantly in the subgroup who did not receive corticosteroids [4 (2; 16.75) to 5.5 (2; 24.5); p = 0.008, n = 36], with a significant difference in CRP variation between the two groups (p = 0.04).
To investigate a larger landscape of immune-related T1D on a national level, we accessed the FPVD. As of 16 December 2016, 18 patients had been reported to the FPVD to have developed immunotherapy-related (ipilimumab, nivolumab or pembrolizumab) diabetes. Their clinical features are listed in Table 3. Of these cases, four patients did not seem to have developed a new case of T1D: two had a personal history of T2D (one presented only with glycemia control impairment without ketoacidosis and in the second one, ketoacidosis could have been precipitated by an infectious disease). For a third patient, diabetes occurred during corticosteroid treatment. A fourth patient already had T1D but developed ketoacidosis during the course of treatment.
Table 3.
Characteristics of the type 1 diabetic patients from the French pharmaco vigilance database
| Variables | Characteristics (n = 14) |
|---|---|
| Sex M/F (n) | 7/7 |
| Age (median) (years) | 69 (range 40–82) |
| BMI (kg/m2) (median) | 24.1 (range 17.6–28.3)/data unknown for 3 patients |
| Cardiovascular risk factors | n = 14 |
| Dyslipidemia | 2 |
| Hypertension | 3 |
| Type 2 diabetes | 1 |
| Obesity | 1 |
| Type of cancer (n) | |
| Melanoma | 8 |
| Lung cancer | 5 |
| Clear renal cell carcinoma | 1 |
| Prior autoimmune disease (n) | 4 patients |
| Thyroiditis | 4 |
| Rheumatoid arthritis | 1 |
| Psoriasis | 1 |
| Treatment (n) | |
| Ipilimumab | 4 |
| Pembrolizumab | 1 |
| Nivolumab | 9 |
| Other immune-related concomitant side effects (n) | |
| Colitis | 2 |
| Hepatitis | 1 |
| Thrombopenia | 1 |
| Pneumonitis | 1 |
| Thyroiditis | 2 |
| Time to onset (weeks) | 8 (range 1.7–63) |
| Symptoms at diagnosis | |
| Polyuria polydispia | 8 |
| Asthenia | 6 |
| Anorexia/weight loss | 6 |
| Neurological disorders | 4 |
| Abdominal disorders | 7 |
| Tachypnea | 2 |
| Ketoacidosis at diagnosis | 12 (N/A data for 2 patients) |
| Glycemia (median) (mmol/l) | 52.78 (range 16.56–106.6) |
| HbA1c (%) at diagnosis | |
| <8.7 | 4/6 |
| >8.7 | 2/6 |
| Autoantibody positivity (n) | |
| GADA | 2/6 performed |
| ZnT8 | 0/0 performed |
| IA2 | 1/4 performed |
| Anti-insulin | 1/3 performed |
| Total | 4/7 tested patients presented positive antibodies |
| Management (n) | |
| Discontinued | 3/14 |
| Stopped and resumed | 3/14 |
| Continued | 4/14 |
| Unknown | 4/14 |
| Insulin therapy (n) | |
| Glycemia control | 12 |
| Death | 1 |
| N/A data | 1 |
| Intensive care unit | 2 |
| Evolution | |
| Glycemia control | 13/14 |
| Remission after treatment discontinuation (n) | 1/14 |
| Death | 1 |
Thus, only 14 cases appeared to be newly diagnosed acute T1D based on T1D criteria (50% men and 50% women). Four were treated with ipilimumab alone and 10 with anti-PD-1 treatment (9 nivolumab and 1 pembrolizumab). It can be noted that one received pembrolizumab before ipilimumab, which was discontinued due to disease progression. The median age was 69 years (range 40–82). Eight cases were treated for melanoma, five for lung cancer, and one for clear renal cell carcinoma. The median time to diabetes onset was 8 weeks (range 1.7–63). Half of the subjects had low HbA1c alongside high glycemia. The median glycemia level at onset was 52.78 mmol/l, and 86% of the patients presented with ketoacidosis at diabetes onset (no data for two subjects), which required admittance to an intensive care unit for two patients. Autoantibodies were positive in 4 of the 7 patients tested. Diabetes occurrence resulted in definitive discontinuation of immunotherapy in 21% (3/14) of cases and discontinuation then resumption in 21% (3/14) of cases. In 29% (4/14) of cases, immunotherapy was maintained without interruption. No details were available for 29% (4/14). For 12 of 14 patients, diabetes was controlled with insulin therapy, whereas one patient died (no data available for one). In addition, an 82-year-old man without a history of T2D presented with fulminant T1D (HbA1c: 7%) with ketoacidosis after 8 weeks of treatment with nivolumab. However, diabetes remission with insulin withdrawal after glucose achievement was noted after immunotherapy discontinuation and without steroid treatment.
Discussion
In this cohort of patients, the occurrence of diabetes appears to be a rare side effect compared to other endocrinopathies, since it occurred in only 2.3% of our patients with a median follow-up of 5 months. However, this is more than twice the proportion previously reported [8, 21].
The main phenotype of immunotherapy-related diabetes has the appearance of T1D at onset with hyperglycemic symptoms and ketosis in the majority of cases. Two patients had autoantibody positivity, confirming the diagnosis of autoimmune diabetes. The third patient was free from autoantibodies. Diabetes was detected from systematic glycemia measures, which were very high. However, C-peptide was undetectable, suggesting total destruction of β-cells at onset. The rapidity of symptom occurrence associated with a relatively low level of HbA1c contrasting with very high blood glucose levels suggests fulminant diabetes (FD) as described in Japanese patients, who do not present autoantibodies in most cases [22, 23]. In addition, 50% of the subjects listed in the FPVD presented with the same profile: acute diabetes onset related to immunotherapy seems to be a fulminant disease in most of the cases, with ketosis or ketoacidosis at presentation. Autoantibodies were positive in 2 of our 3 DT1 patients and in 4 of the 7 patients who were tested for this and reported in the FPVD, with a high suspicion of T1D, as well as in 11 of the 18 Caucasian patients tested and reported in the literature [14].
In our cohort, autoantibodies were found to be positive in two patients prior to anti-PD-1 treatment and diabetes onset, suggesting that they were predisposed to T1D. In general, T1D occurs mainly in childhood and adolescence [24]; however, latent autoimmune diabetes in adults (LADA) also represents approximately 10% of non-insulin-dependent cases, involving 30- to 50-year-old subjects who at a high risk of becoming insulin dependent (usually in 4–6 years) and display several genetic (HLA), immune and metabolic characteristics of T1D. Positive autoantibodies are associated with LADA, especially anti-GAD, as are other antibodies whose association is correlated with a more rapid progression to insulinopenia and, therefore, insulin requirement [25]. Thereby, a specific T CD8+ cell clone could cause islet β-cell destruction, but this would have been controlled by the PD-1/PD-L1 interaction. In one of our cases, the pre-diabetic state could have been triggered beforehand by IFNα treatment introduced some time prior to anti-PD-1 [14]. Thus, in these 2 cases, PD-1/PD-L1 binding seemed to be both necessary and sufficient to control this autoimmunity induced by anti-PD-1 treatment. In mice, an important role of PD-1/PD-L1 binding has been demonstrated in preventing diabetes onset [26, 27], and the existence of PDC1 (PD-1 gene) polymorphisms in humans has been linked to an increased risk of autoimmune diabetes [28].
The absence of autoantibodies in some patients does not exclude autoimmune diabetes. Other factors seem to be involved in anti-diabetic immune tolerance, such as CTLA-4 and T regulatory cells (Treg) via low IL2 production, as shown by Kochupurakkal et al. [29]. Their interactions seem to participate in CD8 T cell downregulation in autoimmune diabetes, and in the absence of negative costimulation induced by the PD-1/PD-L1 pathway, CTLA-4 and IL2 production possibly maintains self-tolerance.
Indeed, in the absence of autoimmune disease, autoreactive T-lymphocytes in healthy subjects are present in the blood circulation, testifying to a silent, “benign autoimmunity” [30], and an intact immune system comprises cells such as CD4+ T cells with the ability to prevent the activation of these autoreactive T-cells [31]. Autoantibody absence could be due to a massive, sudden infiltration and destruction of islet beta cells by a specific CD8+ T cell clone produced because of the concomitant neutralization of several checkpoint inhibitors (CTLA-4, PD-1, IL2/T regulatory cells). Possibly, this sudden event, without a pre-diabetic state and in the absence of previous β-cell destruction, does not enable prior antibody production.
At diabetes onset, in most documented cases, immunotherapy was continued or temporarily discontinued until hyperglycemia resolved after antidiabetic treatment. In the majority of cases, insulin therapy was needed and maintained even if immunotherapy was discontinued. No description was found of hyperglycemia worsening after immunotherapy was resumed. However, in the FPVD, the patient who had preexisting T1D displayed ketoacidosis under immunotherapy. Menzies et al. reported an aggravation of preexisting autoimmune diseases with anti-PD-1 therapy, which could generally be managed with symptomatic treatments without decreasing the anti-PD-1 efficacy and without increased risk of other immune-related side effects [32]. In the case reported by Hansen et al. [4] and one patient in the FPVD, clinical remission of diabetes was observed after anti-PD-1 treatment discontinuation and no steroid prescription, which suggests a potential spontaneous reversible phenomenon. However, corticosteroids do not seem to reverse autoimmune diabetes, which has also been reported in the literature [33] and in one other patient in the FPVD.
In parallel with this acute and severe immune-related side effect, which appears relatively early after the start of anti-PD-1 treatment—according to the literature, cases reported to the FPVD and observed in our institution—we have hypothesized a potential long-term and insidious side effect on glycemia on patients treated with anti-PD-1, with or without prior or concomitant anti-CTLA-4 therapy, due to the potentiation of an abnormal inflammatory state by anti-PD-1/anti-CTLA-4 therapy and because of a prolonged response resulting in a long therapy duration.
In fact, T2D is a metabolic disease that is strongly linked to obesity and often preceded by IR [34]. Nutrient excess and adiposity induce low-grade inflammation [35] which, as it is now well established, characterizes prediabetes and T2D [36]. Previous studies have suggested that chronic subclinical inflammation causes insulin resistance (IR), resulting in an inverse relationship between insulin sensitivity and CRP levels [16]. Moreover, IR and T2D involve different subsets of the innate and adaptive immune systems [37–40]. In our study, a slight but significant increase in CRP levels was observed, which could reflect the inflammatory profile induced by immunotherapy, but we cannot rule out the possibility that this is related to disease progression (70% of our cases presented with progressive disease) rather than to immunotherapy.
Generally, blood glucose levels were not significantly affected by anti-PD-1 treatment. However, the absence of a change could be explained by a short duration of exposure. In fact, the patient who developed T2D had the longest duration of anti-PD-1 treatment (39 months) with diabetes onset at 24 months. However, he was obese, which constitutes a major risk factor for IR and T2D. Like diabetes, atherosclerotic macrovascular disease is associated with abnormal cellular immunity, which may be both a cause and a consequence of immune activation.
The PD-1/PD-L1 pathway has been more thoroughly investigated: PD-1 has an important role in downregulating the pro-atherogenic T-cell response, and thus PD-1 blockade could increase the risk of cardiovascular complications. In addition, the role of PD-1/PD-L1 in the development of T2D with atherosclerotic macrovascular lesions has been studied. It was shown that the soluble form of PD-L1 (released through proteolytic cleavage of membrane-bound PD-L1), was promoted by IFN-γ in the patients with T2D, and this subsequently promoted T-cell proliferation by blocking the PD-1/PD-L1 pathway involved in the pathogenesis and progression of T2D and atherosclerotic macrovascular disease [41]. In our study, the number of patients in the prediabetes state doubled in 3 months (from 5 to 11 patients) on the basis of the HbA1c criterion. This number may be underestimated since we did not perform a fasting blood glucose measurement or an oral glucose tolerance test for every patient. On the other hand, the use of corticosteroid treatment could be a confounding factor, and this treatment was administered temporarily to 3 out of the 6 patients who became prediabetic.
In the group of patients treated previously or concomitantly with anti-CTLA-4 drugs, there was a trend of increased glycemia during anti-PD-1 treatment (the lack of significance might be due to the low power of this analysis). Several studies have highlighted costimulation molecules that seem to impact IR occurrence. Insulin sensitivity improvement has been observed with CTLA-4-IgG administration [42–44] or CD40L deletion/blockade [45] in mice by inducing changes in macrophage polarization (anti-inflammatory M2 polarization from pro-inflammatory M1 polarization). In fact, adipose tissue (AT) is an active site for MHC class I- and II-mediated antigen presentation (probably free fatty acids after adipocyte death [38, 39]) by environmental macrophages, adipocytes and dendritic cells [40], and AT T-cells seem to have a limited T-cell receptor (TCR) repertoire, which suggests a consequence of local antigen presentation [37]. However, despite this solid rational that supposes long-term glycemia impairment with immunotherapy, no significant changes in glycemia were observed, even for fasting glycemia or in patients treated previously or concomitantly with ipilimumab, despite a significant increase in CRP. A longer follow-up and better evaluation criteria (HbA1c) may be necessary to address this issue.
Conclusion
Acute, symptomatic diabetes is an increasingly reported adverse event of anti-PD-1 treatment. Because of the rapidity of occurrence and the severity of the diabetes, physicians in charge of patients on immunotherapy should be aware of the need to immediately check blood glucose levels as the symptoms can be confused with those of cancer progression, such as asthenia, abdominal pain, dehydration and confusion. We need more observational studies to understand how this side effect evolves after anti-PD-1 maintenance or discontinuation and to ascertain the predictive value of T1D-associated autoantibodies.
Asymptomatic abnormal glucose tolerance has not been proven to occur in patients on immunotherapy, whereas changes in inflammatory profiles have been and are known to promote insulin resistance. Whether metabolic syndrome and T2D will prove to be significant long-term side effects of this class of drugs should be explored in further longitudinal studies.
Acknowledgements
We thank the French Network of Pharmacovigilance Centers and all the patients for their contribution.
Abbreviations
- AP-HP
Assistance Publique-Hôpitaux de Paris
- AT
Adipose tissue
- CRP
C-reactive protein
- CTLA-4
Cytotoxic T-lymphocyte-associated protein 4
- FD
Fulminant diabetes
- IL2
Interleukin 2
- IR
Insulin resistance
- PD1
Programmed cell death-1
- PD-L1
Programmed cell death ligand-1
- T1D
Type 1 diabetes
- T2D
Type 2 diabetes
- Th
T-helper
- Treg
regulatory T cells
Author contribution statement
Marie-Léa Gauci, Philippe Boudou, Céleste Lebbé and Jean-François Gautier had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. (1) All authors made substantial contributions to the conception and design, the acquisition of data or analysis and the interpretation of data. (2) All authors contributed to drafting the article or revising it critically for important intellectual content. (3) All authors gave their final approval of the version to be published.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval and ethical standards
There is no trial number. Official approval was obtained on 14 May 2012 from the ethical committee (Comité de Protection des Personnes (CPP)) Ile-de-France XI for the inclusion (and use of clinical and biological data) of patients in Melbase after their consent. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Informed consent
Informed consent was obtained from all participants in the study.
Footnotes
Céleste Lebbé and Jean-François Gautier are co-last authors.
References
- 1.Sakuishi K, Apetoh L, Sullivan JM, et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 4.Hansen E, Sahasrabudhe D, Sievert L. A case report of insulin-dependent diabetes as immune-related toxicity of pembrolizumab: presentation, management and outcome. Cancer Immunol Immunother. 2016;65:765–767. doi: 10.1007/s00262-016-1835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin-Liberal J, Furness AJS, Joshi K, et al. Anti-programmed cell death-1 therapy and insulin-dependent diabetes: a case report. Cancer Immunol Immunother. 2015;64:765–767. doi: 10.1007/s00262-015-1689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes J, Vudattu N, Sznol M, et al. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. 2015;38:e55–e57. doi: 10.2337/dc15-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mellati M, Eaton KD, Brooks-Worrell BM, et al. Anti-PD-1 and Anti-PDL-1 monoclonal antibodies causing type 1 diabetes. Diabetes Care. 2015;38:e137–e138. doi: 10.2337/dc15-0889. [DOI] [PubMed] [Google Scholar]
- 8.Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190–199. doi: 10.1016/j.ejca.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 9.Gaudy C, Clévy C, Monestier S, et al. Anti-PD1 pembrolizumab can induce exceptional fulminant type 1 diabetes. Diabetes Care. 2015;38:e182–e183. doi: 10.2337/dc15-1331. [DOI] [PubMed] [Google Scholar]
- 10.Miyoshi Y, Ogawa O, Oyama Y. Nivolumab, an anti-programmed cell death-1 antibody, induces fulminant type 1 diabetes. Tohoku J Exp Med. 2016;239:155–158. doi: 10.1620/tjem.239.155. [DOI] [PubMed] [Google Scholar]
- 11.Munakata W, Ohashi K, Yamauchi N, Tobinai K. Fulminant type I diabetes mellitus associated with nivolumab in a patient with relapsed classical Hodgkin lymphoma. Int J Hematol. 2016;105:383–386. doi: 10.1007/s12185-016-2101-4. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto M, Okamoto M, Gotoh K, et al. Fulminant type 1 diabetes mellitus with anti-programmed cell death-1 therapy. J Diabetes Investig. 2016;7:915–918. doi: 10.1111/jdi.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teramoto Y, Nakamura Y, Asami Y, et al. Case of type 1 diabetes associated with less-dose nivolumab therapy in a melanoma patient. J Dermatol. 2016;44:605–606. doi: 10.1111/1346-8138.13486. [DOI] [PubMed] [Google Scholar]
- 14.Gauci M-L, Laly P, Vidal-Trecan T, et al. Autoimmune diabetes induced by PD-1 inhibitor-retrospective analysis and pathogenesis: a case report and literature review. Cancer Immunol Immunother. 2017;66:1399–1410. doi: 10.1007/s00262-017-2033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 17.Solomon DH, Massarotti E, Canning C. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525–2531. doi: 10.1001/jama.2011.878. [DOI] [PubMed] [Google Scholar]
- 18.Antohe JL, Bili A, Sartorius J, et al. Diabetes mellitus risk in rheumatoid arthritis: reduced incidence with anti-tumor necrosis factor α therapy. Arthritis Care Res (Hoboken) 2012;64:215–221. doi: 10.1002/acr.20657. [DOI] [PubMed] [Google Scholar]
- 19.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Re- P. Classification and diagnosis of diabetes. Diabetes Care. 2017;40:S11–S24. doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 21.Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens. JAMA Oncol. 2018;4:173–182. doi: 10.1001/jamaoncol.2017.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imagawa A, Hanafusa T, Awata T, et al. Report of the Committee of the Japan Diabetes Society on the Research of Fulminant and Acute-onset Type 1 diabetes mellitus: new diagnostic criteria of fulminant type 1 diabetes mellitus. J Diabetes Investig. 2012;3:536–539. doi: 10.1111/jdi.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka S, Kobayashi T, Momotsu T. A novel subtype of type 1 diabetes mellitus. N Engl J Med. 2000;342:1835–1837. doi: 10.1056/NEJM200006153422413. [DOI] [PubMed] [Google Scholar]
- 24.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473–2479. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laugesen E, Østergaard JA, Leslie RDG. Latent autoimmune diabetes of the adult: current knowledge and uncertainty. Diabet Med. 2015;32:843–852. doi: 10.1111/dme.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajasalu T, Brosi H, Schuster C, et al. Deficiency in B7-H1 (PD-L1)/PD-1 coinhibition triggers pancreatic β-cell destruction by insulin-specific, murine CD8 T-cells. Diabetes. 2010;59:1966–1973. doi: 10.2337/db09-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Yoshida T, Nakaki F, et al. Establishment of NOD-Pdcd1/ mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci USA. 2005;102:11823–11828. doi: 10.1073/pnas.0505497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee YH, Bae SC, Kim JH, Song GG. Meta-analysis of genetic polymorphisms in programmed cell death 1. Z Rheumatol. 2015;74:230–239. doi: 10.1007/s00393-014-1415-y. [DOI] [PubMed] [Google Scholar]
- 29.Kochupurakkal NM, Kruger AJ, Tripathi S, et al. Blockade of the programmed death-1 (PD1) pathway undermines potent genetic protection from type 1 diabetes. PLoS One. 2014;9:e89561. doi: 10.1371/journal.pone.0089561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mouthon L, Lacroix-Desmazes S, Guillevin L, Kaveri SV, Coutinho A, Kazatchkine MD (1999) La reconnaissance immunologique du soi: quelles frontières entre autoréactivité physiologique et pathologie autoimmune? Méd Sci 15:30–37. 10.4267/10608/1193(Article in French)
- 31.Fowell D. Evidence that the T cell repertoire of normal rats contains cells with the potential to cause diabetes. Characterization of the CD4+ T cell subset that inhibits this autoimmune potential. J Exp Med. 1993;177:627–636. doi: 10.1084/jem.177.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2017;28:368–376. doi: 10.1093/annonc/mdx377.007. [DOI] [PubMed] [Google Scholar]
- 33.Aleksova J, Lau PKH, Soldatos G, McArthur G. Glucocorticoids did not reverse type 1 diabetes mellitus secondary to pembrolizumab in a patient with metastatic melanoma. BMJ Case Rep. 2016;2016:bcr2016217454. doi: 10.1136/bcr-2016-217454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes. 2008;32:451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 35.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41:36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Fiorentino TV, Hribal ML, Perticone M, et al. Unfavorable inflammatory profile in adults at risk of type 2 diabetes identified by hemoglobin A1c levels according to the American Diabetes Association criteria. Acta Diabetol. 2014;52:349–356. doi: 10.1007/s00592-014-0647-2. [DOI] [PubMed] [Google Scholar]
- 37.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 38.Deng T, Lyon CJ, Minze LJ, et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab. 2013;17:411–422. doi: 10.1016/j.cmet.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris DL, Cho KW, DelProposto JL, et al. Adipose tissue macrophages function as antigen presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes. 2013;62:2762–2772. doi: 10.2337/db12-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stefanovic-Racic M, Yang X, Turner MS, et al. Dendritic cells promote macrophage infiltration and comprise a substantial proportion of obesity-associated increases in CD11c+ cells in adipose tissue and liver. Diabetes. 2012;61:2330–2339. doi: 10.2337/db11-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi B, Du X, Wang Q, et al. Increased PD-1 on CD4+ CD28– T cell and soluble PD-1 ligand-1 in patients with T2DM: association with atherosclerotic macrovascular diseases. Metabolism. 2013;62:778–785. doi: 10.1016/j.metabol.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Ise W, Kohyama M, Nutsch KM, et al. CTLA-4 regulates pathogenicity of antigen-specific autoreactive T cells by cell-intrinsic and -extrinsic mechanisms. Nat Immunol. 2010;11:129–135. doi: 10.1038/ni.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujii M, Inoguchi T, Batchuluun B, et al. CTLA-4Ig immunotherapy of obesity-induced insulin resistance by manipulation of macrophage polarization in adipose tissues. Biochem Biophys Res Commun. 2013;438:103–109. doi: 10.1016/j.bbrc.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 44.Montes VN, Turner MS, Subramanian S, et al. T cell activation inhibitors reduce CD8+ T Cell and pro-inflammatory macrophage accumulation in adipose tissue of obese mice. PLoS One. 2013;8(7):e67709. doi: 10.1371/journal.pone.0067709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poggi M, Engel D, Christ A, et al. CD40L deficiency ameliorates adipose tissue inflammation and metabolic manifestations of obesity in mice. Arterioscler Thromb Vasc Biol. 2011;31:2251–2260. doi: 10.1161/ATVBAHA.111.231357. [DOI] [PubMed] [Google Scholar]