Abstract
Purpose
To evaluate the clinical–pathological and prognostic significance of the circulating PD-L1 level in patients with surgically treated NSCLC, by combining data for PD-L1 expression with other immune-related markers and tumor metabolism.
Methods
Overall, 40 patients with resected NSCLC (stage Ia–IIIa) who had preoperative blood storage and underwent staging PET/CT were enrolled for the study. In all cases, we determined plasma levels of PD-L1 (pg/ml), immune-reactive areas (IRA %) covered by CD3, CD68, CD20, CD8, PD-1, and PD-L1 in the tumor specimen, and metabolic parameters on PET, i.e., SUVmax, SUVpeak, metabolic tumor volume (MTV), and total lesion glycolysis (TLG). Variables were statistically analyzed to establish their association with disease-free survival (DFS).
Results
The circulating levels of PD-L1 in the bloodstream could be determined in 38/40 (95%) samples. The mean and median expression levels were 34.86 pg/ml and 24.83 pg/ml, respectively. We did not find any statistically significant correlation between circulating PD-L1 and tissue expression of PD-L1/PD-1. Some mild degree of positive correlation was determined between tissue PD-L1 and SUVmax (ρ = 0.390; p = 0.0148). Hierarchical clustering combining circulating, tissue, and metabolic parameters identified clusters with high metabolic tumor burden or high expression of plasma PD-L1 levels (Z score ≥ 2) as having a poor DFS (p = 0.033). The multivariate analysis detected stage and metabolism (i.e., SUVmax and SUVpeak) as independent prognostic factors for DFS.
Conclusion
Plasma levels of PD-L1 are independent of the expression of PD-1/PD-L1 in NSCLC tumor tissue and, when combined with other clinical–pathological parameters, allow for the identification of clusters with different outcomes.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02387-9) contains supplementary material, which is available to authorized users.
Keywords: Non-small cell lung cancer, Circulating PD-L1, PD-L1 expression, Positron emission tomography, Outcome
Introduction
Lung cancer remains the leading cause of cancer-related death worldwide, with non-small cell lung cancer (NSCLC) representing the predominant subtype [1, 2]. Cytotoxic chemotherapy has for decades been the only treatment capable of prolonging survival in advanced NSCLC, although durable responses have been rare [3]. In recent years, however, treatment options for NSCLC have profoundly changed, moving towards an increasingly personalized, molecular-based medicine [4].
The development of immune checkpoint inhibitors and the unprecedented results reported in second/third-line regimens prompted the evaluation of these novel agents in chemotherapy-naïve patients either alone or in combination with platinum-based chemotherapy [5–11]. To date, nivolumab, pembrolizumab, and atezolizumab represent the programmed death-1/programmed death ligand-1 (PD-1/PD-L1) inhibitors approved as monotherapy for the treatment of advanced NSCLC. Along with the approval of these drugs by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA), new diagnostic assays have been implemented to evaluate PD-L1 expression and to improve patient selection [12]. Of the available checkpoint inhibitors, however, only pembrolizumab has been approved for the first-line treatment of NSCLC patients with PD-L1 expression ≥ 50% [7, 8]. Although all clinical trials require either a core needle biopsy or surgical resection to evaluate PD-L1 immunoexpression in tumor cells, variability in tissue preparation and processing as well as cut-off values has complicated the interpretation of these assays and delayed the achievement of a broad consensus [12–14]. Around 80% of advanced NSCLC patients will only have tissue from small biopsies or cytology and as many as 31% of patients do not have adequate tissue, making them ineligible for clinical trials [15].
On the other hand, it is possible to assess soluble PD-L1 in patients with neoplasia. The rationale behind this assay is that serum PD-L1 levels are significantly higher in patients with malignancies than in healthy individuals [16, 17]. Zheng et al. have in addition described an association between serum PD-L1 levels and prognosis in patients with advanced gastric cancer [17, 18]. Other authors have reported similar results for melanoma [19], diffuse large B cell lymphoma [20, 21], pancreatic cancer [22], biliary tract neoplasia [23], renal cell carcinoma [24], hepatocellular carcinoma [25], multiple myeloma [16], and lung cancer [26]. In particular, in NSCLC patients treated with erlotinib, Sorensen et al. [26] have demonstrated that an increase in soluble PD-1 during treatment is predictive of a more favorable outcome.
Based on the above findings, we decided to evaluate the clinical–pathological and prognostic significance of circulating PD-L1 levels in patients with surgically treated NSCLC. In addition, we correlated these data with the tissue expression of PD-L1 and other immune-related markers, as well as with the metabolic parameters measured on fluorine-18 fluorodeoxyglucose (18F-FDG) PET/CT.
Materials and methods
Patient population
We conducted a retrospective analysis of 40 patients with NSCLC who were treated at the Humanitas Clinical and Research Hospital between March 2016 and August 2017. The inclusion criteria were as follows: (a) pathologically confirmed NSCLC (stage Ia–IIIa); (b) 18F-FDG PET/CT performed within 45 days prior to surgery; (c) availability of tumor samples for immunohistochemistry (IHC) staining.
Baseline epidemiologic and clinical characteristics of the study population are shown in Table 1.
Table 1.
Patients’ clinical characteristics
| Variables | ||
|---|---|---|
| Age | Years | |
| Median | 71 | |
| Range | 43.4–84 | |
| Sex | N (%) | |
| Male | 23 | 57.5 |
| Female | 17 | 42.5 |
| Smoker | ||
| Yes | 16 | 40 |
| No | 7 | 17.5 |
| Former | 17 | 42.5 |
| Histology | ||
| ADC | 26 | 65 |
| SCC | 10 | 25 |
| Other | 4 | 10 |
| Grade | ||
| II | 20 | 50 |
| III | 16 | 40 |
| NS | 4 | 10 |
| Stage | ||
| Ia–b | 20 | 50 |
| IIa–b | 11 | 27.5 |
| IIIa–IV | 9 | 22.5 |
| CHT adj. | ||
| Yes | 8 | 20 |
| No | 31 | 77.5 |
| NS | 1 | 2.5 |
| RT adj. | ||
| Yes | 4 | 10 |
| No | 35 | 87.5 |
| NS | 1 | 2.5 |
| Follow-up | Months | |
| Median | 12.5 | |
| Range | 0.4–24.8 | |
NS not specified
Circulating PD-L1 assessment
Peripheral blood samples had been collected in EDTA (ethylene diamine tetra-acetic acid) Vacutainer tubes from patients included in the study before surgery. In accordance with the Humanitas Centre for Biological Resource Standard Operating Procedures, samples were centrifuged at 1700 rpm (revolutions per minute) for 15 min at + 4 °C and the plasma obtained was immediately frozen and stored at − 80 °C. The circulating levels of PD-L1 were assessed in plasma samples using the Human Programmed Death Ligand-1 (PD-L1/B7-H1) ELISA kit (Quantikine ELISA, R&D Systems, Inc). Plasma samples were centrifuged for 15 min at 1000g and the assay was performed according to the manufacturer’s instructions. Briefly, 96-well precoated plates were incubated with standards and plasma samples for 2 h at room temperature. Then, 200 μl of human/cynomolgus monkey B7-H1 conjugate was added to each well. After several aspiration/wash processes, 200-μl substrate solution was added for 30 min at room temperature. Subsequently, 50-μl stop solution was added and sample absorbance was read within 30 min at 450 nm with the Microplate Absorbance Reader (Biorad, Italy). The concentrations of PD-L1 were calculated according to standard curves.
Immunohistochemistry
Tumor specimens were subjected to IHC analyses with monoclonal antibodies against CD3 (F7.2.38 clone, Dako, Glostrup, Denmark), CD8 (C8/144B clone, Dako), CD20 (L26 clone, Dako), PD-1 (NAT105 clone; Abcam, Cambridge, UK), and PD-L1 (22C3 clone, Dako), as previously described [27, 28]. Briefly, two board-certified pathologists, blinded to clinical outcome, evaluated the stained slides and selected non-overlapping and non-contiguous areas for quantity analysis. An ad hoc software quantified the percentage of immune-reactive area (IRA %) covered by CD3 tumor-infiltrating lymphocytes (TILs), CD68 tumor-associated macrophages (TAMs), CD20 B cells, CD8 TILs, PD-1, and PD-L1.
Imaging protocol and tumor delineation
PET/CT scans were performed in fasting patients approximately 60 min after tracer administration. An activity of 2.5–5.0 MBq/kg of 18F-FDG, depending on patient weight, was administered. Two integrated PET/CT systems were used: Siemens Biograph LSO 6 scanner or GE Discovery PET/CT 690. After low-dose CT (30 mA, 120 keV), PET images were obtained from the base of the skull to the mid-thigh. Scanners used in this study were accredited by the EANM Research Ltd (EARL) programme.
All PET/CT images were reviewed by two board-certified nuclear medicine physicians using GE ADW4.6 workstation (GE Healthcare, Waukesha, WI, USA). SUVmax (maximum standardized uptake value) was defined as the pixel with the highest value within the tumor masses outlined on PET images. SUVmean was defined as mean SUV related to the metabolic tumor volume (MTV) outlined by the sum of volumes of interest (VOIs) drawn on tumor volumes with an uptake greater than 42% of SUVmax. SUVpeak was defined as the average activity concentration within a 1-cm3 spherical VOI centered on the hottest focus within the tumor image multiplied by the ratio of lean body mass to injected activity decayed to time of scan. Finally, we computed total lesion glycolysis (TLG) as the product of SUVmean and MTV.
Statistical analysis
Continuous variables were presented for means, medians, range and standard deviation (SD) and compared using an independent t test or Wilcoxon test, when appropriate. Categorical variables were analyzed and compared between two groups by means of the Chi squared test. Spearman’ correlation coefficient (ρ) was used for rank correlation.
The Kaplan–Meier method with log-rank test was adopted to calculate survival probabilities. Primary outcome was disease-free survival (DFS), defined as the time from surgery until relapse, death from any cause, or the last follow-up visit at our institution. Overall survival (OS) was excluded from the analysis, since in our cohort, only one patient died during follow-up. To identify prognostic factors associated with DFS, the Cox proportional hazards regression model was used. The median follow-up of our cohort was 12.5 months (Table 1).
Hierarchical clustering with heatmap visualization was used for circulating and tissue-related immune markers and PET-based semiquantitative parameters. The heatmap representation was obtained by default clustering based on Euclidean distance and scaled by row. The Z scores were computed from the centered data by dividing by the SD. The heatmap was displayed in red (lowest score) and green (highest score) colors. Statistical significance was set at p ≤ 0.05 for each evaluation. Statistical analyses were performed using the Statistical Package for Social Sciences, version 23.0, for Windows (SPSS, Chicago, IL).
Results
Circulating PD-L1 and IHC results
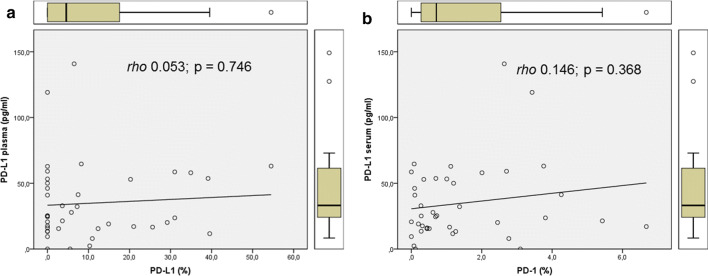
The circulating levels of PD-L1 in the bloodstream could be determined in 38 out of the 40 (95%) samples analyzed. The mean and median expression levels were 34.86 pg/ml and 24.83 pg/ml, respectively. Table 2 summarizes the distribution of all circulating and tissue markers in our study cohort. PD-L1 expression could be visualized in 23 out of 40 tumor specimens (57.5%). The corresponding mean and median values of IRA % were 10.64 and 4.58, respectively. The tissue expression of PD-1 was determined in 37 cases (92.5%), with mean and median IRA % of 1.44 and 0.71, respectively (Table 2). On statistical analyses, we found no correlation between the circulating and the tissue expression of PD-L1, or between plasma PD-L1 and tissue expression of PD-1. Figure 1 illustrates the comparative scatter plots for the above-mentioned parameters.
Table 2.
Distribution of circulating and tissue markers in the study cohort
| Variables | Median | Range | Mean | SD |
|---|---|---|---|---|
| PD-L1 plasma | 24.83 | 0–140.86 | 34.86 | ± 29.37 |
| CD3 (%) | 8.94 | 2.02–41.2 | 11.32 | ± 8.59 |
| CD8 (%) | 5.18 | 1.31–37.21 | 7.84 | ± 7.38 |
| CD20 (%) | 5.04 | 0.11–24.61 | 6.36 | ± 5.2 |
| PD-L1 (%) | 4.58 | 0–54.51 | 10.64 | ± 14.29 |
| PD-1 (%) | 0.71 | 0–6.68 | 1.44 | ± 1.64 |
| SUVmax | 10.3 | 1–34.8 | 11.78 | ± 8.34 |
| SUVmean | 5.7 | 0.9–17.4 | 6.45 | ± 3.94 |
| SUVpeak | 8.05 | 0.9–31.2 | 9.91 | ± 7.46 |
| MTV | 5.75 | 0.4–239.1 | 27.31 | ± 49.46 |
| TLG | 41.9 | 0.4–2775.8 | 275.3 | ± 573.1 |
Fig. 1.
Scatter plots for circulating PD-L1 for tissue PD-L1 (a) and PD-1 (b). As is noticeable from the Spearman coefficient ρ and regression lines, in both cases there was no significant correlation between plasma levels of PD-L1 and tissue expression of checkpoint inhibitors
Correlation with PET-based parameters
Table 2 shows the overall distribution of PET-based parameters analyzed in our cohort. On Pearson’s rank test, we did not find any statistically significant correlation between either circulating PD-L1 or tissue PD-L1 and metabolic parameters. Some mild degree of positive correlation was determined between tissue PD-L1 and SUVmax (ρ = 0.390; p = 0.0148).
Heatmap and hierarchical clustering
The default hierarchical clustering produced two cluster models. The first model was represented by five different clusters (Cluster-5 model) combining all analyzed parameters. Figure 2 illustrates the heatmap obtained from the analysis. The second model was represented by three clusters (Cluster-3 model), defined visually as follows: cluster I (#1; n = 15), high metabolic tumor burden (TLG ≥ Z score 2) with average expression of circulating PD-L1 (− 1 ≤ Z score ≤ 1); cluster II (#2; n = 20), increased expression of circulating PD-L1 (Z score ≥ 1); cluster III (#3; n = 5), all the other combinations of circulating, tissue, and metabolic parameters. Corresponding box plots for the above-mentioned parameters classified in these three clusters are visualized in the Supplementary Fig. 1.
Fig. 2.
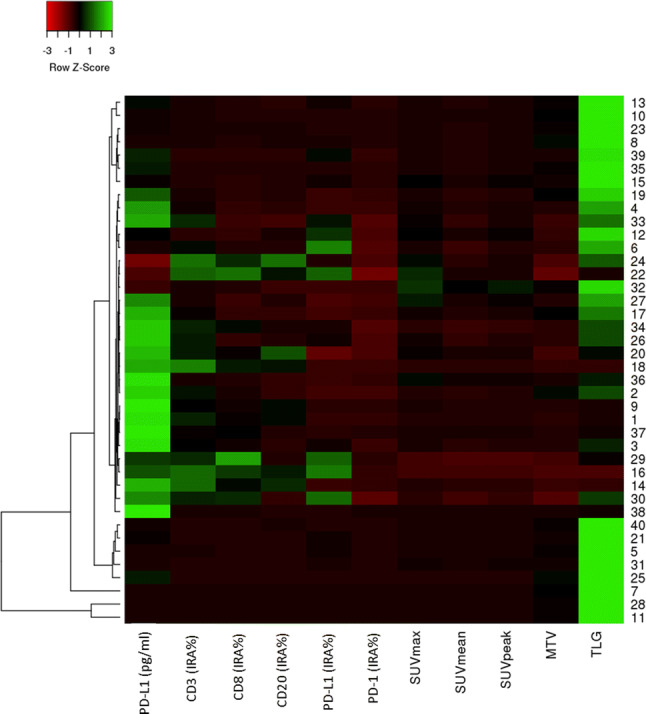
Heatmap with hierarchical clustering obtained based on Euclidean distance and with the option scale by row. The color scale used is red/green based on row Z score
Univariate and multivariate analyses
The different cluster models determined in our analyses were significantly correlated with DFS, as shown on the Kaplan–Meier curves (Fig. 3) (p = 0.000 and 0.033 for Cluster-5 and Cluster-3 models, respectively). Using the Cox proportional hazards regression model, we performed uni- and multivariate analyses of all clinical and study-based parameters (Table 3). Univariate analysis revealed grade, stage, adjuvant radiation treatment, Cluster-3 model, and PET parameters (i.e., SUVmax, SUVpeak, MTV, and TLG) to be significantly associated with DFS. Among these, stage (p = 0.049), SUVmax (p = 0.028), and SUVpeak (p = 0.03) were found to be independent predictive factors for DFS (Table 3).
Fig. 3.
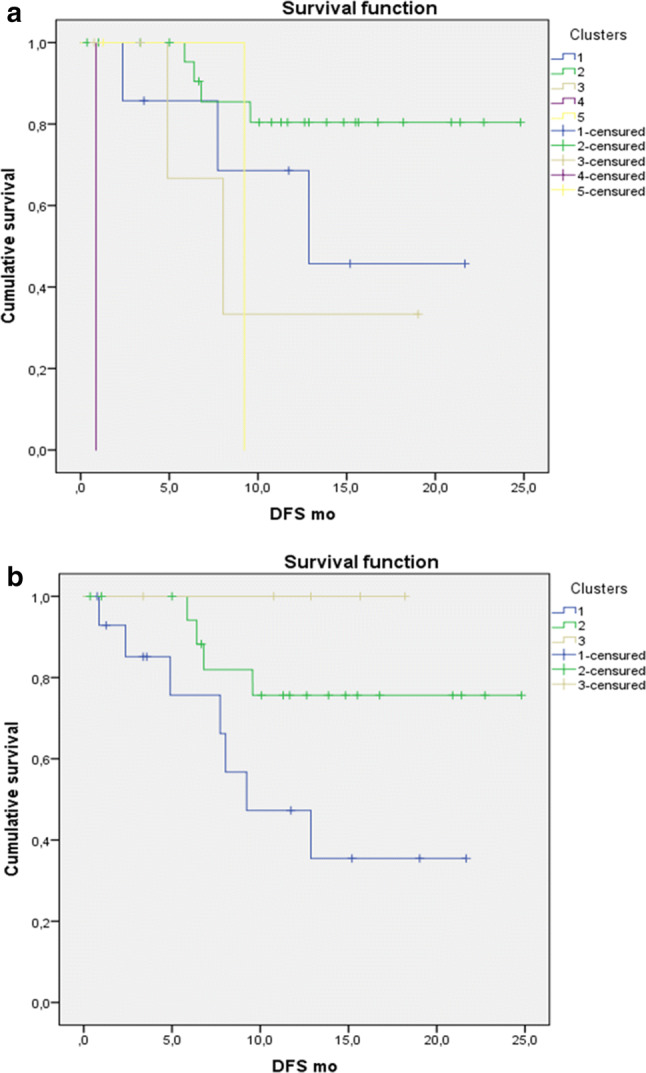
Kaplan–Meier curves with log rank (Mantel–Cox) test obtained from: a cluster-5 model (p = 0.000) and b cluster-3 model (p = 0.033)
Table 3.
Summary of the univariate and multivariate analyses for all study parameters
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR | p value | HR | p value | |
| Smoking status | 1.299 | 0.533 | – | – |
| Histology | 1.720 | 0.188 | – | – |
| Grade | 6.459 | 0.019 | 0.386 | 0.501 |
| Stage | 2.705 | 0.002 | 4.503 | 0.049 |
| CHT adj. | 2.776 | 0.093 | – | – |
| RT adj. | 4.663 | 0.014 | 3.035 | 0.503 |
| PD-L1 plasma | 1.009 | 0.265 | – | – |
| CD3 (%) | 0.942 | 0.258 | – | – |
| CD8 (%) | 1.010 | 0.786 | – | – |
| CD20 (%) | 0.888 | 0.144 | – | – |
| PD-L1 (%) | 1.004 | 0.829 | – | – |
| PD-1 (%) | 1.129 | 0.475 | – | – |
| SUVmax | 1.101 | 0.032 | 3.393 | 0.028 |
| SUVmean | 1.156 | 0.096 | – | – |
| SUVpeak | 1.100 | 0.043 | 0.269 | 0.030 |
| MTV | 1.008 | 0.032 | 0.974 | 0.588 |
| TLG | 1.001 | 0.041 | 1.002 | 0.589 |
| Cluster-5 | 1.594 | 0.104 | – | – |
| Cluster-3 | 0.255 | 0.017 | 0.256 | 0.256 |
Discussion
PD-L1 expression in tumor tissue is regarded as a predictive biomarker for response to immunotherapy, although its use has been widely debated and about 20–25% of patients show clinical benefit and durable response despite low or absent PD-L1 staining [29–31]. In this scenario, there is a compelling need to identify reliable biomarkers able to select patients and predict early tumor response to checkpoint inhibitors.
For this purpose, we evaluated the clinical–pathological and prognostic significance of PD-L1 plasma levels in patients with surgically treated NSCLC, by combining circulating and tissue immune markers with metabolic parameters. Our first observation was the lack of correlation between the circulating and tissue expression of PD-L1 levels (Fig. 1a), or between plasma PD-L1 and tissue PD-1 expression levels (Fig. 1b). This apparent independent expression of tumor and soluble checkpoints might be related to the secretion of PD-L1 by other cells, not just tumor. For example, one possible source of soluble PD-L1 is represented by mesenchymal stromal cells (MSCs) [32]. These multipotent cells typically exert a contact-dependent inhibitory effect on T-cell proliferation by upregulating PD-L1 expression on their cell surface [33–35]. Recently, Davies et al. [32] have demonstrated the ability of MSCs to secrete soluble PD-1 ligands (PD-L1 and PD-L2) for suppression of T-cell activation, especially in response to proinflammatory cytokines. It is noteworthy that the presence of PD-1/PD-L1 has also been acknowledged in peripheral blood mononuclear cells (PBMCs) [36, 37]. This percentage of PBMCs has been documented to be higher in cancer patients than in healthy donors [36]. Patients with increased inflammatory biomarkers in the blood could be more sensitive to chemotherapy combined or not with immune strategies. In addition, patients with a high percentage of PD-1 + PBMCs, PD-1 + CD3 + , PD-L1 + CD3 + , PD-L1 + CD3 + CD8 + , PD-L2 + CD3 + , PD-L2 + CD3 + CD4 + , or PD-L2 + CD3 + CD8 + cells have been reported to have a poorer survival in multivariate analysis [36]. These findings suggest that increased expression of co-inhibitor molecules, i.e., PD-1 and its ligands, on peripheral T cells may represent a further means of tumor escape from immune surveillance, regardless of its correlation with tissue expression at IHC [37]—hence the need for direct sampling of PD-L1 levels in the blood.
Our subsequent analyses compared circulating PD-L1 and tissue PD-L1/PD-1 levels with metabolic parameters. In the current cohort, we found some mild degree of positive correlation between tissue PD-L1 and SUVmax (ρ = 0.390; p = 0.0148). This finding is not completely new in the literature, since previous studies published by our group [27] and Takada et al. [38] have demonstrated a statistically significant correlation between tumor metabolism and tissue expression of checkpoints, such as PD-1 and PD-L1. In particular, tumors presenting with higher levels of PD-1 or PD-L1 tend to have a higher glycolytic metabolism [27, 28, 38, 39]. Similar findings were also reported in two recently published papers [40, 41] for squamous cell carcinoma and adenocarcinoma of the lung. In fact, in these publications [40, 41], apart from correlating with each other, PD-L1 tumor expression and metabolic parameters were prognostic for DFS or OS in NSCLC patients undergoing surgery. Kasahara et al. [40] analyzed 167 patients (153 men and 14 women) with squamous cell carcinoma, reporting a significant correlation between tumor SUVmax and PD-L1 expression (p = 0.02). On multivariate analysis, poor OS was reported for advanced stage, elevated PD-L1 expression, and high SUVmax. Moreover, patients with elevated SUVmax could be stratified for poor prognosis based on advanced stage and high expression of PD-L1. Similar findings were reported by Kaira et al. in 315 surgically resected adenocarcinomas of the lung [41]. PD-L1 expression was significantly correlated with SUVmax, and both were found to be independent prognostic predictors of DFS. Paradoxically, a higher glycolytic metabolism might be associated with a higher probability of patient response to immunotherapy with checkpoint inhibitors, as preliminarily reported by our group recently [39] on the basis of an increased immune infiltrate and a higher PD-L1 expression. Moreover, glycolytic metabolism could be correlated with the oxidative stress, thereby improving the efficacy of immunotherapy.
In view of these previous findings and the known prognostic relevance of PET parameters in NSCLC [42–44], we combined all tissue, circulating, and metabolic variables to test the occurrence of different prognostic clusters in the analyzed NSCLC cohort. In fact, when plotting the parameters in the hierarchical clustering, we could obtain different prognostic groups. The cluster models depicted in our analyses were significantly correlated with DFS (p = 0.000 and 0.033, respectively). In particular, cluster I (Fig. 3b), characterized by 15 patients with a high metabolic tumor burden (TLG ≥ Z score 2) and average plasma levels of PD-L1 (− 1 ≤ Z score ≤ 1), presented with the worst prognosis. The second in order, cluster II (Fig. 3b), was represented by high levels of PD-L1 in plasma; while the good prognostic cluster of patients, cluster III, was characterized by low metabolism and low circulating PD-L1 levels. The univariate analyses in the cohort allowed us to confirm the association between tumor grade and stage, adjuvant radiation treatment, metabolic parameters (i.e., SUVmax, SUVpeak, MTV, and TLG), and the Cluster-3 model and DFS. Of these, the multivariate analysis detected stage, SUVmax, and SUVpeak as independent factors. Previously, our group [28] has combined metabolic parameters, image heterogeneity analysis, and immune markers to develop a comprehensive score for the prediction of survival in surgically resected NSCLC. In that cohort, the scoring system was confirmed to be strongly associated with DFS (median: 19 months; p < 0.004).
Potentially, the contemporaneous detection of soluble PD-L1 levels and glycolytic metabolism might boost prediction of immunotherapy response. This hypothesis is sustained by our observations [39] and recent findings on advanced lung cancer that document a poor prognosis for patients with high plasma levels of PD-L1 [45], even when treated with radiotherapy [46] or when investigated prior to immunotherapy with checkpoint inhibitors [47, 48]. Okuma et al. [45] were the first to report a prognostic role for plasma soluble PD-L1, in a heterogeneous cohort of 96 lung cancer patients (65 chemo-naïve; 73 adenocarcinoma). Although the authors found no correlation of plasma PD-L1 levels with histology, genetic status, smoking history, stage, or laboratory data, there was a significantly reduced OS in patients with high compared with low (< 7.32 ng/ml) plasma PD-L1 levels. The same group later [48] reported on 39 NSCLC patients treated with nivolumab. When using an optimal cut-off value of 3.357 ng/mL, 59% of patients having a baseline PD-L1 plasma level lower than the cut-off achieved a complete or a partial response. Moreover, plasma PD-L1 levels significantly correlated with driver mutation status, patient performance status, and smoking status (p < 0.05) [48]. In parallel with Okuma and colleagues, Zhao et al. [46] investigated plasma PD-L1 levels in 126 clinically inoperable NSCLC patients at baseline, at week 2, and at week 4 during thoracic radiation therapy. The cut-off value in this case was 96.5 pg/ml; again, patients with lower baseline plasma PD-L1 levels had a longer OS than those with higher levels (27.8 months vs 15.5 months, p = 0.005) [46]. In all cases, there was a decrease in soluble PD-L1 during the course of radiotherapy compared to baseline. Interestingly, the authors reported no significant difference between the baseline and post-radiotherapy plasma PD-L1 levels, meaning that after treatment the soluble PD-L1 levels returned to the original levels. The clinical significance of this finding remains to be established. More recently, Costantini et al. [47] monitored several factors, i.e., soluble PD-L1, soluble PD-L2, interleukin-2, interferon-gamma, and Granzyme B, in 43 patients with advanced NSCLC at diagnosis, at the initiation of nivolumab, and 2 months thereafter. The authors for the first time demonstrated the lack of a correlation between PD-L1 expression on tumor samples and the levels of soluble PD-L1. Interestingly, baseline values of plasma PD-L1 and Granzyme B were antithetically correlated with outcome. More specifically, high levels of soluble PD-L1 and low levels of Granzyme B were associated with poor progression-free survival and OS.
Overall, current results and those previously published [36–40] might help us to develop an algorithm that is helpful in clinical practice and capable of overcoming biases recognized in the prediction of the efficacy of checkpoint inhibitors application [28]. The determination of both circulating PD-L1 levels and tumor expression of checkpoint inhibitors, along with clinical and metabolic information, would contribute to a possible scoring system for immunotherapy.
Despite its innovative nature, our study presents some limitations. Firstly, the data presented herein were collected retrospectively. Although it is common practice in our institution to perform a timely staging prior to surgery and to collect blood samples for oncological biobanking, it is still possible to obtain biased data. Secondly, it would have been preferable for the sample size to exceed 40 and to have involved more than one center. Both these limitations, however, are related to the exploratory nature of the study. We hope that larger, multicenter series of patients undergoing immunotherapy will be investigated in the near future, providing robust evidence on the potential predictive markers analyzed in this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Plasma samples for this study were provided by The Center for Biological Resources at Humanitas Clinical and Research Center, IRCCS.
Abbreviations
- 18F-FDG
18F-fluorodeoxyglucose or fluorine-18 fluorodeoxyglucose
- ADC
Adenocarcinoma
- Adj.
Adjuvant
- CHT
Chemotherapy
- DFS
Disease-free survival
- IRA %
Immune-reactive area (%)
- MSC
Mesenchymal stromal cells
- MTV
Metabolic tumor volume
- PBMC
Peripheral blood mononuclear cell
- PET/CT
Positron emission tomography/computed tomography
- RT
Radiation therapy
- SUV
Standardized uptake value
- TAMs
Tumor-associated macrophages
- TLG
Total lesion glycolysis
- VOI
Volume of interest
Author contributions
Study conception and design: EL, FG. Patient recruitment and management: EL, LT, SR, PN, GV, DR, DP, AC. Data acquisition, management, analysis, and interpretation: EL, FG, AC, DQ, SR, DP, VP, GV, PN, SM, RM, DR, LT. Manuscript preparation: EL, FG, AC, DQ, SR, DP, GV, PN, SM, LT.
Funding
The Fondazione AIRC (Associazione Italiana per la Ricerca sul Cancro) is acknowledged for their funding support with the Grant 18923.
Compliance with ethical standards
Conflict of interest
Egesta Lopci has received faculty remuneration from ESMIT (The European School of Multimodality Imaging & Therapy). Giulia Veronesi reports receiving personal fees from Ab Medica SpA. No other potential conflicts of interest relevant to this article exist.
Ethical approval and ethical standards
The Institutional Review Board at the Humanitas Clinical and Research Hospital approved this study (Protocol ONC/NUC/01-2014 version 2.0, approved the 23rd of January 2018), and it was conducted in compliance with the Declaration of Helsinki. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study. The general consent document was signed by the patient prior to any investigation and included approval for research analyses on tumor specimen, blood samples, and imaging procedures.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Brahmer JR, Govindan R, Anders RA, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of non-small cell lung cancer (NSCLC) J Immunother Cancer. 2018;6(1):75. doi: 10.1186/s40425-018-0382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russo A, Franchina T, Ricciardi GRR, et al. The changing scenario of 1(st) line therapy in non-oncogene addicted NSCLCs in the era of immunotherapy. Crit Rev Oncol Hematol. 2018;130:1–12. doi: 10.1016/j.critrevonc.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 8.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 9.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 10.Rittmeyer A, Barlesi F, Waterkamp D, OAK Study Group et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niki M, Nakaya A, Kurata T, et al. Immune checkpoint inhibitor re-challenge in patients with advanced non-small cell lung cancer. Oncotarget. 2018;9(64):32298–32304. doi: 10.18632/oncotarget.25949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel SP, Kurzrock R. PD-L1 Expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14(4):847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 13.Grigg C, Rizvi NA. PD-L1 biomarker testing for non-small cell lung cancer: truth or fiction? J Immunother Cancer. 2016;4:48. doi: 10.1186/s40425-016-0153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carbone DP, Gandara DR, Antonia SJ, et al. Non-small-cell lung cancer: role of the immune system and potential for immunotherapy. J Thorac Oncol. 2015;10(7):974–984. doi: 10.1097/JTO.0000000000000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noll B, Wang WL, Gong Y, et al. Programmed death ligand 1 testing in non-small cell lung carcinoma cytology cell block and aspirate smear preparations. Cancer Cytopathol. 2018;126(5):342–352. doi: 10.1002/cncy.21987. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Wang H, Chen H, et al. Serum levels of soluble programmed death ligand 1 predict treatment response and progression free survival in multiple myeloma. Oncotarget. 2015;6(38):41228–41236. doi: 10.18632/oncotarget.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi N, Iwasa S, Sasaki Y, et al. Serum levels of soluble programmed cell death ligand 1 as a prognostic factor on the first-line treatment of metastatic or recurrent gastric cancer. J Cancer Res Clin Oncol. 2016;142(8):1727–1738. doi: 10.1007/s00432-016-2184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng Zh, Bu Zh, Liu X, et al. Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chin J Cancer Res. 2014;26(1):104–111. doi: 10.3978/j.issn.1000-9604.2014.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou J, Mahoney KM, Giobbie-Hurder A, et al. Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol Res. 2017;5(6):480–492. doi: 10.1158/2326-6066.CIR-16-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossille D, Gressier M, Damotte D, Groupe Ouest-Est des Leucémies et Autres Maladies du Sang; Groupe Ouest-Est des Leucémies et Autres Maladies du Sang et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-cell lymphoma: results from a French multicenter clinical trial. Leukemia. 2014;28(12):2367–2375. doi: 10.1038/leu.2014.137. [DOI] [PubMed] [Google Scholar]
- 21.El-Ghammaz AMS, Gadallah HA, Kamal G, et al. Impact of serum soluble programed death ligand 1 on end of treatment metabolic response of diffuse large B cell lymphoma patients. Clin Exp Med. 2018 doi: 10.1007/s10238-018-0506-5. [DOI] [PubMed] [Google Scholar]
- 22.Kruger S, Legenstein ML, Rösgen V, et al. Serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death ligand 1 (sPD-L1) in advanced pancreatic cancer. Oncoimmunology. 2017;6(5):e1310358. doi: 10.1080/2162402X.2017.1310358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ha H, Nam AR, Bang JH, et al. Soluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget. 2016;7(47):76604–76612. doi: 10.18632/oncotarget.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukuda T, Kamai T, Masuda A, et al. Higher preoperative serum levels of PD-L1 and B7-H4 are associated with invasive and metastatic potential and predictable for poor response to VEGF-targeted therapy and unfavorable prognosis of renal cell carcinoma. Cancer Med. 2016;5(8):1810–1820. doi: 10.1002/cam4.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finkelmeier F, Canli Ö, Tal A, et al. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer. 2016;59:152–159. doi: 10.1016/j.ejca.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Sorensen SF, Demuth C, Weber B, et al. Increase in soluble PD-1 is associated with prolonged survival in patients with advanced EGFR-mutated non-small cell lung cancer treated with erlotinib. Lung Cancer. 2016;100:77–84. doi: 10.1016/j.lungcan.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Lopci E, Toschi L, Grizzi F, et al. Correlation of metabolic information on FD-PET with tissue expression of immune markers in patients with non-small cell lung cancer (NSCLC) who are candidates for upfront surgery. Eur J Nucl Med Mol Imaging. 2016;43:1954–1961. doi: 10.1007/s00259-016-3425-2. [DOI] [PubMed] [Google Scholar]
- 28.Castello A, Grizzi F, Toschi L, et al. Tumor heterogeneity, hypoxia, and immune markers in surgically resected non-small-cell lung cancer. Nucl Med Commun. 2018;39(7):636–644. doi: 10.1097/MNM.0000000000000832. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Dong Zh, Jiang T, et al. Heterogeneity of PD-L1 expression among the different histological components and metastatic lymph nodes in patients with resected lung adenosquamous carcinoma. Clin Lung Cancer. 2018;19(4):e421–e430. doi: 10.1016/j.cllc.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Ilie M, Hofman V, Dietel M, et al. Assessment of the PD-L1 status by immunohistochemistry: challenges and perspectives for therapeutic strategies in lung cancer patients. Virchows Arch. 2016;468:511–525. doi: 10.1007/s00428-016-1910-4. [DOI] [PubMed] [Google Scholar]
- 31.Qiao M, Jiang T, Zhou C. Shining light on advanced NSCLC in 2017: combining immune checkpoint inhibitors. J Thorac Dis. 2018;10(Suppl 13):S1534–S1546. doi: 10.21037/jtd.2018.04.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies LC, Heldring N, Kadri N, Le Blanc K. Mesenchymal stromal cell secretion of programmed death-1 ligands regulates T cell mediated immunosuppression. Stem Cells. 2017;35:766–776. doi: 10.1002/stem.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheng H, Wang Y, Jin Y, et al. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008;18:846–857. doi: 10.1038/cr.2008.80. [DOI] [PubMed] [Google Scholar]
- 34.Stagg J, Pommey S, Eliopoulos N, et al. Interferon-gamma-stimulated marrow stromal cells: a new type of nonhematopoietic antigen-presenting cell. Blood. 2006;107:2570–2577. doi: 10.1182/blood-2005-07-2793. [DOI] [PubMed] [Google Scholar]
- 35.Luz-Crawford P, Noel D, Fernandez X, et al. Mesenchymal stem cells repress Th17 molecular program through the PD-1 pathway. PLoS One. 2012;7(9):e45272. doi: 10.1371/journal.pone.0045272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arrieta O, Montes-Servin E, Hernandez-Martinez JM, et al. Expression of PD-1/PD-L1 and PD-L2 in peripheral T-cells from non-small cell lung cancer patients. Oncotarget. 2017;8(60):101994–102005. doi: 10.18632/oncotarget.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Zhu W, Zhang X, et al. Expression and clinical significance of programmed death-1 on lymphocytes and programmed death ligand-1 on monocytes in the peripheral blood of patients with cervical cancer. Oncol Lett. 2017;14(6):7225–7231. doi: 10.3892/ol.2017.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takada K, Toyokawa G, Okamoto T, et al. Metabolic characteristics of programmed cell death-ligand 1-expressing lung cancer on 18 F-fluorodeoxyglucose positron emission tomography/computed tomography. Cancer Med. 2017;6(11):2552–2561. doi: 10.1002/cam4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grizzi F, Castello A, Lopci E. Is it time to change our vision of tumor metabolism prior to immunotherapy? Eur J Nucl Med Mol Imaging. 2018;45(6):1072–1075. doi: 10.1007/s00259-018-3988-1. [DOI] [PubMed] [Google Scholar]
- 40.Kasahara N, Kaira K, Bao P, et al. Correlation of tumor-related immunity with 18F-FDG-PET in pulmonary squamous-cell carcinoma. Lung Cancer. 2018;119:71–77. doi: 10.1016/j.lungcan.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Kaira K, Shimizu K, Kitahara Sh, et al. 2-Deoxy-2[fluorine-18]fluoro-d-glucose uptake on positron emission tomography is associated with programmed death ligand-1 expression in patients with pulmonary adenocarcinoma. Eur J Cancer. 2018;101:181–190. doi: 10.1016/j.ejca.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 42.Castello A, Lopci E. Non-small cell lung carcinoma: understanding cancer microenvironment to drive immunotherapy and patients’ selection. Transl Cancer Res. 2018;7:S568–S572. doi: 10.21037/tcr.2018.04.10. [DOI] [Google Scholar]
- 43.Lopci E, Rossi S. Tumor metabolism and prognostic role of EZH2 in non-small cell lung cancer. Transl Cancer Res. 2017;6(Suppl 6):S982–S988. doi: 10.21037/tcr.2017.06.42. [DOI] [Google Scholar]
- 44.Rossi S, Castello A, Toschi L, Lopci E. Immunotherapy in non-small-cell lung cancer: potential predictors of response and new strategies to assess activity. Immunotherapy. 2018;10(9):797–805. doi: 10.2217/imt-2017-0187. [DOI] [PubMed] [Google Scholar]
- 45.Okuma Y, Hosomi Y, Nakahara Y, et al. High plasma levels of soluble programmed cell death ligand 1 are prognostic for reduced survival in advanced lung cancer. Lung Cancer. 2017;104:1–6. doi: 10.1016/j.lungcan.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 46.Zhao J, Zhang P, Wang J, et al. Plasma levels of soluble programmed death ligand-1 may be associated with overall survival in nonsmall cell lung cancer patients receiving thoracic radiotherapy. Medicine. 2017;96(7):e6102. doi: 10.1097/MD.0000000000006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Costantini A, Julie C, Dumemil C, et al. Predictive role of plasmatic biomarkers in advanced non-small cell lung cancer treated by nivolumab. OncoImmunology. 2018 doi: 10.1080/2162402x.2018.1452581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okuma Y, Wakui H, Utsumi H, et al. Soluble programmed cell death ligand 1 as a novel biomarker for nivolumab therapy for non-small-cell lung cancer. Clin Lung Cancer. 2018;19:410–417. doi: 10.1016/j.cllc.2018.04.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.