Abstract
The purpose of our study was to assess the immune function of patients with inoperable hepatic malignancies after treatment with selective internal radiotherapy (SIRT) and to identify possible correlations with clinical parameters. In 25 patients receiving SIRT lymphocyte proliferation and the production of pro- and anti-inflammatory cytokines (interferon-γ and interleukin-10) after stimulation with mitogens and microbial antigens were tested prior to therapy, directly after therapy (day 1) and at day 2, 7 and 28 post therapy using the lymphocyte transformation test and enzyme-linked immunospot assays. Absolute counts and percentages of leukocyte and lymphocyte subsets were determined by flow cytometry. The most prominent finding was an immediate and significant (p < 0.05) decrease of lymphocyte proliferation and interferon-γ production directly after therapy which lasted until day 28 and was stronger upon stimulation with microbial antigens than with mitogens. Moreover, lymphopenia was revealed, affecting all lymphocyte subsets (CD3+, CD4+, CD8+ T cells, CD4+ CD8+ T cells, B cells and NK cells). SIRT led to a reduction in the percentage of activated HLA-DR+ monocytes and of CD45R0+ memory T cells. Higher radiation activity, the presence of liver cirrhosis, chronic kidney disease, diabetes mellitus and metastases were unfavorable factors for immunocompetence, while a better Eastern Cooperative Oncology Group performance status was associated with stronger immunological reactions. In conclusion, SIRT leads to severe impairment of cellular in vitro immune responses. Further studies are needed to assess a potential clinical impact.
Keywords: Hepatic malignancy, Selective internal radiotherapy, Lymphocyte proliferation, ELISpot, Interferon-γ, Flow cytometry
Introduction
From radiolabeled peptides and monoclonal antibodies to small particles containing radionuclides, therapeutic radiopharmaceuticals are gaining wider clinical implementation in the field of cancer therapy, providing targeted treatment modalities with fewer potential side effects. Selective internal radiotherapy (SIRT) is one such modality, which offers an alternative in patients with irresectable primary liver cancer and liver metastases [1].
SIRT consists of millions of resin or glass microspheres containing the radioisotope 90Yttrium (90Y) which are delivered by hepatic artery injection directly into the capillary bed. The microspheres preferentially lodge in the microvasculature of the tumor and destroy it mainly by delivering high-dose radiotherapy [2]. The treatment exploits the higher arterial perfusion of the hepatic tumors compared to the liver due to their blood supply from the hepatic artery rather than the portal vein [3], thus resulting in a lower radiation dose to the normal liver parenchyma.
The radioisotope 90Y is a beta-emitter with a half-life of 64.1 h and a maximum tissue penetration of 11 mm (mean 2.5 mm). There has been only sparse research on the effect of beta radiation on the immune system. In a recent study, it could be shown that patients with thyroid carcinoma treated with the beta-emitter radioiodine exhibit no cellular immunodeficiency after the treatment, but a shift from TH-2 to TH-1 response [4]. On the contrary, the lymphocyte proliferation and the pro-inflammatory immune responses decreased after treatment with 90Y radiolabeled somatostatin analogs (90Y DOTATOC) in patients with neuroendocrine tumors, revealing an immunosuppression [5]. In accordance with these results, grade 2–3 lymphocyte toxicity after peptide-receptor radionuclide therapy (PRRT) with 90Y or 177Lutetium radiolabeled somatostatin analogs or both has been reported [6].
Unlike the above-mentioned tumor radiotherapies, where the radionuclides circulate in the bloodstream exposing the whole body to low-dose radiation, in SIRT the 90Y radioactive microspheres are captured in the capillary beds of the tumors and the liver, resulting in a local irradiation of these tissues. Since the liver is a well-perfused organ with a mean total hepatic blood flow of approximately 1 L/min [7–9], the whole blood is irradiated during its passage through the liver circulation. In addition, it is known that the liver apart from being responsible for the metabolism and detoxification of substances exerts many complex immunological functions and continuing research reveals new insights into its important role in the innate and adaptive immune system [10, 11]. The fact that it comes into contact with food antigens and pathogens from the gut and the systemic circulation makes the balance between immune tolerance and response vital for the whole organism. These immunological functions are underpinned by a large and heterogenous population of resident lymphocytes, while at the same time constantly being patrolled by recirculating lymphocytes [11–16]. Furthermore, apart from the fact that hepatocellular carcinoma is characterized by arterial hypervascularisation, it is described that also the portal lymph flow is increased in cirrhosis, which is a common underlying disease in hepatocellular carcinoma (HCC) [17]. Carreira et al. demonstrated the presence of a rich system of lymphatic vessels at the margins of HCC and metastatic disease to the liver [18]. Therefore, in addition to the irradiation of non-tumorous liver tissue, SIRT also results in an inadvertent irradiation of the immune system.
In 2004, Carr was the first to report lymphopenia in a cohort of sixty-five patients with nonresectable HCC treated by SIRT [19]. In this study, more than 75% of the patients exhibited decreased lymphocyte counts after therapy, which in some cases persisted for more than 1 year. Since then, several other studies have reported mild to severe lymphopenia as an adverse event [20, 21]. Nevertheless, since no serious infections had ever been described in the patients after treatment, it has never been subject of further studies.
It was the aim of this prospective study to explore for the first time effects of SIRT on the immune defense against microorganisms, not just by determining immune cell counts but by going beyond and investigating the changes of lymphocyte function. To assess lymphocyte function, the capacity to proliferate was determined by lymphocyte transformation test (LTT) and the capacity to produce pro- and anti-inflammatory cytokines by enzyme-linked immunospot (ELISpot), an assay that measures cytokine production on a single cell level [22]. To determine in detail, phenotypic changes of leukocyte subsets, fluorescence-activated cell sorting (FACS) analyses were performed. In addition, the impact of potential risk factors was investigated.
Patients, material, methods
Patients and sample collection
Between January 2013 and April 2014, 25 patients treated with 90Y radioactive glass microspheres because of irresectable hepatic malignancies were included into this prospective study. Patient characteristics are detailed in Table 1. Since the aim of the study was to assess immunological alterations after radiotherapy, the following exclusion criteria were applied: known autoimmune disease, immunosuppressive medications, previous liver transplantation, and previous treatment with sorafenib. Depending on the clinical condition of the patient and the tumor response, a patient can receive more than one cycle of radiotherapy. In our study, 21 patients underwent their first cycle, 3 were recruited at their second cycle and 1 at the third cycle (Table 1). To rule out a possible influence from the last therapy cycle, patients were included only if the last cycle was applied more than 1 year ago. Blood sampling was scheduled prior to radiotherapy (day 0), directly after radiotherapy (day 1), at 24 h (day 2), at day 7 and day 28 post therapy. Strictly speaking, blood samples of the last two time points were drawn on day 7.3 ± 1.3 (mean and standard error of the mean) and on day 27.4 ± 1.1. Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood after density gradient centrifugation.
Table 1.
Characteristics of 25 patients with hepatic malignancies treated by selective internal radiotherapy
| Female/male | 5/20 |
| Median age (range), years | 73 (33–84) |
| Median administered activity (range), GBq | 2.92 (1.01–6.42) |
| Median of target volume doses (range), Gy | 119 (110–282) |
| Median lung dosea (range), Gy | 6.45 (1–21.4) |
| Median pulmonary shunt (range) % | 4.4 (1.3–28.1) |
| No. of treatment cycle | |
| 1st | 21 |
| 2nd | 3 |
| 3rd | 1 |
| Primary tumor | |
| Liver | 24 |
| CUP | 1 |
| Metastases | |
| Without | 10 |
| Lungs | 4 |
| Adrenal gland | 2 |
| Bones | 2 |
| Pancreas | 1 |
| Stomach | 1 |
| Other | 5 |
| Without/with bone metastases | 23/2 |
| Child–Pugh classa | |
| No liver cirrhosis = 0 | 3 |
| A = 1 | 21 |
| Without/with chronic kidney disease | 20/5 |
| Without/with diabetes mellitus type II | 12/13 |
| ECOG performance status | |
| 0 | 14 |
| 1 | 9 |
| 2 | 2 |
| Without/with arterial hypertension | 3/22 |
| Without/with coronary artery disease | 14/11 |
| Laboratory-chemical values prior to SIRT | |
| Glomerular filtration rate prior to therapyb | |
| < 90 mL/min/1.73 m2 | 20 |
| > 90 mL/min/1.73 m2 | 3 |
| Quick value | |
| Within/below lower limit of normal | 20/5 |
| Serum creatinineb | |
| Within/above or below sex-dependent norm | 14/9 |
| Bilirubin total | |
| Within/above upper limit of normal | 17/8 |
| Bilirubin directa | |
| Within/above upper limit of normal | 1/23 |
| Albuminb | |
| Within/above upper limit of normal | 22/1 |
| Aspartate aminotransferaseb | |
| Within/above sex-dependent upper limit of normal | 10/13 |
| Alanine aminotransferaseb | |
| Within/above sex-dependent upper limit of normal | 12/11 |
| Alkaline phosphataseb | |
| Within/above sex-dependent upper limit of normal | 8/15 |
| Gamma-glutamyl transferaseb | |
| Within/above sex-dependent upper limit of normal | 1/22 |
| α-fetoprotein prior to SIRT | |
| < 10 IU/mL | 12 |
| > 10 IU/mL | 13 |
| α-fetoprotein after SIRTc | |
| < 10 IU/mL | 5 |
| > 10 IU/mL | 17 |
CUP cancer of unknown primary, ECOG Eastern Cooperative Oncology Group
aOne unknown value
bTwo unknown values
cThree unknown values
Lymphocyte transformation test
The LTT was used to quantify the in vitro proliferation of lymphocytes upon stimulation by four mitogens [phytohaemagglutinin (PHA), concanavalin A (ConA), pokeweed mitogen (PWM) and anti-CD3 monoclonal antibody (OKT3)] and twelve recall antigens [Purified protein derivate (PPD), tetanus toxoid, diphtheria toxoid, Candida albicans, cytomegalovirus, herpes simplex virus type 1, varicella zoster virus, rubella virus, mumps virus, measles virus, influenza A and B virus]. As described previously in greater detail [4], 50 000 PBMC in 200 µL of cell culture medium per well of microtiter plates were incubated with mitogens and antigens for 3 and 5 days, respectively. 16 h before harvesting 37 kBq 3H thymidine was added. The cultures were harvested onto filter pads, and the incorporated radioactivity was measured in a liquid scintillation counter.
ELISpot assay
Our focus of interest was the quantitative assessment of the pro-inflammatory cytokine IFN-γ and the counter-regulatory, anti-inflammatory cytokine IL-10. Four mitogens (PHA, ConA, PWM and OKT3) and three antigens (PPD, tetanus toxoid and Candida albicans) were applied as stimuli of cytokine production. As described thoroughly in a previous publication [4], 200,0000 or 400,0000 PBMC in 200 µL culture medium per well of microtiter plates were stimulated for 1 day with various mitogens or antigens, respectively. They were then added to a 96-well plate, which was pre-coated with a monoclonal antibody specific for the secreted cytokine. After 2 days of incubation, the cells were washed away and a second detection antibody was added. The resulting complex was then colored using avidin–biotin peroxidase and the substrate 3-amino-9-ethyl-carbazole. After 12 min, the reaction could be seen as spots on the surface of each well. The number of spots was counted by an ELISpot Reader (AID Fluorospot; Autoimmun Diagnostika GmbH, Strassberg, Germany).
Flow cytometry
For the phenotypic characterization of cells pre- and post-radiotherapy using multicolor flow cytometry, 50 µL of EDTA-treated whole blood was incubated with 20 µL of fluorochrome-conjugated monoclonal antibodies for 10 min in the dark at 20 °C. Cells were surface-stained with the following antibodies: CD45, CD14, CD3, CD4, CD8, CD16+ 56, CD19, CD45RA, CD45RO, HLA-DR, IgG1 and IgG2a (BD Biosciences, Heidelberg, Germany). Subsequently, the red blood cells were lysed with FACS Lysing Solution (BD Biosciences) and the samples were analyzed on a FACSCalibur flow cytometer (BD Biosciences). This procedure was combined with beads containing defined amounts of a fluorophore (True Count Beads, BD Biosciences) to determine the absolute numbers of stained cells. Flow data were analyzed using BD CellQuest Pro™ software.
Statistical analysis
For the evaluation of LTT responses, the second highest 3H thymidine uptake was considered as described previously [23]. Counts per minute (cpm), referring to 3H thymidine uptake, and spots per cell were the measurement units for LTT and ELISpot, respectively. The cumulative antigen score was generated by an algorithm as published [23]; it sums up the scores of each reaction towards the twelve recall antigens tested by LTT. Reactions pre- and post-SIRT were compared by Wilcoxon matched pairs test. The statistical analysis of all these data was performed using GraphPad Prism software (version 5.03). For the correlation of numerical variables, the Spearman Rho test was applied using SPSS software (version 22). Correlation of categorical variables with the immune function was analyzed by Mann–Whitney test. P values of < 0.05 were considered statistically significant.
Results
Immune response after SIRT
In 25 patients receiving SIRT proliferative lymphocyte responses as well as IFN-γ production upon stimulation with different mitogens and recall antigens decreased rapidly after the treatment and remained considerably low during the whole period of follow-up, showing a strong impairment of the immune system.
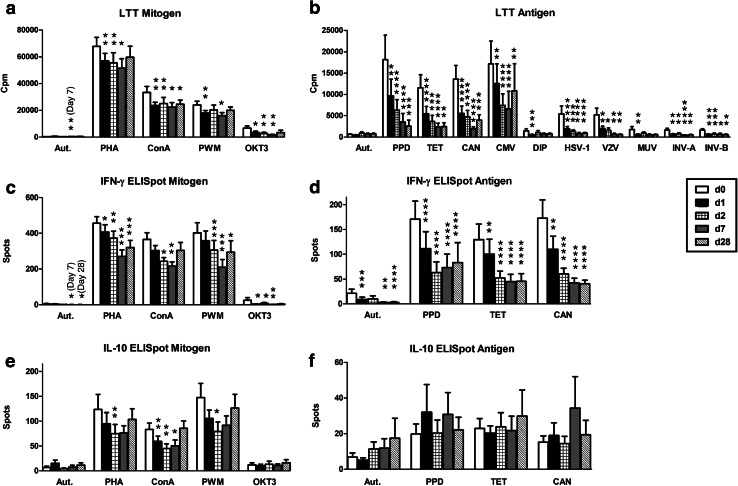
In detail, lymphocyte proliferation as determined by LTT towards all mitogens and ten out of twelve recall antigens (Fig. 1a, b) was significantly (p < 0.05) reduced at day 1 and kept on decreasing on day 2 reaching a minimum at day 7. The remaining two recall antigens, rubella and measles virus, showed the same tendency. Twenty-eight days after therapy, the proliferative responses to all mitogens and antigens were still lower compared to the pretreatment levels. Towards the mitogen ConA and eight out of twelve recall antigens, the proliferation remained significantly (p < 0.05) lower than prior to therapy.
Fig. 1.
Cellular in vitro reaction toward mitogens and recall antigens in patients with hepatic tumors receiving selective internal radiotherapy (n = 22–25). Data represent mean and standard error of the mean (SEM), either pre therapy (day 0, white bars), directly after therapy (day 1, black bars), at 24 h (day 2, hatched bars), at day 7 (gray bars) or at day 28 (striped bars) post therapy. LTT mitogen responses and LTT antigen responses (a, b) are given as counts per minute of 3H thymidine uptake (cpm), IFN-γ and IL-10 secretion to the ELISpot (c–f) as spots per culture. The Wilcoxon matched pairs test was used for comparisons; the level of significance is indicated in comparison with day 0 (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). LTT lymphocyte transformation test, Aut autologous, PHA phythohemagglutinin, ConA concanavalin A, PWM pokeweed mitogen, OKT3 anti-CD3 monoclonal antibody, PPD purified protein derivate (tuberculin), TET tetanus toxoid, CAN Candida albicans, CMV cytomegalovirus, DIP diphtheria toxoid, HSV-1 Herpes simplex virus type 1, VZV Varicella zoster virus, MUV mumps virus, INV-A influenza virus A, INV-B influenza virus B
Similarly, IFN-γ secretion after stimulation with all mitogens and microbial antigens (Fig. 1c, d) dropped constantly after therapy until day 7 and at day 28 was still below the pretreatment levels. For the mitogens PHA and PWM and all antigens (PPD, tetanus toxoid, Candida albicans) the reaction was significantly (p < 0.05) weaker compared to that prior to the therapy. Moreover, a decrease of IL-10 secretion (p < 0.05) was observed at day 2 for the mitogens PHA and PWM and at day 1, 2 and 7 for ConA (Fig. 1e).
Influence of SIRT on peripheral blood leukocytes
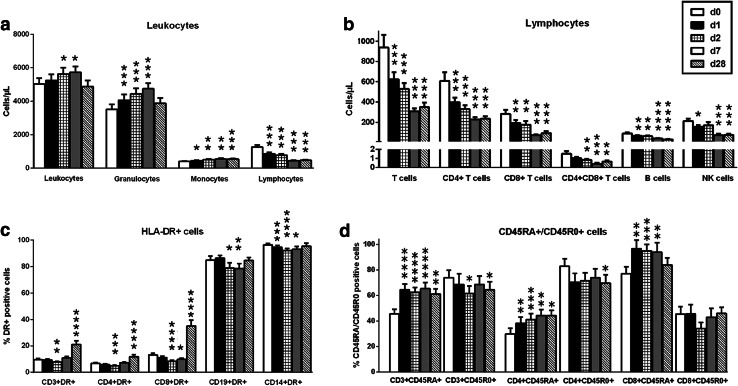
To investigate the influence of SIRT on the immune cell counts and their subpopulations in the peripheral blood, different markers were used in flow cytometric analysis pre- and post-treatment. Overall, treated patients exhibited a profound lymphopenia directly after therapy, whereas granulocytes and monocytes increased rapidly, overall leading to an increase of the total number of leukocytes (Fig. 2a). Granulocytes fell to the pretreatment levels at day 28 while the monocyte counts remained at high level.
Fig. 2.
Effect of selective internal radiotherapy on peripheral blood cells in patients with hepatic tumors (n = 21–25). a The number of granulocytes and monocytes increased after therapy, consequently leading to an increase of the total number of leukocytes, whereas lymphocytes decreased. b, c SIRT had a suppressive effect on all types of peripheral lymphocytes as well as HLA-DR-positive cells. d The effects of SIRT on peripheral naïve (CD45RA+) and memory (CD45R0+) T cells were diverse. Data represent mean and standard error of the mean (SEM), either pre therapy (day 0, white bars), directly after therapy (day 1, black bars), at 24 h (day 2, hatched bars), at day 7 (gray bars) or at day 28 (striped bars) post therapy. Numbers of cells are given as cells/µL. The Wilcoxon matched pairs test was used for comparisons; the level of significance is indicated in comparison with day 0 (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001)
Significant (p < 0.05) lymphopenia was observed affecting all the subpopulations, i.e. CD3+, CD4+, CD8+ T cells, CD4+ CD8+ T cells, CD19+ B cells and NK cells (Fig. 2b). On the last day of follow-up (day 28), all lymphocyte subpopulations remained decreased with the T cell subsets showing a tendency for recovery. The analyses of the percentage of HLA-DR+ T cells (CD3+, CD4+, CD8+) revealed an interesting pattern: after decreasing directly after therapy and reaching a minimum (p < 0.01) at day 2, all HLA-DR+ T cells started increasing at day 7 and were approximately two-fold higher at day 28 compared to pretherapy levels (< 0.0001) (Fig. 2c). The effect of SIRT on the percentage of HLA-DR+ antigen-presenting cells (CD19+ B cells and CD14+ monocytes) was also suppressive reducing their counts at day 2. But both populations returned to pretherapy levels after 28 days. Furthermore, T cells were analyzed for alterations after SIRT according to their naïve (CD45RA+) or memory (CD45R0+) phenotype. Taken together, memory T cells tended to decrease while their naïve counterparts moved profoundly in the opposite direction (Fig. 2d). The proportion of CD3+ memory T cells was significantly (p < 0.05) depleted at day 2 and 28 and of CD4+ memory T cells at day 28. A tendency for lower cell counts was observed at day 2 also for CD8+ memory T cells (p = 0.07). Conversely, naïve CD3+, CD4+ and CD8+ T cells were significantly (p < 0.05) augmented rapidly after SIRT and remained elevated for the whole time of follow-up.
Correlation of clinical parameters and cellular in vitro immune responses
To assess the relationship between immune function and clinical parameters, all patient characteristics as given in Table 1 were included into the analyses. Tables 2 and 3 present the most significant results.
Table 2.
Correlation analysis of numerical variables (Spearman’s Rho)
| Parameter | Time point (day) | r | p | |
|---|---|---|---|---|
| Clinical | Immune system | |||
| Administered radiation activity (GBq) | Granulocyte count | 0 | 0.40 | 0.045 |
| 7 | 0.43 | 0.04 | ||
| 28 | 0.46 | 0.038 | ||
| Lymphocyte count | 7 | − 0.56 | 0.005 | |
| CD8 + T cells | 7 | − 0.52 | 0.011 | |
| B cells | 7 | − 0.51 | 0.013 | |
| 28 | − 0.63 | 0.002 | ||
| HLA-DR + T cells | 2 | − 0.42 | 0.042 | |
| 7 | − 0.45 | 0.03 | ||
| HLA-DR + B cells | 2 | − 0.46 | 0.023 | |
| 7 | − 0.54 | 0.008 | ||
| 28 | − 0.62 | 0.003 | ||
| CD45RA + T cells | 7 | − 0.57 | 0.005 | |
| CD8 + CD45RA + cells | 7 | − 0.63 | 0.001 | |
| 28 | − 0.44 | 0.049 | ||
| CD8 + CD45R0 + cells | 7 | − 0.50 | 0.016 | |
| PHA LTT | 2 | − 0.46 | 0.024 | |
| 7 | − 0.43 | 0.039 | ||
| LTT Cum. antigen score | 7 | − 0.64 | 0.001 | |
| PHA IFN-γ ELISpot | 2 | − 0.49 | 0.017 | |
| 7 | − 0.50 | 0.014 | ||
| ConA IFN-γ ELISpot | 2 | − 0.45 | 0.031 | |
| 7 | − 0.48 | 0.021 | ||
| PWM IFN-γ ELISpot | 2 | − 0.45 | 0.032 | |
| TET IFN-γ ELISpot | 7 | − 0.53 | 0.009 | |
| CAN IFN-γ ELISpot | 2 | − 0.45 | 0.03 | |
| Target volume dose (Gy) | B cells | 1 | − 0.52 | 0.009 |
| 7 | − 0.46 | 0.028 | ||
| HLA-DR + B cells | 1 | − 0.55 | 0.005 | |
| 2 | − 0.63 | 0.001 | ||
| 7 | − 0.52 | 0.01 | ||
| HSV-1 LTT | 7 | − 0.58 | 0.003 | |
| PHA IFN-γ ELISpot | 2 | − 0.44 | 0.037 | |
| Lung dose (Gy) | CD8 + T cells | 7 | − 0.44 | 0.039 |
| CD8 + CD45RA + cells | 7 | − 0.50 | 0.019 | |
| LTT Cum. Antigen score | 7 | − 0.46 | 0.03 | |
| TET IFN-γ ELISpot | 28 | − 0.54 | 0.018 | |
| GFR (mL/min/1.73 q) | PHA LTT | 2 | 0.47 | 0.02 |
| 7 | 0.44 | 0.04 | ||
| ConA LTT | 2 | 0.41 | 0.048 | |
| PHA IFN-γ ELISpot | 0 | 0.53 | 0.006 | |
| 2 | 0.66 | 0.001 | ||
| PWM IFN-γ ELISpot | 0 | 0.44 | 0.03 | |
| 1 | 0.46 | 0.02 | ||
| 2 | 0.60 | 0.002 | ||
| OKT3 IFN-γ ELISpot | 7 | 0.52 | 0.01 | |
| PHA IL-10 ELISpot | 0 | 0.56 | 0.005 | |
| 1 | 0.57 | 0.005 | ||
| 2 | 0.56 | 0.008 | ||
| 7 | 0.75 | < 0.0001 | ||
| 28 | 0.47 | 0.04 | ||
| ConA IL-10 ELISpot | 0 | 0.55 | 0.007 | |
| 1 | 0.44 | 0.04 | ||
| 2 | 0.47 | 0.03 | ||
| 7 | 0.74 | < 0.0001 | ||
| PWM IL-10 ELISpot | 0 | 0.54 | 0.007 | |
| 1 | 0.62 | 0.002 | ||
| 2 | 0.60 | 0.005 | ||
| 7 | 0.68 | 0.001 | ||
| Child–pugh score (no liver cirrhosis = 0, A = 1, B = 2, C = 3 | PPD IL-10 ELISpot | 0 | − 0.43 | 0.046 |
| 1 | − 0.54 | 0.01 | ||
| TET IL-10 ELISpot | 1 | − 0.56 | 0.008 | |
| CAN IL-10 ELISpot | 0 | − 0.45 | 0.04 | |
| 1 | − 0.46 | 0.04 | ||
| 2 | − 0.50 | 0.03 | ||
PHA phythohemagglutinin, LTT lymphocyte transformation test, Cum. antigen score Cumulative antigen score (sum of reactions toward antigens), IFN interferon, ConA concanavalin A, PWM pokeweed mitogen, TET tetanus toxoid, CAN Candida albicans, HSV-1 Herpes simplex virus type 1, GFR glomerular filtration rate, OKT3 anti-CD3 monoclonal antibody, PPD purified protein derivate (tuberculin), IL interleukin
Table 3.
Correlation with categorical variables (Mann–Whitney test)
| Category | Parameter | Time point (day) | Mean values of each group | p |
|---|---|---|---|---|
| Without/with chronic kidney disease | PHA IFN-γ ELISpot | 2 | 415/221 | 0.048 |
| CAN IFN-γ ELISpot | 2 | 70/25 | 0.04 | |
| TET IL-10 ELISpot | 0 | 28/5 | 0.04 | |
| ConA IL-10 ELISpot | 7 | 59/13 | 0.02 | |
| PWM IL-10 ELISpot | 7 | 109/14 | 0.02 | |
| PHA IL-10 ELISpot | 7 | 89/19 | 0.03 | |
| Without/with DM | ConA IFN-γ ELISpot | 2 | 302/189 | 0.01 |
| ECOG: 0/1 or 2 | PWM IFN-γ ELISpot | 2 | 463/134 | 0.002 |
| PHA IL-10 ELISpot | 0 | 181/62 | 0.01 | |
| 1 | 126/58 | 0.04 | ||
| 7 | 110/36 | 0.009 | ||
| ConA IL-10 ELISpot | 7 | 75/21 | 0.006 | |
| PWM IL-10 ELISpot | 0 | 198/92 | 0.03 | |
| 7 | 142/31 | 0.007 | ||
| 28 | 166/83 | 0.04 | ||
| OKT3 IL-10 ELISpot | 0 | 20/4 | 0.04 | |
| Without/with metastases | OKT3 LTT | 0 | 5760/2216 | 0.049 |
| 1 | 3635/1435 | 0.01 | ||
| CMV LTT | 7 | 7835/1343 | 0.03 |
PHA phythohemagglutinin, IFN interferon, CAN Candida albicans, TET tetanus toxoid, IL interleukin, ConA concanavalin A, PWM pokeweed mitogen, DM diabetes mellitus type II, ECOG Eastern Cooperative Oncology Group, OKT3 anti-CD3 monoclonal antibody, LTT lymphocyte transformation test
The radiation activity correlated positively with the granulocyte count post therapy. Patients receiving higher radiation activity had higher granulocyte counts for the whole course after therapy. On the contrary, lymphocytes decreased with higher radiation activity. The radiation effects on lymphocytes were most profound at day 7. At that point in time, the radiation activity correlated negatively (p ≤ 0.005) with the absolute lymphocyte number, different lymphocyte subpopulations (such as CD8+ T cells, B cells and activated B cells), lymphocyte proliferation after stimulation with a set of twelve recall antigens (cumulative antigen score) and secretion of the pro-inflammatory cytokine IFN-γ after stimulation with mitogens and antigens (PHA, ConA, PWM, tetanus toxoid and Candida albicans).
Cellular in vitro immune responses were positively (p < 0.05) influenced by glomerular filtration rate (GFR) and negatively (p < 0.05) by Child–Pugh score, i.e., better kidney and liver function correlated with stronger immune responses pre- and post treatment.
As expected, the presence of concomitant diseases such as chronic kidney disease or diabetes mellitus led to significantly reduced (p < 0.05) immune responses, as indicated by lower levels of IFN-γ and IL-10 production. A similar result was observed for patients with poorer baseline Eastern Cooperative Oncology Group (ECOG) performance status (1 or 2 versus 0). Moreover, the presence of metastases was associated with decreased T-cell responses. Specifically, these patients had lower T-cell proliferation (p < 0.05) upon stimulation with OKT3 before therapy and at day 1 and upon stimulation with cytomegalovirus at day 7.
Summarizing, factors contributing to impaired immune function were higher 90Y activity, the presence of liver cirrhosis, chronic kidney disease, diabetes mellitus and metastases, whereas better ECOG score was a favorable factor.
Discussion
Increased evidence discloses the important role of the liver as an immunological organ which regulates the homeostasis of the whole organism [10, 24, 25]. Therefore, it is reasonable to assume that the irradiation of the liver could have systemic implications on the immune system. Studies have already shown that SIRT leads to lymphopenia [19, 20]. Our study focuses on the functional properties of remaining lymphocytes and indicates that therapy with SIRT for the treatment of hepatic malignancies leads to rapid cellular immunodeficiency. The capacity of the immune system to proliferate upon stimulation with non-specific and specific stimuli (mitogens and microbial antigens, respectively) as well as to produce inflammatory cytokines was markedly reduced directly after therapy and maintained low for as long as 1 month after treatment. In accordance with the present study, we observed a decrease in in vitro antimicrobial immune responses with another tumor therapy containing 90Y [5]. After treatment with 90Y radiolabeled somatostatin analogs (90Y DOTATOC) in patients with neuroendocrine tumors lymphocyte proliferation and IFN-γ secretion was reduced at day 1 and day 7 [5]. Nevertheless, in DOTATOC study, the biodistribution of the radionuclide is different.
In the present study, mitogens and recall antigens were used for the stimulation of lymphocytes. Mitogens can directly stimulate lymphocytes and show evidence of the total lymphocyte-mediated immunity, whereas recall antigens require the presence of antigen-presenting cells which stimulate lymphocytes through the T-cell receptor and reflect the antigen-specific immunity. Interestingly, our results showed that lymphocyte proliferation and cytokine production were even weaker upon stimulation with recall antigens than mitogens. The combination of proportional decreases of memory CD45RO+ T cells and professional antigen-presenting cells (monocytes) expressing HLA-DR could be one reason for this phenomenon. Moreover, preliminary experiments in our laboratory where antigen-presenting cells of healthy volunteers were co-cultured with CD3 T-cells isolated from patients after SIRT, showed that T-cells had decreased capacity to secrete IFN-γ, indicating that T-cell function mediated by the T-cell receptor was also impaired.
Apart from the alterations of lymphocyte function, SIRT also affected the absolute numbers of peripheral blood mononuclear cells. Patients having received SIRT displayed significant lymphopenia together with an increase in granulocyte and monocyte counts. This observation is in line with the results of a recent study by Carr et al. reporting asymptomatic lymphopenia following SIRT treatment [21], which led the researchers to further investigate alterations in the lymphocyte subsets of 25 patients up to 24 months post treatment. Notably, we found exactly the same trend for all lymphocyte subpopulations at 24 h and 1 month post therapy, i.e., a prompt and prolonged depletion of the absolute counts of CD3+, CD4+, CD8+ T cells and CD19 + B cells. Similar to our results, NK cells tended to decrease at least until month 1 following treatment, but recovered eventually. The decrease in these lymphocyte subsets seems to be a direct effect of the therapy, but we cannot exclude that factors such as hepatic inflammation and lymphocyte recruitment to the liver contribute to this result. Furthermore, common findings in both studies were elevated absolute numbers of granulocytes post treatment. In a study by Shi et al., it has been proposed that the liver is involved in the apoptosis of circulating neutrophils [26]. Therefore, it remains unclear whether the increase in granulocytes occurred as a compensative reaction for the depletion of lymphocytes or whether the therapy itself reduced the capacity of the liver to regulate the number of neutrophils in the systemic circulation. Another possible explanation for the increase of granulocytes in the peripheral blood post treatment could be the production of certain chemokines which inhibit the neutrophil transendothelial migration. It has been reported by Fernandez et al. that radioembolization lead to a significant increase of IL-8 [27], a chemokine that in a soluble form can inhibit the adhesion of neutrophils to endothelial cells in vitro or to the endothelium in vivo [28]. The previous study by Carr et al. [21] did not investigate numbers of monocytes, HLA-DR expression, CD45RA+ naive and CD45RO+ memory T cells.
In our study, the effects of SIRT on peripheral CD45RA+ naïve and CD45RO+ memory T cells were diverse, with the percentage of CD45RO+ memory T cells decreasing after therapy while the percentage of their CD45RA+ naïve counterparts increased. An explanation for this phenomenon could be based on the distinct recirculation pattern of naïve versus memory T cells and the strictly local type of SIRT irradiation. It is known that naïve and memory T cells display different recirculation pathways with naïve T lymphocytes trafficking predominantly to secondary lymphoid organs, while primed T cells prefer the nonlymphoid tissues [29, 30]. Moreover, several studies have reported that the majority of intrahepatic T cells express the CD45RO+/CD45RA- isoform, indicating that they are memory T cells [13, 16, 31, 32]. Although the interaction between resident and recirculating lymphocytes after irradiation is not yet clear, it is conceivable that the high radiation doses delivered intrahepatically by SIRT damage memory T cells to a greater extent than naïve T cells and may consequently reduce their percentage in the periphery. This in turn could be the cause of a compensative increase of the percentage of peripheral naïve T cells. Another factor contributing to the decrease of memory T cells in the periphery could be the production of certain chemokines which cause a preferential migration of this T cell subpopulation in the liver. For example, CCR1, CCR2 and CCR5 are all chemokine receptors expressed by memory T-cells and have been all found to be elevated in hepatic inflammation [33]. Such factors remain to be elucidated and future studies on chemokines could shed light to their crucial role in hepatic inflammation and lymphocyte recruitment.
Furthermore, our study showed a decrease in the percentage of peripheral monocytes and B cells expressing HLA-DR. The downregulation of HLA-DR on dendritic cells and on B cells of mice after gamma irradiation has been reported [34, 35], but as far as we know, this is the first time to report similar results for the effect of beta rays. On the contrary, the initial depletion of the percentage of HLA-DR positive T cells (CD3+, CD4+ and CD8+ T cells) was followed by a significant increase 1 month after therapy, although the respective absolute numbers of HLA-DR+ T cells decreased or remained unchanged (data not shown). This increase could reflect an enhancement of HLA-DR expression caused by irradiation or a greater depletion of HLA-DR negative versus HLA-DR-positive T cells. In accordance with our study, increased percentages of CD4+ HLA-DR+ and CD8+ HLA-DR+ T lymphocytes were demonstrated 2 weeks after the completion of thoracic irradiation in patients with lung cancer [36].
The radioactivity of 90Y declines with time and is at its peak shortly after the injection into the capillary bed. Beta particles are energetic electrons which induce ionization of atoms and can act directly on the cellular components or on water molecules leading to the production of radicals. Radicals in turn affect cells by causing DNA breaks. Whereas double-strand DNA breaks usually lead to cell death, most single-strand breaks can be repaired at the expense of cell proliferation and function. We propose that the very early drop of lymphocyte counts (at day 1 and 2) as well as the prompt deterioration of their function is a result of radiation-induced DNA damages on the circulating lymphocytes.
In spite of the profound impairment of lymphocyte function, we did not observe increased risk for viral infections. This finding is in line with other studies that reported significant lymphopenia after beta irradiation without clinical sequela [5, 6, 21] and underlines the importance of further studies applying systematic surveillance protocols in a larger cohort of patients to elicit further clinically relevant information.
Acknowledgements
This article is a partial fulfillment of requirements for the doctor’s degree at the Medical Faculty, University of Duisburg-Essen, for Mrs. A. Domouchtsidou. We thank M. Huben, B. Nyadu and M. Praast for their excellent technical assistance.
Abbreviations
- CAN
Candida albicans
- Cpm
Counts per minute
- DOTATOC
DOTA(0)-Phe(1)-Tyr(3)-octreotide
- ECOG
Eastern Cooperative Oncology Group
- HCC
Hepatocellular carcinoma
- HSV-1
Herpes simplex virus type 1
- LTT
Lymphocyte transformation test
- OKT3
Anti-CD3 monoclonal antibody
- PPD
Purified protein derivate
- PWM
Pokeweed mitogen
- SIRT
Selective internal radiotherapy
- TET
Tetanus toxoid
- 90Y
Yttrium-90
Author contributions
AD, VB, SPM, AB and ML contributed to the conception and design of the study. AD, PAH and ML wrote the report. AD, JB, JE and SB recruited the patients and took blood samples. AD performed the cellular in vitro assays. All authors read and approved the final manuscript.
Funding
No relevant funding.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
The study was institutional review board approved by the ethics committee of the Medical Faculty, University Hospital Essen (approval number 09-3991), and carried out in accordance with the 1964 Helsinki Declaration.
Informed consent
All patients provided written informed consent prior to their inclusion in the study.
References
- 1.Lewandowski RJ, Salem R. Yttrium-90 radioembolization of hepatocellular carcinoma and metastatic disease to the liver. Semin Interv Radiol. 2006;23:64–72. doi: 10.1055/s-2006-939842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang LM, Jani AR, Hill EJ, Sharma RA. Anatomical basis and histopathological changes resulting from selective internal radiotherapy for liver metastases. J Clin Pathol. 2013;66:205–211. doi: 10.1136/jclinpath-2012-201231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969–985. [PMC free article] [PubMed] [Google Scholar]
- 4.Barsegian V, Müller SP, Horn PA, Bockisch A, Lindemann M. Lymphocyte function following radioiodine therapy in patients with thyroid carcinoma. Nuklearmedizin. 2011;50:195–203. doi: 10.3413/nukmed-04241108. [DOI] [PubMed] [Google Scholar]
- 5.Barsegian V, Hueben C, Mueller SP, Poeppel TD, Horn PA, Bockisch A, Lindemann M. Impairment of lymphocyte function following yttrium-90 DOTATOC therapy. Cancer Immunol Immunother. 2015;64:755–764. doi: 10.1007/s00262-015-1687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sierra ML, Agazzi A, Bodei L, et al. Lymphocytic toxicity in patients after peptide-receptor radionuclide therapy (PRRT) with 177 lu-DOTATATE and 90Y-DOTATOC. Cancer Biother Radiopharm. 2009;24:659–665. doi: 10.1089/cbr.2009.0641. [DOI] [PubMed] [Google Scholar]
- 7.Greenway CV, Stark RD. Hepatic vascular bed. Physiol Rev. 1971;51:23–65. doi: 10.1152/physrev.1971.51.1.23. [DOI] [PubMed] [Google Scholar]
- 8.Schenk WG, Jr, Mc DJ, Mc DK, Drapanas T. Direct measurement of hepatic blood flow in surgical patients: with related observations on hepatic flow dynamics in experimental animals. Ann Surg. 1962;156:463–471. doi: 10.1097/00000658-196209000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yzet T, Bouzerar R, Baledent O, Renard C, Lumbala DM, Nguyen-Khac E, Regimbeau J-M, Deramond H, Meyer M-E. Dynamic measurements of total hepatic blood flow with Phase Contrast MRI. Eur J Radiol. 2010;73:119–124. doi: 10.1016/j.ejrad.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 10.Parker GA, Picut CA. Immune functioning in non lymphoid organs: the liver. Toxicol Pathol. 2012;40:237–247. doi: 10.1177/0192623311428475. [DOI] [PubMed] [Google Scholar]
- 11.Nemeth E, Baird AW, O’Farrelly C. Microanatomy of the liver immune system. Semin Immunopathol. 2009;31:333–343. doi: 10.1007/s00281-009-0173-4. [DOI] [PubMed] [Google Scholar]
- 12.Norris S, Collins C, Doherty DG, Smith F, McEntee G, Traynor O, Nolan N, Hegarty J, O’Farrelly C. Resident human hepatitis lymphocytes are phenotypically different from circulating lymphocytes. J Hepatol. 1998;28:84–90. doi: 10.1016/S0168-8278(98)80206-7. [DOI] [PubMed] [Google Scholar]
- 13.Pruvot FRNF, Janin A, Labalette M, Masy E, Lecomte-HouckeM, et al. Characterization, quantification, and localization of passenger T lymphocytes and NK cells in human liver before transplantation. Transpl Int. 1995;8:273–279. doi: 10.1111/j.1432-2277.1995.tb01521.x. [DOI] [PubMed] [Google Scholar]
- 14.Doherty DG, O’Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunol Rev. 2000;174:5–20. doi: 10.1034/j.1600-0528.2002.017416.x. [DOI] [PubMed] [Google Scholar]
- 15.Xu XD, Ueta H, Zhou S, Shi C, Koga D, Ushiki T, Matsuno K. Trafficking of recirculating lymphocytes in the rat liver: rapid transmigration into the portal area and then to the hepatic lymph. Liver Int. 2008;28:319–330. doi: 10.1111/j.1478-3231.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- 16.Tu Z, Bozorgzadeh A, Crispe IN, Orloff MS. The activation state of human intrahepatic lymphocytes. Clin Exp Immunol. 2007;149:186–93. doi: 10.1111/j.1365-2249.2007.03415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohtani O, Ohtani Y. Lymph circulation in the liver. Anat Rec (Hoboken) 2008;291:643–52. doi: 10.1002/ar.20681. [DOI] [PubMed] [Google Scholar]
- 18.Mouta Carreira C, Nasser SM, di Tomaso E, Padera TP, Boucher Y, Tomarev SI, Jain RK. LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res. 2001;61:8079–8084. [PubMed] [Google Scholar]
- 19.Carr BI. Hepatic arterial 90Yttrium glass microspheres (Therasphere) for unresectable hepatocellular carcinoma: Interim safety and survival data on 65 patients. Liver Transpl. 2004;10:S107-S110. doi: 10.1002/lt.20036. [DOI] [PubMed] [Google Scholar]
- 20.Salem R, Lewandowski RJ, Atassi B, Gordon SC, Gates VL, Barakat O, Sergie Z, Wong CY, Thurston KG. Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol. 2005;16:1627–1639. doi: 10.1097/01.RVI.0000184594.01661.81. [DOI] [PubMed] [Google Scholar]
- 21.Carr BI, Metes DM. Peripheral blood lymphocyte depletion after hepatic arterial 90Yttrium microsphere therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;82:1179–1184. doi: 10.1016/j.ijrobp.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 22.Czerkinsky C, Andersson G, Ekre HP, Nilsson LA, Klareskog L, Ouchterlony O. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J Immunol Methods. 1988;110:29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- 23.Lindemann M, Witzke O, Winterhagen T. T-cell function after interleukin-2 therapy in HIV-infected patients is correlated with serum cortisol concentrations. AIDS. 2004;18:2001–2007. doi: 10.1097/00002030-200410210-00004. [DOI] [PubMed] [Google Scholar]
- 24.Robinson MW, Harmon C, O’Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol. 2016;13:267–276. doi: 10.1038/cmi.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 26.Shi J, Gilbert GE, Kokubo Y, Ohashi T. Role of the liver in regulating numbers of circulating neutrophils. Blood. 2001;98:1226–1230. doi: 10.1182/blood.V98.4.1226. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Ros N, Inarrairaegui M, Paramo JA, et al. Radioembolization of hepatocellular carcinoma activates liver regeneration, induces inflammation and endothelial stress and activates coagulation. Liver Int. 2015;35:1590–1596. doi: 10.1111/liv.12592. [DOI] [PubMed] [Google Scholar]
- 28.Marshall LJ, Ramdin LS, Brooks T, PC DP, Shute JK. Plasminogen activator inhibitor-1 supports IL-8-mediated neutrophil transendothelial migration by inhibition of the constitutive shedding of endothelial IL-8/heparan sulfate/syndecan-1 complexes. J Immunol. 2003;171:2057–2065. doi: 10.4049/jimmunol.171.4.2057. [DOI] [PubMed] [Google Scholar]
- 29.Mackay CR, Marston WL, Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990;171:801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu H, Ward EJ, Marelli-Berg FM. Mechanisms of T cell organotropism. Cell Mol Life Sci. 2016;73:3009–3033. doi: 10.1007/s00018-016-2211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lalor PF, Shields P, Grant AJ, Adams DH. Recruitment of lymphocytes to the human liver. Immunol Cell Biol. 2002;80:52–64. doi: 10.1046/j.1440-1711.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 32.Grayson JM, Harrington LE, Lanier JG, Wherry EJ, Ahmed R. Differential sensitivity of naive and memory CD8+ T cells to apoptosis in vivo. J Immunol. 2002;169:3760–3770. doi: 10.4049/jimmunol.169.7.3760. [DOI] [PubMed] [Google Scholar]
- 33.Oo YH, Adams DH. The role of chemokines in the recruitment of lymphocytes to the liver. J Autoimmun. 2010;34:45–54. doi: 10.1016/j.jaut.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Cao MD, Chen ZD, Xing Y. Gamma irradiation of human dendritic cells influences proliferation and cytokine profile of T cells in autologous mixed lymphocyte reaction. Cell Biol Int. 2004;28:223–228. doi: 10.1016/j.cellbi.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Ashwell MKJaRHS JD. Effect of gamma radiation on resting B lymphocytes. II. Functional characterization of the antigen-presentation defect. J Immunol. 1988;141:2536–2544;. [PubMed] [Google Scholar]
- 36.Nakayama Y, Makino S, Fukuda Y, Min KY, Ikemoto T, Shimizu A, Ohsawa N. Varied effects of thoracic irradiation on peripheral lymphocyte subsets in lung cancer patients. Intern Med. 1995;34:959–965. doi: 10.2169/internalmedicine.34.959. [DOI] [PubMed] [Google Scholar]