Abstract
Relapsed/refractory B-precursor acute lymphoblastic leukemia (pre-B ALL) remains a major therapeutic challenge. Chimeric antigen receptor (CAR) T cells are promising treatment options. Central memory T cells (Tcm) and stem cell-like memory T cells (Tscm) are known to promote sustained proliferation and persistence after T-cell therapy, constituting essential preconditions for treatment efficacy. Therefore, we set up a protocol for anti-CD19 CAR T-cell generation aiming at high Tcm/Tscm numbers. 100 ml peripheral blood from pediatric pre-B ALL patients was processed including CD4+/CD8+-separation, T-cell activation with modified anti-CD3/-CD28 reagents and transduction with a 4-1BB-based second generation CAR lentiviral vector. The process was performed on a closed, automated device requiring additional manual/open steps under clean room conditions. The clinical situation of these critically ill and refractory patients with leukemia leads to inconsistent cellular compositions at start of the procedure including high blast counts and low T-cell numbers with exhausted phenotype. Nevertheless, a robust T-cell product was achieved (mean CD4+ = 50%, CD8+ = 39%, transduction = 27%, Tcm = 50%, Tscm = 46%). Strong proliferative potential (up to > 100-fold), specific cytotoxicity and low expression of co-inhibitory molecules were documented. CAR T cells significantly released TH1 cytokines IFN-γ, TNF-α and IL-2 upon target-recognition. In conclusion, partly automated GMP-generation of CAR T cells from critically small blood samples was feasible with a new stimulation protocol that leads to high functionality and expansion potential, balanced CD4/CD8 ratios and a conversion to a Tcm/Tscm phenotype.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2155-7) contains supplementary material, which is available to authorized users.
Keywords: CAR T cells, GMP production, Tscm/cm, Pediatric ALL
Introduction
Treatment with genetically modified T cells has the potential to induce sustained clinical remissions even in patients with relapsed or primary refractory disease. In particular, T cells genetically directed against the B-cell lineage antigen CD19 are currently evaluated in more than 40 clinical phase I/II trials. Preliminary results underline their enormous potential in B-lineage malignancies. Especially in pediatric pre-B ALL, response rates of up to 95% have been shown in refractory, heavily pre-treated patients [5–10]. Those T cells are typically generated by retro- or lentiviral transduction of patient-derived autologous T cells with a vector encoding for a chimeric antigen receptor (CAR) consisting of the anti-CD19 single-chain variable fragment (ScFv) fused to CD3ζ as a domain of the T-cell receptor (TCR) complex [11]. However, low cell number in pediatric samples, exhausted T cells under treatment protocols and the presence of leukemic cells in the starting fraction are challenges for stable production of functionally active CAR T-cell products.
Although the vast majority of pediatric ALL patients respond to second generation anti-CD19 CAR T-cell treatment, there are patients not responding or relapsing after T-cell therapy. Leukemia relapse or initial non-response is typically caused by early loss or non-engraftment of anti-CD19 CAR T cells [6, 12, 13]. Although associated with B-cell aplasia, Maude et al. underlined the impact of prolonged anti-CD19 CAR T-cell persistence through sustained in vivo proliferation on long-term outcome of pediatric ALL patients [12]. Thus, we aimed at generating CAR T cells with a T-cell phenotype in favor of high proliferative potential. Central memory (Tcm) or stem cell-like memory (Tscm) T cells are known to be best candidates for a sustained in vivo response after adoptive T-cell transfer as they display good effector functions while maintaining their high proliferative capacity [14–16]. Moreover, leukemic cells are known to up-regulate inhibitory molecules such as PD-L1 to circumvent T-cell mediated TH1 attack [17]. Generation of anti-CD19 CAR T cells with low sensitivity to inhibitory checkpoint signals thus might play a crucial role for sustained remissions after T-cell therapy [18].
Although CAR T-cell generation is well established in many laboratories, it is a complex procedure originally involving many hands-on/open steps [19]. To simplify and to facilitate standardization of the process, a protocol for partly automated large scale CAR T-cell manufacturing was established on a closed, GMP-compatible device requiring only few open hands-on steps [20]. As the activity protocol has a modular structure, it can be adapted for various conditions such as different starting cell counts, varied CD4/8 proportion, feeding or washing steps and final formulation. Here, we demonstrate feasibility of partly automated, GMP-compatible clinical-scale manufacturing of anti-CD19 CAR T cells on a closed device even from very small pediatric patients’ samples.
Materials and methods
Patients
Patients’ characteristics are shown in Table 1.
Table 1.
Characteristics of patients and anti-CD19 CAR T-cell products
| Patient CAR001 | Patient CAR002 | Patient CAR003 | Patient CAR004 | |
|---|---|---|---|---|
| Patient-related information | ||||
| Gender | Female | Female | Female | Female |
| Age at time-point of blood sampling | 18 | 15 | 14 | 18 |
| Diagnosis | cALL | cALL | cALL | cALL relapse |
| Age at diagnosis | 17 | 15 | 14 |
18 Initial diagnosis: 13 |
| CNS state | Negative | Negative | Negative | Negative |
| Cytogenetics |
TEL deletion 12p13 MLL deletion 11q23 ABL trisomy 9q34 |
BCR-ABL fusion | My+ | High-hyperdiploid karyotype |
| Treatment protocol | CoALL Protocol 08/09 | EsPhALL-CoALL Protocol 08/09 | CoALL Protocol 08/09 | ALL REZ BFM Protocol |
| Day of blood sampling according to protocol | Before start of day 92 treatment | Before treatment | Before treatment | After first R2 block |
| Systemic chemotherapy received for current diagnosis until date of blood sampling |
Prednisolone DNR, VCR, HIDAC, PEG-ASP, MTX, VP16, ARA-C, 6-MP, 6-TG |
None | None |
Dexamethasone VCR, MTC, PEG-ASP, ARA-C IDA, CPM, 6-TG, 6-MP, VDS, IFO, DNR |
| Disease activity at time-point of blood sampling | MRD negative | 71% blasts in PB | 64% blasts in PB | MRD negative |
| Process-related information | ||||
| Volume of blood sample (ml) | 120 | 100 | 100 | 100 |
| T-cell count after CD4/8 separation | 1.74 × 107 | 4.89 × 107 | 3.86 × 107 | 9.36 × 106 |
| T-cell count of final product | 3.35 × 109 | 1.53 × 109 | 4.87 × 108 | 8.87 × 108 |
| T-cell transduction rate (%) | 40.4 | 19.6 | 19.3 | 28.5 |
| Absolute number of CAR+ T cells in final product | 1.35 × 109 | 3.00 × 108 | 9.40 × 107 | 2.53 × 108 |
cALL common acute lymphoblastic leukemia, CNS central nervous system, DNR daunorubicin, VCR vincristine, HIDAC high-dose cytarabine, PEG-ASP pegylated asparaginase, MTX methotrexate, VP16 etoposide, ARA-C cytarabine, 6-MP 6-mercaptopurine, 6-TG 6-thioguanine, MRD minimal residual disease, PB peripheral blood
Peripheral blood mononuclear cell (PBMC) generation
PBMCs were generated from 100 to 120 ml PB of pediatric ALL patients by density gradient centrifugation (Biocoll, Biochrom, Berlin, Germany). Cells were frozen in 5% human serum albumin (HSA) (Biotest, Dreieich, Germany) containing 10% DMSO (Sigma, Taufkirchen, Germany).
Flow cytometry
Cells were stained for CD3-FITC, CD8-APC-Vio 770, CD4-VioGreen, CD69-PE-Vio 770, CD25-PE, CD137-APC, CD56-PE, CD14-PerCP, CD19-PE-Vio 770, 7-AAD, CD45RO-PE-Vio 770, CD62L-VioBlue, CD95-APC, CD95-PE, OX-40-PE-Vio 770 (Miltenyi Biotec), CD4-BV650, CTLA-4-APC, CD3-BUV395 (Becton, Dickinson and Company (BD), Franklin Lakes, NJ, USA), CD8-PE/Cy7, CD137-BV421, CD56-BV421, 2B4-APC, BTLA-BV421, CD10-APC, TIM-3-BV785, PD-1-BV421, CTLA-4-PE/Cy7, PD-L1-BV421, CD8-PerCP/Cy5.5 (Biolegend, San Diego, CA, USA), VISTA-APC (R&D Systems, Minneapolis, MN, USA), TIGIT-APC, LAG-3-PE, Fixable Viability Dye eFluor 780 (eBioscience/Thermo Fisher Scientific, Waltham, MA, USA). Transduction rate was evaluated by staining with 5 ng/ml Biotin-Protein L (GenScript, Piscataway, NJ, USA) and anti-Biotin-APC or -PE (Miltenyi Biotec). Intracellular stains for IFN-γ-PE (BD) and TNF-α-PacificBlue (Biolegend) were performed using Fix and Perm Cell Permeabilization Kit (Thermo Fisher Scientific). Flow cytometric measurements were performed on a MACSQuant Analyzer 10 (Miltenyi Biotec) or BD LSRFortessa (BD).
T-cell transduction process
Material of the CliniMACS process was from Miltenyi Biotec, Bergisch Gladbach, Germany, unless otherwise stated. The CliniMACS Prodigy is an automated, closed system constructed for large-scale generation of immunotherapeutic T-cell products in a GMP-accredited way. It consists of 24 valves into which a sterile, closed tubing set is inserted without direct contact to the device. All parts intended to come into contact with the cellular product are sterile and single-use materials. The tubing set contains a single-use culture chamber which is fixed into the CentriCult Unit of the CliniMACS Prodigy where optimal culturing conditions of 37 °C/5% CO2 are achieved and the cell product can be centrifuged and shaken during the process.
After written informed consent from patients/their parents, 100–120 ml PB were taken from the patients’ central venous access (Hickman catheter/port), PBMCs were generated and frozen. The frozen product was thawed in pre-warmed TexMACS GMP medium and immediately transferred to the CliniMACS Prodigy on day 0 by sterile tube welding without additional washing steps. Tubing set CliniMACS Prodigy TS520 was used together with TCT software version 1.0. CliniMACS PBS/EDTA supplemented with 0.5% HSA (Grifols, Barcelona, Spain) served as process buffer for separation with CliniMACS CD4/CD8 reagent. Cell culture was set-up after selection for CD4+/CD8+ cells by modular programming the activity matrix of the process (Supplementary Table 1). Culture medium consisted of TexMACS GMP medium, 3% human AB serum (Institute for Clinical Transfusion Medicine, Ulm, Germany), 12.5 ng/ml MACS GMP Recombinant Human IL-7 and IL-15. Medium bag was exchanged for a fresh one on day 6 containing only interleukins, no serum was added. For activation, one vial TransAct T Cell Reagent-Large Scale, human was used according to manufacturer’s recommendations (working dilution of 17.5 of the vial). Activation reagent was washed out together with the lentivirus on day 2 of the process, no magnetic bead removal step was required. One day after activation, T-cell transduction was performed with a second generation CAR lentiviral vector encoding for anti-CD19 single-chain variable fragment (scFv), 4-1BB (CD137) co-stimulatory domain and CD3ζ chain. ScFv sequence was derived from mouse hybridoma FMC-63 (AA 1-267, GenBank ID: HM852952.1), (GGGGS)3 was used as linker domain as described by Schneider et al. [21]. Lentivirus was thawed at room temperature and diluted with TexMACS GMP medium to a final volume of 10 ml. The suspension was transferred to a 150 ml Transfer Bag which was welded to the tubing set. Multiplicity of infection (MOI) rates are shown in Supplementary Table 2. Activation reagent/virus were washed out on day 2. On day 12, cells were harvested in a final volume of 100 ml TexMACS GMP medium. Cells were frozen in 5% human serum albumin (Grifols, Barcelona, Spain) containing 10% DMSO (Sigma, Taufkirchen, Germany). Flow cytometric/functionality analyses were either performed immediately after harvest or from frozen cells which were thawed in warm TexMACS GMP medium. Untransduced MOCK controls were taken from reapplication bag after separation for CD4/8. MOCK cells were cultured in 48-well plates (Corning, Corning, New York, USA) and treated similar to large scale runs except for transduction. Samples were taken whenever needed using the automated sterile sampling system. In case three sampling pouches were not sufficient, an additional Triple Sampling Adapter was welded to the tubing set. Sterile tube welding was performed with an SC-201AH TSCD Sterile Tube Welder (Terumo).
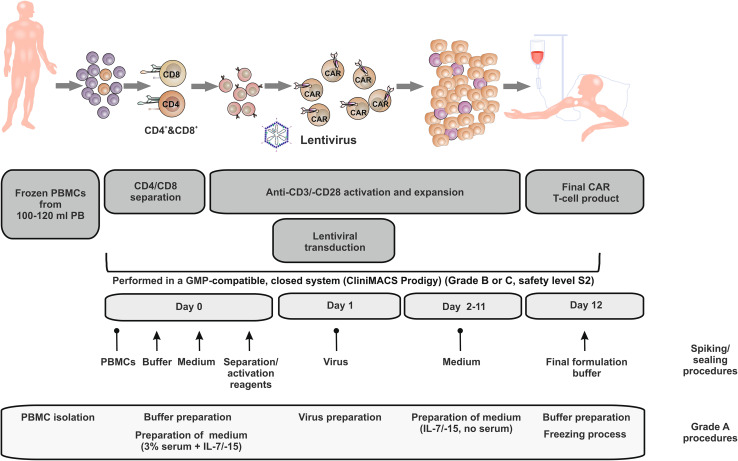
PBMC isolation, buffer/medium/virus preparation and cryopreservation required grade A clean room conditions (open/manual steps). Spiking and sealing procedures are indicated in Fig. 1. All other steps were performed in the closed device (CliniMACS Prodigy placed in clean room grade B or C).
Fig. 1.
Schematic representation of T-cell transduction (TCT) process on the CliniMACS Prodigy: PBMCs from pediatric ALL patients were thawed and immediately transferred to the device on day 0. After automated CD4/8 separation and CD3/28 activation, transduction with the lentiviral anti-CD19 CAR vector was performed on day 1. CAR T cells were expanded for 12 days until final CAR T-cell product was harvested from the device. For the clinical setting, the CliniMACS Prodigy is currently located in GMP grade B or C. Open steps (GMP grade A), spiking (↑) and sealing ( ) procedures are indicated in the lower part of the figure. Cells are cultured in safety level S2. Due to multiple washing and dilution steps, the final product is downscaled to safety level S1. PBMC peripheral blood mononuclear cells, PB peripheral blood, CAR chimeric antigen receptor, GMP good manufacturing practice
) procedures are indicated in the lower part of the figure. Cells are cultured in safety level S2. Due to multiple washing and dilution steps, the final product is downscaled to safety level S1. PBMC peripheral blood mononuclear cells, PB peripheral blood, CAR chimeric antigen receptor, GMP good manufacturing practice
Small scale runs to compare CD4/CD8-separated cells with PBMCs were performed similar to the large scale protocol. The amount of virus for transduction was calculated based on the T-cell count. 1 × 106 T cells were transduced with 2 × 106 particles.
Cytotoxicity assay
Target cells were labeled according to CellTrace Violet Cell Proliferation Kit (Thermo Fisher Scientific) and co-cultured with CAR T cells for 24 h. Effector-cell count was calculated based on transduction rate (CAR+ cells) which was analyzed by flow-cytometric staining for Protein-L. Absolute count of remaining CellTrace Violet-positive cells was calculated with the MACSQuant Analyzer 10 (Miltenyi Biotec) and set into relation to the count of CellTrace Violet-positive cells of control wells (target cells only).
Proliferation assay
T cells were labeled according to CellTrace Violet Cell Proliferation Kit (Thermo Fisher Scientific) and co-cultured with Jeko cells at an E:T ratio of 5:1 (2 × 104 target cells). Effector-cell count was calculated based on transduction rate (CAR+ cells) which was analyzed by Protein-L stain. T cells were re-stimulated every 24 h with 2 × 104 target cells. Cell proliferation was measured on a MACSQuant Analyzer 10 (Miltenyi Biotec) before stimulation with targets and 24 h after each re-stimulation.
MACSPlex assay
CAR T cells were co-cultured with target cells at an E:T ratio of 1:2. Effector-cell count was calculated based on transduction rate (CAR+ cells) analyzed by flow-cytometric staining for Protein-L. Supernatant of co-cultures was harvested after 24 h and frozen at − 20 °C. For analysis, supernatant was thawed and diluted 1:5 with buffer supplied with the MACSPlex Cytokine 12 kit (Miltenyi Biotec). Flow-cytometric measurement was performed according to supplier’s information.
qPCR
To determine lentiviral copy numbers, real-time qPCR was used. Amplification of vector-specific sequences (gag) was compared to host-specific sequences (PTBP2) using Taqman Fast Advanced Master Mix (Thermo Fisher Scientific) and the BIO-RAD CFX96 Touch Real-Time PCR Detection System.
Statistics
Statistical analyses were performed using Graphpad Prism 7.
Results
Anti-CD19 CAR T-cell generation using small peripheral blood samples from pediatric ALL patients
Autologous anti-CD19 chimeric antigen receptor (CAR) T-cell products were generated on the CliniMACS Prodigy from 100 to 120 ml peripheral blood of four different pediatric patients. The CliniMACS Prodigy is an automated, closed system constructed for large-scale generation of T-cell products in a GMP-accredited way (Fig. 1). Patients’ characteristics are shown in Table 1. Patients’ age ranged from 14 to 18 years (mean 16.25 years). All four patients were female and suffered from B-precursor acute lymphoblastic leukemia (common ALL, cALL), either after relapse (n = 1) or after primary diagnosis (n = 3). None of the patients had central nervous system (CNS) disease. Patients were either pre-treated with standard chemotherapy according to CoALL 08/09 protocol (n = 1) or ALL REZ BFM 2012 protocol (n = 1), respectively, or evaluated at time-point of diagnosis, and thus prior to chemotherapeutic treatment (n = 2). Blood of pre-treated patients was taken between two cycles of chemotherapy after lymphocyte counts had resolved to > 500/µl. Blood was not taken during treatment with corticosteroids to avoid inhibitory effects on the T cells. At time-point of sampling, minimal residual disease of two patients was lower than detection limit by polymerase chain reaction (PCR) in the bone marrow, whereas the other two patients suffered from morphological disease (71 or 64% blasts in peripheral blood, respectively).
Initial T-cell count of the starting fraction of the four patients ranged between 9.36 × 106 and 4.89 × 107 T cells (mean 2.86 × 107) after CD4/8 separation. Although the process is optimized for an initial cell product of 1 × 108 T cells, generation of sufficient amounts of anti-CD19 CAR T cells was feasible from these very low T-cell numbers (Table 1). After 12 days of expansion on the CliniMACS Prodigy, a mean of 4.99 × 108 CAR+ T cells (range 9.4 × 107 to 1.35 × 109) was achieved, suitable for the treatment of a 100 kg patient with a dose of up to 4.99 × 106 CAR+ T cells/kg body weight.
Anti-CD19 CAR T cells derived from pediatric patients show high expansion potential and transduction rates
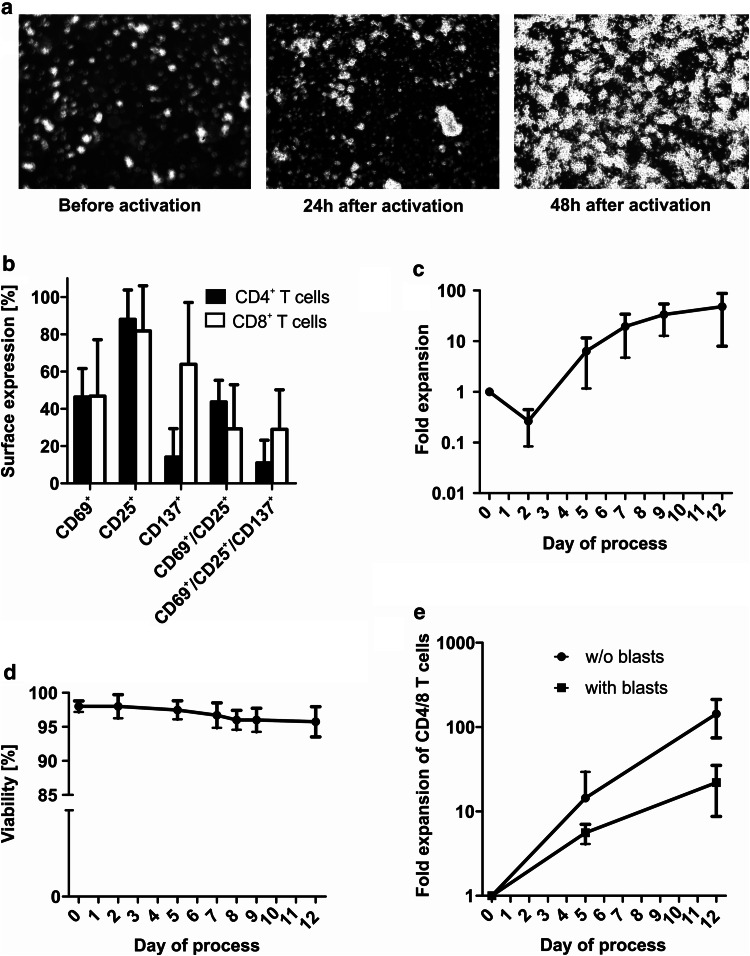
T cells were activated on day 0 of the process with a new anti-CD3/-CD28-based activation reagent (TransAct T Cell Reagent-Large Scale, human) and were cultured in the presence of IL-7 and IL-15. Microscope pictures of the integrated microscope showed clustering of T cells 24 and 48 h after activation indicating efficient T-cell activation (Fig. 2a). When analyzed by flow cytometry 48 h after activation, T cells expressed activation markers CD69, CD25 and CD137 (Fig. 2b). Cell count/viability were evaluated on day 0, 2, 5, (6), 7, 9 and at the end of the process by sterile sampling from the device. Mean expansion rate of lymphocytes within the culture was 48-fold (range 14.7–102.4) although two out of four patients had received corticosteroids in previous chemotherapy cycles (Fig. 2c). A slight decrease in cell count was detectable on day 2 of the process possibly due to cell death caused by thawing, activation and transduction. Viability did not fall below a mean of 95.8% during the process (Fig. 2d). For CAR002, cell count decreased below detection threshold on day 2 of the process and survival of T cells was confirmed by re-concentration through additional centrifugation of the material provided in the sampling pouch. Microscope pictures of the culture chamber showed massive cell clumps associated with the destruction of leukemic cells. Viability and cell count resumed within the next three days of culture. A trend towards decreased T-cell expansion in the presence of blasts was observed (Fig. 2e).
Fig. 2.
Activation, expansion and viability during TCT process. a Exemplary microscope pictures of the T-cell culture before, 24 and 48 h after activation with CD3/CD28-based TransAct T Cell Reagent showed clustering of T cells as a sign of T cell activation (400-fold magnification, taken with microscope included in the CliniMACS Prodigy). b T cells expressed various activation markers 48 h after CD3/CD28 activation (n = 2). c A mean expansion rate of 47.9-fold was achieved during the process (overall cell count in culture chamber, n = 4). d Mean viability was higher than 95% throughout the protocol (n = 4). For CAR002, cell count decreased below detection threshold on day 2 of the process and survival of T cells was only confirmed by re-concentration through additional centrifugation of the material provided in the sampling pouch. e A trend towards decreased T-cell expansion in the presence of blasts was observed (gated on CD4+/CD8+ T cells, n = 2 for each group)
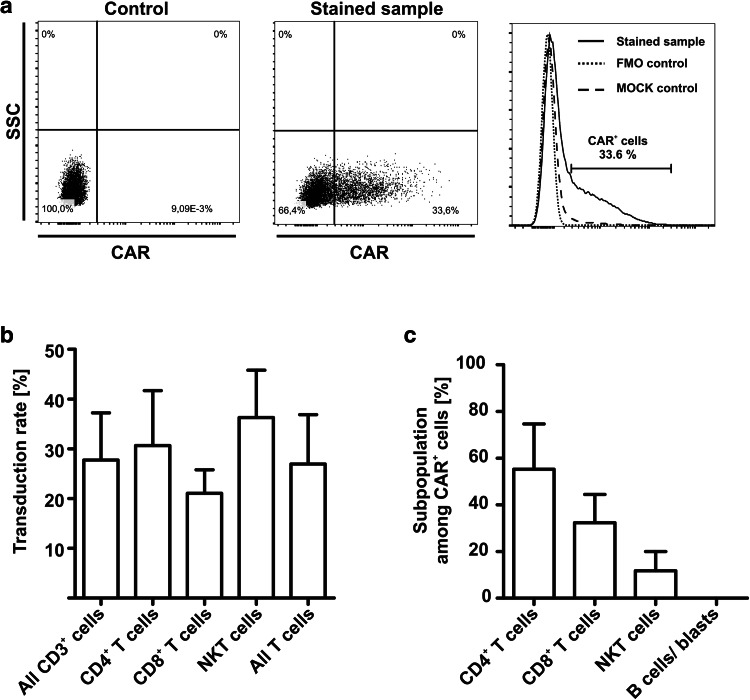
Lentiviral transduction was performed on day 1 after sterile welding the virus-containing bag to the tubing set of the device. A viral concentration of 2 × 108 particles per 100 ml was used, irrespective of T-cell counts. Frequency of CAR+ T cells was analyzed by flow-cytometric stain for Biotin-protein L and detected by anti-Biotin-APC or -PE (Fig. 3a). Mean T-cell transduction rate at the end of the process was 26.95% (Fig. 3b). Multiplicity of infection (MOI) and corresponding transduction rates are shown in Supplementary Table 2. Transduced cells consisted of mainly CD4 and CD8 T cells, although a small amount of CD3+/CD56+ NKT cells was also transduced. The cellular composition among CAR+ cells showed a slight but not statistically significant predominance of CD4+ T cells followed by CD8+ T cells (Fig. 3c). No B cells or leukemic blasts were transduced. Transduced NKT cells displayed a predominant CD3+/CD8+ NKT-cell phenotype (Supplementary Figure 1). The number of viral copies (vector copy number) determined by real-time PCR of genomic DNA did not exceed 2.3 per cell (mean 1.72), and thus met the requirements of authorities to decrease the potential risk of insertional mutagenesis.
Fig. 3.
T-cell transduction with anti-CD19 CAR lentiviral vector. a Transduction rate was analyzed by flow cytometric stain for Biotin-Protein L and secondary stain for anti-Biotin-PE or -APC. Cells stained only with the secondary antibody (FMO) or untransduced T cells were used as controls. b Transduction rate among different cell subsets was analyzed by flow cytometry and showed no significant difference between the subsets. Mean T-cell transduction rate was 26.95% (n = 4). Two-tailed unpaired t test was performed to determine statistical significance. c Cellular composition of CAR+ cells was analyzed by flow cytometry (n = 4). Transduced T cells consisted of CD4+ T cells, CD8+ T cells and NKT cells. No transduced B cells or blasts were detected. Two-tailed unpaired t test was performed to determine statistical significance. FMO: fluorescence minus one
Balanced CD4/CD8 ratio with predominance of Tcm and Tscm T-cell phenotype and low expression of inhibitory checkpoint molecules
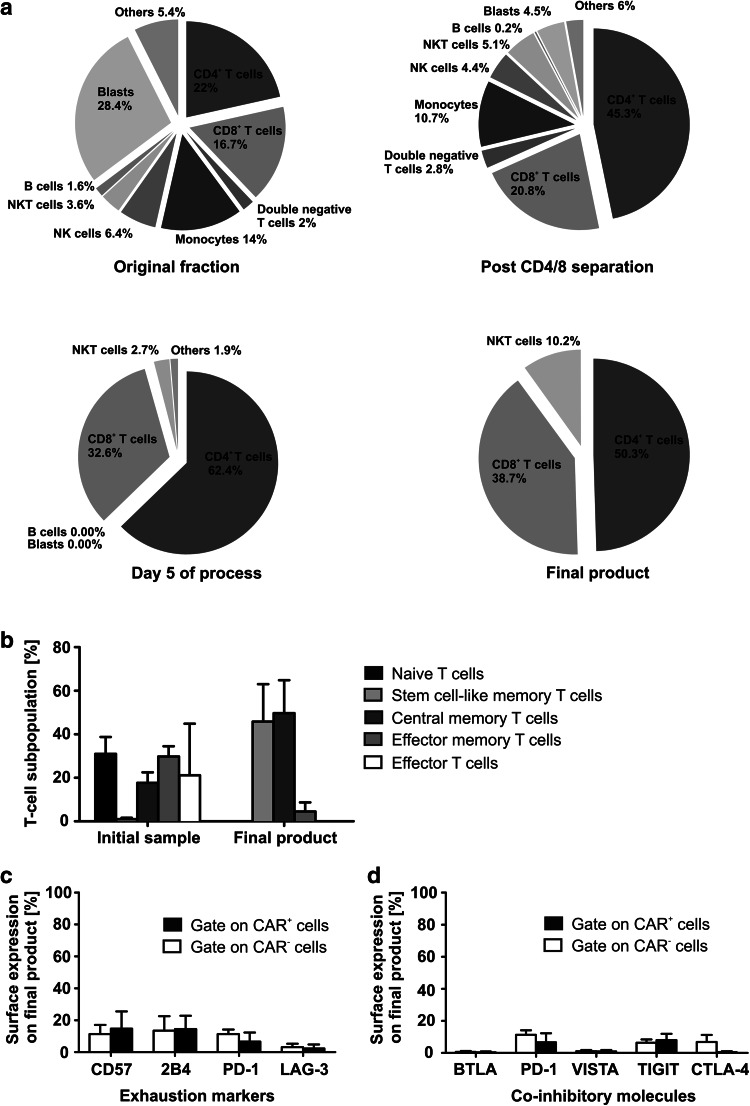
Cellular composition was evaluated by flow cytometry prior to and after CD4/CD8 separation on day 0 as well as on day 5 and at the end of the process (Fig. 4a). Gating strategies/exemplary plots are shown in Supplementary Figure 4. The patient-derived initial product showed a huge variety in cellular composition including high blast and monocyte counts and a mean of only 22% CD4+ T cells (range 2.74–39.5%) and 16.73% CD8+ T cells (range 1.7–28%). Although CD4/8 separation led to enrichment of both cell types (mean of 45.25% for CD4+ cells and 20.84% for CD8+ cells), monocytes and blasts were still present in the culture after separation. Already after five days of IL-7/-15-based expansion, no B cells, leukemic blasts or monocytes were detectable anymore. The final product contained a robust cellular composition with a mean of 50.3% CD4+ and 38.7% CD8+ cells. A mean of 10.2% of the cells were NKT cells. The T-cell phenotype of the initial and the final product was analyzed by flow cytometric stain for CD62L, CD45RO and CD95 (Fig. 4b). Gating strategy is shown in Supplementary Figure 5. Whereas the initial product showed a broad variety and rather exhausted phenotype, the final CAR T-cell product consisted mostly of Tcm and Tscm cells which are known to have excellent proliferative potential and functionality in vivo. No increased expression of exhaustion/senescence markers CD57, 2B4, PD-1 and LAG-3 was measurable on the CAR+ or CAR− T cells of the final product (Fig. 4c). Only isolated expression of TIM-3 was found on CAR− and CAR+ T cells (data not shown). Low sensitivity to inhibitory signals caused by leukemic cells was detected by low surface expression of inhibitory checkpoint molecules BTLA, PD-1, VISTA, TIGIT and CTLA-4 (Fig. 4d). A significantly different expression between CAR+ and CAR− cells was not detectable.
Fig. 4.
Cellular composition and T-cell phenotype of CAR T cells. a Despite broad variety in cellular composition of the initial blood sample and after CD4/8 separation, a robust final product was achieved consisting of CD4+, CD8+ T cells and NKT Cells. No blasts or B cells were detectable on day 5 of the process and in the final product. b Despite a rather exhausted phenotype of the initial blood product, a Tcm and Tscm T-cell phenotype of the final product was reached. Effector T cells: CD62L−, CD45RO−, CD95+; effector memory T cells: CD62L−, CD45RO+, CD95+; central memory T cells: CD62L+, CD45RO+, CD95+; stem cell-like memory T cells: CD62L+, CD45RO−, CD95+; naïve T cells: CD62L+, CD45RO−, CD95−. c, d Anti-CD19 CAR T cells were stained for extracellular expression of T-cell exhaustion/senescence markers and co-inhibitory molecules. No significant differences in expression between CAR+ and CAR− T cells were detectable. Wilcoxon signed rank test was performed to determine statistical significance
TH1 driven specific functionality of anti-CD19 CAR T cells
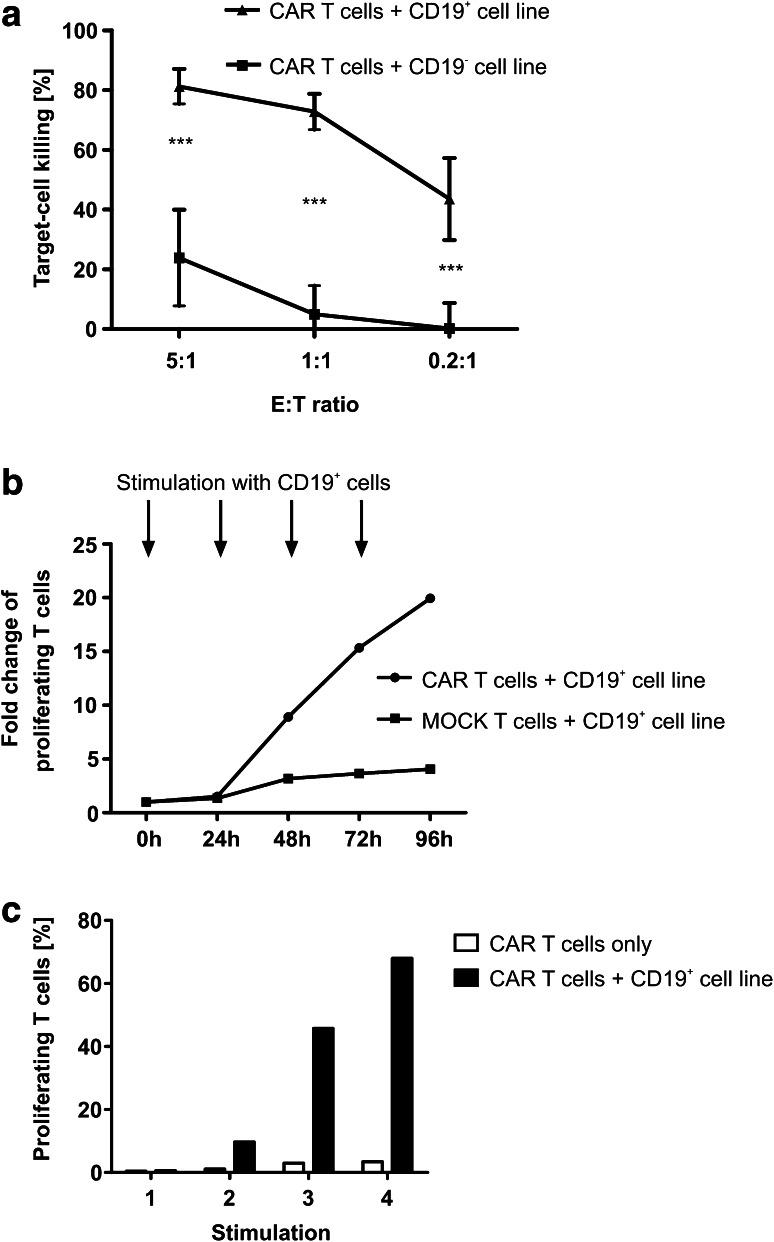
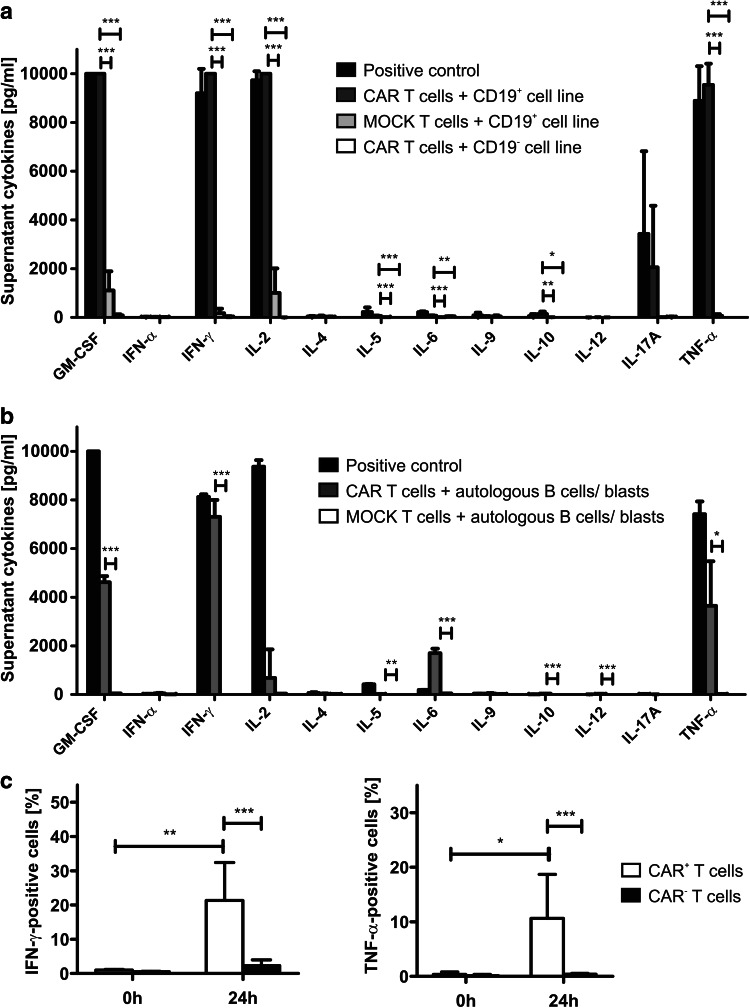
After co-culture with a CD19+ cell line, anti-CD19 CAR T cells dose-dependently killed up to 80% of the target cells at an E:T ratio of 5:1 (Fig. 5a). Anti-CD19 CAR T cells co-cultured with a CD19− cell line served as control. CAR T cells proliferated up to 20-fold when co-cultured with a CD19+ cell line even after several re-stimulations (Fig. 5b, c). In a second step, cytokine release was measured in the co-culture supernatant by flow cytometry (Fig. 6a). After contact to a CD19+ cell line, CAR T cells were able to secrete GM-CSF, IFN-γ, IL-2, and TNF-α, indicating a strong TH1 response of the CAR T cells. IL-5 and IL-10 were also secreted significantly higher compared to negative controls but stayed at very low concentrations (mean of 63.36 pg/ml for IL-5, mean of 138.79 pg/ml for IL-10). Interestingly, although many clinical studies detect high amounts of IL-6 after anti-CD19 CAR T-cell infusion, a significant but only very low production of IL-6 was detectable after co-culture with target cell lines (mean 72.07 pg/ml). PMA/Ionomycin-stimulated T cells served as positive, CAR T cells co-cultured with a CD19− cell line and untransduced T cells co-cultured with a CD19+ cell line served as negative controls. For further analysis, we co-cultured CAR T cells with autologous target cells using the negative fraction derived from CD4/8 separation on day 0 of the process (termed here “autologous B cells/blasts”). The negative fraction consisted of mainly CD19+ B cells and CD19+/CD10+ leukemic blasts (Supplementary Figure 2). When co-cultured with these autologous B cells/blasts, CAR T cells showed a similar TH1-based cytokine release profile (Fig. 6b). Interestingly, compared to experiments with target cell lines, the supernatant of those co-cultures showed a higher amount of IL-6 (mean of 1702.67 pg/ml) supporting recent findings that not the CAR T cells but other immune cells such as antigen-presenting cells might be responsible for the high IL-6 levels associated with CAR T-cell treatment. PMA/Ionomycin-stimulated T cells served as positive, untransduced T cells co-cultured with autologous B cells/blasts as negative control. To confirm the specific cytokine release of CAR T cells, an intracellular cytokine stain of CAR T cells after contact to a CD19+ target cell line was performed for the two most relevant TH1 cytokines IFN-γ and TNF-α. A significant increase of IFN-γ- and TNF-α-positive cells was detected among CAR+ cells after contact to the antigen, whereas CAR− cells secreted IFN-γ and TNF-α to a significantly lower amount (Fig. 6c).
Fig. 5.
Functionality analysis of CAR T cells. a CAR T cells showed dose-dependent killing of the CD19+ leukemic target cell line Raji determined by flow cytometry-based functionality assay (n = 4 CAR T-cell products, technical duplicates or triplicates). CD19− cell lines U-266 and Molm-13 served as control (n = 3 CAR T-cell products, technical duplicates or triplicates). Two-tailed unpaired t test was performed to determine statistical significance. b Proliferation results of patient-derived CAR T cells: CAR T cells or untransduced T cells were co-cultured with CD19+ cell line Jeko and re-stimulated with the same cell line every 24 h. Proliferation assay was performed before stimulation and every 24 h after stimulation. The figure shows fold change of proliferating CAR T cells after co-culture compared to T cells only. c Proliferation in percentage of proliferating cells 24 h after stimulation 1–4 compared to CAR T cells without stimulation
Fig. 6.
Functionality and target cell-dependent TH1 cytokine release of CAR T cells. a CAR T cells were co-cultured with the CD19+ target cell line Raji. After 24 h, supernatant was analyzed for cytokine concentrations using a flow cytometry-based assay. Untransduced T cells of the same donor and CD19− cell lines (U-266 and Molm-13) served as negative, stimulation with PMA/ionomycine as positive control (n = 3 CAR T-cell products, technical duplicates or triplicates). b Exemplary cytokine release assay of run CAR003: CAR T cells were co-cultured with the negative fraction of CD4/CD8 separation performed on day 0 of the process. After 24 h, supernatant was analyzed for cytokine concentrations using a flow cytometry-based assay. Untransduced T cells of the same patient co-cultured with the same autologous target-cell fraction served as negative, stimulation with PMA/ionomycine as positive control (technical triplicates). c Intracellular cytokine stain of anti-CD19 CAR T cells for IFN-γ and TNF-α was performed 24 h after contact to the CD19+ cell line Raji. A significant increase of IFN-γ and TNF-α positivity was detected for CAR+ cells (n = 4 CAR T-cell products). Ratio paired two-tailed t test was performed to determine statistical significance
Discussion
Several phase I/II trials have demonstrated strong anti-leukemic potency of T cells genetically modified to express a chimeric antigen receptor (CAR) against the B-cell lineage antigen CD19 [5, 6]. Anti-CD19 CAR T cells are able to induce complete remissions in up to 95% of patients suffering from relapsed/refractory B-cell malignancies after several courses of second or even third/fourth line therapy [22]. A major hurdle that limited broad availability of this highly effective treatment was the lack of simplified production of a functionally active CAR T-cell product. In this study, we provide a solution to produce CAR T cells against CD19+ malignancies in GMP centers with clean room grade B/C for closed steps and grade A for open steps.
We used challenging blood products from pediatric ALL patients with high variability in cellular composition including high blast counts and low T-cell frequencies. A balanced and stable CD4/8 ratio of the final CAR T-cell product was achieved. No T-cell exhaustion was seen in the final T-cell product. The majority of T cells in the product showed a Tscm/Tcm phenotype determined by expression of CD62L and CD95 or additional expression of CD45RO, respectively. Although repeated stimulation does not reflect in vivo persistence and clinical outcome, we confirm a sustained proliferation after short term re-stimulation in vitro.
The generation of anti-CD19 CAR T cells is a time-consuming and technically challenging procedure consisting of T-cell separation, activation, transduction with a retro- or lentiviral vector and expansion. Current standard manufacturing protocols use large culture flasks or bioreactors for expansion and include many open steps with hands-on time of the personnel [19]. To facilitate CAR T-cell generation, the lentiviral transduction process on the CliniMACS Prodigy was previously described by Mock et al. and Lock et al. [20, 23]. Mock and colleagues used healthy donor leukapheresis to compare the performance of nanomatrix stimulation with conventional bead-based stimulation [20]. Lock et al. used an anti-CD20 CAR with a variety of starting materials (leukapheresis, buffy coat, a single sample whole blood—either derived from healthy donors, melanoma or adult diffuse large B-cell lymphoma patients) [23]. Here, we transfer the process to small blood samples of pediatric patients. In contrast to Mock et al. and Lock et al., we use PBMCs of pediatric patients suffering from acute lymphoblastic leukemia which are transduced with a second generation anti-CD19 CAR virus designed for clinical application. Encouraged by Lock and colleagues who already described one successful run with a blood sample derived from an adult melanoma patient [23], we used 100–120 ml of peripheral blood from pediatric ALL patients. 2/4 initial samples contained residual leukemic blasts. Since the transfer of the protocol towards a clinical ALL trial is planned, the present study was designed to achieve closest possible approximation of the starting material to reflect the clinical situation of the pediatric ALL patients. Due to ethical restrictions, for research use only, a leukapheresis from children has not been possible. For clinical studies, especially in patients who will not tolerate blood sampling of 100 ml or suffer from very low T-cell counts, a leukapheresis still might be inevitable to standardize T-cell counts and guarantee sufficient CAR T-cell numbers. Nevertheless, our work describes the potential of this process to generate functional CAR T-cell products even from very small, unstandardized and challenging pediatric ALL patient samples.
T-cell separation, activation, transduction, expansion and harvest were performed in a closed sterile tubing set. Open steps are isolation of PBMCs, preparation of buffers, vector and media and cryopreservation of the final product. All reagents are connected to the device by spiking or sterile tube welding. Labeling of the cells with anti-CD4/-CD8 microbeads was performed in the culture chamber followed by separation with the incorporated MACS column and the magnet of the device. Small scale experiments from healthy donors showed no significant impact of CD4/CD8 separation concerning expansion, transduction and T-cell phenotype. Nevertheless, B-cell counts of the starting fraction were significantly higher in the un-separated samples (Supplementary Figure 3). Especially for patients with residual leukemic blasts, CD4/CD8 separation is regarded as a safety procedure to decrease blast counts in the culture. Although sufficient enrichment of the patient-derived CD4/CD8 T cells was observed after magnetic separation, effort needs to be spent on improving CD4/8 purity especially of samples with high blast frequencies. Nevertheless, already five days after separation, no malignant cells or B cells were detectable in the culture chamber anymore, proofing complete elimination of leukemic cells and thus excluding a re-infusion of leukemic cells. Activation was performed using a modified CD3/CD28 T-cell reagent, which is not based on conventional magnetic bead technology but uses a polymeric nanomatrix structure which is conjugated to CD3/28 agonist. Thus, it does not require a magnetic bead removal step but is washed out by centrifugation two days later. This procedure is anyway required to remove residual lentivirus from the culture one day after transduction. As lentiviral vectors are known to have a lower risk for mutational oncogenesis than γ-retroviral vectors [24], transduction was performed with a lentiviral vector encoding the anti-CD19 CAR one day after activation and yielded sufficient transduction rates. Because the process was initially standardized for 1 × 108 T cells on day 0, a viral concentration of 2 × 108 particles/100 ml was used to reach an MOI of 2. Here, due to extremely high variety of T-cell counts and to achieve a constant concentration of viral particles, we transduced with a standard dose of 2 × 108 viral particles, irrespective of T-cell counts. Estimated MOI rates and corresponding transduction rates are shown in Supplementary Table 2. Despite high variability in MOI rates, the vector copy number did not exceed 2.3 per T cell, and thus met the authority’s requirements. For use in clinical trials the T-cell count can be adjusted to 1 × 108 T cells/100 ml with a viral concentration of 2 × 108 particles/100 ml, resulting in a standardized MOI of 2. In contrast to other manufacturing protocols [25], CD4 and CD8 T cells were not cultured separately but within one culture chamber in the presence of IL-7 and IL-15. T-cell proliferation within the culture was up to > 100 fold despite challenging blood samples with low initial T-cell counts. Nevertheless, especially in the presence of blasts, T-cell numbers might decrease to levels below detection limit and could only be identified in the sampling pouch after additional re-concentration. This decrease in T-cell count might be caused by blast destruction and the formation of free DNA. Fortunately, T-cell counts robustly recovered from day 5 of the process until final formulation.
The final CAR T-cell product described here consists of highly functional CAR T cells which were able to specifically lyse CD19+ cell lines and secrete IFN-γ and TNF-α upon target-cell contact. Interestingly, IL-6 was not released by CAR T cells co-cultured with leukemic target-cell lines underlining recent findings that not CAR T cells but antigen-presenting cells might be responsible for high IL-6 levels observed during CAR-triggered cytokine release syndrome [26]. We recently reported that the expression of exhaustion/activation marker PD-1 is significantly higher on ALL patients’ T cells compared to healthy donors and is induced by T-cell attack against blasts [17]. The CAR T cells produced with the current protocol expressed rather low levels of inhibitory checkpoint molecules such as PD-1, CTLA-4, 2B4 and LAG-3 on their surface indicating a low susceptibility for inhibitory signals derived by the malignant cell and confirming their non-exhausted, highly functional phenotype.
In conclusion, a partly automated protocol for GMP-compatible generation of CAR T cells in a closed system was developed which yields high CAR T-cell counts even from small pediatric patient blood samples. In case a leukapheresis cannot be performed due to ethical restrictions or the current clinical condition of the patient, this protocol enables the generation of a sufficient number of CAR T cells even from 100 to 120 ml peripheral blood. The final CAR T-cell product has a robust CD4/8 composition with a favorable Tcm and Tscm phenotype, good functionality and low expression of inhibitory checkpoint molecules. The manufacturing protocol described here will enable safe multi-center manufacturing of CAR T cells. The process fulfils current requirements of regulatory authorities and simplifies the complex procedure of CAR manufacturing while minimizing the risk for contamination and operator-to-operator variability. In vivo efficacy and safety of the cells produced here will be evaluated in subsequent clinical trials.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all patients and their parents for participating in the study. Nadine Stoll, Tanja Weisser, Nicola Habjan, Florian Jurgeleit, Carola Barth and Daniela Mauer are acknowledged for excellent technical assistance. The authors thank Katharina Drechsel and Nadine Mockel-Tenbrinck for helpful advice.
Abbreviations
- 6-MP
6-Mercaptopurine
- 6-TG
6-Thioguanine
- ARA-C
Cytarabine
- B-NHL
B-cell non-Hodgkin’s lymphoma
- cALL
Common acute lymphoblastic leukemia
- CNS
Central nervous system
- DNR
Daunorubicin
- FMO
Fluorescence minus one
- HIDAC
High-dose cytarabine
- HSA
Human serum albumin
- MOI
Multiplicity of infection
- MTX
Methotrexate
- PB
Peripheral blood
- PEG-ASP
Pegylated asparaginase
- Pre-B ALL
B-precursor acute lymphoblastic leukemia
- Tcm
Central memory T cells
- TCT
T-cell transduction
- Teff
Effector T cells
- Tem
Effector memory T cells
- Tscm
Stem cell-like memory T cells
- VCN
Vector copy number
- VCR
Vincristine
- VP16
Etoposide
Author contributions
Experiments were designed by TF, FB and ADK; the automated process was developed by ADK and MA; patient samples were provided by TF, SW and MD; experiments were performed by FB, DS and TK; JF set up experiments and provided protocols; RL provided healthy donor starting fractions and human serum. Data analysis was done by FB, DS and TF; the manuscript was written by FB and TF and was reviewed by all co-authors.
Funding
This work was supported by Elterninitiative Ebersberg, Elterninitative Intern3 and Bettina Braeu Stiftung, Adler Stiftung and the Care for Rare Foundation. Miltenyi Biotec provided reagents free of charge.
Compliance with ethical standards
Conflict of interest
Andrew Didier Kaiser and Mario Assenmacher are employees of Miltenyi Biotec. This work has been performed as a collaboration between Tobias Feuchtinger, Franziska Blaeschke and Miltenyi Biotec. Miltenyi Biotec provided reagent free of charge. All other authors declare that they have no conflict of interest.
Ethical approval and ethical standards
This study was approved by the Institutional Ethical Review Board (“Ethikkommission bei der LMU München”), approval number 435 − 15, and was performed in accordance with the Declaration of Helsinki.
Informed consent
Patients/their representatives gave written informed consent according to the guidelines and approval of the Institutional Ethical Review Board.
Cell line authentication
Cell lines Raji, Jeko, Molm-13, U-266 were routinely tested for identity by short-tandem repeat analyses (DSMZ, Braunschweig, Germany).
Footnotes
Poster presentation at 58th ASH Annual Meeting and Exposition (American Society of Hematology), December 3–6 2016, San Diego, USA [1].
Poster presentation at Cellular Therapy, International Symposium Erlangen, March 16–17 2017, Erlangen, Germany [2].
Oral presentation at 43rd Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT), March 26–29 2017, Marseille, France [3].
Oral presentation at Annual Meeting of Paediatrische Arbeitsgemeinschaft für Stammzelltransplantation and Zelltherapie (PAS&ZT), September 14–15 2017, Hamburg, Germany [4].
References
- 1.Blaeschke F, Kaeuferle T, Feucht J, Weber D, Lotfi R, Kaiser A, Assenmacher M, Doering M, Feuchtinger T (2016) Defined central memory and stem memory T cell phenotype of CD4 and CD8 CAR T cells for the treatment of CD19+ acute lymphoblastic leukemia in an automated closed system. In: 58th ASH annual meeting and exposition. Blood, 128(22) (Abstract 4558)
- 2.Stenger D, Blaeschke F, Kaeuferle T, Willier S, Lotfi R, Kaiser A, Assenmacher M, Doering M, Feucht J, Feuchtinger T (2017) Automated generation of central memory and stem cell-like memory CD19-specific CAR T cells in a closed, GMP compatible system. In: Cellular therapy, international symposium Erlangen, Erlangen (Abstract 134)
- 3.Blaeschke F, Stenger D, Kaeuferle T, Willier S, Lotfi R, Kaiser A, Assenmacher M, Doering M, Feucht J, Feuchtinger T (2017) CD19-specific CAR T cells with a central memory and stem memory phenotype—automated generation in a closed, GMP-compatible system from peripheral blood of pediatric patients with acute lymphoblastic leukemia. In: 43rd annual meeting of the European Society for Blood and Marrow Transplantation (EBMT), Marseille (Abstract)
- 4.Blaeschke F, Stenger D, Kaeuferle T, Willier S, Lotfi R, Kaiser A, Assenmacher M, Doering M, Feucht J, Feuchtinger T (2017) Induction of a central memory and stem cell memory phenotype in functionally active CD4+ and CD8+ CAR T cells produced in an automated GMP system for the treatment of CD19+ acute lymphoblastic leukemia. In: Annual meeting of Paediatrische Arbeitsgemeinschaft fuer Stammzelltransplantation and Zelltherapie (PAS&ZT), Hamburg (Abstract) [DOI] [PMC free article] [PubMed]
- 5.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, Steinberg SM, Stroncek D, Tschernia N, Yuan C, Zhang H, Zhang L, Rosenberg SA, Wayne AS, Mackall CL. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ, Hughes MS, Sherry RM, Raffeld M, Feldman S, Lu L, Li YF, Ngo LT, Goy A, Feldman T, Spaner DE, Wang ML, Chen CC, Kranick SM, Nath A, Nathan DA, Morton KE, Toomey MA, Rosenberg SA. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orlowski RJ, Porter DL, Frey NV. The promise of chimeric antigen receptor T cells (CARTs) in leukaemia. Br J Haematol. 2017;177(1):13–26. doi: 10.1111/bjh.14475. [DOI] [PubMed] [Google Scholar]
- 10.Maude S, Barrett DM. Current status of chimeric antigen receptor therapy for haematological malignancies. Br J Haematol. 2016;172(1):11–22. doi: 10.1111/bjh.13792. [DOI] [PubMed] [Google Scholar]
- 11.Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3(4):388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, Sussman R, Lanauze C, Ruella M, Gazzara MR, Martinez NM, Harrington CT, Chung EY, Perazzelli J, Hofmann TJ, Maude SL, Raman P, Barrera A, Gill S, Lacey SF, Melenhorst JJ, Allman D, Jacoby E, Fry T, Mackall C, Barash Y, Lynch KW, Maris JM, Grupp SA, Thomas-Tikhonenko A. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5(12):1282–1295. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacoby E, Nguyen SM, Fountaine TJ, Welp K, Gryder B, Qin H, Yang Y, Chien CD, Seif AE, Lei H, Song YK, Khan J, Lee DW, Mackall CL, Gardner RA, Jensen MC, Shern JF, Fry TJ. CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity. Nat Commun. 2016;7:12320. doi: 10.1038/ncomms12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Popplewell LL, Wagner JR, Naranjo A, Blanchard MS, Mott MR, Norris AP, Wong CW, Urak RZ, Chang WC, Khaled SK, Siddiqi T, Budde LE, Xu J, Chang B, Gidwaney N, Thomas SH, Cooper LJ, Riddell SR, Brown CE, Jensen MC, Forman SJ. Phase 1 studies of central memory-derived CD19 CAR T-cell therapy following autologous HSCT in patients with B-cell NHL. Blood. 2016;127(24):2980–2990. doi: 10.1182/blood-2015-12-686725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17(10):1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biasco L, Scala S, Basso Ricci L, Dionisio F, Baricordi C, Calabria A, Giannelli S, Cieri N, Barzaghi F, Pajno R, Al-Mousa H, Scarselli A, Cancrini C, Bordignon C, Roncarolo MG, Montini E, Bonini C, Aiuti A. In vivo tracking of T cells in humans unveils decade-long survival and activity of genetically modified T memory stem cells. Sci Transl Med. 2015;7(273):273ra213. doi: 10.1126/scitranslmed.3010314. [DOI] [PubMed] [Google Scholar]
- 17.Feucht J, Kayser S, Gorodezki D, Hamieh M, Doring M, Blaeschke F, Schlegel P, Bosmuller H, Quintanilla-Fend L, Ebinger M, Lang P, Handgretinger R, Feuchtinger T. T-cell responses against CD19+ pediatric acute lymphoblastic leukemia mediated by bispecific T-cell engager (BiTE) are regulated contrarily by PD-L1 and CD80/CD86 on leukemic blasts. Oncotarget. 2016;7(47):76902–76919. doi: 10.18632/oncotarget.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, Sadelain M, Adusumilli PS. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Investig. 2016;126(8):3130–3144. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollyman D, Stefanski J, Przybylowski M, Bartido S, Borquez-Ojeda O, Taylor C, Yeh R, Capacio V, Olszewska M, Hosey J, Sadelain M, Brentjens RJ, Riviere I. Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy. J Immunother. 2009;32(2):169–180. doi: 10.1097/CJI.0b013e318194a6e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mock U, Nickolay L, Philip B, Cheung GW, Zhan H, Johnston IC, Kaiser AD, Peggs K, Pule M, Thrasher AJ, Qasim W. Automated manufacturing of chimeric antigen receptor T cells for adoptive immunotherapy using CliniMACS prodigy. Cytotherapy. 2016;18(8):1002–1011. doi: 10.1016/j.jcyt.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Schneider D, Xiong Y, Wu D, Nlle V, Schmitz S, Haso W, Kaiser A, Dropulic B, Orentas RJ. A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines. J Immunother Cancer. 2017;5:42. doi: 10.1186/s40425-017-0246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, Bleakley M, Brown C, Mgebroff S, Kelly-Spratt KS, Hoglund V, Lindgren C, Oron AP, Li D, Riddell SR, Park JR, Jensen MC. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322–3331. doi: 10.1182/blood-2017-02-769208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lock D, Mockel-Tenbrinck N, Drechsel K, Barth C, Mauer D, Schaser T, Kolbe C, Al Rawashdeh W, Brauner J, Hardt O, Pflug N, Holtick U, Borchmann P, Assenmacher M, Kaiser A. Automated manufacturing of potent CD20-directed chimeric antigen receptor T cells for clinical use. Hum Gene Ther. 2017;28(10):914–925. doi: 10.1089/hum.2017.111. [DOI] [PubMed] [Google Scholar]
- 24.Montini E, Cesana D, Schmidt M, Sanvito F, Bartholomae CC, Ranzani M, Benedicenti F, Sergi LS, Ambrosi A, Ponzoni M, Doglioni C, Di Serio C, von Kalle C, Naldini L. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J Clin Investig. 2009;119(4):964–975. doi: 10.1172/JCI37630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, Robinson E, Steevens NN, Chaney C, Soma L, Chen X, Yeung C, Wood B, Li D, Cao J, Heimfeld S, Jensen MC, Riddell SR, Maloney DG. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Investig. 2016;126(6):2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett DM, Singh N, Hofmann TJ, Gershenson Z, Grupp SA (2016) Interleukin 6 is not made by chimeric antigen receptor T cells and does not impact their function. In: 58th ASH annual meeting and exposition. Blood, 128(22) (Abstract 654)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.