Abstract
Background
Nasopharyngeal carcinoma (NPC) is an EBV-associated neoplasm occurring endemically in Southeast Asia and sporadically all over the world. In children and adolescents, high cure rates have been obtained using chemotherapy, radiochemotherapy and maintenance therapy with interferon beta (IFNβ). The mechanism by which IFNβ contributes to a low systemic relapse rate has not yet been fully revealed.
Patients and methods
NK cells and serum samples from two patients with NPC were analyzed before and at different time points during IFNβ therapy, for assessment of TRAIL expression and NK cell cytotoxicity. Cytotoxicity was measured using the calcein release assay and the contribution of different death effector pathways was analyzed using specific inhibitors.
Results
Treatment with IFNβ induced TRAIL expression on patients’ NK cells and increased their cytotoxicity against NPC targets in vitro. NK cell-mediated cytotoxicity was predominately mediated via TRAIL. IFNβ also induced the production of soluble TRAIL (sTRAIL) by NK cells and its release upon contact with NPC cells. IFNβ treatment increased serum levels of sTRAIL in patients. Moreover, sTRAIL concentrated from patients’ serum samples induced apoptosis ex vivo in NPC cells from a patient-derived xenograft.
Conclusion
Increased cytotoxicity of NK cells against NPC cells and increased serum levels of biologically active TRAIL in patients treated with IFNβ could be a means to eliminate micrometastatic disease and explain the low systemic relapse rate in this patient group.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02368-y) contains supplementary material, which is available to authorized users.
Keywords: Nasopharyngeal carcinoma, Interferon beta, Natural killer cells, Tumor necrosis factor apoptosis-inducing ligand, Adolescents, Children
Introduction
Nasopharyngeal carcinoma (NPC) is an EBV-associated neoplasm mainly occurring in adolescents/young adults and in people above 60 years of age [1, 2]. It is endemic in Southeast Asia, occurs less frequently in Northern Africa, and is rarely seen in Europe and the Americas. Tumors in the endemic region and the young age group are usually undifferentiated and characterized by a marked lymphomononuclear infiltrate [3, 4]. State-of-the-art treatment in adolescents and young adults consists of the combination of neoadjuvant chemotherapy and radiochemotherapy resulting in 5-year event-free survival rates of around 80% in patients with localized disease [5–8]. Relapse occurs in about 20% of patients and is almost exclusively metastatic. The addition of interferon beta (IFNβ) as maintenance therapy has led to event-free survival rates above 90% as demonstrated by two prospective multicenter studies, NPC-GPOH-91 and NPC-GPOH-2003 [6, 7]. Though there is sufficient clinical evidence about the effectiveness of IFNβ in the treatment of NPC [6, 7, 9–12], studies exploring the mechanisms of the anti-tumor effects of IFNβ against NPC have only started recently. In a first study, we could show that IFNβ induces apoptosis in NPC cell lines and cells of a patient-derived xenograft at concentrations achievable in humans [13]. Apoptosis was dependent on the induction of surface expression of the death ligand TRAIL on NPC cells and subsequent activation of the TRAIL signaling pathway. However, the anti-tumor effect of IFNβ, in general, is not only mediated by its direct action on tumor cells but also indirectly by activating an anti-tumor immune response [14, 15]. IFNβ has been shown to induce TRAIL expression on NK cells [16, 17]. NK cells play a major role in protecting against tumor initiation and metastasis [18–21]. Recently, a higher extent of tumor infiltration by NK cells was shown to be associated with a better overall and progression-free survival in NPC patients [22]. There is evidence that the anti-metastatic effect of NK cells is mediated by TRAIL and that this effect can be augmented by type I interferons [23, 24]. In a mouse model of hepatic metastasis, injection of IFNα into mice led to activation of liver NK cells and metastasis rejection [25].
In this study, we have analyzed the effects of IFNβ treatment on two NPC patients with regard to the expression of TRAIL and its influence on NK cell cytotoxicity.
Materials and methods
Patients
Blood samples from two patients with EBV-positive nasopharyngeal carcinoma were used in this study. Patients were two 18-year-old females who were coincidentally diagnosed with EBV-positive NPC at the same time, which was classified by histology as nonkeratinizing squamous cell carcinoma of undifferentiated subtype in both cases. Both patients had locoregional NPC with stage II (T2N1M0) in one patient and stage III (T3N1M0) in the other one. Patients were treated according to the recommendations of the Nasopharyngeal Carcinoma Study Section of the German Society of Pediatric Oncology and Hematology (GPOH) which propose three cycles of chemotherapy with 5-fluorouracil and cisplatin, followed by radiochemotherapy and then maintenance therapy with IFNβ over 6 months [26]. Therapy with IFNβ was started with 3 Mio U of human recombinant IFNβ s.c. three times a week for the 1st week, and was afterwards increased to 6 Mio U s.c. three times a week. Blood samples were drawn at 0 h, 6 h and 24 h on day 1 of weeks 1, 2 and 4 of maintenance treatment with IFNβ. Both patients received recombinant human interferon beta 1a (Rebif®, Merck, Darmstadt, Germany). For comparison, peripheral blood mononuclear cells (PBMC) from healthy volunteers were obtained.
Purification of NK cells
PBMC were obtained from EDTA blood samples by Ficoll density gradient centrifugation. NK cells were isolated from PBMC by positive magnetic selection of CD56+ cells according to the manufacturer’s instructions (Miltenyi, Bergisch Gladbach, Germany). Purified NK cells were used immediately for flow cytometric analysis, cytotoxicity assays and RNA sequencing. NK cells were held in RPMI1640 medium (Gibco, Paisley, UK) supplemented with 10% FCS and 100 U/ml penicillin and 100 mg/ml streptomycin (Gibco).
Cell lines
The NPC cell line C666-1 and the nasopharyngeal epithelial cell line NP69 were used. C666-1 cells were maintained in RPMI1640 medium (Gibco) supplemented with 10% fetal bovine serum (Gibco), 100 U/ml penicillin, 100 mg/ml streptomycin (Gibco), and NP69 cells in keratinocyte serum-free medium (Gibco). Cells were cultured in a humidified incubator with 95% air and 5% CO2 at 37 °C. Cell line C666-1 expresses FAS and TRAIL-R2, cell line NP-69 FAS and TRAIL-R1 as previously shown [13].
Patient-derived xenograft
The xenograft C17 was established from a patient with an EBV-positive metastatic NPC in nude mice [27]. For the experiment described, single cells suspensions were derived from freshly isolated C17 tumor fragments as described before [13]. C17 cells express FAS and TRAIL-R2 as reported previously [13].
Animal studies
Swiss nude mice were bred in the animal facility at Gustave Roussy and housed in pathogen-free conditions in filter cap cages holding a maximum of five animals with irradiated aspen chip bedding and cotton fiber nesting material. They were maintained on a 12/12 light/dark cycle, with ad libitum UV-treated water and RM1 rodent diet. Typically, xenografts were performed on 6–8 female mice by subcutaneous introduction of tumor fragments (about 200 mg) under general anesthesia. They were sacrificed when the total tumor volume reached 1700 mm3. The animals were monitored for signs of pain, such as immobility or restlessness, reduction of drinking and food intake. The persistence of abnormal behaviors led to the euthanasia of animals with suffering presumption. Prior to tumor collection, mice were sacrificed by cervical dislocation. Otherwise, mice were euthanatized by carbon dioxide asphyxiation.
Reagents
For cell culture studies, human recombinant interferon beta (IFNβ) was obtained from R&D System, the primary mouse monoclonal antibody against TRAIL, clone 2E5, from Enzo Life Science (Paris, France), anti-FAS antibody, clone ZB4, from Millipore (Temecula, USA) and concanavalin A from Sigma (St. Louis, USA). For immunohistochemistry, the following antibodies were used: mouse anti-human TRAIL-R1 monoclonal antibody (Enzo Life Science, Clone TR1.02), mouse anti-human TRAIL-R2 monoclonal antibody (Enzo Life Science, Clone DJR2-2), mouse anti-human TRAIL monoclonal antibody (Enzo Life Science, Clone III6F) as well as mouse anti-pan Keratin-, anti-CD3- and anti-CD56 antibodies (DAKO-Agilent).
Immunohistochemistry
Immunohistochemistry was performed on 3 μm sections of formalin-fixed, paraffin-embedded tissue samples as previously described [13].
Calcein release assay
A standard fluorescence-based calcein-AM release assay was used to determine the cytotoxic activity, using patients’ and healthy donors’ NK cells as effector cells and C666-1, C17 and NP69 cells lines as a source of target cells. Target cells were washed and resuspended in 15 µM calcein-AM (Thermo Fisher, Eugene, USA) for 30 min at 37 °C, before co-incubation with NK cells at different effector to target (E:T) ratios as indicated for 4 h at 37 °C. 4% Triton (Merck, Darmstadt, Germany) was added to ensure maximum calcein release in controls. After centrifugation, cell-free supernatant was transferred to a Cell Carrier Plate (Sarstedt, Nümbrecht, Germany) to measure relative fluorescence units (RFU) using a spectrophotometer (TECAN Infinite 200 Pro, Tecan, Männedorf, Switzerland). The percentage of specific lysis was calculated as follows: [(RFU value in respective treatment − RFU value in control (spontaneous release))/(RFU value Triton (maximum release) − RFU value in control (spontaneous release)) × 100].
Analysis of NK cell cytotoxicity
To analyze the contribution of death ligands in NK cell-mediated killing, NK cells were incubated with the blocking anti-TRAIL mAb, clone 2E5 (100 ng/ml) and NPC cells were incubated with the anti-FAS antibody, clone ZB4 (100 ng/ml); cells were pretreated for 1 h with the respective antibodies before co-culture. In some experiments, NK cells were pretreated with 2.5 µg/ml concanavalin A (ConA; Sigma) for 2 h to inactivate the perforin/granzyme B pathway. Cytotoxicity was determined via calcein release assay as described above.
Flow cytometric analysis
NK cells from patients were suspended at a density of 1 × 105 cells in 500 μl of medium. Additionally, NK cells from healthy donors had been pretreated with or without IFNβ at 1000 U/ml for 24 h. Analysis of surface expression of TRAIL was done as previously described [13].
RNA extraction, library construction and sequencing
Total RNA was isolated from patients’ NK cells and NK cells of healthy donors treated with 1000 U/ml IFNβ for 0 h (control), 6 h or 24 h using the Maxwell RSC Simply RNA Tissue kit (Promega, Mannheim, Germany) according to the manufacturer´s instructions. RNA quality was evaluated using the Agilent 4200 Tape Station (RNA screen tape assay) (Agilent, Santa Clara, USA) and quantification was done using the Quantus Fluorometer (Promega). Libraries were generated from 1 µg of total RNA with the TrueSeq Stranded Total RNA Library Prep Kit (Illumina, San Diego, USA) and Ribo-Zero Gold Kit (Illumina) as described by the manufacturer. Quality and quantity of the RNA libraries were assessed using the 4200 Tape Station (D1000 screen tape assay) and the Quantus Fluorometer, respectively. The libraries were run on an Illumina NextSeq 500 platform using the High Output 150 cycles Kit (2 × 76 cycles, paired-end reads, single index) (Illumina), resulting in 101.5 M reads per sample in average. Data were analyzed with an inhouse pipeline embedded in the workflow management system of the Quick NGS environment.
Determination of sTRAIL serum levels
Serum samples from patients were obtained at the time points described above and were stored at − 80° C until analysis. Supernatants from 4 h co-cultures of NK cells, either stimulated or not with 1000 U/ml IFNβ, with C666-1 cells, were collected and stored in − 80° C until analysis. In all samples, levels of soluble TRAIL (sTRAIL) were measured using a commercial ELISA kit (R&D Systems, Minneapolis, USA) according to the manufacturer’s instructions.
Concentration of sTRAIL and cytotoxicity assay
Serum from both patients obtained at 24 h after IFNβ injection on day 1 of week 2 was concentrated for protein by centrifugation using Vivaspin columns (Sartorius, Göttingen, Germany). Total protein concentration was then determined by the NanoDrop method [28] (Peqlab, Erlangen, Germany) and sTRAIL concentration was calculated by the initial TRAIL concentration multiplied by the total protein concentration factor. Supernatants or PBS as a control were then added to C17 cells labeled with calcein as above. Where indicated, C17 cells were preincubated with a blocking anti-TRAIL mAb. After incubation for 24 h, cells were centrifuged and cell-free supernatant was analyzed as described before for the calcein assay. As a control, supernatant from PBMC and NK cells of healthy controls incubated for 24 h with 1000 U/ml IFNβ was used.
Confocal microscopy
NK cells were allowed to settle onto poly-l-lysine (Sigma)-coated coverslips for 15 min and were then fixed with 4% paraformaldehyde (Sigma). Staining for TRAIL was done as previously described with a monoclonal antibody recognizing TRAIL (Alexis Biochemicals, San Diego, CA, USA; 1:200) [13].
Statistical analysis
Data are represented as a mean ± SE. Each set of data represents the mean from at least three independent experiments conducted in quintuplicates for calcein release assays and triplicates for flow cytometric analyses. Differences between groups were examined for significant differences by unpaired t test. The level of statistical significances was set at p < 0.05.
Results
Expression of TRAIL receptors on patients’ tumor cells and detection of NK cells in tumor infiltrates
Tumors of both patients were analyzed by immunohistochemistry. Staining for both TRAIL-R1 and -R2 revealed a low to moderate membranous expression pattern in carcinoma cells and histiocytic cells. Inflammatory infiltrates contained mainly CD3-positive T lymphocytes and few NK cells, both expressing TRAIL. The staining patterns were similar for both patients and results are shown for patient 1 (Supplementary figure 1).
IFNβ administered to patients with nasopharyngeal carcinoma induces expression of TRAIL in NK cells
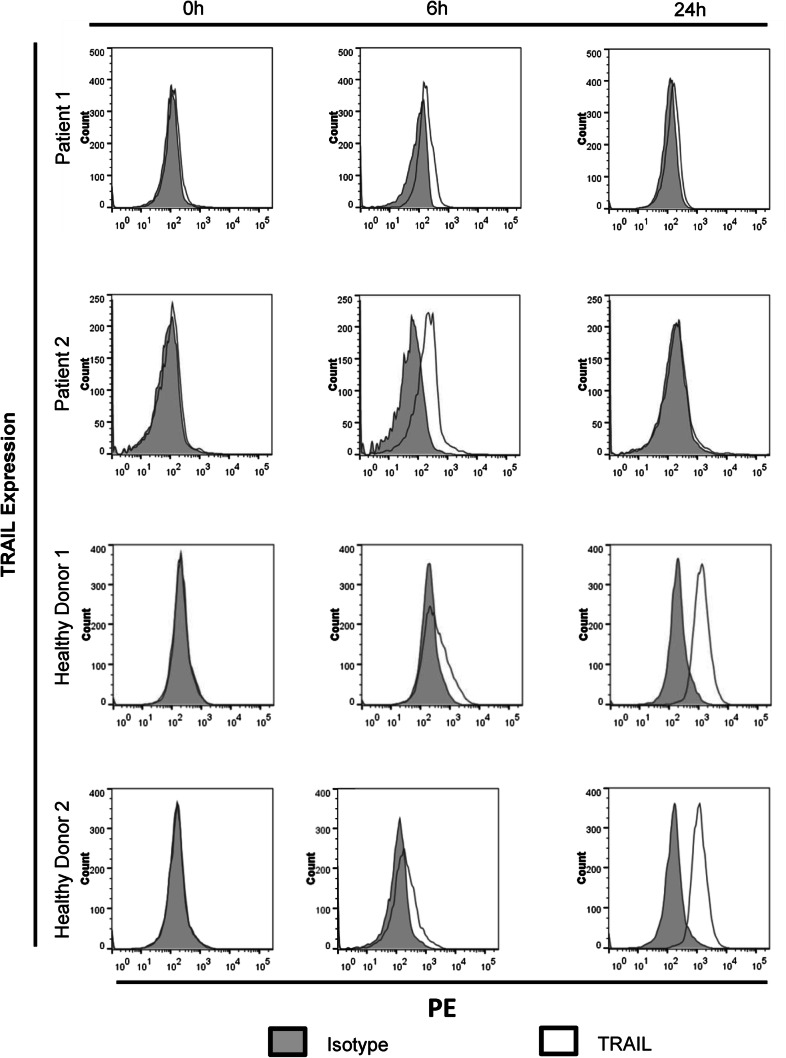
As IFNβ induces the expression of TRAIL on lymphomononuclear cells [16, 17] and nasopharyngeal carcinoma cells have been shown to be susceptible to TRAIL-mediated apoptosis [13], we investigated whether the dosage of IFNβ administered to NPC patients was able to induce TRAIL expression on patients’ NK cells. At the beginning of maintenance therapy, PBMCs from two NPC patients were obtained before, 6 h and 24 h after the subcutaneous injection of the first dose of 3 Mio U human recombinant IFNβ. NK cells were isolated and TRAIL surface expression was analyzed by flow cytometry. NK cells isolated from two healthy donors treated in vitro with 1000 U/ml IFNβ served as controls.
As shown in Fig. 1, TRAIL surface expression was not detectable at baseline in NK cells from patients and controls. 6 h after IFNβ administration, TRAIL could be detected on NK cells of both patients, decreasing again at 24 h. Similarly, in NK cells of healthy controls, in vitro incubation with IFNβ led to the appearance of TRAIL after 6 h, further increasing after 24 h. Upregulation of TRAIL by NK cells after in vivo exposure to IFNβ in patients or in vitro exposure in controls was also noted at the mRNA level through RNAseq analysis (Supplementary table 1). Interestingly, the surface expression of TRAIL in patients’ NK cells almost disappeared 24 h after administration of IFNβ, but the TRAIL mRNA expression at 24 h was higher than at 6 h.
Fig. 1.
Induction of TRAIL expression on NK cells of NPC patients after subcutaneous administration of IFNβ. Two patients with NPC received 3 Mio U IFNβ s.c. at day 1 of week 1 of IFNβ maintenance therapy. NK cells were isolated before, 6 h and 24 h after IFNβ administration. TRAIL expression was measured by flow cytometry. As controls, NK cells from two healthy volunteers were incubated in vitro with 1000 U/ml IFNβ. Representative histograms of triplicates are shown
Increased TRAIL expression of NK cells during IFNβ maintenance therapy
In the GPOH treatment recommendations for NPC, maintenance therapy with IFNβ started with 50% of the IFNβ dose in the 1st week, for optimal monitoring of side effects [6, 7, 26]. If IFNβ is well tolerated, as it was the case for these two patients, the dosage is increased to 100%, equaling 6 Mio U s.c. three times a week, for a total of 6 months. To investigate whether a dose-dependent increase in TRAIL expression of NK cells was seen under the full dose of IFNβ and whether this effect prevailed in the course of maintenance therapy, TRAIL expression on NK cells was also studied on day 1 of weeks 2 and 4 after the start of IFNβ therapy. As shown in Table 1, at week 2, NK cells of both patients already expressed TRAIL at baseline, prior to the next injection of IFNβ, and baseline levels were further increased at week 4. Though the baseline expression of TRAIL at day 1 of weeks 2 and 4 was higher in NK cells of patient 1, TRAIL expression levels on NK cells 6 h after IFNβ administration were in a similar range for both patients, with 88.1% for patient 1 and 87.0% for patient 2 at week 4.
Table 1.
Induction of TRAIL on the surface of NK cells from NPC patients treated with IFNβ
| Patient 1 | Patient 2 | |||||
|---|---|---|---|---|---|---|
| 0 h | 6 h | 24 h | 0 h | 6 h | 24 h | |
| Week 1 | 0.05 ± 2.4 | 9.9 ± 3.0 | 2.3 ± 2.9 | 0.04 ± 2.7 | 18.8 ± 3.1 | 0.04 ± 3.3 |
| Week 2 | 22.0 ± 3.3 | 28.9 ± 2.9 | 20.1 ± 2.7 | 11.0 ± 2.5 | 21.2 ± 3.1 | 9.1 ± 3.0 |
| Week 4 | 79.1 ± 3.0 | 88.1 ± 3.6 | 83.4 ± 3.0 | 61.1 ± 3.3 | 87.0 ± 3.7 | 84.4 ± 3.1 |
Values are net fluorescent intensities between mean fluorescent intensity of anti-TRAIL antibody and mean fluorescent intensity of the respective isotype
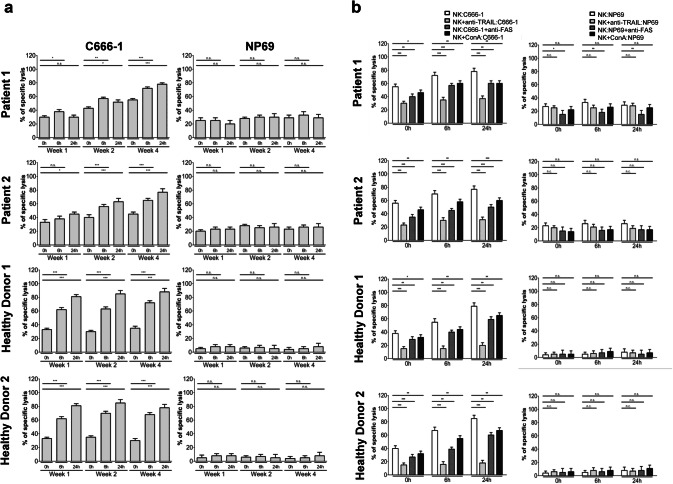
IFNβ administered to patients with nasopharyngeal carcinoma increases NK cell killing of NPC cells in vitro
Since NK cells can be induced to express TRAIL and NPC cells are susceptible to TRAIL-mediated apoptosis, we investigated whether NK cells from NPC patients were able to kill NPC cells, and whether killing could be augmented by IFNβ. Therefore, NK cells isolated from the two patients were incubated with calcein-labeled cells of NPC cell line C666-1 at an effector target (E:T) ratio of 6:1. Cells of the non-malignant nasoepithelial cell line NP69 served as controls. After 4 h of incubation, calcein release of NPC cells was measured as a means of cell death. In addition, NK cells isolated from two healthy donors were stimulated with IFNβ in vitro as above and used as controls.
Figure 2a shows that NK cells from both patients were able to kill NPC cells. Specific killing by NK cells of patient 1 was 30.2 ± 2.4% and of patient 2 34.9 ± 3.2% and was similar to killing by NK cells isolated from two healthy volunteers (32.4 ± 2.9% and 33.9 ± 3.3%). Killing significantly increased when NK cells were isolated from patient 1 after 6 h and patient 2 after 24 h of the first subcutaneous injection of IFNβ. Interestingly, similar to the increase of TRAIL expression on NK cells at weeks 2 and 4, baseline cytotoxicity of NK cells from both patients against C666-1 cells rose 2 and 4 weeks after the start of maintenance therapy and cytotoxicity was even further increased 6 h and 24 h after application of IFNβ, reaching levels of 78.8 ± 3.5% in patient 1 and 75.5 ± 4.9% in patient 2 24 h after IFNß application on day 1 of week 4. Addition of IFNβ in vitro to NK cells isolated from healthy volunteers increased their cytotoxicity to NPC cells after 6 h and 24 h. In contrast, NK cells from patients and controls, either unexposed or exposed to IFNβ were unable to kill nasoepithelial cells NP69. Taken together, these results show that NK cells isolated from patients with NPC are able to kill NPC cells in vitro and that killing is increased when NK cells have been exposed to IFNβ in vivo.
Fig. 2.
Increased NK cell cytotoxicity against NPC cells after administration of IFNβ to patients. Patients received 3 Mio U IFNβ at day 1 of week 1 and 6 Mio U IFNβ at day 1 of weeks 2 and 4. NK cells were isolated before, 6 h and 24 h after IFNβ administration and co-cultured at an E:T ratio of 6:1 with calcein-labeled C666-1 cells. NK cells from healthy volunteers, incubated in vitro with IFNβ were used as positive controls for effectors and nasoepithelial cells NP69 for targets. a Data are presented as means ± SEM (Student’s t test; *P < 0.05; **P < 0.01; ***P < 0.001). b Killing of NPC cells by NK cells is predominately mediated via TRAIL. NK cells from patients isolated before, 6 h and 24 h after IFNβ administration on day 1 of week 4 of IFNβ maintenance therapy were incubated as above with calcein-labeled NPC cells with or without an anti-TRAIL antibody, anti-FAS antibody or concanavalin A. NK cells from healthy donors, incubated in vitro with 1000 U/ml IFNβ were used as controls for effectors and nasoepithelial cells NP69 for targets. Data are presented as means ± SEM (Student’s t test; *P < 0.05; **P < 0.01; ***P < 0.001)
In the next step, we analyzed the contribution of the different death effector pathways in NK cell killing against NPC cells. NK cells kill target cells via two major pathways, the granzyme/perforin pathway and the death ligand pathway with FasL and TRAIL as main effectors in the latter one [29, 30]. To block the granzyme/perforin and TRAIL pathway, NK cells from both patients isolated at day 1 of week 4 and from two healthy volunteers were incubated with concanavalin A and an anti-TRAIL antibody, respectively, before coculturing them with NPC cells; for blockage of the FasL/Fas pathway, NPC cells were incubated with a FAS blocking antibody before co-culture with NK cells. Cytotoxicity was measured as shown above using the calcein assay. Figure 2b demonstrates that blocking of TRAIL reduced cytotoxicity of NK cells the most, followed by blockade of FAS; no major effect of concanavalin A was observed. When patients’ NK cells were isolated 6 and 24 h after injection of IFNβ, the contribution of TRAIL to NK cell-mediated cytotoxicity was even higher. This suggests that the increase in NK cell cytotoxicity induced by IFNβ results to a large extent from the increase in TRAIL expression on NK cells as shown in Fig. 1. A similar pattern was observed in NK cells isolated from two healthy volunteers stimulated with IFNβ in vitro or when NPC cells were exposed to IFNβ before co-culture with NK cells (data not shown).
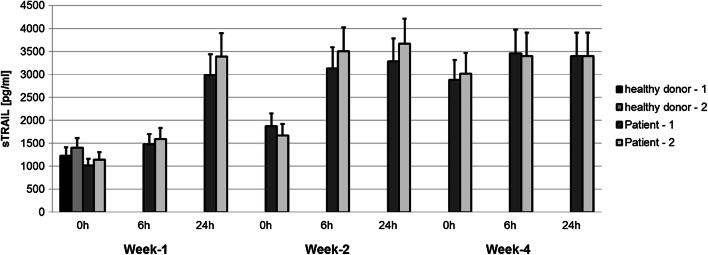
Increased serum levels of soluble TRAIL during IFNβ maintenance therapy
TRAIL also exists in a soluble form (sTRAIL) which is able to induce apoptosis in susceptible cells [31]. As IFNβ has been previously shown to promote the release of sTRAIL from cell populations such as neutrophils, monocytes, T and B lymphocytes [32], we investigated whether sTRAIL could be detected in the serum of the two patients treated with IFNβ. As shown in Fig. 3, serum sTRAIL levels were in a similar low range for the two NPC patients before the start of IFNβ treatment and the two healthy controls. sTRAIL levels slightly rose 6 h after injection of IFNβ in both patients and doubled after 24 h. Baseline levels of sTRAIL before injection of IFNβ were higher in the 2nd week of maintenance therapy compared to the 1st week, and increased further in the 4th week. These results go along with the previous observations underlining that the effect of IFNβ on expression of membrane-bound TRAIL on NK cells and the release of sTRAIL increases during the 1st month of maintenance therapy.
Fig. 3.
Increased detection of soluble TRAIL in the serum of NPC patients treated with IFNβ. Serum from two patients with NPC, treated with IFNβ was obtained at the indicated time points; sTRAIL was measured by ELISA. Serum from two healthy volunteers was used as a control. Data are presented as means ± SEM
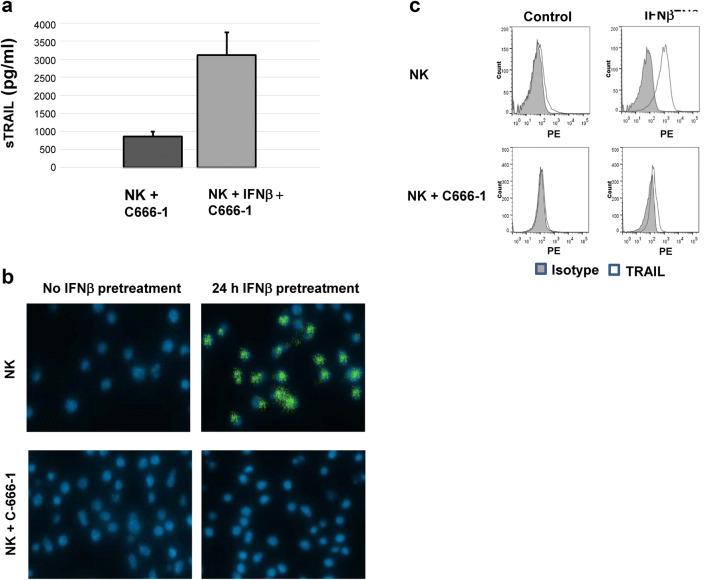
IFNβ induces the production of soluble TRAIL in NK cells which gets released upon interaction with NPC cells
We were then interested in knowing whether NK cells also produced sTRAIL. For this, NK cells of a healthy volunteer were cultured in the presence or absence of IFNβ for 24 h and then co-cultured with NPC cells C666-1 at an E:T ratio of 6:1. After 4 h of co-culture, supernatant was analyzed for the presence of sTRAIL. sTRAIL was already detectable in the supernatant of unstimulated NK cells and was about four times higher in NK cells incubated with IFNβ (Fig. 4a). When NK cells treated as above were stained for TRAIL and analyzed by confocal microscopy, a predominately cytoplasmatic staining pattern was detected in cells incubated for 24 h with IFNβ but not in unstimulated NK cells. Interestingly, when staining of NK cells was done after 4 h of co-culture with NPC cells, TRAIL could not be detected in IFNβ-stimulated NK cells anymore, indicating that the contact with NPC cells lead to the release of intracellularly stored TRAIL and the loss of surface expression of TRAIL (Fig. 4b). This loss of surface expression of TRAIL could be confirmed by flow cytometry of NK cells treated as above (Fig. 4c), as treatment of NK cells with IFNβ for 24 h lead to marked upregulation of surface TRAIL which then markedly diminished after 4 h of co-culture with NPC cells. Interestingly, downregulation of TRAIL on NK cells was also observed in patients’ NK cells obtained 24 h after application of IFNβ compared to 6 h (Fig. 1), suggesting that during the time period of 24 h NK cells had encountered TRAIL receptor-positive target cells inducing the release of sTRAIL as well as leading to the loss of TRAIL surface expression.
Fig. 4.
IFNβ-stimulated NK cells produce soluble TRAIL which gets released upon co-culture with NPC cells. a Supernatant from NK cells stimulated or not for 24 h with IFNβ at 1000 U/ml and then co-cultured for 4 h with NPC cells C666-1, and then was analyzed for sTRAIL by ELISA. NK cells treated as above and co-cultured or not for 4 h with NPC cells C666-1 were stained with anti-TRAIL antibody and analyzed by b confocal microscopy or c flow cytometry
Induction of apoptosis by sTRAIL from serum of NPC patients treated with IFNβ
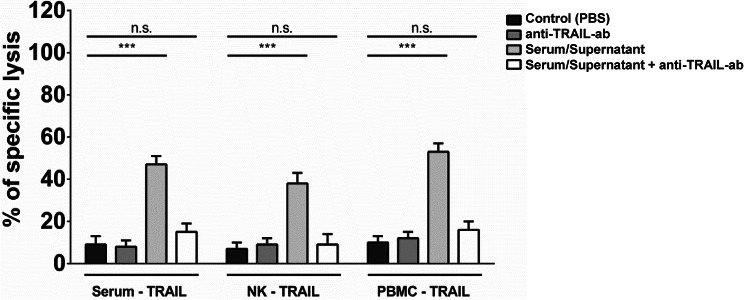
As sTRAIL levels increased in the serum of NPC patients after application of IFNβ, we questioned, whether such sTRAIL was able to induce apoptosis in NPC cells. Therefore, serum from both patients obtained 24 h after IFNβ injection on day 1 in week 4 was concentrated for protein using Vivaspin columns. C17 cells isolated from a patient-derived NPC xenograft and labeled with calcein were then incubated with protein-concentrated serum at 30 ng/ml sTRAIL. As shown in Fig. 5, sTRAIL-containing serum specifically killed NPC cells as killing could be greatly impaired by a TRAIL-blocking antibody. Supernatant of PBMC and NK cells stimulated in vitro with IFNβ also killed C17 cells in a TRAIL-specific manner, indicating that IFNβ induces expression functional sTRAIL by NK cells.
Fig. 5.
Soluble TRAIL from the serum of NPC patients kills cells of a patient-derived NPC xenograft. Serum obtained 24 h after injection of IFNβ at d1 of week 4 of IFNβ maintenance therapy was concentrated about tenfold via Vivaspin centrifugation and added to calcein-labeled C17 PDX cells for 24 h. Lysis of target cells was determined by measurement of calcein in collected supernatants by an ELISA reader. Killing could be inhibited by anti-TRAIL antibody. As a control supernatant from PBMC or NK cells incubated in vitro with 1000 U/ml IFNβ was used
Discussion
In this manuscript, we have shown that IFNβ applied to two patients with NPC (1) induced TRAIL expression on patients’ NK cells, (2) increased their cytotoxicity against NPC cells, and (3) that this killing effect was largely mediated by TRAIL. Furthermore, treatment of patients with IFNβ increased serum levels of biologically functional soluble TRAIL (sTRAIL) indicating an additional way to induce apoptosis in NPC cells.
Malignant NPC cells are known to express TRAIL receptors in situ. Examining 174 tumor biopsy specimens from patients with NPC by immunohistochemistry, Wang et al. detected TRAIL-R1 in 29.9% and TRAIL-R2 in 36.6% of tumors [33]; the percentage of biopsy specimens which expressed at least one TRAIL receptors was not given, but one can assume that it ranged between 36.6% and 66.5%. As the activation of either TRAIL-R1 or -R2 is sufficient for eliciting apoptosis, this suggests that a large number of NPC patients bear tumors which are sensitive to apoptosis induction by TRAIL. The authors also found that the expression of TRAIL-R2 was associated with a better survival rate, indicating that the TRAIL signaling pathway is of importance in the elimination of NPC tumor cells [33]. Using a panel of six different NPC cell lines and cells of one patient-derived xenograft (PDX) we have previously shown that all NPC cell lines studied and PDX cells expressed TRAIL receptors and that they were susceptible to TRAIL-mediated apoptosis [13]. In addition, IFNβ-induced expression of TRAIL in all cell lines except for cell line C666-1 and led to the induction of apoptosis in an autocrine way. We were also able to show that IFNβ markedly increased the expression of TRAIL-R2 in NPC cells including PDX cells, suggesting that the clinical use of IFNβ in patients could sensitize tumors to the induction of apoptosis by TRAIL. As IFNβ has been shown to induce TRAIL expression in lymphomononuclear cells in vitro [16, 17], such cells could be an additional means to induce apoptosis in TRAIL-sensitive NPC cells, especially in NPC cells which are refractory to induction of endogenous TRAIL expression like C666-1 cells.
Investigating the expression of TRAIL on NK cells of two patients who started maintenance therapy with IFNβ, we demonstrate that the dosages applied were able to induce TRAIL expression. Upregulation of TRAIL expression on NK cells was already seen when patients received 3 Mio U IFNβ s.c., which was given as a 50% starting dose in the 1st week of maintenance treatment. TRAIL expression on NK cells of both patients was higher when patients got 6 Mio U at week 2 and week 4. Induction of TRAIL in NK cells has also been shown on the mRNA level in patients with chronic HepC infection receiving IFNα which like IFNβ belongs to the type I IFNs [34]; IFNα and IFNβ both signal mainly through IFNAR1 and -AR2 [35].
NK cells have been previously shown to kill NPC cells in vitro [36]. However, killing was only studied after culture of cells for 4 weeks. Killing of NPC cell lines CNE2 and 915 used as targets in that study was at E:T ratios of 3:1 and 10:1 around 20% and 30%, respectively, and in a similar range as for NK cells of the two patients in this study before start of IFNβ maintenance therapy. In our experiments, NK cells isolated from both patients before start of IFNβ treatment killed cells of the NPC cell line C666-1 to a similar extent as did NK cells isolated from two healthy volunteers. When NK cells were isolated from patients after injection of IFNβ, killing of NPC cells by patients’ NK cells increased with respect to NK cells from healthy controls. Similarly to the increase in TRAIL expression during the course of maintenance therapy, an increase in the killing ability of patients’ NK cells was observed at week 2, when the IFNβ dose was doubled and at week 4 of maintenance therapy. Increased killing of NK cells isolated from cancer patients when treated with IFNβ has been described before by Fujimiya et al., using the CML cell line K562 as a target [37]. In that study, NK cell cytotoxicity increased 24 h after injection of IFNβ; in contrast to our observations, NK cytotoxic activity fell below the original baseline levels after 2 weeks of alternate daily injections of IFNβ in the Fujimiya study.
To kill their targets, NK cells use diverse effectors which belong to two main categories: the granzyme B/perforin and the death ligand pathway with FASL and TRAIL as its major constituents for killing [38]. We investigated the contribution of these different pathways in the killing of NPC cells by NK cells. Our results show that TRAIL is the main effector of apoptosis induction in NPC cells. The contribution of the TRAIL pathway to NPC killing increased when NK cells were isolated after injection of IFNβ or NK cells from healthy volunteers were incubated in vitro with IFNβ. This is in contrast to the killing of other targets by NK cells. The killing of K562 cells by NK cells has been shown to be completely dependent on the granzyme B/perforin pathway [38]. Also, in the killing of neuroblastoma cell lines by NK cells, the granzyme B/perforin pathway represents the major pathway of killing and is supplemented by the TRAIL signaling pathway in TRAIL-sensitive cell lines [39]. In contrast, killing of FAS-positive Jurkat cells by NK cells is only partly dependent on the granzyme B/perforin system and mainly mediated by FASL [40]. The contribution of different death effector pathways probably reflects the sensitivity of target cells to the corresponding death mechanism.
TRAIL is expressed as a membrane-bound protein on the cell surface. It also exists in a soluble form (sTRAIL), generated through enzymatic shedding of surface-anchored TRAIL or by cellular secretion [32]. Type I interferons have been shown to increase the intracellular production of TRAIL in neutrophils, monocytes and T cells [41, 42]. Though only a minor part of TRAIL is secreted after exposure to type I interferons, additional stimuli like TNFα or lipopolysaccharides in neutrophiles or PHA in T cells lead to a rapid release of intracellularly stored TRAIL [42, 43]. The role of sTRAIL derived from NK cells has not been studied so far. Here, we show that IFNβ induces production of TRAIL in NK cells, and that TRAIL is being stored intracellularly and is expressed on the cell membrane, similarly to neutrophils, monocytes and T cells. Contact with NPC cells as targets then leads to the release of intracellularly stored TRAIL and its detection in the supernatant. Interestingly, co-culture with NPC cells also leads to the loss of membranous expression of TRAIL in NK cells, a phenomenon which has not been previously described in other cell systems, especially T cells. The loss of surface expression of TRAIL from NK cells when co-cultured with NPC cells in vitro is consistent with the observation made in vivo that circulating NK cells lose their TRAIL expression 24 h after the application of IFNβ. One possible explanation could be that in vivo as in vitro NK cells lose their surface TRAIL molecules after the interaction with TRAIL receptor-bearing target cells. As surface-anchored TRAIL has been shown to be cleaved by cathepsin E [44], one can speculate that either NPC cells do express cathepsin on their cell surface or that cathepsin is secreted by NK cells or NPC cells upon cell to cell interaction.
Increased levels of sTRAIL have been measured in patients treated with type I interferons [45, 46]. Treatment of melanoma patients with IFNα resulted in TRAIL plasma levels around 3000 pg/ml [46] which were in a similar range as the levels in our two NPC patients. sTRAIL isolated from PBMC stimulated in vitro with IFNα has been shown to be biologically active and to induce apoptosis in leukemic cells [46]. In our experiments, we directly demonstrate that sTRAIL from serum of NPC patients was able to kill NPC cells of a patient-derived xenograft. We can therefore speculate that the clinical efficacy of IFNβ in patients with NPC might also rely on the induction of apoptosis by circulating sTRAIL in NPC cells.
In summary, IFNβ could mediate an anti-tumor effect in patients with nasopharyngeal carcinoma through induction of TRAIL via three distinct pathways: (1) induction of TRAIL expression on NPC cells and subsequent activation of the TRAIL pathway in an autocrine and paracrine manner [13], (2) induction of TRAIL on NK cells and elimination of NPC cells by activated NK cells, and (3) direct induction of apoptosis in NPC cells via soluble TRAIL. As relapse in the treatment of NPC is nowadays mainly systemic, the activation of these pathways by IFNβ could explain the clinical observation that NPC patients participating in the GPOH studies have an increased disease-free survival and lower risk of distant failure compared to patients on other pediatric studies without adjuvant IFNβ [5–8]. Distant failure rates are even higher in treatment protocols for adult NPC patients which mainly rely on radiochemotherapy [47]. Therefore, the extension of maintenance therapy with IFNβ to adults with NPC is expected to decrease the risk for metastatic relapse and should ideally be tested in a randomized clinical trial. In addition, our study suggests a potential therapeutic benefit of IFNβ-activated NK cells in patients with NPC. The adoptive transfer of NK cells has been shown to be safe and efficacious in various malignancies refractory to standard treatment [48]. A clinical trial using high levels of NK cells in patients with small NPC metastases is currently ongoing in patients with relapsed disease in Guangzhou, China [NCT03007836].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Anshu Babbar who carefully proofread the manuscript.
Abbreviations
- Calcein-AM
Calcein-acetyoxymethyl
- EBV
Epstein–Barr virus
- FAS
First apoptosis signal
- FASL
FAS ligand
- GPOH
German Society of Pediatric Oncology and Hematology
- IFNα, -β
Interferon alpha, -beta
- IFNAR1, -2
Interferon alpha and beta receptor subunit 1, -2
- NGS
Next-generation sequencing
- NPC
Nasopharyngeal carcinoma
- PDX
Patient-derived xenograft
- RFU
Relative fluorescence units
- sTRAIL
Soluble TRAIL
- TRAIL-R1, -R2
TRAIL receptor 1, -2
Author contribution
Conception and design: AM, UK; development of methodology: AM, BD, VB, PB; acquisition of data: AM, SF, TB, LS, BD; analysis and interpretation of data: AM, TB, BD, PB, UK; writing and review of the manuscript: AM, PB, UK; material support: BD, PB; study supervision: UK.
Funding
The study has been funded internally by the Medical Faculty, Rhenish-Westphalian Technical University Aachen, Germany.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical standards
The final protocol was approved by the ethics committee of the Rhenish-Westphalian Technical University, Aachen, Germany [EK 005/18]. The study was conducted in accordance with the Declaration of Helsinki (2013 revision).
Ethical approval
Procedures for mouse handling and xenografts were reviewed and approved by the Ethics Committee for animal experimentation n°26 (Gustave Roussy, Villejuif, France), in accordance with the European directive 2010/63/EU and the decrees of the French ministry of Agriculture R. 214-87 to R. 214-126. The approval was given in November 26, 2015, under the Number Apafis #1605—2015090216498538-v2.
Informed consent
Written informed consent was obtained from all individual participants included in the study. Patients were treated at the Uniklinik RWTH Aachen. Informed consent including use of biological specimen (tumor, peripheral blood, urine) and data acquisition and processing was obtained from patients prior to the initiation of the study. Informed consent including the use of peripheral blood and anonymous processing of data was obtained from healthy volunteers who were part of the laboratory staff.
Animal source
Swiss nude mice were bred in the animal facility at Gustave Roussy.
Cell line authentication
C666-1 was a gift from Prof. Fei–Fei Liu, University of Toronto, Canada [49] and the SV40T-antigen immortalized nasopharyngeal epithelial cell line NP69 [50] was obtained from Prof. George Tsao (The Chinese University of Hong Kong, Hong Kong, China). Cell authentication was done using short tandem repeated profiles as described previously [13], and cell lines were tested at regular intervals by PCR to rule out mycoplasma contamination. Authentication of C17 cell was done by checking of HLA class I alleles by PCR (A02.01/A26.01–B44.02/B51.01).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 2.Chua M, Wee J, Hui E, Chan A. Nasopharyngeal carcinoma. Lancet. 2016;387(10022):1012–1024. doi: 10.1016/S0140-6736(15)00055-0. [DOI] [PubMed] [Google Scholar]
- 3.Jayasurya A, Bay BH, Yap WM, Tan NG. Lymphocytic infiltration in undifferentiated nasopharyngeal cancer. Arch Otolaryngol Head Neck Surg. 2000;126(11):1329–1332. doi: 10.1001/archotol.126.11.1329. [DOI] [PubMed] [Google Scholar]
- 4.Huang S, Tsao S, Tsang C. Interplay of viral infection, host cell factors and tumor microenvironment in the pathogenesis of nasopharyngeal carcinoma. Cancers (Basel) 2018;10(4):106. doi: 10.3390/cancers10040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Galindo C, Wofford M, Castleberry RP, et al. Preradiation chemotherapy with methotrexate, cisplatin, 5-fluorouracil, and leucovorin for pediatric nasopharyngeal carcinoma. Cancer. 2005;103:850–857. doi: 10.1002/cncr.20823. [DOI] [PubMed] [Google Scholar]
- 6.Mertens R, Granzen B, Lassay L, et al. Treatment of nasopharyngeal carcinoma in children and adolescents. Definitive results of a multicenter study (NPC-91-GPOH) Cancer. 2005;104:1083–1089. doi: 10.1002/cncr.21258. [DOI] [PubMed] [Google Scholar]
- 7.Buehrlen M, Zwaan CM, Granzen B, et al. Multimodal treatment, including interferon beta, of nasopharyngeal carcinoma in children and young adults. Cancer. 2012;118:4892–4900. doi: 10.1002/cncr.27395. [DOI] [PubMed] [Google Scholar]
- 8.Casanova M, Bisogno G, Gandola L, et al. A prospective protocol for nasopharyngeal carcinoma in children and adolescents. Cancer. 2012;118:2718–2725. doi: 10.1002/cncr.26528. [DOI] [PubMed] [Google Scholar]
- 9.Treuner J, Niethammer D, Dannecker G, Hagmann R, Neef V, Hofschneider P. Successful treatment of nasopharyngeal carcinoma with interferon. Lancet. 1980;1(8172):817–818. doi: 10.1016/S0140-6736(80)91308-2. [DOI] [PubMed] [Google Scholar]
- 10.Connors JM, Andiman WA, Howarth CB, Liu E, Merigan TC, Savage ME, Jacobs C. Treatment of nasopharyngeal carcinoma with human leukocyte interferon. J Clin Oncol. 1985;3(6):813–817. doi: 10.1200/JCO.1985.3.6.813. [DOI] [PubMed] [Google Scholar]
- 11.Mertens R, Lassay L, Heimann G. Combined treatment of nasopharyngeal cancer in children and adolescents-concept of a study. Klin Padiatr. 1993;205(4):241–248. doi: 10.1055/s-2007-1025233. [DOI] [PubMed] [Google Scholar]
- 12.Wolff HA, Rödel RM, Gunawan B, et al. Nasopharyngeal carcinoma in adults: treatment results after long-term follow-up with special reference to adjuvant interferon-beta in undifferentiated carcinomas. J Cancer Res Clin Oncol. 2010;136:89–97. doi: 10.1007/s00432-009-0640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makowska A, Wahab L, Braunschweig T, Kapetanakis N, Vokuhl C, Denecke B, Shen L, Busson P, Kontny U. Interferon beta induces apoptosis in nasopharyngeal carcinoma cells via the TRAIL-signaling pathway. Oncotarget. 2018;9(18):14228–14250. doi: 10.18632/oncotarget.24479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker BS, Rautela J, Hertzog PJ. Antitumour actions of interferons: implications for cancer therapy. Nat Rev Cancer. 2016;16:131–144. doi: 10.1038/nrc.2016.14. [DOI] [PubMed] [Google Scholar]
- 15.Bekisz J, Sato Y, Johnson C, Husain SR, Puri RK, Zoon KC. Immunomodulatory effects of interferons in malignancies. J Interf Cytokine Res. 2013;33:154–161. doi: 10.1089/jir.2012.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kayagaki N, Yamaguchi N, Nakayama M, Eto H, Okumura K, Yagita H. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells: a novel mechanism for the antitumor effects of type I IFNs. J Exp Med. 1999;189(9):1451–1460. doi: 10.1084/jem.189.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato K, Hida S, Takayanagi H, Yokochi T, Kayagaki N, Takeda K, Yagita H, Okumura K, Tanaka N, Taniguchi T, Ogasawara K. Antiviral response by natural killer cells through TRAIL gene induction by IFN-alpha/beta. Eur J Immunol. 2001;31:3138–3146. doi: 10.1002/1521-4141(200111)31:11<3138::AID-IMMU3138>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 18.Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer. 2016;16(1):7–19. doi: 10.1038/nrc.2015.5. [DOI] [PubMed] [Google Scholar]
- 19.Smyth MJ, Crowe NY, Godfrey DI. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol. 2001;13(4):459–463. doi: 10.1093/intimm/13.4.459. [DOI] [PubMed] [Google Scholar]
- 20.Street SE, Hayakawa Y, Zhan Y, Lew AM, MacGregor D, Jamieson AM, Diefenbach A, Yagita H, Godfrey DI, Smyth MJ. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J Exp Med. 2004;199(6):879–884. doi: 10.1084/jem.20031981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorelik E, Wiltrout RH, Okumura K, Habu S, Herberman RB. Role of NK cells in the control of metastatic spread and growth of tumor cells in mice. Int J Cancer. 1982;30(1):107–112. doi: 10.1002/ijc.2910300118. [DOI] [PubMed] [Google Scholar]
- 22.Lu J, Chen XM, Huang HR, Zhao FP, Wang F, Liu X, Li XP. Detailed analysis of inflammatory cell infiltration and the prognostic impact on nasopharyngeal carcinoma. Head Neck. 2018;40(6):1245–1253. doi: 10.1002/hed.25104. [DOI] [PubMed] [Google Scholar]
- 23.Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H, Okumura K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7(1):94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- 24.Müller L, Aigner P, Stoiber D. Type I interferons and natural killer cell regulation in cancer. Front Immunol. 2017;8:304. doi: 10.3389/fimmu.2017.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takehara T, Uemura A, Tatsumi T, Suzuki T, Kimura R, Shiotani A, Ohkawa K, Kanto T, Hiramatsu N, Hayashi N. Natural killer cell-mediated ablation of metastatic liver tumors by hydrodynamic injection of IFNalpha gene to mice. Int J Cancer. 2007;120(6):1252–1260. doi: 10.1002/ijc.22152. [DOI] [PubMed] [Google Scholar]
- 26.Kontny U, Franzen S, Behrends U, Bührlen M, Christiansen H, Delecluse H, Eble M, Feuchtinger T, Gademann G, Granzen B, et al. Diagnosis and treatment of nasopharyngeal carcinoma in children and adolescents—recommendations of the GPOH-NPC study group. Klin Padiatr. 2016;228(3):105–112. doi: 10.1055/s-0041-111180. [DOI] [PubMed] [Google Scholar]
- 27.Gressette M, Vérillaud B, Jimenez-Pailhès A, Lelièvre H, Lo K, Ferrand F, Gattolliat C, Jacquet-Bescond A, Kraus-Berthier L, Depil S, et al. Treatment of nasopharyngeal carcinoma cells with the histone-deacetylase inhibitor abexinostat: cooperative effects with cisplatin and radiotherapy on patient-derived xenografts. PLoS One. 2014;9(3):e91325. doi: 10.1371/journal.pone.0091325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desjardins P, Hansen JB, Allen M. Microvolume protein concentration determination using the NanoDrop 2000c spectrophotometer. J Vis Exp. 2009;33:1610. doi: 10.3791/1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martínez-Lostao L, Anel A, Pardo J. How do cytotoxic lymphocytes kill cancer cells? Clin Cancer Res. 2015;21(22):5047–5056. doi: 10.1158/1078-0432.CCR-15-0685. [DOI] [PubMed] [Google Scholar]
- 30.Guicciardi M, Gores GJ. Life and death by death receptors. FASEB J. 2009;23(6):1625–1637. doi: 10.1096/fj.08-111005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi J, Zheng D, Liu Y, Sham MH, Tam P, Farzaneh F, Xu R. Overexpression of soluble TRAIL induces apoptosis in human lung adenocarcinoma and inhibits growth of tumor xenografts in nude mice. Cancer Res. 2005;65(5):1687–1692. doi: 10.1158/0008-5472.CAN-04-2749. [DOI] [PubMed] [Google Scholar]
- 32.Ehrlich S, Infante-Duarte C, Seeger B, Zipp F. Regulation of soluble and surface-bound TRAIL in human T cells, B cells, and monocytes. Cytokine. 2003;24(6):244–253. doi: 10.1016/S1043-4666(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Li J, Wen Q, Luo J, Chu S, Chen L, Qing Z, Xie G, Xu L, Alnemah MM, Li M, Fan S, Zhang H. 4EGI-1 induces apoptosis and enhances radiotherapy sensitivity in nasopharyngeal carcinoma cells via DR5 induction on 4E-BP1 dephosphorylation. Oncotarget. 2016;7:21728–21741. doi: 10.18632/oncotarget.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stegmann KA, Björkström NK, Veber H, Ciesek S, Riese P, Wiegand J, Hadem J, Suneetha PV, Jaroszewicz J, Wang C, et al. Interferon-alpha-induced TRAIL on natural killer cells is associated with control of hepatitis C virus infection. Gastroenterology. 2010;138(5):1885–1897. doi: 10.1053/j.gastro.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 35.Medrano RFV, Hunger A, Mendonça SA, Barbuto JAM, Strauss BE. Immunomodulatory and antitumor effects of type I interferons and their application in cancer therapy. Oncotarget. 2017;8(41):71249–71284. doi: 10.18632/oncotarget.19531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng Y, Cao KY, Ng SP, Chua DT, Sham JS, Kwong DL, Ng MH, Lu L, Zheng BJ. Complementary activation of peripheral natural killer cell immunity in nasopharyngeal carcinoma. Cancer Sci. 2006;97(9):912–919. doi: 10.1111/j.1349-7006.2006.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujimiya Y, Wagner RJ, Groveman S, Sielaff K, Kohsaka T, Nakayama M. In vivo priming effects of interferon-beta ser on NK activity of peripheral blood mononuclear cells in cancer patients. Ther Immunol. 1995;2(1):15–22. [PubMed] [Google Scholar]
- 38.Vrazo AC, Hontz AE, Figueira SK, Butler BL, Ferrell JM, Binkowski BF, Li J, Risma KA. Live cell evaluation of granzyme delivery and death receptor signaling in tumor cells targeted by human natural killer cells. Blood. 2015;126(8):e1–e10. doi: 10.1182/blood-2015-03-632273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheard MA, Asgharzadeh S, Liu Y, Lin TY, Wu HW, Ji L, Groshen S, Lee DA, Seeger RC. Membrane-bound TRAIL supplements natural killer cell cytotoxicity against neuroblastoma cells. J Immunother. 2013;36(5):319–329. doi: 10.1097/CJI.0b013e31829b4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zamai L, Ahmad M, Bennett IM, Azzoni L, Alnemri ES, Perussia B. Natural killer (NK) cell-mediated cytotoxicity: differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J Exp Med. 1998;188(12):2375–2380. doi: 10.1084/jem.188.12.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halaas Ø, Liabakk NB, Vik R, Beninati C, Henneke P, Sundan A, et al. Monocytes stimulated with group B streptococci or interferons release tumour necrosis factor-related apoptosis-inducing ligand. Scand J Immunol. 2004;60:74–81. doi: 10.1111/j.0300-9475.2004.01448.x. [DOI] [PubMed] [Google Scholar]
- 42.Cassatella MA, Huber V, Calzetti F, Margotto D, Tamassia N, Peri G, et al. Interferon-activated neutrophils store a TNF-related apoptosis-inducing ligand (TRAIL/Apo-2 ligand) intracellular pool that is readily mobilizable following exposure to proinflammatory mediators. J Leukoc Biol. 2006;79:123–132. doi: 10.1189/jlb.0805431. [DOI] [PubMed] [Google Scholar]
- 43.Monleón I, Martínez-Lorenzo MJ, Monteagudo L, Lasierra P, Taulés M, Iturralde M, et al. Differential secretion of Fas ligand- or APO2 ligand/TNF related apoptosis-inducing ligand-carrying microvesicles during activation-induced death of human T cells. J Immunol. 2001;167:6736–6744. doi: 10.4049/jimmunol.167.12.6736. [DOI] [PubMed] [Google Scholar]
- 44.Kawakubo T, Okamoto K, Iwata J, Shin M, Okamoto Y, Yasukochi A, et al. Cathepsin E prevents tumor growth and metastasis by catalyzing the proteolytic release of soluble TRAIL from tumor cell surface. Cancer Res. 2007;67:10869–10878. doi: 10.1158/0008-5472.CAN-07-2048. [DOI] [PubMed] [Google Scholar]
- 45.Buttmann M, Merzyn C, Hofstetter HH, Rieckmann P. TRAIL, CXCL10 and CCL2 plasma levels during long-term Interferon-beta treatment of patients with multiple sclerosis correlate with flu-like adverse effects but do not predict therapeutic response. J Neuroimmunol. 2007;190(1–2):170–176. doi: 10.1016/j.jneuroim.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 46.Tecchio C, Huber V, Scapini P, Calzetti F, Margotto D, Todeschini G, Pilla L, Martinelli G, Pizzolo G, Rivoltini L, Cassatella MA. IFNalpha-stimulated neutrophils and monocytes release a soluble form of TNF-related apoptosis-inducing ligand (TRAIL/Apo-2 ligand) displaying apoptotic activity on leukemic cells. Blood. 2004;103:3837–3844. doi: 10.1182/blood-2003-08-2806. [DOI] [PubMed] [Google Scholar]
- 47.Blanchard P, Lee A, Marguet S, Leclercq J, Ng WT, Ma J, Chan AT, Huang PY, Benhamou E, Zhu G, Chua DT, Chen Y, Mai HQ, Kwong DL, Cheah SL, Moon J, Tung Y, Chi KH, Fountzilas G, Zhang L, Hui EP, Lu TX, Bourhis J, Pignon JP, MAC-NPC Collaborative Group Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16(6):645–655. doi: 10.1016/S1470-2045(15)70126-9. [DOI] [PubMed] [Google Scholar]
- 48.Dahlberg C, Sarhan D, Chrobok M, Duru A, Alici E. Natural killer cell-based therapies targeting cancer: possible strategies to gain and sustain anti-tumor activity. Front Immunol. 2015;6:605. doi: 10.3389/fimmu.2015.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheung S, Huang D, Hui A, Lo K, Ko C, Tsang Y, Wong N, Whitney B, Lee J. Nasopharyngeal carcinoma cell line (C666-1) consistently harbouring Epstein–Barr virus. Int J Cancer. 1999;83(1):121–126. doi: 10.1002/(SICI)1097-0215(19990924)83:1<121::AID-IJC21>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 50.Tsao S, Wang X, Liu Y, Cheung Y, Feng H, Zheng Z, Wong N, Yuen P, Lo A, Wong Y, et al. Establishment of two immortalized nasopharyngeal epithelial cell lines using SV40 large T and HPV16E6/E7 viral oncogenes. Biochim Biophys Acta. 2002;1590(1–3):150–158. doi: 10.1016/S0167-4889(02)00208-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.