Abstract
The perspective of combining cancer vaccines with immunomodulatory drugs is currently regarded as a highly promising approach for boosting tumor-specific T cell immunity and eradicating residual malignant cells. The efficacy of dendritic cell (DC) vaccination in combination with lenalidomide, an anticancer drug effective in several hematologic malignancies, was investigated in a follicular lymphoma (FL) model. First, we evaluated the in vitro activity of lenalidomide in modulating the immune responses of lymphocytes co-cultured with a new DC subset differentiated with IFN-α (IFN-DC) and loaded with apoptotic lymphoma cells. We next evaluated the efficacy of lenalidomide and IFN-DC-based vaccination, either alone or in combination, in hu-PBL-NOD/SCID mice bearing established human lymphoma. We found that lenalidomide reduced Treg frequency and IL-10 production in vitro, improved the formation of immune synapses of CD8 + lymphocytes with lymphoma cells and enhanced anti-lymphoma cytotoxicity. Treatment of lymphoma-bearing mice with either IFN-DC vaccination or lenalidomide led to a significant decrease in tumor growth and lymphoma cell spread. Lenalidomide treatment was shown to substantially inhibit tumor-induced neo-angiogenesis rather than to exert a direct cytotoxic effect on lymphoma cells. Notably, the combined treatment with the vaccine plus lenalidomide was more effective than either single treatment, resulting in the significant regression of established tumors and delayed tumor regrowth upon treatment discontinuation. In conclusion, our data demonstrate that IFN-DC-based vaccination plus lenalidomide exert an additive therapeutic effect in xenochimeric mice bearing established lymphoma. These results may pave the way to evaluate this combination in the clinical ground.
Keywords: Cancer vaccines, Immunotherapy, Lymphomas, Dendritic cells, Combination therapy, Lenalidomide
Introduction
Follicular lymphoma (FL) is the most common indolent non-Hodgkin lymphoma in Europe and in the United States [1, 2]. Over the past several decades, substantial advances have been made in the progression-free survival (PFS) and overall survival (OS) of patients with FL. However, despite the improved effectiveness of chemo-immunotherapy regimens, FL is still considered an incurable disease [3, 4]. Recently, immunotherapeutic approaches have revolutionized cancer treatments, and the chance to combine cancer vaccines with immunomodulatory drugs appears as a highly promising approach [5, 6].
Dendritic cells (DC) have been widely used over the last decade in clinical trials in patients with different types of hematologic malignancies [7], including FL, with some encouraging results [8, 9]. We described an effective modality of differentiation of partially mature DC (IFN-DC) from human monocytes in the presence of GM-CSF and IFN-α [10]. IFN-DC are characterized by the capacity to release a unique array of cytokines and chemokines known to favor Th1 type response and to powerfully stimulate cellular CD8 + T cell immune responses [11–13]. We previously showed, in vitro and in immunodeficient NOD/SCID mice reconstituted with human PBL, that antigen-pulsed IFN-DC are endowed with an enhanced cross-priming activity and immunostimulatory activities against viral and tumor antigens as compared to conventional myeloid DC obtained with IL-4 and GM-CSF [12–16]. Recently, we evaluated in preclinical models a new DC-based vaccination protocol, exploiting the specific features of IFN-DC loaded with FL cells undergoing immunogenic cell death [17]. In particular, we demonstrated that IFN-DC loaded with apoptotic lymphoma cells from FL patients cultured for 2 weeks with autologous lymphocytes led to massive IFN-γ production, Th1 response skewing and enhanced cytotoxic effector function toward autologous lymphoma cells [17]. Although the use of IFN-DC in pre-clinical studies has led to encouraging results, it should be noted that long-term clinical benefit rarely occurs in clinical protocols involving cancer vaccination alone. This is possibly due to the induction of T cell regulatory response and immune tolerance. Therefore, we assumed that the combination with novel immunotherapeutics or immune modulators might improve the effectiveness of IFN-DC-based therapeutic vaccination, antagonizing negative immune regulation and enhancing the strength and persistence of antitumor effector cells.
Lenalidomide represents an attractive drug, due to its original mechanism of action. Its activity against several hematologic malignancies is mediated by pleiotropic effects, including the activation of anti-tumor immunity and the modification of tumor microenvironment [18]. In refractory/relapsed FL patients, lenalidomide has proven effective in determining an overall response rate of 29% [19]. Higher response rates can be achieved when it is administered in combination with rituximab [20]. Here, we have investigated both in in vitro and in vivo models whether an improved anti-tumor activity can be achieved by combining an IFN-DC-based vaccine with lenalidomide, which has been shown to dampen immunoregulatory responses and to enhance immune functions in many tumor models [21–23].
Studies on the immunotherapy of FL have been hampered so far by the lack of validated syngeneic mouse models of FL and the impossibility to engraft immunodeficient mice with primary human FL cells [24–26]. Thus, we evaluated the antitumor effect of lenalidomide administration combined with IFN-DC-based vaccine in humanized NOD/SCID mice bearing Karpas-422 human FL cells, a FL model utilized with success in our previous studies [27].
Materials and methods
Cell preparation and culture
Peripheral blood mononuclear cells (PBMC) were obtained from buffy coats of healthy blood donors. The HLA-A2 haplotype of the subjects was assessed by FACS analysis, using anti A2-specific mAbs (BD Biosciences, USA). PBMC were purified from blood by Ficoll density gradient centrifugation (Biochrom, Germany). IFN-DC were obtained from purified CD14 + monocytes according to our procedure previously described elsewhere [17] and plated at the concentration of 2 × 106 cells/mL in CellGro DC medium (Cell Genix, USA), supplemented with 500 U/mL GM-CSF (Genzyme Corporation, UK) and 10,000 U/mL IFN-α2b (Intron A; Schering Plough, USA) for 3 days. PBL were obtained from PBMC following CD14 + monocyte depletion [17]. Karpas-422 cells were cultured in RPMI-1640 medium (Gibco, USA) supplemented with 20% FBS, 2 mM l-glutamine, 100 U/mL penicillin and 100 µg/mL streptomycin.
Tumor cell death induction and PBL/DC co-cultures
Apoptotic cell bodies were generated by exposing Karpas-422 cell suspensions to heat shock, γ-irradiation, and UVC rays, as already described [17]. IFN-DC were pulsed with apoptotic tumor cells at a 1:2 ratio for 16 h at 37 °C and then cultured with autologous PBL at a cell ratio of 1:4. The medium was supplemented with human IL-2 (50 U/mL, Roche, Germany), starting from day 3 of co-culture, once every 3 days. At day 7 of co-culture, PBL were restimulated with frozen-stocks of apoptotic cell-loaded IFN-DC and 7 days later the effector cells were analyzed. Lenalidomide was added to the culture medium from the step of antigen presentation and every 3 days for 14 days at the concentration of 2 µM.
Flow cytometry
Cells were stained with fluorochrome-conjugated mAbs specific for DC, T and NK cells: anti-CD11c, anti-CD80, anti-CD86, anti-CD83, anti-CD40, HLA-ABC, HLA-DR, anti-CD54, anti-CD70, anti-CD3, anti-CD4, anti-CD25, anti-CD8, anti-CD56 (BD Biosciences, USA) and anti-FOXP3 (eBiosciences, USA). PBL functional tests were performed on day 14 of PBL/DC co-cultures, as previously described [28].
Apoptosis and proliferation assays
Karpas-422 cells were incubated with Annexin V/7-Amino-Actinomycin D (7-AAD) and analyzed by flow cytometry using the Kaluza 1.3 software. Cell proliferation was evaluated by CFSE (2.5 µM) dilution assay.
Cytokine assay
Cytokine quantification was performed by commercial ELISA kits for: IFN-γ (15.6 pg/mL), IL-10 (3.9 pg/mL) (Biolegend Inc., USA), IL-15 (15.6 pg/mL), IL-12 (7.8 pg/mL) and TNF-α (15.6 pg/mL) (Boster Biological Technology, USA), according to manufacturer instruction.
Cytotoxicity assay
Cytotoxicity was tested against Karpas-422 or K562 by Calcein-AM release test. Target cells were labelled with 15 µM calcein-acetoxymethyl (Calcein-AM, USA) for 30 min at 37 °C. Tests were performed V-bottom 96-well plates for 4 h at 37° as previously described [17].
Conjugation assay and confocal laser scanning microscopy (CLSM) analysis
Cell conjugates were obtained culturing freshly purified or stimulated PBL with Karpas-422 target cells (E:T 2:1) for 20 min, as previously reported [16]. Cells were stained for CD8 and CD56 detection (PE-conjugated anti-CD8 and anti-CD56 mAbs, BD Biosciences, USA), then fixed and permeabilized, followed by actin staining with Alexa Fluor®-488-conjugated phalloidin (Thermo-Fisher Scientific, USA). CLSM observations were performed with a Leica TCS SP2 AOBS apparatus, using a 63 ×/1.40 NA oil objective and excitation spectral laser lines at 405, 488 and 594 nm.
Staining of tumor sections
Hematoxylin and eosin staining (H&E) and immunofluorescent labeling for CD31, CD8, Granzyme B, CD4 and FOXP3 detection were performed on Formalin Fixed Paraffin Embedded (FFPE) tissue sections. Slides (5 µm thick) were deparaffinized, hydrated through graded alcohols and subjected to a heat-induced Epitope Retrieval step by citrate pH 6 (Novus) for 3 × 3 min in a microwave oven. Sections were washed with PBS-T (0.01% Tween 20) and blocked in PBS-BSA 3% for 60 min at 37 °C. Primary Abs (rabbit anti-Pecam-1 or CD31, LifeSpan BioSciences, USA; mouse anti-CD8 clone C8/144B, Dako; rabbit anti-Granzyme-B-AlexaFluor®-647, Novus Biologicals; mouse anti-CD4 clone 4B12, Dako; rabbit anti-FOXP3 clone 1054C, R&D Systems) were incubated for 30 min at 37 °C, followed by secondary Abs plus DAPI (Thermo Fisher Scientific, USA). Image acquisition was performed with a Leica TCS SP2 AOBS apparatus and the fluorescence intensity of CD31, was determined by the confocal software (Leica 2.6 rel 1537), using the parameter Sum of intensity [SUM (I)]. Equivalent sized regions for each experimental condition were analyzed (the mean value of CD31 immunofluorescence staining in tissue sections from control untreated mice was normalized to 1.0). Microvessel density was also evaluated by H&E staining.
Therapeutic vaccination of tumor-bearing hu-PBL-NOD/SCID mice
NOD/SCID mice were injected subcutaneously (s.c.) in the shoulder with 5 × 106 Karpas-422 cells re-suspended in 0.2 mL RPMI 1640 medium. Lymphoma cells required about 14 days to become palpable masses. NOD/SCID mice were reconstituted intraperitoneally (i.p.) with 20-30 × 106 HLA-A2 + human PBL, re-suspended in 0.5 mL RPMI medium [25] before tumor masses became evident (day 10). The resulting hu-PBL-NOD/SCID mice were vaccinated (i.p.) 4 days later with 2 × 106 IFN-DC loaded with apoptotic Karpas-422 cells, received lenalidomide or were left untreated (CTR). Mice received boost immunizations at days 21 and 28. Tumor growth was followed for 70 days. The two major diameters of each tumor nodule were measured by calipers and the mean tumor diameter was calculated for each tumor.
Lenalidomide treatment of hu-PBL-NOD/SCID mice
Lenalidomide was dissolved in dimethyl sulfoxide (DMSO) at a stock concentration of 10.36 mg/mL. Vaccinated mice received lenalidomide i.p. (10 mg/kg) starting on the day of immunization, and every 3 days or equal volume vehicle control for 3 weeks. Treatment control groups consisted in tumor-bearing mice receiving only PBL and either unloaded IFN-DC or lenalidomide.
Statistical analysis
All results are expressed as mean ± SD, except where indicated, whereas the statistical analyses were performed by Mann–Whitney or Wilcoxon test and Spearman’s rank correlation test.
Results
Lenalidomide does not affect IFN-DC phenotype and activity when added during the DC differentiation step
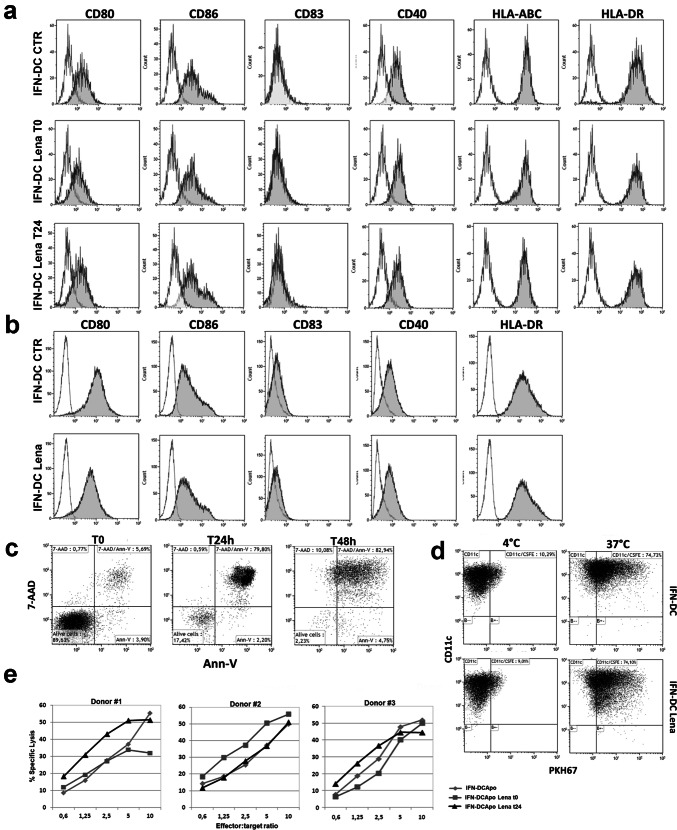
We first evaluated the phenotype and quality of IFN-DC generated in the presence of lenalidomide. IFN-DC were obtained from peripheral monocytes cultured for 3 days in the presence of GM-CSF and IFN-α, with or without lenalidomide (10 μM/mL) added from the beginning or after 24 h of culture. Lenalidomide did not influence IFN-DC viability and yield. However, FACS analysis did not reveal any significant difference in the expression of DC-associated surface markers in the different culture conditions (Fig. 1a). Analogous results were obtained with IFN-DC from FL patients (Fig. 1b). No significant differences in IL-12, IL-15 and TNF-α production, nor in IL-10 release by lenalidomide-treated IFN-DC could be detected by ELISA test (data not shown). Then, we loaded IFN-DC from HLA-A2 + healthy donors with apoptotic Karpas-422 lymphoma cells induced to undergo immunogenic apoptotic cell death (Fig. 1c, d) as previously described [17]. Tumor cell-loaded IFN-DC were used to stimulate autologous PBL in vitro to induce antigen-specific CTLs. No clear improvements of cytotoxic activity could be detected in cultures stimulated with IFN-DC differentiated in the presence of lenalidomide, as depicted in Fig. 1e, which shows results from cytotoxic assays toward Karpas-422 cells, performed with three different donors. Overall, our results indicated that lenalidomide pre-treatment of IFN-DC did not provide significant improvement of PBL immune response against lymphoma cells with respect to IFN-DC differentiated by the standard protocol.
Fig. 1.
Immunophenotypic pattern and activity of IFN-DC differentiated in the presence of lenalidomide. a Phenotypic characterization of IFN-DC differentiated in the presence or not of lenalidomide added at the beginning of the culture (t0) or for the last 24 h of culture (t24). b Representative dot histogram FACS profiles of IFN-DC from one FL patient. c Dot plot representation of typical apoptotic and necrotic Karpas-422 cell frequency detected by annexin V-FITC and 7-AAD staining at 24 h and 48 h after apoptosis induction. d Phagocytosis of apoptotic Karpas-422 cells labelled with PHK67 (green fluorescence) by purified IFN-DC (stained with anti-CD11c) after 90 min of co-culture. One representative experiment is shown. e Cytotoxic activity of PBL isolated from healthy blood donors and cultured for 14 days with IFN-DC differentiated in the presence or not of lenalidomide and loaded with apoptotic tumor cells. Cytotoxic activity was tested by Calcein-AM assay against Karpas-422 target cell line. Three different representative experiments are shown
Lenalidomide modulates immune responses induced by IFN-DC: effects on functional activity
A second set of experiments was performed by adding lenalidomide to the culture medium from the step of antigen presentation and throughout the whole IFN-DC/PBL co-culture period. Lenalidomide was used at the concentration of 2.0 µM/mL, since higher concentrations resulted in decreased lymphocyte viability and yield over a 2 weeks co-culture. Karpas-422 cells (HLA-A2 +) were induced to undergo immunogenic apoptosis, loaded onto IFN-DC from a small group of HLA-matched healthy donors and used to stimulate autologous PBL.
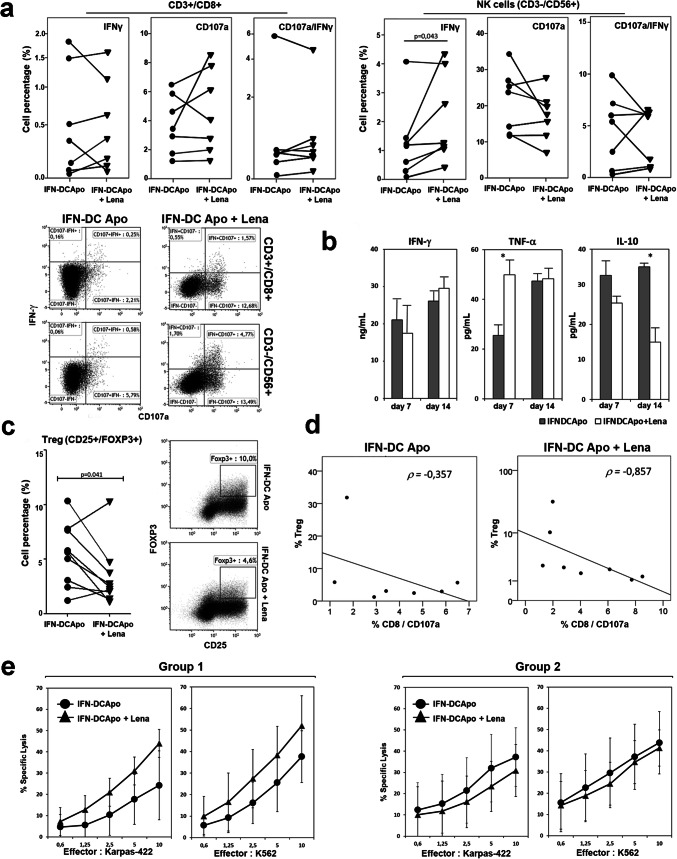
We measured CD8 T cell response to Karpas-422 cells in terms of IFN-γ production and ectopic expression of CD107a, as a marker of cytolytic activity by FACS analysis, showing that lenalidomide can determine an increase in the frequency of IFN-γ −/CD107a + and IFN-γ +/CD107a + effector CD8 + T cells. However, as some donors appeared not to benefit from lenalidomide addition to their PBL cultures, the overall difference between the two culture conditions was not statistically significant. On the contrary, FACS analysis of NK cell response showed a statistically significant lenalidomide-induced increase of IFN-γ production in response to Karpas-422 cells (p = 0.043), while the lytic activity, as measured by the detection of CD107a ectopic expression, did not differ in the two culture conditions (Fig. 2a). Cytokine detection in culture supernatants at day 7 and 14 showed that IFN-γ production was not modulated by lenalidomide treatment (Fig. 2b). However, lenalidomide was shown to significantly improve early TNF-α release (day 7) and to reduce IL-10 accumulation in culture supernatants (Fig. 2b), which is a key immune-suppressive cytokine produced by helper T cells and regulatory T cells (Tregs). Notably, lower levels of this cytokine were detected in the presence of lenalidomide at both days 7 and 14 in supernatants from PBL co-cultivated with tumor cell-loaded IFN-DC with respect to control cultures.
Fig. 2.
Modulation of antitumor immune responses of PBL from HLA-A2 + healthy donors, stimulated by IFN-DC loaded with apoptotic Karpas-422 cells (HLA-A2 + FL cells) in the presence of lenalidomide. a Frequencies of Karpas-422-responsive CD3 +/CD8 + lymphocytes and NK cells (CD3 −/CD56 +), as determined by FACS analysis for IFN-γ intracellular staining and degranulation (CD107a membrane mobilization) in PBL cultured for 14 days with autologous IFN-DC loaded with apoptotic Karpas-422 cells in the presence or not of 2 µM lenalidomide. Representative dot plot graphs from one representative experiment are shown. b Modulation of IFN-γ, TNF-α and IL-10 release as evaluated in culture supernatants by ELISA test at day 7 and 14. The data represent the mean value ± SD of 4 independent experiments. Statistically significant differences were calculated using Wilcoxon test (*p ≤ 0.05). c Frequency of CD25 +/FOXP3 + Tregs in CD4 + lymphocyte, as assessed by flow cytometric analysis. The dot-plot analysis of CD25 +/FOXP3 + expression in CD4 + cells from one representative experiment is shown d Spearman’s rho correlation calculation between Treg frequency and the percentage of degranulating CD8 + cells in response to lymphoma cells, measured as CD107a mobilization to the cell membrane by FACS analysis. An inverse correlation (ρ = − 0.857) is shown in lenalidomide-exposed cultures (ρ = − 0.857). e Cytotoxic activity against Karpas-422 and NK-sensitive K562 target cells as assessed by Calcein-AM assay in PBL cultures, showing Treg frequency reduction (group 1) or irrelevant changes (group 2) in response to lenalidomide treatment (see “Results”)
Effects on Tregs frequency
We have recently demonstrated that IFN-DC induce Treg expansion with a lower efficiency as compared to conventional DC obtained with GM-CSF and IL-4, thus potentially reducing the negative impact of Treg responses induced by vaccination [17]. Likewise, lenalidomide has been reported to inhibit Treg expansion and suppressive function [28]. Thus, we assessed whether lenalidomide can contribute to further reduce Treg frequency in cultures stimulated with tumor cell-loaded IFN-DC. The results of Tregs analysis are summarized in the column chart of Fig. 2c, which shows Treg percentage after two cycles of PBL stimulation with tumor cell-loaded autologous IFN-DC in the presence or absence of lenalidomide, respectively. Overall, Treg frequency, determined as the percentage of CD4 + lymphocytes expressing CD25 and FOXP3, was significantly reduced in the presence of lenalidomide (p = 0.041 Wilcoxon rank test). Reduced Treg frequency and lower IL-10 levels in culture supernatants (Fig. 2b, c) suggest that lenalidomide may interfere with immunosuppressive mechanisms, thus potentially improving the activity of IFN-DC. Consistently with this assumption, in lenalidomide-exposed cultures, we found an inverse correlation, as calculated by Spearman’s rho rank correlation coefficient (ρ = − 0.857), between Treg frequency and the rate of CD8 + cell degranulation in response to lymphoma cells measured by FACS analysis as CD107a mobilization to the cell membrane (Fig. 2d). In contrast, such correlation was not found in the basal culture condition without lenalidomide (ρ = − 0.357).
Cytotoxic response to lymphoma cells
We then evaluated whether lenalidomide was able to improve lymphocyte cytotoxic activity toward target lymphoma cells. As some healthy blood donors appeared not to benefit from lenalidomide addition to their PBL co-cultures with IFN-DC, cytotoxic activity toward lymphoma cells was evaluated on the basis of Treg reduction in response to lenalidomide. Accordingly, PBL cultures showing at least twofold reduction of Treg frequency (6.7% ± 1.5–2.7% ± 0.9) and defined as group 1 were assayed for cytotoxicity distinctly from those showing irrelevant changes in Treg percentage (3.8% ± 2.0–4.6% ± 2.7) and defined as group 2 (Fig. 2e).
T cell cytotoxicity was determined to evaluate specific anti-tumor activity against Karpas-422 cells and against K562 cells to assess natural killer activity (Fig. 2e). Of note, PBL from lenalidomide-treated cultures showing reduced Treg frequency (group 1) exhibited improved cytotoxic activity toward both Karpas-422 and K562 target cells. On the contrary, no significant improvement of cell cytotoxicity could be detected in the cultures showing irrelevant Treg modulation (Fig. 2e, left panels).
Effects on immune synapse
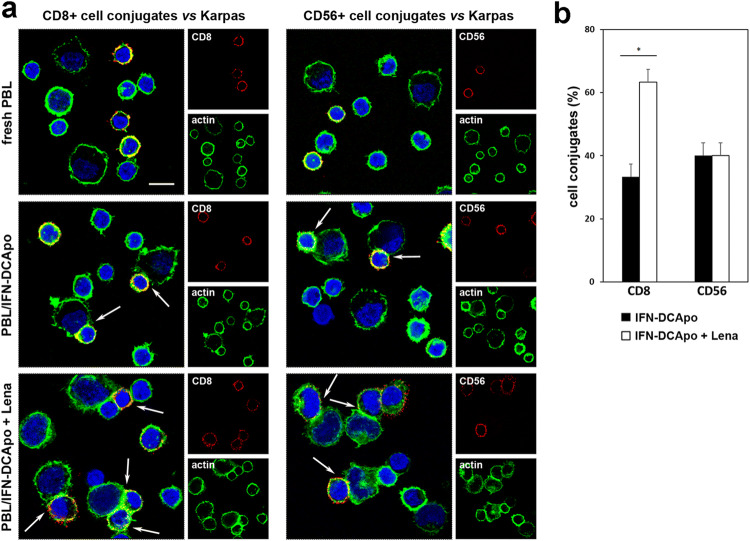
To assess whether lenalidomide was able to improve immune effector cell capability to form conjugates with lymphoma target cells, confocal images of PBL allowed to conjugate with Karpas-422 target cells and stained for CD8 or CD56 were acquired. As shown in Fig. 3a, both CD8 + and CD56 + cells from IFN-DC-stimulated PBL cultures were able to conjugate with Karpas-422 cells, at variance with freshly purified PBL from the same donor. Of note, the median percentage of target cells conjugated to PBL was increased from 33.3 ± 1.9 to 50.0 ± 2.0% (median ± SE) upon lenalidomide addition to PBL co-cultured with lymphoma cell-loaded IFN-DC. In particular, the frequency of CD8 + cell conjugates with lymphoma cells appeared increased in lenalidomide-treated PBL, which also displayed binding of multiple CD8 + effector cells to single target cells (Fig. 3a). Actually, visual counts of the overall cell conjugates in distinct microscopic fields showed a rise in CD8 + cell conjugates from 33.3% ± 5.6 to 63.3% ± 4.0 (median ± SE) when lenalidomide was added to PBL co-cultured with lymphoma cell-loaded IFN-DC (Fig. 3b), while no significant modulation of CD56 + NK cell capability to conjugate with target lymphoma cells was detected (Fig. 3b).
Fig. 3.
Effects of lenalidomide on conjugate formation. a CLSM examinations of lymphoma co-cultures (central optical sections) with freshly purified PBL or PBL cultured for 14 days with tumor cell-loaded IFN-DC in the absence or presence of lenalidomide. Cells were stained for CD8 or CD56 (red) and for actin detection (green). DAPI was used to stain nuclei (blue). Arrows indicate CD8 + or CD56 + cell conjugates. Scale bars 10 µm. Images are representative of three independent experiments. b Quantitative analysis of cell conjugates expressed as the median percentage of CD8 + or CD56 + cell conjugates from PBL/lymphoma co-cultures treated or not with lenalidomide (median ± SE of three independent experiments). Statistically significant differences were estimated by the Wilcoxon test (*p < 0.05)
Lenalidomide does not induce apoptosis and cell growth inhibition in vitro, while it impairs tumor development in lymphoma-bearing mice
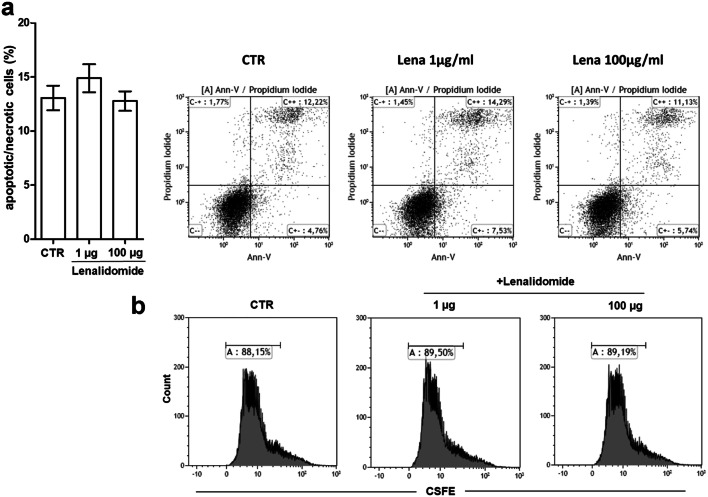
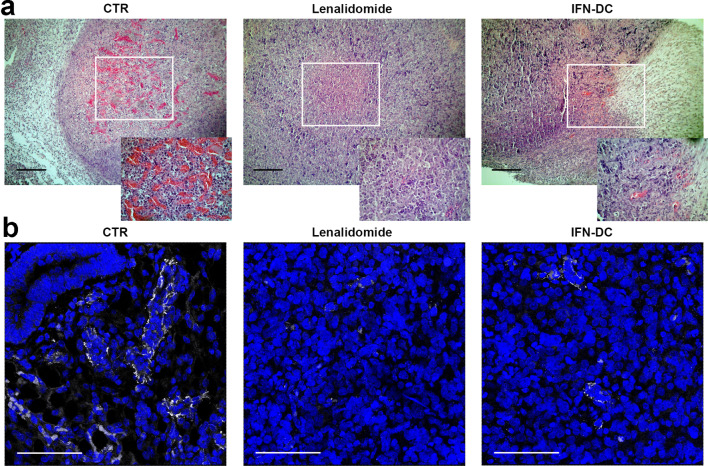
First, we assessed whether lenalidomide could exert any possible direct cytotoxic effect on FL cells cultured up to 72 h in the presence of escalating drug concentrations using annexin-V/Propidium iodide staining. FACS analysis of stained cells showed no appreciable rise in apoptotic or necrotic cell percentage as compared to untreated cultures (Fig. 4a). Likewise, no effects on Karpas-422 cell division rate could be evidenced by CSFE dilution assay (Fig. 4b). We then evaluated the ability of lenalidomide to alter lymphoma tissue microenvironment in NOD/SCID mice engrafted with Karpas-422 cells in the absence of human PBL. Preliminary experiments in NOD/SCID mice demonstrated that subcutaneous inoculation of 5 × 106 Karpas-422 cells led to the development of tumor masses becoming palpable after 14 days from implantation. Thus, mice were inoculated subcutaneously with Karpas-422 cells in the right flank, tumors were allowed to engraft and grow. To evaluate drug direct effect on tumor masses and neo-angiogenesis, on day 14 after tumor injection, mice were divided into the following groups: (1) control lymphoma-bearing mice injected with reference volume vehicle; (2) lymphoma-bearing mice injected with lenalidomide every 3 days for six cycles; (3) lymphoma-bearing mice injected with 2 × 106 IFN-DC every 7 days for three cycles. Tumor growth was monitored for 70 days. Mice were then euthanized, tumors were harvested, the necrotic and vascularized areas were evaluated on Formalin-Fixed Paraffin-Embedded (FFPE) tissue sections by hematoxylin/eosin staining (Fig. 5a). Tumor tissue sections were scanned at low power (10 ×). Despite tumor masses were similarly reduced in the treated mice with respect to the control group, no major differences in the extension of necrotic areas could be detected among the different groups (data not shown). Remarkably, the density of blood vessels in the tumor masses, as detected by hematoxylin/eosin staining, was strongly reduced in lenalidomide-treated animals and to a lesser extent in mice treated with IFN-DC as compared to the control group (Fig. 5a). To better evaluate neo-angiogenesis, the expression of the pan-endothelial marker CD31 in tumor tissue was assessed by CLSM examinations of FFPE sections after immunofluorescent staining (Fig. 5b). Consistently with hematoxylin/eosin staining, intratumoral expression of CD31-positive areas in tissue sections from lenalidomide-treated mice was considerably lower as compared to control animals (Fig. 5b). By normalizing to 1.0 the median value of CD31 immunofluorescence staining (sum of intensity) in tissue sections from control mice, CD31 staining intensity dropped to 0.23 ± 0.06 in tumor tissues from lenalidomide-treated mice. These results confirm that in our xenochimeric mouse model lenalidomide can exert its actions at least in part by altering tissue vascularization rather than exhibiting direct apoptotic effects on lymphoma cells. Interestingly, a substantial reduction in microvasculature density was also shown in xenochimeric mice injected with IFN-DC (CD31 median value of 0.37 ± 0.12), as compared to control lymphoma-bearing mice.
Fig. 4.
Evaluation of lenalidomide direct effect on Karpas-422 cell viability and proliferative activity. a Bar graph resuming FACS analysis of apoptotic/necrotic Karpas-422 cells cultured for 72 h in the presence of 1 or 100 µg lenalidomide, as determined by Annexin-V/propidium iodide staining. Dot-plot analysis of apoptotic/necrotic cells from one representative experiment are shown b relative proliferative activity of Karpas-422 cells cultured for 4 days with or without the addition of lenalidomide, as determined by CFSE dilution assay. Cytometric analyses are representative of three experiments
Fig. 5.
Effects of lenalidomide on tissue vascularization of lymphoma-bearing NOD/SCID mice. a Hematoxylin and eosin (H&E) staining of FFPE tissue sections of Karpas-422 lymphoma bearing NOD/SCID mice. Tissues from untreated tumor-bearing mice (on the left) are compared to tumor sections from lenalidomide (in the middle) or IFN-DC-treated mice (on the right). Blood vessel density is particularly evident in insets. Results of one representative mouse out of three are shown. Bars correspond to 100 μm. b Detection of intratumoral CD31 (marker of vascular endothelial cells) by CLSM examinations of FFPE tissue sections from untreated mice (left panel), lenalidomide (middle panel) or IFN-DC-treated xenochimeric mice (right panel). Images (three-dimensional reconstruction images) show the merged fluorescent signal from CD31 (pseudocolor gray) and DAPI (blue) staining. Panels are representative of three independent experiments. Scale bars 50 µm
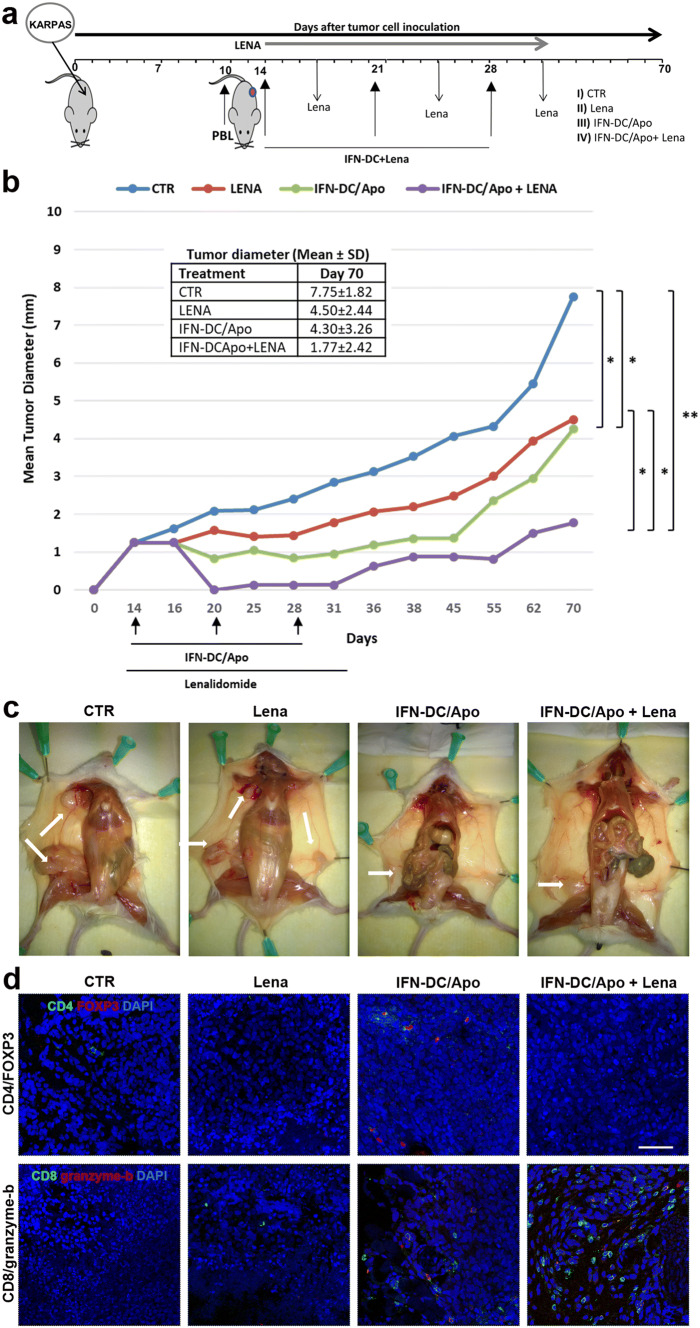
Combining lenalidomide with the IFN-DC vaccine results in a potent therapeutic antitumor effect against established lymphomas in hu-PBL-NOD/SCID mice
To assess the feasibility and efficacy of the combined treatment in vivo, we tested tumor cell-pulsed-IFN-DC vaccination in a therapeutic setting using a hu-PBL-NOD/SCID mouse model. Lymphoma bearing mice were reconstituted with human PBL on day 10 and subsequently exposed to three cycles of vaccination (Fig. 6a). We compared four groups of human PBL-reconstituted xenochimeric mice: (1) untreated tumor-bearing control mice, (2) tumor-bearing mice treated with lenalidomide; (3) mice vaccinated with IFN-DC loaded with apoptotic-Karpas-422 cells; (4) mice receiving the combined therapy of both therapeutic vaccination and lenalidomide administration. None of the engrafted mice developed any sign of GVHD in long term follow-up. Treatments were well tolerated, and all mice survived until sacrifice with no changes in any vital organs among the different treatment groups. Mouse survival and tumor growth were followed for 70 days. Mice receiving IFN-DC vaccination or lenalidomide showed significant and comparable inhibition of tumor growth with respect to the control group. However, tumor growth was inhibited without obvious regression upon either single treatment, while the combination of active immunization with lenalidomide was markedly more effective, resulting in the rapid reduction of tumor size and delayed tumor regrowth upon treatment discontinuation, as compared to the other treatment groups (Fig. 6b). Necroscopic gross examination of control tumor-bearing mice revealed lymphoma cell spread from the injection site to the axillary nodes forming visible tumor masses, while lymphatic and blood vessels proximal to the tumor appeared thicker and/or dilated (Fig. 6c). On the contrary, examination of mice in the treatment groups, revealed reduced lymphoma cell dissemination to axillary lymph nodes and smaller tumor masses. Nonetheless, complete tumor eradication could not be achieved and tumors eventually resumed growth after treatment discontinuation in the majority of treated mice. Of note, the combination treatment appeared far more effective than either single therapy intervention in inhibiting lymphoma cell spread and growth. We next analyzed the extent of tumor-infiltrating human CD8 + T lymphocytes and CD4 + Tregs, which could be involved in tumor growth inhibition. Thus, tumor tissues were analyzed 14 days after the last immunization by immunofluorescent labeling for CD8, granzyme-b, CD4 and FOXP3 detection. Only scarce human FOXP3 + Tregs were detectable in tumor tissue from mice treated with the IFN-DC vaccine alone. Of note, no FOXP3 + cells could be detected in the corresponding samples from mice receiving the combination therapy (Fig. 6d). Conversely, the median number (± SE) of tumor-infiltrating CD8 + T cells in the combination therapy group (whose great majority was also granzyme-b positive) was markedly increased (57 ± 4.25 per field), as compared to the group receiving only vaccination (12 ± 6.03) (Fig. 6d).
Fig. 6.
In vivo evaluation of the antitumor effects of lenalidomide and IFN-DC-based vaccination in hu-PBL-NOD/SCID mice bearing FL tumors. a Therapeutic vaccination schedule. All NOD/SCID mice were engrafted with Karpas-422 lymphoma cells, reconstituted with human PBL and divided into four groups. Hu-PBL-NOD/SCID mice were vaccinated 4 days later and received boost immunizations at day 21 and day 28. Lenalidomide was administered as described in “Materials and methods”. b Evaluation of tumor-growth inhibition. The graph represents Karpas-422 cell growth rate in the different treatment groups. Four mice per group were treated as reported and observed until day 70. The mean tumor growth is shown as a compendium of three separate experiments. Statistically significant differences were estimated at day 70 by the non-parametric Mann–Whitney test (*p < 0.05, **p < 0.01). Mean tumor diameters ± SD at day 70 in the different treatment groups are also shown. c Representative necroscopic examinations of mice from different treatment groups, showing lymphoma cell masses and spreading from the injection site to axillary nodes (arrows). d Evaluation of Treg and CD8 + lymphocyte infiltration in tumor sections. Tumors were harvested and analyzed 14 days after the last immunization. FFPE tissue sections were stained for CD4 (detected in green) and FOXP3 (red) expression (upper panels) or for CD8 (detected in green) and granzyme-b (red) detection (lower panels). DAPI was used to stain nuclei (blue). Scale bars 50 µm
Discussion
In the era of targeted therapies and personalized medicine, the development of a new generation of DC-based vaccines and their combination with immune check-point inhibitors and immunomodulatory drugs is regarded as a promising approach to defeat cancer [5]. IFN-DC can represent a valuable DC candidate subset [10, 15, 29] to be tested for the development of effective cancer vaccines. We have recently demonstrated that IFN-DC from FL patients loaded with autologous lymphoma cells can efficiently activate the cellular arm of the immune response to kill lymphoma cells in vitro [17].
Lenalidomide as single agent exhibits activity in different subtypes of non-Hodgkin lymphoma and recent clinical trials suggest that its combination with rituximab can substitute standard chemotherapy in the FL treatment [31]. Furthermore, lenalidomide can effectively be combined with other chemo-free approaches and used as immune adjuvant with therapeutic cancer vaccines [32].
Here, we report on the effects of IFN-DC-based vaccination in combination with lenalidomide in both in vitro and in vivo models of FL. Differently from a recent study in multiple myeloma patients [23], we could not show any effect of lenalidomide on DC functional activity or phenotype. We showed that lenalidomide can reduce Treg frequency in vitro, reporting an inverse correlation between the percentage of Tregs and CD8 + T cell degranulation activity in response to Karpas-422 cells. Notably, an enhanced lytic activity toward lymphoma cells was detected only in PBL cultures exhibiting a significant reduction of Treg frequency in response to lenalidomide treatment. Although our results substantiate the notion of lenalidomide as an immune adjuvant, we currently cannot explain the variable sensitivity of PBL from different blood donors to lenalidomide. Likewise, it remains unclear whether the enhancement of CD8 + T cell response is directly correlated to Treg reduction or instead both are independently due to the pleiotropic activity of lenalidomide. Although lenalidomide was previously reported to inhibit both Treg expansion and suppressive function [30], existing reports on Treg reduction in lenalidomide-treated patients are conflicting [33–39]. Notably, non-responding patients exhibiting no Treg decline under lenalidomide therapy have also been described [40]. Importantly, our results also show that lenalidomide has effects on the immune synapse formation, increasing the frequency of CD8 + T cell conjugates with lymphoma cells, in accordance with previous observations of lenalidomide reversal of immune synapse alterations in FL [41].
Here, we have also reported the therapeutic efficacy of the lymphoma vaccine and lenalidomide in NOD/SCID mice bearing established human lymphomas, showing that both treatments can markedly inhibit the growth and spread of established tumors. Noteworthy, while lenalidomide did not exert any direct anti-proliferative or cytotoxic effect on Karpas-422 cells under in vitro conditions, it inhibited in vivo the tumor growth in lymphoma-bearing mice, even in the absence of human PBL. Remarkable inhibition of blood microvessel formation was demonstrated in tumor tissue sections from these mice. This effect was not unexpected and can partly account for tumor growth inhibition seen in reconstituted mice, since lenalidomide is known to inhibit VEGF-induced endothelial cell cord formation [42] as well as functional tumor lymphangiogenesis in a mouse xenochimeric model of mantle cell lymphoma [43]. Interestingly, we also found that the sole IFN-DC administration in lymphoma-bearing mice could slightly reduce microvascular density in tumor tissues. Emerging data indicate that angiogenesis may be important in the progression and maintenance of lymphoid malignancies [44]. Our observation on the IFN-DC-induced inhibition of tumor angiogenesis strengthens the notion that DC can promote or inhibit angiogenic processes/mediators depending on their activation status and cytokine production [45]. Noteworthy, the combination showed a significant additive therapeutic effect on human lymphoma cell growth, rapidly reducing tumor burden and effectively delaying tumor regrowth after the discontinuation of the therapy, demonstrating that the combination is far more effective than either single therapeutic approach. On these grounds, we may speculate that tumor eradication can be achieved by administering an appropriate number of combined therapy cycles, with short intervals between administrations to minimize tumor regrowth. Our results are consistent with previous studies demonstrating that a combination of DC vaccination and lenalidomide can efficiently enhance antitumor immune response in murine models of multiple myeloma and colon cancer, via the inhibition of immunosuppressor cells and the enhancement of CD8 cell responses [21–23].
Unloaded IFN-DC have recently been utilized by our group in a phase I clinical study in patients with advanced melanoma in combination with dacarbazine [46] and, more recently, in another phase I trial (EudraCT no. 2013-003158-25) of sequential intranodal injection of low dose rituximab followed by the injection of unloaded IFN-DC in FL patients, exploiting the ADCC-mediated lymphoma cell killing by rituximab and the capacity of IFN-DC to take up apoptotic lymphoma cells in vivo [47]. While the results of this pilot trial are encouraging, the next logical step is to evaluate lymphoma cell-loaded IFN-DC as a personalized therapeutic vaccine in clinical settings of combination therapies. Even though a cautious approach should be maintained due to the possible outbreak of autoimmune diseases [32], our results support the hypothesis that lenalidomide can improve the clinical efficacy of therapeutic DC cancer vaccines. Proof-of-concept trials are eagerly awaited to assess the clinical efficacy of this new and promising combination therapy.
Acknowledgements
The authors thank Mr. Daniele Macchia for extensive help with animal care and technical assistance in studies with xenochimeric mice.
Abbreviations
- ADCC
Antibody-dependent cell cytotoxicity
- CLSM
Confocal laser scanning microscopy
- DC
Dendritic cells
- FL
Follicular lymphoma
- FOXP3
Forkhead box P3
- GM-CSF
Granulocyte macrophage colony-stimulating factor
- IFN-DC
IFN-α-conditioned dendritic cells
- IFN-α
Interferon alpha
- IFN-γ
Interferon gamma
- IL-4
Interleukin-4
- NK
Natural killer
- NHL
Non-Hodgkin lymphoma
- NOD-SCID
Nonobese diabetic/severe combined immunodeficiency
- PBL
Peripheral blood lymphocytes
- TNF-α
Tumor necrosis factor α
Author contributions
Conception and design: SMS, CL; Development of methodology: SMS, CL, FU, IM; Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): SMS, CL, SD, FS, PS, FU, IM, LL, MS; Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): SMS, CL, SD, FS, PS, FU; Writing, review, and/or revision of the manuscript: SMS, CL, FB; Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): SMS, CL; Study supervision: SMS, CL, FB, MCC.
Funding
The research was supported by Grants from Celgene Corporation (Stefano M. Santini) (Grant no. ITA-017) and the Italian Association for Research against Cancer (AIRC IG16891) (Filippo Belardelli).
Compliance with ethical standards
Conflict of interest
Stefano M. Santini received research funding from Celgene. All other authors declare that they have no conflict of interest. Celgene had no role in study design, data collection, data interpretation, writing of the manuscript.
Ethical approval
All experiments utilizing PBMC from healthy donors were conducted in accordance with the ethical standards of the Ethics Committee of Istituto Superiore di Sanità and the Declaration of Helsinki. Institutional Review Board approval was not required for this kind of study. Buffy coat supply for our studies was approved by Azienda Ospedaliera Policlinico Umberto I on 24/02/2014 (aut.6802). All experiments utilizing blood samples from FL patients were conducted in accordance with the declaration of Helsinki. Study and procedures were approved by the Ethics Committee of Azienda Ospedaliera Sant’Andrea (aut.169/2011). All experiments on mice were executed in compliance with the Istituto Superiore di Sanità Service for Animal Welfare guidelines and after approval from the Italian Ministry of Health (aut. 296/2015-PR). Mice were housed according to Legislative Decree 26/2014 guideline.
Informed consent
PBMC were freshly isolated from peripheral blood samples of anonymous volunteer healthy donors at the Transfusion Center of of Policlinico Umberto I—University “La Sapienza”, Rome. Written informed consent was obtained from all blood donors to the use of their blood for research and scientific purposes. Blood samples from FL patients were anonymously provided by the Hematology Unit at the Azienda Ospedaliera Sant’Andrea Rome, Italy. Written informed consent was obtained from FL patients for the use of blood and lymph node specimens in IFN-DC-based vaccine researches.
Animal source
NOD/SCID (NOD.CB17-Prkdcscid/NCrHsd) female mice were purchased from Envigo (Italy), used at 3–4 weeks of age.
Cell line authentication
Karpas-422 FL cell line was purchased from the cell bank Interlab Cell Line Collection (ICLC). Human K562 erythroleukemic cell line was purchased from the European Collection of Authenticated Cell Culture (ECACC). Both cell lines were authenticated by the suppliers by short tandem repeat (STR) profiling. The cell lines were initially grown and cryopreserved into multiple aliquots. All the experiments were performed with cells at low passage numbers (≤ 10).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Caterina Lapenta, Email: caterina.lapenta@iss.it.
Stefano M. Santini, Email: stefano.santini@iss.it
References
- 1.Kahl BS, Yang DT. Follicular lymphoma: evolving therapeutic strategies. Blood. 2016;127:2055–2063. doi: 10.1182/blood-2015-11-624288. [DOI] [PubMed] [Google Scholar]
- 2.Freedman A. Follicular lymphoma: 2018 update on diagnosis and management. Am J Hematol. 2018;93:296–305. doi: 10.1002/ajh.24937. [DOI] [PubMed] [Google Scholar]
- 3.Federico M, Bellei M, Marcheselli L, et al. Follicular Lymphoma International Prognostic Index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol. 2009;27:4555–4562. doi: 10.1200/JCO.2008.21.3991. [DOI] [PubMed] [Google Scholar]
- 4.Sarkozy C, Trneny M, Xerri L, et al. Risk factors and outcomes for patients with follicular lymphoma who had histologic transformation after response to first-line immunochemotherapy in the PRIMA trial. J Clin Oncol. 2016;34:2575–2582. doi: 10.1200/JCO.2015.65.7163. [DOI] [PubMed] [Google Scholar]
- 5.Garg AD, Coulie PG, Van den Eynde BJ, Agostinis P. Integrating next-generation dendritic cell vaccines into the current cancer immunotherapy landscape. Trends Immunol. 2017;38:577–593. doi: 10.1016/j.it.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Garg AD, More S, Rufo N, et al. Trial watch: immunogenic cell death induction by anticancer chemotherapeutics. Oncoimmunology. 2017;6:e1386829. doi: 10.1080/2162402X.2017.1386829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinstock M, Rosenblatt J, Avigan D. Dendritic cell therapies for hematologic malignancies. Mol Ther Methods Clin Dev. 2017;5:66–75. doi: 10.1016/j.omtm.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Nicola M, Zappasodi R, Carlo-Stella C, et al. Vaccination with autologous tumor-loaded dendritic cells induces clinical and immunologic responses in indolent B-cell lymphoma patients with relapsed and measurable disease: a pilot study. Blood. 2009;113:18–27. doi: 10.1182/blood-2008-06-165654. [DOI] [PubMed] [Google Scholar]
- 9.Kolstad A, Kumari S, Walczak M, et al. Sequential intranodal immunotherapy induces antitumor immunity and correlated regression of disseminated follicular lymphoma. Blood. 2015;125:82–89. doi: 10.1182/blood-2014-07-592162. [DOI] [PubMed] [Google Scholar]
- 10.Santini SM, Lapenta C, Belardelli F. Type I interferons as regulators of the differentiation/activation of human dendritic cells: methods for the evaluation of IFN-induced effects. Methods Mol Med. 2005;116:167–181. doi: 10.1385/1-59259-939-7:167. [DOI] [PubMed] [Google Scholar]
- 11.Lapenta C, Santini SM, Spada M, et al. IFN-alpha-conditioned dendritic cells are highly efficient in inducing cross-priming CD8+ T cells against exogenous viral antigens. Eur J Immunol. 2006;36:2046–2060. doi: 10.1002/eji.200535579. [DOI] [PubMed] [Google Scholar]
- 12.Parlato S, Santini SM, Lapenta C, et al. Expression of CCR-7, MIP-3beta, and Th-1 chemokines in type I IFN-induced monocyte-derived dendritic cells: importance for the rapid acquisition of potent migratory and functional activities. Blood. 2001;98:3022–3029. doi: 10.1182/blood.V98.10.3022. [DOI] [PubMed] [Google Scholar]
- 13.Lapenta C, Santini SM, Logozzi M, et al. Potent immune response against HIV-1 and protection from virus challenge in hu-PBL-SCID mice immunized with inactivated virus-pulsed dendritic cells generated in the presence of IFN-alpha. J Exp Med. 2003;198:361–367. doi: 10.1084/jem.20021924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santini SM, Lapenta C, Donati S, et al. Interferon-α-conditioned human monocytes combine a Th1-orienting attitude with the induction of autologous Th17 responses: role of IL-23 and IL-12. PLoS One. 2011;6:e17364. doi: 10.1371/journal.pone.0017364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spadaro F, Lapenta C, Donati S, et al. IFN-α enhances cross-presentation in human dendritic cells by modulating antigen survival, endocytic routing, and processing. Blood. 2012;119:1407–1417. doi: 10.1182/blood-2011-06-363564. [DOI] [PubMed] [Google Scholar]
- 16.Lattanzi L, Rozera C, Marescotti D, et al. IFN-α boosts epitope cross-presentation by dendritic cells via modulation of proteasome activity. Immunobiology. 2011;216:537–547. doi: 10.1016/j.imbio.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Lapenta C, Donati S, Spadaro F, et al. NK cell activation in the antitumor response induced by IFN-α dendritic cells loaded with apoptotic cells from follicular lymphoma patients. J Immunol. 2016;197:795–806. doi: 10.4049/jimmunol.1600262. [DOI] [PubMed] [Google Scholar]
- 18.Witzig TE, Nowakowski GS, Habermann TM, et al. A comprehensive review of lenalidomide therapy for B-cell non-Hodgkin lymphoma. Ann Oncol. 2015;26:1667–1677. doi: 10.1093/annonc/mdv102. [DOI] [PubMed] [Google Scholar]
- 19.Ahmadi T, Chong EA, Gordon A, et al. Combined lenalidomide, low-dose dexamethasone, and rituximab achieves durable responses in rituximab-resistant indolent and mantle cell lymphomas. Cancer. 2014;120:222–228. doi: 10.1002/cncr.28405. [DOI] [PubMed] [Google Scholar]
- 20.Chong EA, Ahmadi T, Aqui NA, et al. Combination of lenalidomide and rituximab overcomes rituximab resistance in patients with indolent B-cell and mantle cell lymphomas. Clin Cancer Res. 2015;21:1835–1842. doi: 10.1158/1078-0432.CCR-14-2221. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen-Pham T-N, Jung S-H, Vo M-C, et al. Lenalidomide synergistically enhances the effect of dendritic cell vaccination in a model of murine multiple myeloma. J Immunother. 2015;38:330–339. doi: 10.1097/CJI.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 22.Vo M-C, Nguyen-Pham T-N, Lee H-J, et al. Combination therapy with dendritic cells and lenalidomide is an effective approach to enhance antitumor immunity in a mouse colon cancer model. Oncotarget. 2017 doi: 10.18632/oncotarget.15917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vo M-C, Anh-NguyenThi T, Lee H-J, et al. Lenalidomide enhances the function of dendritic cells generated from patients with multiple myeloma. Exp Hematol. 2017;46:48–55. doi: 10.1016/j.exphem.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Epron G, Ame-Thomas P, Le Priol J, et al. Monocytes and T cells cooperate to favor normal and follicular lymphoma B-cell growth: role of IL-15 and CD40L signaling. Leukemia. 2012;26:139–148. doi: 10.1038/leu.2011.179. [DOI] [PubMed] [Google Scholar]
- 25.Mourcin F, Pangault C, Amin-Ali R, et al. Stromal cell contribution to human follicular lymphoma pathogenesis. Front Immunol. 2012;3:280. doi: 10.3389/fimmu.2012.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amé-Thomas P, Tarte K. The yin and the yang of follicular lymphoma cell niches: role of microenvironment heterogeneity and plasticity. Semin Cancer Biol. 2014;24:23–32. doi: 10.1016/j.semcancer.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Montico B, Lapenta C, Ravo M, et al. Exploiting a new strategy to induce immunogenic cell death to improve dendritic cell-based vaccines for lymphoma immunotherapy. Oncoimmunology. 2017;6:e1356964. doi: 10.1080/2162402X.2017.1356964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macchia I, Urbani F, Proietti E. Immune monitoring in cancer vaccine clinical trials: critical issues of functional flow cytometry-based assays. Biomed Res Int. 2013 doi: 10.1155/2013/726239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santini SM, Lapenta C, Santodonato L, et al. IFN-alpha in the generation of dendritic cells for cancer immunotherapy. Handb Exp Pharmacol. 2009 doi: 10.1007/978-3-540-71029-5_14. [DOI] [PubMed] [Google Scholar]
- 30.Galustian C, Meyer B, Labarthe M-C, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother. 2009;58:1033–1045. doi: 10.1007/s00262-008-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morschhauser F, Fowler NH, Feugier P, et al. Rituximab plus lenalidomide in advanced untreated follicular lymphoma. N Engl J Med. 2018;379:934–947. doi: 10.1056/NEJMoa1805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palma M, Hansson L, Mulder TA, et al. Lenalidomide as immune adjuvant to a dendritic cell vaccine in chronic lymphocytic leukemia patients. Eur J Haematol. 2018;101:68–77. doi: 10.1111/ejh.13065. [DOI] [PubMed] [Google Scholar]
- 33.Lee B-N, Gao H, Cohen EN, et al. Treatment with lenalidomide modulates T-cell immunophenotype and cytokine production in patients with chronic lymphocytic leukemia. Cancer. 2011;117:3999–4008. doi: 10.1002/cncr.25983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minnema MC, van der Veer MS, Aarts T, et al. Lenalidomide alone or in combination with dexamethasone is highly effective in patients with relapsed multiple myeloma following allogeneic stem cell transplantation and increases the frequency of CD4 + Foxp3 + T cells. Leukemia. 2009;23:605–607. doi: 10.1038/leu.2008.247. [DOI] [PubMed] [Google Scholar]
- 35.Kneppers E, van der Holt B, Kersten M-J, et al. Lenalidomide maintenance after nonmyeloablative allogeneic stem cell transplantation in multiple myeloma is not feasible: results of the HOVON 76 Trial. Blood. 2011;118:2413–2419. doi: 10.1182/blood-2011-04-348292. [DOI] [PubMed] [Google Scholar]
- 36.Lioznov M, El-Cheikh J, Hoffmann F, et al. Lenalidomide as salvage therapy after allo-SCT for multiple myeloma is effective and leads to an increase of activated NK (NKp44+) and T (HLA-DR+) cells. Bone Marrow Transplant. 2010;45:349–353. doi: 10.1038/bmt.2009.155. [DOI] [PubMed] [Google Scholar]
- 37.Busch A, Zeh D, Janzen V, et al. Treatment with lenalidomide induces immunoactivating and counter-regulatory immunosuppressive changes in myeloma patients. Clin Exp Immunol. 2014;177:439–453. doi: 10.1111/cei.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muthu Raja KR, Kovarova L, Hajek R. Induction by lenalidomide and dexamethasone combination increases regulatory cells of patients with previously untreated multiple myeloma. Leuk Lymphoma. 2012;53:1406–1408. doi: 10.3109/10428194.2011.652106. [DOI] [PubMed] [Google Scholar]
- 39.Tzankov A, Meier C, Hirschmann P, et al. Correlation of high numbers of intratumoral FOXP3 + regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin’s lymphoma. Haematologica. 2008;93:193–200. doi: 10.3324/haematol.11702. [DOI] [PubMed] [Google Scholar]
- 40.Aqui N, Leinbach L, Chong EA, et al. Changes in regulatory T-cells in responding and non-responding patients with indolent B-cell or mantle cell lymphomas during treatment with lenalidomide, dexamethasone, and rituximab. J Clin Oncol. 2010 doi: 10.1200/jco.2010.28.15_suppl.8085. [DOI] [Google Scholar]
- 41.Ramsay AG, Clear AJ, Kelly G, et al. Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: implications for the tumor microenvironment and immunotherapy. Blood. 2009;114:4713–4720. doi: 10.1182/blood-2009-04-217687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu L, Payvandi F, Wu L, et al. The anti-cancer drug lenalidomide inhibits angiogenesis and metastasis via multiple inhibitory effects on endothelial cell function in normoxic and hypoxic conditions. Microvasc Res. 2009;77:78–86. doi: 10.1016/j.mvr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Song K, Herzog BH, Sheng M, et al. Lenalidomide inhibits lymphangiogenesis in preclinical models of mantle cell lymphoma. Cancer Res. 2013;73:7254–7264. doi: 10.1158/0008-5472.CAN-13-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ribatti D, Nico B, Ranieri G, et al. The role of angiogenesis in human non-Hodgkin lymphomas. Neoplasia. 2013;15:231–238. doi: 10.1593/neo.121962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sozzani S, Rusnati M, Riboldi E, et al. Dendritic cell-endothelial cell cross-talk in angiogenesis. Trends Immunol. 2007;28:385–392. doi: 10.1016/j.it.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Rozera C, Cappellini GA, D’Agostino G, et al. Intratumoral injection of IFN-alpha dendritic cells after dacarbazine activates anti-tumor immunity: results from a phase I trial in advanced melanoma. J Transl Med. 2015;13:139. doi: 10.1186/s12967-015-0473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cox MC, Castiello L, Mattei M, et al. Clinical and antitumor immune responses in relapsed/refractory follicular lymphoma patients after intranodal injections of IFNα-dendritic cells and rituximab. Clin Cancer Res. 2019 doi: 10.1158/1078-0432.CCR-19-0709. [DOI] [PubMed] [Google Scholar]