Abstract
Purpose
It is now recognized that solid tumors encroach on the host’s immune microenvironment to favor its own proliferation. Strategies to enhance the specificity of the endogenous T-cell population against tumors have been met with limited clinical success. We aimed to devise a two-tier protocol coupling in vivo whole antigen priming with ex vivo cellular expansion to clinically evaluate survival in patients following re-infusion of primed, autologous T cells, thereby determining treatment efficacy.
Experimental design
Treatment commenced with the acquisition of whole tumor antigens from tumor cell lines corresponding with patients’ primary malignancy. Lysate mixture was inoculated intradermally, while peripheral blood mononuclear cells (PBMCs) were periodically extracted via phlebotomy and expanded in culture ex vivo for re-infusion. Post-treatment tumor-specific T-cell response and cytotoxicity was confirmed via Elispot and real-time cell analyzing (RTCA) assay. Serum cytokine levels and cytotoxicity scores were evaluated for associations with survival status and duration.
Results
There was a significant increase in cytotoxicity exhibited by T cells measured using both Elispot and RTCA following treatment. Correlation analysis determined significant association between higher post-treatment cytotoxicity scores and survival status (R = 0.52, p = 0.0028) as well as longer survival duration in months (R = 0.59, p = 0.005).
Conclusions
Our treatment protocol successfully demonstrated significant correlation between tumor-associated antigen-specific immune response and objective prolongation of survival. Whole-cell cancer antigen priming and adoptive T-cell therapy is, therefore, a highly feasible clinical model which can be easily replicated to positively influence outcome in end-stage malignancy.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2142-z) contains supplementary material, which is available to authorized users.
Keywords: Combination, Cancer antigen, In vivo priming, Autologous T cell, Terminal cancer
Introduction
Persistent cancer progression following surgical and oncological interventions is evidence of therapeutic failure, indicating an urgent need for a novel therapy to be developed in the management of terminal disease. Therapeutic cancer vaccines currently under development aim to enhance the body’s immune response sufficiently to impede cancer cell growth. This, however, is difficult to achieve as cancer cells are: (1) highly heterogeneous with both inter and intracellular antigen variability; (2) capable of concealing themselves from the immune surveillance via their continuous mutation of their genetic materials through a process termed immunoediting, thereby taking advantage of the loopholes in one’s natural immune system; as well as (3) able to generate a tumor microenvironment which actively downregulates the potency of native anti-tumor cells [1–5]. As such, it is imperative for a cancer vaccine to be personalized to the patient’s unique cancer antigen repertoire to capture the antigenic diversity of the specific malignancy in context.
Whole cancer cell antigens and adoptive T-cell therapy are two of the most frequently engaged techniques attempting to counter the aforementioned immune-diversity of cancer. In both animal studies and human clinical trials, active immunization with allogeneic tumor lysates has shown significant potentials [6]. Treatment with tumor cell lysate-loaded antigen presenting cells, such as dendritic cells (DCs), was found to generate tumor-specific immune response without any therapeutic side effect [7]. However, Obtaining a tumor antigen-specific immune response in clinical settings presents a marked challenge as neither cell-associated markers nor in vivo biological responses reflective of treatment efficacy has been identified [8–10]. Infusion of tumor-specific T cells obtained from the tumor itself or via regional lymph nodes has demonstrated therapeutic potential. However, the technique is limited by (1) the physical inability to gain access to tumor-infiltrating lymphocytes in individuals with the most frequently encountered malignancies; and (2) the ex vivo difficulty in cell expansion with low number of tumor-specific lymphocyte to achieve a sizeable population sufficient to produce discernable clinical effects [11]. To overcome these obstacles, tumor antigen-specific T-cell expansion following in vivo priming with active vaccination was attempted. This technique was subsequently shown to be very successful, allowing a large number of antigen-specific T cells to be acquired from the peripheral blood, greatly facilitating tumor antigen-specific T-cell expansion [12].
In this preliminary clinical study, we developed a two-tiered treatment strategy, consisting of active immunization with whole cancer cell antigen made available through lysed whole tumor cells, followed by adoptive T-cell therapy performed ex vivo via cell expansion using patient’s peripheral blood. We aimed to demonstrate detectable biological changes in T-cell tumor-specific cytotoxicity as well as a clear survival advantage in individuals who had undergone this novel treatment.
Materials and methods
Participants and setting
Patients with solid organ tumors who had failed to benefit from at least one cycle of the conventional therapy (surgery, chemotherapy, radiotherapy, target therapy, or interventional radiological procedure) were identified in the Xiamen 5th Hospital and Zhang Zhou Xing Pu Hospital, Fujian Province, China from January 2010 to January 2016. All individuals were above the age of 18 and had biopsy-proven end-stage disease determined via TNM staging (stage ≥ III), with an expected survival of at least 3 months. Of note, if patients were on schedule for further conventional therapy, such regimens were not halted and were concurrently administered with our protocol, unless the patient explicitly requested otherwise. This was in keeping with stipulations laid out by the clinical ethics committee. Although this inevitably exposed participants to treatments outside of our protocol, it ensured that they remained on gold-standard therapy while volunteering in our study.
Study design
The primary objective of this multi-center study was to demonstrate an improvement in overall survival (months) in end-stage cancer patients on this novel protocol who have failed to benefit from the conventional therapies. Our secondary objective sought to show that cancer antigen priming via lysed whole tumor cells and subsequent adoptive T-cell therapy enhanced tumor-specific cytokine expression and T-cell tumor-specific cytotoxicity. The feasibility of this two-tiered treatment protocol was also evaluated in the clinical setting retrospectively upon treatment termination. All interventions were performed and specimens were acquired after obtaining written informed consent from study subjects in accordance with the Declaration of Helsinki. The trial was registered to the World Health Organization International Clinical Trials Registry Platform (ChiCTR-OPC-15006703) and was approved by the Xiamen 5th Hospital and Zhang Zhou Xing Pu Hospital Ethics Committee (Project 03/200909).
Antigen preparation
Selected cancer cell lines (Cell Bank, Chinese Academy of Sciences, Shanghai, China) corresponding with patients’ malignancies were identified in accordance to individual biopsy reports (Supplementary Table 1). Cells were permitted to grow to near confluence in cell culture flask in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% fetal bovine serum (FBS). Before harvesting, the cells were grown in RPMI medium without FBS for 3–4 days. Whole antigens of each cancer cell line were obtained via mechanical disruption of 1 × 107 cancer cells in the cold with a stainless steel high speed tissue homogenizer, followed by three cycles of freezing with liquid nitrogen and thawing to room temperature. The presence exclusively of disrupted cancer cells was confirmed by microscopic examination of random samples of the lysate mixture. The absence of viable cancer cells in our preparations was demonstrated by inoculation of 0.2 mL of the lysate mixture into each of the five nude mice, which showed no growth of tumor over the course of the next 4 weeks. The lysate mixture preparations were further confirmed sterile, mycoplasma and endotoxin free, non-pyrogenic, and devoid of both hepatitis and AIDS viruses. The above were achieved through lysate-inoculation on agar-based growth medium for 1 week, mycoplasma staining assay (pyrogen), tachypleus amebocyte lysate assay, and immunogold labeling technique respectively.
Therapeutic protocol
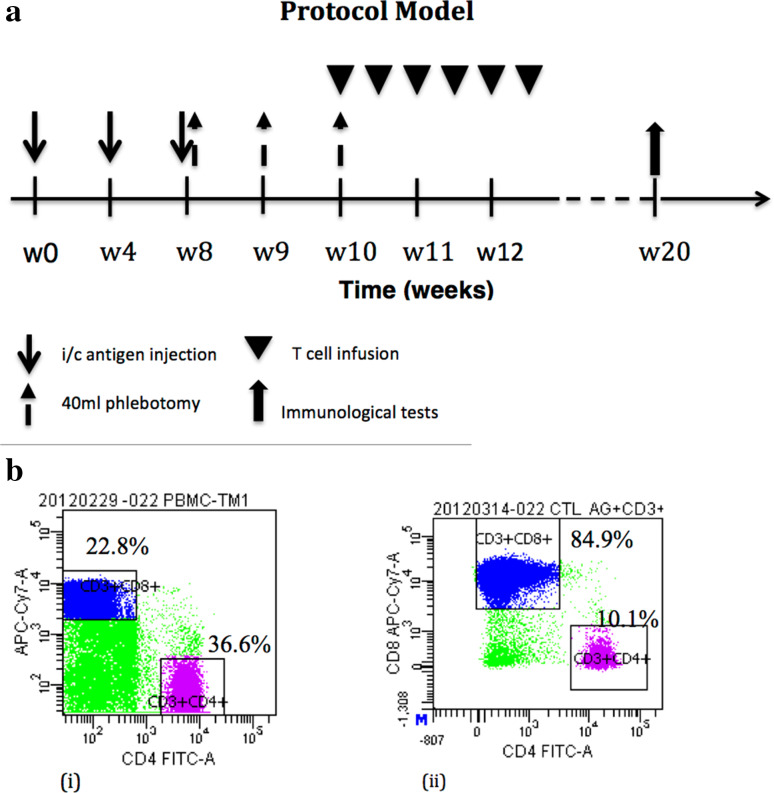
Three milliliter of lysate mixture containing pooled whole antigens derived from 1 × 107 cancer cells of each cell line and 100 µg of recombinant human GM-CSF were administered intradermally into the inguinal region once a month for 3 months. At least two cell lines were simultaneously used. This was followed by twice-weekly infusion of 2–3 × 109 of autologous T-lymphocyte expanded ex vivo for 2 weeks with specificity confirmed via flow cytometry. Our standardized protocol of administration is shown in Fig. 1a. After completion of the protocol, patients were followed every 3 months post-discharge to assess for clinical progress.
Fig. 1.
a Therapeutic model comprising of in vivo priming, peripheral blood collection, and ex vivo T-cell expansion followed by re-infusion; b (i) Representative flow cytometry demonstrating pre-culture CD8 + lymphocyte at 22.8% and (ii) post-culture CD8 + cells at 84.9%
Generation of autologous CD8+ T-lymphocyte
Peripheral blood mononuclear cells (PBMCs) were obtained from 40 mL of whole blood collected once a week for 3 consecutive weeks following the last immunization. Cytotoxic T-cell (CTL) population was expanded by placing PBMCs into X-vivo15 T-cell medium (Longza, Basel, Switzerland) containing anti-human CD3 (Biolegend, San Diego, CA, USA), CD28 antibodies (Ancell Corporation, MN, US), and recombinant human IL-2 (SiHuan pharmaceutical company, Beijing, China) and IL-15 (Peprotech, NJ, USA) ex vivo. The culture is maintained for 14 days prior to being ready for infusion.
Cytotoxicity assays
To determine if the lymphocytes expanded ex vivo and post-treatment were tumor-specific, cytotoxicity assay against the cancer cells from which the antigens were made was performed with either morphological observation or real-time cell analysis (RTCA).
For morphological observation, target cancer cells (1 × 105) were allowed to attach and grow for 24 h in a 6-well tissue culture plate where a clearly visible marking was made. The field of cancer cell growth was localized by a mark under an inverted microscope before and after the expanded lymphocytes or PBMC (1 × 106) were added and co-cultured for a further 24 h. The target cancer cell growth was then compared at the site of the constant field before and after the lymphocytes were added.
For RTCA, an xCelligence real-time cell analyzer S16 (ACEA Bioscience Inc., San Diego, CA, USA) was used as previously described by Kho et al [13] in 2015. Briefly, the E16 xCELLigence plates were prepared via addition of 50 µL of culture medium to every well and insertion of wells into the xCELLigence station in a CO2 incubator (Thermo Electron LDD GmbH, Langenselbold, Germany) set at 37 °C with 5% CO2 atmosphere. The baseline impedance was measured to ensure that all wells and connections were working within acceptable limits. Following harvesting and counting, cancer cells were diluted to a concentration of 105 cells/mL and 100 µL were seeded to the wells. Real-time variations in impedance due to the adhesion, growth, and morphological changes of cancer cells were recorded during interaction with gold-microelectrodes placed at the bottom of the E-plates. The impedance signal was converted to arbitrary “cell index” (CI) units, which were recorded and analyzed through the RTCA software 1.2.1 (ACEA Bioscience). To determine the cytotoxicity, either 2 × 105 PBMCs or ex vivo expanded T cells in 100 µL of complete media were added to the wells at the normalized CI value of 1 and monitored for 12 h. Percentage of cancer cell death was represented by the mean of CI values at 12 h of control wells less the experimental wells over the mean of CI values at 12 h of control wells.
Antigen-specific immune response assay
IFN-γ ELISPOT assay was used to determine the frequencies of lysate mixture specific T cells before and 8 weeks after the treatment was completed. Briefly, on day 1, PBMCs were re-suspended at a concentration of 1 × 106/mL in fresh T-cell medium, and 100 µL was placed in the well of 96-well tissue culture plate. The cells were incubated with or without 100 µL lysate mixture of 1 × 105 cancer cells (three replicates per condition) at 37 °C in 5% CO2 overnight. On day 2, the stimulated cells were transferred into anti-human IFN-γ monoclonal antibody-coated 96-well nitrocellulose plates (Millipore Corp, Bedford, MA) and further incubated overnight. On day 3, PBMCs were replaced by a solution of 50 µL of anti-human IFN-γ biotinylated monoclonal antibody at 1 µg/mL in PBS. Two hours after incubation at 37 °C, the plates were washed and developed for 1 h at room temperature with 50 µL of streptavidin–alkaline phosphatase (diluted to the ratio 1:1000 in PBS; Bio-Rad Lab, Hercules, CA). After washing, substrate (bio-Rad Lab) was added and incubated for 20 min. The resultant spots were then counted using an ELISPOT reader (ImmunoSpot, CTL). Data were then calculated using the IFN-γ specific frequency (in 105 cells) averaged from three-well replicates, while subtracting from it the mean value from non-antigenic wells.
Serum IFN-γ and IL-10 and peripheral TIFN−γ and Treg
Cytokines IFN-γ and IL-10 in the serum were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits, according to the manufacturer’s instructions (eBioscience) before the treatment and 8 weeks after the last session of lymphocyte infusion.
For intracellular cytokine assay, harvested PBMCs were permeabilized and stained with intracellular markers IFN-γ-PE and Human Treg Flow Kit (BioLegend Inc., San Diego, CA, USA) according to the manufacturer’s instructions. The cells were then acquired and a minimum of 5000 CD4+/CD8+ T-cell events were collected per sample. Analysis was conducted using Guava EasyCyte (Millipore Corporation, Hayward, CA, USA).
Statistical test
Primary end points of post-procedure survivability as well as survival duration in months were defined as dependent variables. Post-procedure serum cytokines IFN-γ and IL-10, CTL and Treg counts, IFN-γ Elispot score, as well as PBMC cytotoxicity score were selected as potential factors of correlation to defined primary end points. Pre- and post-treatment serum IFN-γ, IL-10, CTL, and Treg levels were analyzed for significant differences via Wilcoxon matched-pairs signed rank test. IFN-γ as a fraction of IL-10 (IFN-γ/IL-10) and CTL as a fraction of Treg (TIFN−r/Treg) were then computed and given scores of either 1 (for value > 1) or 0 (for value < 1). Pre- and post-treatment scores were again compared for significant differences in median using the Chi-square test. PBMC cytotoxicity was taken as the difference between pre- and post-treatment co-cultured cancer cell counts. PBMC cytotoxicity score was defined by assigning a numerical value from 1 to 7 to cytotoxic PBMC responses post-procedure towards each cancer cell line used and obtaining an average based on the summation of scores for every patient. One stands for a post-procedure cytotoxicity response of < 0% (higher cancer cell count than pre-procedure), while 7 points to a > 100% increase in co-cultured cancer cell reduction. Similarly, IFN-γ positive Elispot score was measured the same way with pre- and post-treatment count differences being assigned scores ranging from 1 (< 0) to 37 (> 350). A mean Elispot score was then computed for each patient corresponding to the number of stimulatory tumor cell lines used in separate PBMC co-cultures. Spearman correlation analysis was carried out to investigate this relationship between survivability (categorical and duration in months) and the treatment associated independent factors (IFN-γ/IL-10 score, TIFN−r/Treg score, Elispot score, and cytotoxicity score). The level of significance was set at a p value of < 0.05. All statistical tests were run on Graph Pad Prism 6TM (GraphPad Software, Inc., San Diego, CA).
Results
Study group demographics
Thirty-one patients met inclusion criteria and consented for protocol administration, consisting of 19 male and 12 female patients with median age of 52 (IQR 21). Eighty-seven point one percent (n = 27) of patients in the study had stage IV cancer at the point of presentation with the remainder being stage III. The distribution of the types of malignancies and associated TNM stages were reflected in Table 1. All patients had undergone at least one conventional therapy modality, with surgery being the most common at 80.6% (n = 25). Seventeen patients had at least three different procedures performed prior to commencement of our protocol (Supplementary Table 2).
Table 1.
Patient demographics
| Characteristics | n | % | Median (IQR) |
|---|---|---|---|
| Age | 52 (21) | ||
| < 40 | 2 | 6.5 | |
| 40–49 | 8 | 25.8 | |
| 50–59 | 11 | 35.5 | |
| 60–69 | 6 | 19.4 | |
| 70–79 | 3 | 9.7 | |
| ≥ 80 | 1 | 3.2 | |
| Gender | N/A | ||
| Male | 19 | 61.3 | |
| Female | 12 | 38.7 | |
| Malignancy | N/A | ||
| Gastroesophageal cancer | 3 | 9.7 | |
| Colorectal cancer | 2 | 6.5 | |
| Lung cancer | 7 | 22.6 | |
| Liver cancer | 8 | 25.8 | |
| Breast cancer | 5 | 16.1 | |
| Others | 6 | 19.4 | |
| Malignancy stage | N/A | ||
| III | 4 | 12.9 | |
| IV | 27 | 87.1 | |
| Conventional therapies | N/A | ||
| Surgery | 25 | 80.6 | |
| Radiotherapy | 17 | 54.8 | |
| Chemotherapy | 18 | 58.1 | |
| Target therapy | 10 | 32.3 | |
| Interventional radiology | 10 | 32.3 | |
| Therapy modalities undergone | 3 (2) | ||
| One therapy | 6 | 19.4 | |
| Two therapies | 8 | 25.8 | |
| Three therapies | 8 | 25.8 | |
| Four therapies | 9 | 29.0 | |
Our protocol was found to be feasible, safe, and well tolerated. There were no significant adverse events with regards to the treatment according to the Common Toxicity Criteria of the National Cancer Institute. Survival of our patient population in months ranged from 4 to 39 after therapy at the time of data collection, attaining a median of 10 (IQR 13) months. The 10–20 and above 20 month survival rates were 32.3 and 19.3% respectively (Table 2). Minor complications were observed in a small number of subjects. Two patients with non-small cell lung cancer developed induration and erythema at the site of the intradermal injections. Skin eruption over the torso was also observed in one of the patients, which self-resolved over 1 month (Supplementary Fig. 1). Both patients were alive and well at the point of data collection, having survived 41 and 30 months respectively.
Table 2.
Patient survival
| Survival measures | n | % | Median (IQR) |
|---|---|---|---|
| Survival (categorical) | |||
| Alive | 13 | 41.9 | |
| Deceased | 18 | 58.1 | |
| Survival duration (months) | 10 (13) | ||
| < 10 | 15 | 48.4 | |
| 10–20 | 10 | 32.3 | |
| 20–30 | 5 | 16.1 | |
| > 30 | 1 | 3.2 | |
IQR interquartile range
Serum IFN-γ/IL-10 and peripheral TIFN − γ/Treg measurement
Post-treatment serum IFN-γ (p = 0.17), IL-10 (p = 0.92), and counts of CTL (p = 0.48) and Treg (p = 0.44) did not significantly change following our protocol (Table 3). Statistical tests for post-procedure differences in IFN-γ/IL-10 (p = 0.093) and TIFN−r/Treg (p = 0.088) ratios also failed to attain significance.
Table 3.
Pre- and post-treatment serum cytokine and cell count
| Serum cytokine/cell count | Pre-treatment median (IQR) | Post-treatment median (IQR) | p |
|---|---|---|---|
| IFN-γ | 1 (19.78) | 1 (19.78) | 0.17 |
| IL-10 | 6.01 (17.75) | 1 (20.5) | 0.92 |
| TIFN−γ | 1.85 (4.16) | 2.3 (4.75) | 0.48 |
| Treg | 0.18 (1.92) | 0.83 (2.04) | 0.44 |
IQR interquartile range
Antigen-specific immune response and cytotoxic assays
The median IFN-γ Elispot score obtained per individual was 4.5 (IQR 8) with a range of 1–20, suggesting a median increase in raw cell count post-treatment of between 21,000 and 40,000 per tumor cell line. This IFN-γ Elispot score bore no significant association with categorical survival (p = 0.089) (Table 4a), but was found to be positively associated with survival duration in months (R = 0.37, p = 0.04) on Spearman correlation analysis (Table 4b).
Table 4.
Spearman correlation analysis for (a) categorical survival (alive/ deceased); (b) survival duration (months)
| Spearman r | p | |
|---|---|---|
| (a) Factors correlating with survival status (alive) | ||
| IFN-r/IL-10 score | 0.14 | 0.46 |
| TIFN−γ/Treg score | − 0.25 | 0.18 |
| IFN-γ Elispot score | 0.31 | 0.089 |
| PBMC cytotoxicity score | 0.52 | 0.0028 |
| (b) Factors correlating with longer survival duration | ||
| IFN-r/IL-10 score | − 0.011 | 0.95 |
| TIFN − γ/Treg score | − 0.25 | 0.17 |
| IFN-γ Elispot score | 0.37 | 0.04 |
| PBMC cytotoxicity score | 0.59 | 0.005 |
Significant values are in bold (p < 0.05)
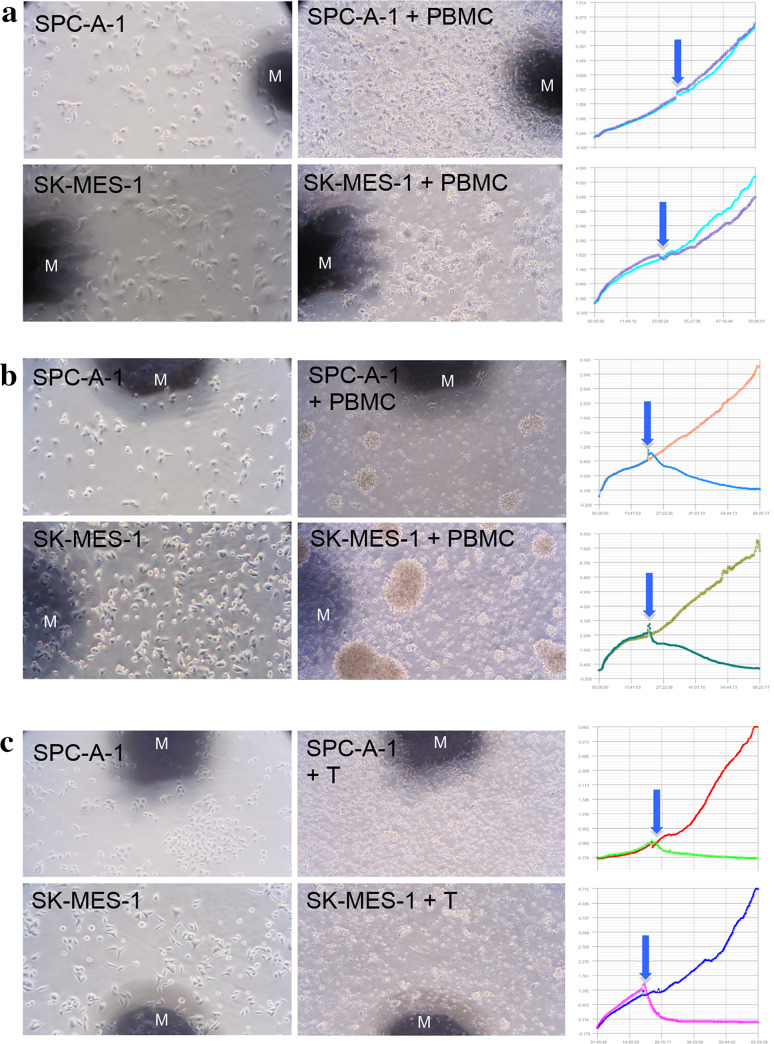
The antigen-specific cytotoxicity of ex vivo expanded T cells and PBMC from the patients after the therapy was confirmed by both morphological observation and RTCA (Fig. 2). The number of cancer cell lines used in the analysis of cytotoxic response in each patient ranged from 2 to 5, with its median being 4 (IQR 3). The median cytotoxic score achieved per patient is 1.75 (IQR 2.1) with a range of 1–6, reflecting an approximate increase in post-procedure cytotoxicity by 0–20% (p = 0.037) per patient, attaining significance on Wilcoxon test. Running Spearman correlation analysis revealed a strong positive correlation between the PBMC cytotoxicity score with categorical survivability of the patient being deceased or alive (R = 0.52, p = 0.0028) as well as survival duration in months (R = 0.59, p = 0.005) (Table 4a, b). Therefore, the higher the PBMC cytotoxicity score, the more likely the patient is alive and has a longer duration of survival.
Fig. 2.
Morphological cytotoxicity and RTCA assay of pre- and post-immunotherapy in a representative patient with NSCLC. Antigen-associated SPC-A-1 and SK-MES-1 cancer cells were allowed to attach and grow for 24 h before primed peripheral lymphocytes or ex vivo expanded lymphocytes were added to the culture. Cancer cell viability was recorded for 24 h with RTCA and morphology observed 24 h subsequent to that. Each panel shows cytotoxicity 48 h after cancer cell growth (left); 24 h after lymphocyte addition (middle); cell index curves recorded by RTCA (right). a Cytotoxicity of PBMC before immunotherapy; b cytotoxicity of PBMC 8 weeks after immunotherapy; c cytotoxicity of 12-day ex vivo expanded PBMC after immunotherapy. M marker; arrow: addition of T/PBMC; microphotography at 200 X
Discussion
Despite both concepts of in vivo cancer antigen priming and adoptive T -cell therapy having been evaluated before, our study presents the first clinical series of sequential in vivo vaccination using allogeneic whole-cell antigens followed by adoptive T-cell therapy after ex vivo antigen-specific T-cell expansion. Furthermore, efficacy in control of actively progressing end-stage diseases measured by survival rate from a wide spectrum of malignant primaries has never been previously demonstrated.
Our protocol aptly presented the feasibility of tumor-specific CTL response that can be achieved with active immunization as shown in the existing literature [14–17]. Although not demonstrable in our model, it has been suggested that active immunization can even prevent subsequent recurrence of diseases [18]. Lysate-based vaccines are adept at capturing the breadth of the tumor antigen repertoire regardless of the HLA type or prior host exposure to other specific antigen targets. It is particularly advantageous in cancers such as non-small cell lung carcinoma (NSCLC), where patients often have extensive mutanomes encoding neoantigens not amenable to host immune tolerance, thereby facilitating rapid immune response to vaccination [19, 20].
We have shown that the mixture of cancer cell lysate with GM-CSF is highly immunogenic in patients of a variety of advanced cancer not only by increasing the frequency of antigen-specific effector seen in IFN-γ ELISPOT, but also through enhanced cytotoxicity against lysate-associated cancer cells detected by RTCA. Enhancement of lysate antigenicity may be due in large part to GM-CSF used as adjuvant with our lysate mixture, as well as our technique of intradermal, instead of subcutaneous injection. This phenomenon has been previously demonstrated where immunization with whole tumor cells genetically engineered to express GM-CSF enhances activation of anti-tumor immunity and inhibits production of immunosuppressive cytokines, such as TGF-β [21]. Vaccination with autologous ascites-derived exosomes (Aex) also mirrored this observation, where patients who received Aex with GM-CSF but not Aex alone showed a beneficial tumor-specific CTL response [22]. In addition, clinical data have shown that the intradermal route of vaccination is more effective than the conventional subcutaneous and intramuscular routes in inducing protective immunity against various infectious diseases [23–25]. The justification being that a larger DC population present in the dermis compared to the subcutaneous fat and muscles would facilitate the antigen uptake, processing, and migration to the draining lymph nodes to elicit a potent antigen-specific T-cell response. Therefore, in the clinical setting, intradermal injection should be conscientiously performed to maximize immune response to the cancer antigens. Intradermal injection in the inguinal region chosen in our practice aimed to reduce pain caused by the tension generated by 3 mL volume of lysate mixture containing GM-CSF.
Taking into consideration the above, it is counterintuitive that our data did not show significant positive shift of immunosuppressive cytokines measured, such as IL-10. This may be attributed to the different method of lysate preparation, which is deemed an important determinant of lysate immunogenicity. Chiang et al. [6] compared whole tumor lysate preparations subjected to either ultraviolet B (UVB) ray-irradiation, repeat cycles of freezing and thawing, or hypochlorous acid (HOCI) treatment. They demonstrated, in both mice and humans, that immunization with autologous DCs loaded with whole tumor cell lysate prepared with HOCI oxidation elicited the strongest anti-tumor T-cell responses and the greatest reduction of IL-10 production in sera compared to DCs loaded with UVB-irradiated or freeze-thawed whole tumor cell lysate [26–28]. Further clinical trial is needed to determine if the use of HOCI prepared whole tumor cell lysate will enhance tumor-specific CTL response through our protocol.
The conceptual justification for T-cell expansion came about through the observation that clinical response can be stimulated in cancer patients refractory to the conventional therapies by the infusion of tumor-specific T cells derived from tumor tissues [29, 30]. Clinical application of this therapeutic strategy is limited by the difficulty in acquiring a large enough number of tumor-specific T cells sufficient to attain therapeutic significance [31, 32]. The previous protocols employed in vitro stimulation, dilution cloning, screening, and expansion, taking in excess of 12 weeks to process. Subsequent use of clinical-grade cell sorter managed to reduce the time from leukapheresis to infusion by approximately 50%. Current systems pursue the integration of cell selection with expansion in a closed-path manner, minimizing human involvement in the maintenance of steps such as pH maintenance and nutrient replacement, further reducing the expansion process to 2–4 weeks [33]. Our protocol is comparable in the level of sophistication and detail, and is advantageous in requiring a relatively shorter amount of time (14 days) to complete the preparations of CTL for infusion. This has positive implications in terms of cost-effectiveness and the efficiency of service delivery.
Overall, our use of whole-cell antigens proved effective in its task of in vivo priming, thereby greatly facilitating the ex vivo cell expansion as previously noted by Dang et al. [12] The unique PBMC culture system used in this study achieved over 84% CD8+ lymphocyte concentration post-expansion (Fig. 1b). Our data showed a clear increase in tumor-specific cytotoxicity post-treatment (p = 0.037), which directly translated to an improved survival rate both categorically (p = 0.0028) as well as duration in months (p = 0.005). This same trend was backed up by the IFN-γ Elispot count which demonstrated a positive association of the IFN-γ Elispot score with survival duration (p = 0.04). The above is a good reflection of successful vaccine-induced tumor-specific T-cell expansion achieved by our culture system which conceptually favored alteration of the tumor microenvironment to promote tumor destruction [34].
Finally, our study was constructed more in keeping with that of a pilot model without rigid mechanisms of blinding or control. The limitations of our study stem from the continued use of conventional therapy with the implementation of our protocol, which may confound our results discussed above, since synergistic effects of chemotherapy and radiotherapy with immunotherapy have been previously discussed in the literature [35]. The study subject with NSCLC was one of our patients who voluntarily curtailed all the conventional therapies on commencement of our protocol, and his clinical results post-treatment were very encouraging as exemplified in Fig. 2. The broad range of malignancies covered by our study attributed strength in variety but weakened consistency through the use of multiple tumor cell lines, necessitating the employment of our scoring system in the calculation of association. In the future, dedicated studies in the recruitment of sufficient patients of specific cancers will streamline this process and allow more in-depth analysis of post-treatment changes segregated by each defined tumor cell line chosen. Conduction of formal randomized-controlled trials will definitely be the direction to take in future studies.
Conclusion
Immunotherapy is a highly promising modality of treatment in end-stage malignancies with many mechanisms of interest under intense study. Our treatment protocol of in vivo whole cancer cell antigen priming and adoptive T-cell therapy has demonstrated a highly feasibility clinical model which can be easily replicated, with efficacy observable on molecular and cellular levels as well as in the direct prolongation of survival. Our protocol has set the stage for the conduction of future randomized-controlled trials to further substantiate our positive clinical findings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- AIDS
Acquired immune deficiency syndrome
- CI
Cell index
- CTL
Cytotoxic T cell
- DCs
Dendritic cells
- FBS
Fetal bovine serum
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- IL
Interleukin
- IQR
Interquartile range
- NSCLC
Non-small cell lung carcinoma
- PBMCs
Blood mononuclear cells
- RPMI
Roswell Park Memorial Institute
- RTCA
Real-time cell analysis
- TGF
Transforming growth factor
- Treg
Regulatory T cell
- TIFN−r
Interferon γ T cell
Author contribution statement
Qing Zhao Ruan: study design, data analysis, and drafting and revision of manuscript. Jian Qian Fu: study conduction and data collection. Xiao Xuan Wu: study conduction and data collection. Li Ping Huang: study conduction and data collection. Run Sheng Ruan: study design, study conduction, data collection, and drafting and revision of manuscript.
Funding
This work was supported by research grants from Xiamen Key Laboratory for Clinical Translation of Cancer Theranostics.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The trial was registered to the World Health Organization International Clinical Trials Registry Platform (ChiCTR-OPC-15006703) and was approved by the Xiamen 5th Hospital and Zhang Zhou Xing Pu Hospital Ethics Committee (Project 03/200909).
Informed consent
Written informed consent from study subjects was obtained in accordance with the Declaration of Helsinki. All study subjects were capable of giving informed consent. The participating patients signed printed consent forms after the trial procedure was explained. Care was taken to have patient repeat the procedure in his/ her own words. The attending clinician signed on the consent form after the patient as the responsible physician in this trial. A separate clinician acted as witness and countersigned the document to officially complete it for filing.
References
- 1.Baitsch L, Fuertes-Marraco SA, Legat A, Meyer C, Speiser DE. The three main stumbling blocks for anticancer T cells. Trends Immunol. 2012;33(7):364–372. doi: 10.1016/j.it.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases—elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16–25. doi: 10.1016/j.coi.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 6.Chiang CL, Coukos G, Kandalaft LE. Whole tumor antigen vaccines: where are we? Vaccines (Basel) 2015;3(2):344–372. doi: 10.3390/vaccines3020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabado RL, Meseck M, Bhardwaj N. Dendritic cell vaccines. Methods Mol Biol. 2016;1403:763–777. doi: 10.1007/978-1-4939-3387-7_44. [DOI] [PubMed] [Google Scholar]
- 8.Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol. 2014;11(1):24–37. doi: 10.1038/nrclinonc.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsueh EC, Essner R, Foshag LJ, Ollila DW, Gammon G, O’Day SJ, Boasberg PD, Stern SL, Ye X, Morton DL. Prolonged survival after complete resection of disseminated melanoma and active immunotherapy with a therapeutic cancer vaccine. J Clin Oncol. 2002;20(23):4549–4554. doi: 10.1200/JCO.2002.01.151. [DOI] [PubMed] [Google Scholar]
- 10.Trzaskowska-Komon E, Wasiak M, Rolinski J, Klatka J. Dendritic cells generated from peripheral blood monocytes (Mo-DCs) and stimulated with laryngeal cancer cell lysates are not good enough in stimulating anti-tumor immunity. Oral Oncol. 2016;55:e2–e3. doi: 10.1016/j.oraloncology.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg SA. Cell transfer immunotherapy for metastatic solid cancer—what clinicians need to know. Nat Rev Clin Oncol. 2011;8(10):577–585. doi: 10.1038/nrclinonc.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dang Y, Knutson KL, Goodell V, dela Rosa C, Salazar LG, Higgins D, Childs J, Disis ML. Tumor antigen-specific T-cell expansion is greatly facilitated by in vivo priming. Clin Cancer Res. 2007;13(6):1883–1891. doi: 10.1158/1078-0432.CCR-06-2083. [DOI] [PubMed] [Google Scholar]
- 13.Kho D, MacDonald C, Johnson R, Unsworth CP, O’Carroll SJ, du Mez E, Angel CE, Graham ES. Application of xCELLigence RTCA biosensor technology for revealing the profile and window of drug responsiveness in real time. Biosensors (Basel) 2015;5(2):199–222. doi: 10.3390/bios5020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber J, Sondak VK, Scotland R, Phillip R, Wang F, Rubio V, Stuge TB, Groshen SG, Gee C, Jeffery GG, Sian S, Lee PP. Granulocyte-macrophage-colony-stimulating factor added to a multipeptide vaccine for resected stage II melanoma. Cancer. 2003;97(1):186–200. doi: 10.1002/cncr.11045. [DOI] [PubMed] [Google Scholar]
- 15.Rubio V, Stuge TB, Singh N, Betts MR, Weber JS, Roederer M, Lee PP. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9(11):1377–1382. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 16.Letsch A, Keilholz U, Kern F, Asemissen AM, Thiel E, Scheibenbogen C. Specific central memory T cells in the bone marrow of patients immunized against tyrosinase peptides. J Immunother. 2006;29(2):201–207. doi: 10.1097/01.cji.0000180903.73965.72. [DOI] [PubMed] [Google Scholar]
- 17.Slingluff CL, Jr, Petroni GR, Yamshchikov GV, Barnd DL, Eastham S, Galavotti H, Patterson JW, Deacon DH, Hibbitts S, Teates D, Neese PY, Grosh WW, Chianese-Bullock KA, Woodson EM, Wiernasz CJ, Merrill P, Gibson J, Ross M, Engelhard VH. Clinical and immunologic results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte-macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cells. J Clin Oncol. 2003;21(21):4016–4026. doi: 10.1200/JCO.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Peoples GE, Gurney JM, Hueman MT, Woll MM, Ryan GB, Storrer CE, Fisher C, Shriver CD, Ioannides CG, Ponniah S. Clinical trial results of a HER2/neu (E75) vaccine to prevent recurrence in high-risk breast cancer patients. J Clin Oncol. 2005;23(30):7536–7545. doi: 10.1200/JCO.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 19.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160(1–2):48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 21.Oh J, Barve M, Matthews CM, Koon EC, Heffernan TP, Fine B, Grosen E, Bergman MK, Fleming EL, DeMars LR, West L, Spitz DL, Goodman H, Hancock KC, Wallraven G, Kumar P, Bognar E, Manning L, Pappen BO, Adams N, Senzer N, Nemunaitis J. Phase II study of vigil(R) DNA engineered immunotherapy as maintenance in advanced stage ovarian cancer. Gynecol Oncol. 2016;143(3):504–510. doi: 10.1016/j.ygyno.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. 2008;16(4):782–790. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldwin SL, Bertholet S, Kahn M, Zharkikh I, Ireton GC, Vedvick TS, Reed SG, Coler RN. Intradermal immunization improves protective efficacy of a novel TB vaccine candidate. Vaccine. 2009;27(23):3063–3071. doi: 10.1016/j.vaccine.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO Publication Hepatitis B vaccines: WHO position paper—recommendations. Vaccine. 2010;28(3):589–590. doi: 10.1016/j.vaccine.2009.10.110. [DOI] [PubMed] [Google Scholar]
- 25.Wu X, Franka R, Svoboda P, Pohl J, Rupprecht CE. Development of combined vaccines for rabies and immunocontraception. Vaccine. 2009;27(51):7202–7209. doi: 10.1016/j.vaccine.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 26.Chiang CL, Kandalaft LE, Tanyi J, Hagemann AR, Motz GT, Svoronos N, Montone K, Mantia-Smaldone GM, Smith L, Nisenbaum HL, Levine BL, Kalos M, Czerniecki BJ, Torigian DA, Powell DJ, Jr, Mick R, Coukos G. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: from bench to bedside. Clin Cancer Res. 2013;19(17):4801–4815. doi: 10.1158/1078-0432.CCR-13-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiang CL, Ledermann JA, Aitkens E, Benjamin E, Katz DR, Chain BM. Oxidation of ovarian epithelial cancer cells by hypochlorous acid enhances immunogenicity and stimulates T cells that recognize autologous primary tumor. Clin Cancer Res. 2008;14(15):4898–4907. doi: 10.1158/1078-0432.CCR-07-4899. [DOI] [PubMed] [Google Scholar]
- 28.Chiang CL, Ledermann JA, Rad AN, Katz DR, Chain BM. Hypochlorous acid enhances immunogenicity and uptake of allogeneic ovarian tumor cells by dendritic cells to cross-prime tumor-specific T cells. Cancer Immunol Immunother. 2006;55(11):1384–1395. doi: 10.1007/s00262-006-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3(9):666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23(10):2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mesel-Lemoine M, Cherai M, Le Gouvello S, Guillot M, Leclercq V, Klatzmann D, Thomas-Vaslin V, Lemoine FM. Initial depletion of regulatory T cells: the missing solution to preserve the immune functions of T lymphocytes designed for cell therapy. Blood. 2006;107(1):381–388. doi: 10.1182/blood-2005-07-2658. [DOI] [PubMed] [Google Scholar]
- 32.Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25 T cells. J Clin Invest. 2003;112(9):1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yee C, Lizee GA. Personalized therapy: tumor antigen discovery for adoptive cellular therapy. Cancer J. 2017;23(2):144–148. doi: 10.1097/PPO.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 34.van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer. 2016;16(4):219–233. doi: 10.1038/nrc.2016.16. [DOI] [PubMed] [Google Scholar]
- 35.Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39(1):74–88. doi: 10.1016/j.immuni.2013.06.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.