Abstract
Background
To characterize the expression of co-signaling molecules PD-L1, PD-1, and B7-H3 in cutaneous squamous cell carcinoma (cSCC) by immune status.
Methods
We retrospectively analyzed 66 cases of cSCC treated with surgical resection from 2012 to 2015. Immunostained tumor sections were analyzed for percent of tumor cells expressing PD-L1 (Tum-PD-L1%), B7-H3 (Tum-B7-H3%), density of peri and intratumoral CD8 T cells (CD8 density), proportion of CD8 T cells expressing PD-1 (CD8-PD-1%) and of tumor-infiltrating immune cells (TII) expressing PD-L1 (TII-PD-L1%).
Results
Of 66 cases, 42 were immunocompetent, 24 immunosuppressed (13 organ transplant, 8 HIV+, 3 other). Defining positive expression at > 5%, 26% of tumors were positive for PD-L1, 85% for B7-H3, 80% had CD8 T cells that expressed PD-1 and 55% had TII that expressed PD-L1. Tum-B7-H3% was significantly higher (median 60 vs. 28%, p = 0.025) in immunocompetent vs. immunosuppressed patients, including when factoring in cause of immunosuppression. No significant difference in Tum-PD-L1%, TII-PD-L1%, CD8 density, or CD8-PD-1% was observed. Tumors from HIV+ patients lacked PD-L1 expression, and had lower B7-H3% (median 2.5 vs. 60%, p = 0.007), and higher CD8 density (median 75% vs. 40%, p = 0.04) compared to immunocompetent patients. Higher tumor grade (Rs = 0.34, p = 0.006) and LVI (Rs = 0.61, p < 0.001) were both associated with higher Tum-PD-L1%.
Conclusion
cSCC showed expression of PD-L1 on tumor in 26% of cases, and high tumor B7-H3 expression (85%) and PD-1 expression on CD8 TILs (80%). Tumor B7-H3 expression was significantly higher in immunocompetent vs. immunosuppressed patients, largely driven by very low expression in HIV+ patients.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2138-8) contains supplementary material, which is available to authorized users.
Keywords: Squamous cell carcinoma, Cutaneous, PD-1, PD-L1, B7-H3
Introduction
There are an estimated 700,000 cases of cutaneous squamous cell carcinoma (cSCC) each year in the United States [1]. While most patients have an excellent prognosis, as many as 12,572 patients per year present with nodal metastasis and an estimated 8791 patients will die of cSCC each year in the USA [1, 2]. Etiological risk factors include ultraviolet light exposure as well as immunosuppression, for example organ transplant patients on immunosuppressive therapy are up to 100 times more likely to develop non-melanoma skin cancers than the general population [3]. Treatment options are very limited for patients with unresectable or metastatic cSCC. Current systemic options include traditional cytotoxic chemotherapy regimens used for other tumors of squamous cell carcinoma histology, as well as agents targeting the epidermal growth factor receptor (EGFR). Data on the efficacy of traditional chemotherapy for advanced cSCC are limited, and most published studies are case reports or small case series [4]. A prospective phase II trial of IgG1 mAb Cetuximab, which targets EGFR, showed a RR of 27% and DCR of 70%; however, duration of activity was very short with a median PFS and OS of only 4 and 8 months, respectively [5]. There is a desperate need in advanced cSCC for better systemic therapy options.
The programmed death ligand 1 (PD-L1): Programmed death 1 (PD-1) pathway has proven to be important in tumor immunology. PD-1 belongs to the CD28 family and is expressed on the cell surface of B and T cells as well as natural killer cells and dendritic cells [6]. PD-L1 (B7-H1) is a member of the B7 superfamily and can be expressed by immune cells as well as many solid tumors including melanoma, renal cell carcinoma, lung, GI, bladder, and head and neck cancers, as well as hematological malignancies [7–9]. Ligation of PD-1 on effector T cells by PD-L1 expressed by tumor can lead to T cell anergy and death, thus protecting the tumor from immune mediated attack [10–13]. Manipulation of this co-signaling pathway by drugs blocking the interaction of PD-1 and PD-L1 has led to significant clinical efficacy, with anti-PD-1 monoclonal antibodies now approved in NSCLC, melanoma, squamous cell carcinoma of the head and neck, and renal cell cancers [14–16]. B7-H3 is a transmembrane glycoprotein which is also part of the B7 superfamily. This co-signaling molecule is less well characterized, and has been associated with stimulatory and inhibitory effects [17]. Similar to PD-L1 it is expressed by numerous cancers including breast, pancreatic, urothelial, ovarian, renal, and head and neck, and in the majority of cases expression has been associated with more aggressive features and poor prognosis, [17, 18] Enoblituzumab, a monoclonal antibody targeting B7-H3 was well tolerated in a phase I study in advanced solid tumors with some observed tumor regression, and is being studied in ongoing clinical trials both as a single agent and in combination with other agents [19].
Expression of these co-signaling molecules have not been well characterized in cSCC and the effect of immune status on expression has not been evaluated. Therefore we evaluated tumor samples from both immunocompetent and immunosuppressed patients with cSCC for tumor expression of PD-L1 and B7-H3, and PD-1 and PD-L1 expression on tumor-infiltrating immune cells.
Methods
After obtaining approval by the University of Maryland, Baltimore Institutional Review Board (IRB), we conducted a retrospective study of patients with cSCC treated with surgical resection at the University of Maryland Medical Center between 2012 and 2015. Baseline demographics collected by chart review included anatomic primary site, stage, age, gender, race, and immune status. Pathologic features including tumor diameter, depth of invasion, differentiation, presence of perineural invasion (PNI), and lymph node involvement were collected. Patients’ immune status was characterized as immunocompetent, HIV-positive, and other immunosuppressed (organ transplant and other reasons for immunosuppression).
Pathology methods
Following review of the hematoxylin and eosin-stained sections from the excision specimens, representative blocks were selected for immunohistochemical staining. Slides were prepared using 5 µm sections of formalin-fixed, paraffin-embedded tissue, deparaffinized, and rehydrated in ethanol. Antigen retrieval was performed by heating tissue sections in 1 mmol/L EDTA (pH 8) to 121 °C using a Digital Decloaking Chamber (Biocare Medical), cooling to 90 °C, and incubating for 5 min. Immunostaining was performed on the Autostainer Plus (Dako). Briefly, endogenous peroxidase was blocked with Endogenous Blocking Solution (Dako); then the sections were washed twice and incubated in Serum-Free Protein Block (Dako) for 5 min; then incubated for 60 min with the following antibodies: purified goat anti-human B7-H3 antibody (clone MM0104-2OJ12, Abcam Inc.) diluted 1:400 with DaVinci antibody diluents (Abcam), purified anti-human PD-1 antibody (clone NAT105, Cell Marque) diluted 1:40, purified anti-human CD8 antibody (clone 2B11, Leica Biosystems) diluted 1:100, and purified anti-human PD-L1 antibody (clone SP142, Ventana Medical Systems, Inc.) diluted 1:50. Tissue sections were incubated in probe from Goat horseradish peroxidase Polymer kit (Biocare Medical #GHP516L) for 15 min. Immunostained sections were counterstained with hematoxylin, dehydrated in ethanol, cleared in xylene, and coverslipped using mounting medium.
The immunostained tumor sections were evaluated for the expression of all four markers in the following manner:
Tumor-infiltrating lymphocytes expressing CD8 were scored based on the density of the lymphoid infiltrate as follows: no CD8-positive lymphocytes: 0; 1–10 CD8-positive lymphocytes per high power field: 1; 11–20 CD8-positive lymphocytes per high power field: 2; 21–30 CD8-positive lymphocytes per high power field: 3, more than 30 CD8-positive lymphocytes per high power field: 4. The percentage of peritumoral and tumor stroma infiltrated by CD8-positive lymphocytes was scored separately in 5% increments. The density of CD4 expressing tumor-infiltrating lymphocytes was scored the same way as CD8 tumor-infiltrating lymphocytes
Tumor- infiltrating CD8 T cells which expressed PD-1 and tumor-infiltrating CD4 T cells which expressed PD-1 were scored based on the density of the lymphoid infiltrate as follows: no PD-1 positive lymphocytes—0, 1–10 PD-1 positive lymphocytes per high power field—1, 11–20 PD-1 positive lymphocytes per high power field—2, 21–30 PD-1 positive lymphocytes per high power field—3, more than 30 PD-1 positive lymphocytes per high power field—4. The percentage of CD8-positive tumor-infiltrating lymphocytes which co-expressed PD-1 was scored in 5% increments.
The percentage of tumor cells that stained positive for B7-H3 was quantified in 5% increments. In addition, the intensity of staining was scored from 0 (negative) to 1 (weak) to 2 (moderate) to 3 (intense). Tumors with < 5% of cells staining for B7-H3 were called negative. Peritumoral neovasculature expression of B7-H3 was scored similarly from 0 to 3 based on intensity of staining.
The percentage of tumor cells that stained positive for PD-L1 was quantified in 5% increments. In addition, the intensity of staining was scored from 0 (negative) to 1 (weak) to 2 (moderate) to 3 (intense). Tumors with < 5% of cells staining for PD-L1 were considered negative.
Tumor- infiltrating immune cells (TII) which expressed PD-L1 were scored based on the density of the immune cell infiltrate as follows: no PD-L1 positive immune cells—0, 1 to 10 PD-L1 positive immune cells per high power field—1, 11–20 PD-L1 positive immune cells per high power field—2, 21–30 PD-L1 positive immune cells per high power field—3, more than 30 PD-L1 positive immune cells per high power field—4. The percentage of tumor-infiltrating immune cells which expressed PD-L1 was scored in 5% increments.
The pathologist reviewers were blinded to patients’ immune status. Examples of immunostained tumor sections are found in Fig. 1. In patients who were immunosuppressed due to solid organ transplant, a search of the surgical pathology archives was performed to identify representative surveillance biopsies of the allografts. Immunohistochemical staining and analysis of immune cell infiltrates within the allografts was performed in the manner described above.
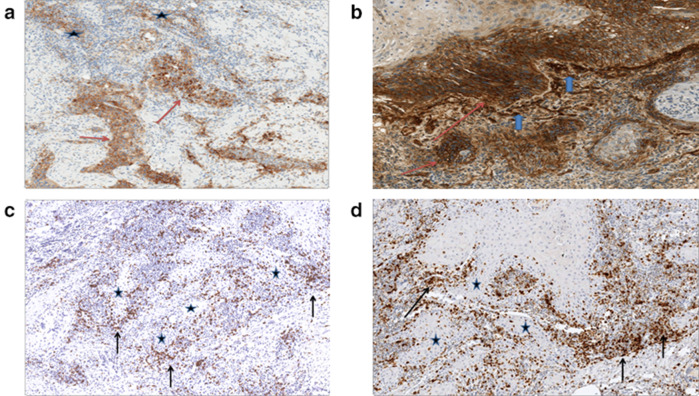
Fig. 1.
Immunostained tumor sections. a PD-L1 expression by the tumor (red arrows) and by tumor-infiltrating immune cells (stars). b B7-H3 expression by the tumor at the advancing tumor edge (red arrows) and in the neovasculature at interface with tumor (blue arrows). c CD8-positive lymphocytes (arrows), Tumor (stars). d PD-1 expressing lymphocytes in the same distribution as CD8-positive lymphocytes (arrows), Tumor (stars)
Statistical methods
Categorical variables were summarized as frequency distributions with indication of the relative frequency and its 95% confidence limits in parenthesis when relevant. Distributions of categorical variables were compared between groups using the Chi-square test or, in case of a 2 × 2 table, Fisher’s exact test.
Box-and-whisker plots were generated as a non-parametric representation of the distribution of a scale variable in a group; the sides of the box indicate the 1st and 3rd quartile of the population distribution with a vertical bar indicating the median. The whiskers extend to 1.5 times the height of the box or, if no case has a value in that range, to the minimum or maximum values. In case of a normally distributed variable, approximately 95% or the data are expected to lie inside the whiskers. Outlying data outside this interval are marked with an asterisk. The non-parametric Wilcoxon rank sum test statistics was used to compare the expression of co-signaling molecules among two groups, and the Kruskal–Wallis test was used for comparing three or more groups.
Co-expression of IHC markers or associations between clinical/pathological characteristics and marker expression were quantified using the non-parametric Spearman’s rank correlation coefficient, Rs, with the corresponding p-value for rejecting the null hypothesis, Rs = 0, of no statistical association between the two variables. Negative values of Rs indicate an inverse association between the two variables compared.
All statistical analyses were conducted using IBM® SPSS® Statistics, version 24.0.
Results
A total of 66 patients with cSCC who had tissue available for analysis were identified between 2012 and 2015. Baseline demographics are shown in Table 1. The male:female ratio was 2:1, and three-quarters of the patients were Caucasian (76%). Forty-two (64%) were immunocompetent and 24 (36%) were immunosuppressed. The immunosuppressed group comprised of 12 (50%) organ transplant patients, 8 (33%) who were HIV-positive, and 4 (17%) who were immunosuppressed for other reasons [CLL/lymphoma (N = 3), inflammatory bowel disease on immunosuppressants (N = 1)]. CD4 counts were available for five of the eight HIV-positive patients, the median was 173 and the range 118–554 cells/mm. Eighty-nine percent of our organ transplant patients were on two or more different immunosuppressive medications (median 2, range 1–3) at the time of their diagnosis of cSCC. Therefore, both our HIV-and organ transplant patients were significantly immunosuppressed. The primary anatomical site was the head and neck in 56% of cases, with 24% originating on the torso/extremities, and 20% from the skin in the anogenital area.
Table 1.
Patients’ characteristics
| Baseline characteristics | Patients (N = 66) |
|---|---|
| Gender | |
| Male | 44 (67%) |
| Female | 22 (33%) |
| Age (years) | |
| Median (range) | 67 (31–88) |
| Race | |
| Caucasian | 50 (76%) |
| African American | 11 (17%) |
| Other | 5 (8%) |
| Immune status | |
| Immunocompetent | 42 (64%) |
| Immunosuppressed | 24 (36%) |
| Organ transplant | 12 (18%) |
| HIV | 8 (12%) |
| Othera | 4 (6%) |
| Site | |
| Head/neck | 37 (56%) |
| Torso/extremities | 16 (24%) |
| Anogenital | 13 (20%) |
aOther = 4 patients with other etiology for immunosuppression (CLL, inflammatory bowel disease on immunosuppression)
High risk pathologic features were common, with 58% of all cases having one or more high-risk feature. In the entire cohort, 41% of patients had a tumor diameter > 2 cm and 50% had tumor thickness greater than 2 mm. Poorly differentiated histology was found in 20% of patients, perineural invasion (PNI) in 17% and lymphovascular invasion (LVI) in 12%. Tumor grade varied significantly by primary site (head/neck vs. torso/extremity vs. anogenital area, p = 0.049), with significantly higher tumor grade and a strong trend toward more patients with LVI (0.06) and PNI (0.06) in cSCC of the head and neck. The proportion of cases with at least one high-risk feature was 72% in cSCC of the head and neck as compared with 37% in the anogenital and 46% in torso/extremities (p = 0.039). Immunosuppressed patients had a greater percentage of poorly differentiated tumors and lymph node involvement while immunocompetent patients had a higher percentage of tumors with PNI and tumor diameter > 2 cm. Overall, the proportion of cases with at least one high-risk feature was the same (58%) irrespective of immune status (Table 2).
Table 2.
High risk pathological features by immune status
| Pathological feature | All patients N = 66 |
Immunocompetent N = 42 |
Immunosuppressed N = 24 |
HIV N = 8 |
Other immunosuppreseda N = 16 |
|---|---|---|---|---|---|
| Tumor diameter > 2 cm | 27 (41%) | 19 (45%) | 8 (33%) | 2 (25%) | 6 (38%) |
| Tumor thickness > 2 mm | 33 (50%) | 21 (50%) | 12 (50%) | 3 (38%) | 9 (56%) |
| PNI | 11 (17%) | 10 (24%) | 1 (4%) | 0 (-) | 1 (6%) |
| Poorly differentiated histology | 13 (20%) | 6 (14%) | 7 (29%) | 1 (13%) | 6 (38%) |
| LN involved | 2 (3%) | 0 (–) | 2 (8%) | 1 (13%) | 1 (6%) |
| LVI | 8 (12%) | 5 (12%) | 3 (13%) | 0 (0%) | 3 (19%) |
PNI perineural invasion, LVI lymphovascular invasion
aOther immunosuppressed consists of 12 organ transplant patients and 4 of other etiology (CLL, inflammatory bowel disease on immunosuppression)
Defining positive as expression in ≥ 5% of tumor cells or tumor-infiltrating immune cells (TIIs), 26% of all tumors were positive for PD-L1, 85% for B7-H3, 80% had CD8 T cells that expressed PD-1, 73% had CD4 T cells that expressed PD-1, and 55% had TIIs that expressed PD-L1. Percentage of patients with positive expression of these co-signaling molecules stratified by immune status is shown in Table 3. Among immunocompetent patients, 27% of tumors were PD-L1 positive, 88% for B7-H3, 80% had CD8 T cells that expressed PD-1, 67% had CD4 T cells that expressed PD-1, and 60% had TIIs that expressed PD-L1 Among immunosuppressed patients, 19% of tumors were PD-L1 positive, 75% for B7-H3, and 88% had CD8 T cells that expressed PD-1, and 46% had TIIs that expressed PD-L1.
Table 3.
Prevalence of expression of co-signaling molecules on tumor and tumor-infiltrating immune cells by immune status
| Patients | Tumor PD-L1 | Tumor B7-H3 | PD-1 CD8 TILs | PD-1 CD4 TILs | PD-L1 TIIs |
|---|---|---|---|---|---|
| All patients (n = 66) | 26% (17%, 37%) | 85% (74%, 92%) | 80% (69%, 88%) | 73% (61%, 82%) | 55% (43%, 66%) |
| Immunocompetent (n = 42) | 26% (15%, 41%) | 88% (75%, 95%) | 81% (67%, 90%) | 67% (52%, 79%) | 60% (44%, 73%) |
| Immunosuppressed (n = 24) | 21% (9%, 40%) | 75% (55%, 88%) | 88% (69%, 96%) | 83% (64%, 93%) | 46% (28%, 65%) |
| HIV (n = 8) | 0% (0%, 37%) | 50% (22%, 78%) | 100% (64%, 100%) | 88% (53%, 98%) | 50% (22%, 78%) |
| Other immunosuppresseda (n = 16) | 31% (14%, 56%) | 88% (64%, 97%) | 81% (57%, 93%) | 81% (57%, 93%) | 44% (23%, 67%) |
Positive expression defined as ≥ 5% expression by tumor cells, tumor-infiltrating CD8 T cells, or tumor-infiltrating immune cells (TII). 95% confidence intervals are in parentheses
aOther immunosuppressed includes 12 organ transplants and 4 of other etiology (CLL, inflammatory bowel disease on immunosuppression)
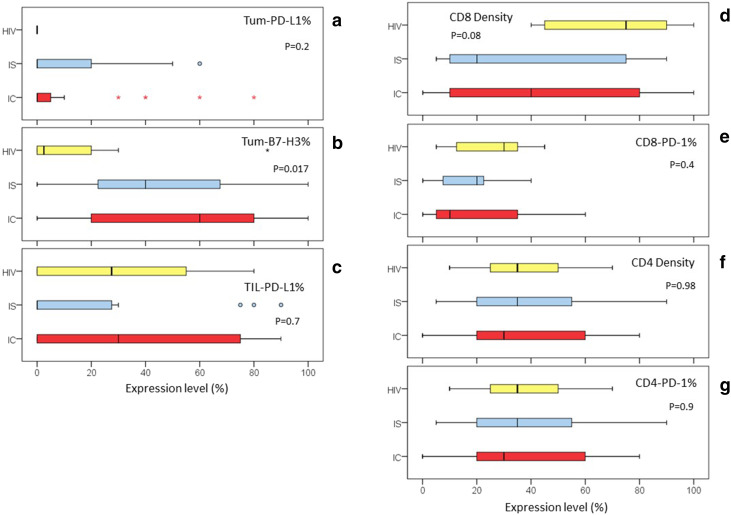
We additionally quantified the percent of tumor cells expressing PD-L1 (Tum-PD-L1%) and B7-H3 (Tum-B7-H3%), the percentage of peritumoral and tumor stroma infiltrated by CD8-positive lymphocytes (CD8 density) and CD4-positive lymphocytes(CD4 density), percentage of CD8-positive tumor-infiltrating lymphocytes that expressed PD-1 (CD8-PD-1%), percentage of CD4-positive tumor-infiltrating lymphocytes that expressed PD-1 (CD4-PD-1%), and TIIs which expressed PD-L1 (TII-PD-L1%). These values are shown in supplemental table 1 with comparative analysis shown in Table 4. Comparing immunocompetent and immunosuppressed patients, Tum-B7-H3% was significantly higher (Median 60 vs. 28%, p = 0.025) in immunocompetent patients, including when factoring in the cause of immunosuppression (immunocompetent vs. HIV vs. other immunosuppressed, p = 0.017) (Fig. 2). There was no significant difference in Tum-PD-L1%, CD8 density, CD4 density, CD8-PD-1%, CD4-PD-1%, or TII-PD-L1% between all immunocompetent and all immunosuppressed patients. Compared to immunocompetent patients, tumors from HIV-positive patients lacked tumor PD-L1 expression, and had significantly lower Tum-B7-H3% (Median 2.5 vs. 60%, p = 0.007). However, HIV-positive patients had a significantly higher CD8 density compared to immunocompetent patients (Median 75 vs. 40%, p = 0.039). There was a significant difference in Tum-PD-L1% by primary site (head/neck vs. torso/extremity vs. anogenital area, p = 0.043), with higher expression in cSCC of the head and neck. Also, the proportion of PD-L1 positive tumors was significantly higher (35%) in cSCC of the head and neck compared with anogenital (12.5%) and torso/extremities (0%), p = 0.014.
Table 4.
Comparison of quantitative expression of co-signaling molecules by immune status
| Tum-PD-L1%a | Tum-B7-H3%b | CD 8 densityc | CD4 densityd | CD8-PD-1%e | CD4-PD-1% f | TII-PD-L1%g | |
|---|---|---|---|---|---|---|---|
| Immunocompetent vs. immunosuppressed | p = 0.5 | p = 0.025 | p = 0.5 | p = 0.88 | p = 0.5 | p = 0.6 | p = 0.3 |
| Immunocompetent vs. HIV | p = 0.2 | p = 0.007 | p = 0.039 | p = 0.9 | p = 0.2 | p = 0.6 | p = 0.4 |
| Immunocompetent vs. HIV vs. other immunosuppressedh | p = 0.2 | p = 0.017 | p = 0.08 | p = 0.98 | p = 0.4 | p = 0.9 | p = 0.7 |
P values in bold are statistically significant at the < 0.05 level based on Kruskal–Wallis tests which was used to compare the distribution by immune status between groups
aTum-PD-L1% = percentage of tumor cells expressing PD-L1
bTum-B7-H3% = percentage of tumor cells expressing B7-H3
cCD8 density = the percentage of peritumoral stroma infiltrated by CD8-positive lymphocytes
dCD4 density = the percentage of peritumoral stroma infiltrated by CD4-positive lymphocytes
eCD8-PD-1% = the percentage of CD8-positive tumor-infiltrating lymphocytes, which expressed PD-1
fCD4-PD-1% = the percentage of CD4-positive tumor-infiltrating lymphocytes, which expressed PD-1
gTII-PD-L1% = percentage of tumor-infiltrating immune cells that express PD-L1
hOther immunosuppressed includes 12 organ transplants and 4 of other etiology (CLL, inflammatory bowel disease on immunosuppression)
Fig. 2.
Distribution of co-signaling molecule expression and CD8 density by immune status. Box-and-Whiskers plot of the distribution of tumor immunology markers according to immune status: HIV = Yellow, IS = Other Immunosuppressed in Blue, IC = Immunocompetent in Red. a Tum-PD-L1% = percentage of tumor cells expressing PD-L1. b Tum-B7-H3% = percentage of tumor cells expressing B7-H3. cTII-PD-L1% = percentage of tumor-infiltrating immune cells that express PD-L1. d CD8 density = the percentage of peritumoral stroma infiltrated by CD8-positive lymphocytes. e CD8-PD-1% = the percentage of CD8-positive tumor-infiltrating lymphocytes, which expressed PD-1. f CD4 density = the percentage of peritumoral stroma infiltrated by CD4-positive lymphocytes. g CD4-PD-1% = the percentage of CD4-positive tumor-infiltrating lymphocytes, which expressed PD-1
We examined the correlation between these co-signaling molecules as well as with pathologic features. Increased Tum-PD-L1% correlated with increased CD8 Density (Rs = 0.32, p = 0.011), CD4 density (Rs = 0.316, p = 0.01), and CD8-PD-1% (Rs = 0.29, p = 0.02). TII-PD-L1% showed positive correlation with Tum-PD-L1% (Rs = 0.35, p = 0.004) CD4 density (Rs = 0.32, p = 0.007), and CD8-PD-1% (Rs = 0.35, p = 0.005). There were also positive correlations between CD8 density and CD4-PD-1% (Rs = 0.41, p = 0.001), as well as CD4 density (Rs = 0.47, p = < 0.0001). There was no correlation between Tum-B7-H3% and Tum-PD-L1%, CD8 density, CD4 density, TII-PD-L1%, CD4-PD-1% or CD8-PD-1%. Tum-B7-H3% correlated with intensity of B7-H3 expression on vessels (Rs = 0.36, p = 0.003). LVI (Rs = 0.61, p = < 0.001) and higher tumor grade (Rs = 0.34, p = 0.006) were both associated with higher Tum-PD-L1%. There were no other significant correlations between co-signaling molecules and pathologic features.
Eight organ transplant patients with cSCC also had available tissue from the transplanted organ as a result of graft biopsies taken during their standard care. We quantified the percent expression of PD-L1 (IC-PD-L1%) and B7-H3 (IC-B7-H3%) in immune cells, PD-1 in CD8 T cells (CD8-PD-1%), and CD8 density in the graft specimens. Values are shown in supplemental table 2. CD 8 T cells were present in all graft samples with co-expression of PD-1 in most cases (86%). Immune cells that expressed PD-L1 were present in fewer specimens (42%). While B7-H3 was expressed by most tumors in these patients, immune cells in the graft did not express B7-H3.
Discussion
We analyzed samples from 66 cSCC patients who underwent surgical resection at the University of Maryland Medical Center for PD-L1 and B7-H3 expression in tumor cells, PD-1 expression in tumor-infiltrating CD8 T cells and CD4 T cells, and PD-L1 expression in tumor-infiltrating immune cells. With positive defined as expression on ≥ 5% of tumor cells and TIIs, 26% of all tumors were positive for PD-L1, 85% for B7-H3, 80% had CD8 T cells that expressed PD-1, 73% had CD4 T cells that expressed PD-1, and 55% had TIIs that expressed PD-L1. We additionally quantified the percentage of expression of these co-signaling molecules by tumors and TIIs as well as CD8 density and CD4 density in the tumor microenvironment, and correlated the degree of expression to immune status. There was no difference in these parameters between immunocompetent and immunosuppressed patients, except for tumor B7-H3 expression, which was significantly higher in immunocompetent patients.
About a quarter (26%) of all tumors expressed PD-L1 using a cut-off of ≥ 5% positive tumor cells. This prevalence of PD-L1 expression is comparable to that seen in NSCLC and HNSCC patients in phase III trials with Nivolumab, using the same cut-off [20, 21]. The proportion in our study falls between the 10 and 38% positive expression in primary cSCC reported prior by Schaper and Slater et al., respectively [22, 23]. Slater et al. found higher tumor PD-L1 expression in patients with high risk pathologic features. We similarly found increased PD-L1 expression in tumor cells in patients with LVI or higher tumor grade, two high risk pathologic features in cSCC [2, 24]. Available data limit adequate comparison of pathologic features of our population to that of Slater and Schaper to determine if this accounts for differences in PD-L1 expression. Additionally, different antibodies were used for PD-L1 analysis in these studies. Cutaneous SCC originating on the head and neck has been associated with increased risk of metastasis [2]. In the analysis by Slater, primary cSCC of the head and neck made up the majority of “high risk” cSCC based on pathologic features, which was associated with increased tumor PD-L1 expression, [23] and the prevalence of PD-L1 positivity in Slater’s study is in good agreement with our prevalence of 35% in cSCC of the head and neck region. In our population, primary cSCC of the head and neck was associated with increased tumor grade and LVI, as well as higher tumor PD-L1 expression compared to other primary sites. It is unclear whether increased tumor PD-L1 expression in head and neck cSCC is independent of high risk pathologic features.
Our study also uniquely analyzed PD-1 expression by CD8 T cells and PD-L1 expression in TII in cSCC. Overall there was a high density of tumor-infiltrating CD8 T cells with expression of PD-1 in the tumor microenvironment of these patients. Congruent with known mechanisms of T cell interaction and IFNγ mediated PD-L1 upregulation in tumors,[7] there was a significant positive correlation between tumor PD-L1 expression and both CD8 density and percentage of CD8 T cells expressing PD-1. We also found that the majority of patients had TII that expressed PD-L1, which combined with tumor PD-L1 expression has been found to be associated with the increased efficacy of anti-PD-1 mAb treatment [25].
To our knowledge, our study is the first to stratify these co-signaling molecules by immune status in cSCC. We found no difference in the degree of expression of PD-L1 by tumor cells or TIIs, PD-1 by CD8 T cells, or CD8 density between all immunocompetent and immunosuppressed patients. However, immunocompetent patients had significantly higher tumor B7-H3 expression compared to immunosuppressed, which appears to have been driven by a lower expression of B7-H3 in HIV-positive patients (median 2.5 vs. 35% in other immunosuppressed patients). No difference in PD-L1 expression in tumor cells or TIIs, and PD-1 expression in CD8 T cells was found between HIV-positive and immunocompetent patients. The expression of these co-signaling molecules has not been well characterized in immunosuppressed patients, including HIV-positive patients, with other malignancies. Evaluation of anal SCC samples in HIV-positive and HIV-negative patients revealed similar tumor cell PD-L1 expression (52 vs. 47%, respectively, p = 0.76) and similar density of CD3+ T lymphocytes in the tumor microenvironment. Our group previously evaluated HIV-positive patients with NSCLC and HNSCC with 23 and 53% of patients positive for PD-L1 (≥ 5% cut-off), respectively [26]. In the current study of cSCC, no tumors from HIV-positive patients expressed PD-L1. However, it should be noted that with 0/8 HIV-positive cases expressing PD-L1 the upper limit of the 95% confidence interval on the positive proportion is 37%. Thus, the proportion seen in HNSCC is within the 95% confidence limit estimated for our 8 HIV-positive cSCC cases. Studies of HIV-positive anal, NSCLC, and HNSCC patients also found a robust infiltration by CD8 TILs and high percentage of CD8 TILs that expressed PD-1 [26, 27]. This is similar to the HIV-positive cSCC in this study, where all had CD8 TILs that were positive for PD-1 expression, with a higher CD8 T cell density than in immunocompetent patients. What accounts for a higher CD8 T cell density in our HIV-positive patients is not clear; however, in anal SCC patients, IL-18, which has been associated with CD8 T cell proliferation, was observed to be higher in HIV-positive than in HIV-negative cases [27, 28]. However, it is unclear why despite a robust CD8 T cell infiltrate, PD-L1 expression by tumor was absent in our cSCC patients with HIV. While functional studies were not possible in this study, one hypothesis would be that these CD8 T cells were already anergic and therefore unable to produce IFNγ, accounting for the absence of PD-L1 expression on tumor. Further evaluation of HIV-positive cSCC is needed before any definitive conclusions can be made, given the low number of HIV-positive patients in this study.
In regards to organ transplant patients, Krynitz et al. compared the peritumoral inflammatory cells in cSCC from organ transplant and immunocompetent patients. They found no significant difference in the density of inflammatory cells, or CD8 T cells [29]. Similarly, in our patient population, when cause of immunosuppression was considered, including organ transplant patients, there was no difference compared to immunocompetent patients in CD8 density as well as tumor PD-L1 expression, PD-1 expression by CD8 TILs, or PD-L1 expression in TIIs.
To our knowledge this is also the first evaluation of B7-H3 in cSCC and in immunosuppressed patients. We found that 85% of patients were positive for B7-H3, which is comparable to findings in other tumor types including NSCLC, pancreatic cancer, HNSCC, and colorectal cancer [17, 30, 31]. Interestingly, B7-H3 expression was the only parameter that was significantly different in immunocompetent compared to immunosuppressed patients as discussed above. While B7-H3 expression in tumors correlated with expression of B7-H3 on vessels, there was no correlation between tumor B7-H3 expression and high risk pathologic features. This is in contrast to other tumor types such as pancreatic cancer and colorectal cancer, where B7-H3 expression has been associated with high risk features, such as lymph node metastasis and higher tumor grade [31, 32]. While an inverse relationship between tumor B7-H3 expression and TILs has been observed [33, 34], data are lacking on whether B7-H3 correlates with PD-L1 and PD-1 expression by tumor cells and TIIs. In our analysis we found no significant correlation between tumor B7-H3 and PD-L1 expression on TII, CD8 or CD4 density, or PD-1 expression on CD8 or CD4 T cells. While the exact mechanism of induction of B7-H3 on tumor has not been elucidated, it has been reported that IFNγ may play a role [35]. However, we found no significant correlation between tumor B7-H3 and tumor PD-L1 expression, which is also upregulated by IFNγ.
In the organ transplant patient population, cSCC accounts for significant morbidity and mortality [36]. The PD-L1:PD-1 pathway has a role in induction and maintenance of graft tolerance, and acute graft rejection with subsequent kidney failure has been observed in published case reports of kidney transplant patients treated with anti-PD-1 mAbs, including one patient with advanced cSCC [37–40] In our population, most organ transplant patients had PD-L1 expression on immune cells, or PD-1 expression on CD8 T cells in the examined graft specimen. However, while the majority of these patients had tumor expression of B7-H3, immune cells in the graft were devoid of B7-H3 expression. Further analysis of the role of B7-H3 in transplant rejection is needed to determine whether or not blockade of B7-H3 could be safe in organ transplant patients with advanced cSCC.
Our analysis has a number of limitations. The patients we analyzed had undergone surgical resection of their cSCC and therefore were earlier stage than more advanced cSCC patient that would require systemic therapy. That being said, these were all patients who underwent surgical resection in the hospital and had high risk pathologic features, therefore they are representative of more aggressive disease than cSCC treated in the dermatology office. Also, given this was a retrospective analysis and the only available tissue was archival formalin fixed paraffin embedded tumor samples, we were not able to perform functional studies to characterize the tumor microenvironment. We acknowledge that this would have enhanced this analysis, and is planned for future study. Lastly, we were unable to correlate co-signaling molecule expression with overall survival because patients’ samples were from more recent years and, therefore, the number of events (deaths) was too low.
Treatment options are very limited for patients with inoperable or metastatic cSCC, with currently no FDA approved therapies specific for advanced cSCC. Our data provide support for clinical trials in cSCC with checkpoint inhibitors targeting the PD-1: PD-L1 pathway or B7-H3. Additional rationale for checkpoint blockade in cSCC comes from mutational analysis. Higher somatic mutational burden has been shown as a strong predictor of response to checkpoint blockade retrospectively in melanoma and NSCLC and prospectively in mismatch repair deficient colon cancer [41–43]. Whole exome sequencing of aggressive cSCC samples showed a high mutational rate with a median of 61.2 mutations/Mb, and mutations were largely clonal [44]. The efficacy of anti-PD-1 mAbs Nivolumab and Pembrolizumab has been reported in advanced cSCC in three case reports on a total of seven patients, most of them had failed prior chemotherapy, cetuximab or both. A total of four patients achieved a PR and three patients had SD [45–47]. Preliminary data with anti-PD-1 mAb REGN2810 in 26 patients with advanced cSCC were presented at the ASCO annual meeting 2017, and showed a response rate of 46% [48].
In conclusion, there were no significant differences in expression of PD-L1 by tumor cells or TII, and PD-1 by CD8 or CD4 T cells according to patients’ immune status. Thus, our data support the development of clinical trials with checkpoint inhibitors targeted against the PD-1:PD-L1 pathway in immunosuppressed as well as in immunocompetent cSCC patients, with the exception of organ transplant patients because of safety concerns. Tumor cell B7-H3 expression was significantly higher in immunocompetent patients compared to immunosuppressed largely driven by lower B7-H3 expression in HIV-positive patients. Our data suggest that targeting of B7-H3 may also be of interest in immunocompetent cSCC patients, and possibly in organ transplant patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- CD4 density
The percentage of peritumoral and tumor stroma infiltrated by CD4-positive lymphocytes
- CD4-PD-1%
Percentage of CD4-positive tumor-infiltrating lymphocytes that expressed PD-1
- CD8 density
The percentage of peritumoral and tumor stroma infiltrated by CD8-positive lymphocytes
- CD8-PD-1%
Percentage of CD8-positive tumor-infiltrating lymphocytes that expressed PD-1
- cSCC
Cutaneous squamous cell carcinoma
- DCR
Disease control rate
- GI
Gastrointestinal
- HNSCC
Squamous cell carcinoma of the head and neck
- IC-B7-H3%
Percentage of immune cells that expressed B7-H3
- IC-PD-L1%
Percentage of immune cells that expressed PD-L1
- IFNγ
Interferon gamma
- IRB
Institutional review board
- LVI
Lymphovascular invasion
- Mb
Megabase
- NSCLC
Non small cell lung cancer
- PFS
Progression free survival
- pH
Potential of hydrogen
- PNI
Perineural invasion
- RR
Response rate
- TII
Tumor-infiltrating immune cell
- TII-PD-L1%
Percentage of tumor-infiltrating immune cells that expressed PD-L1
- Tum-B7-H3%
Percent of tumor cells expressing B7-H3
- Tum-PD-L1%
Percent of tumor cells expressing PD-L1
- SCC
Squamous cell carcinoma
Author contributions
All authors contributed to data analysis, writing, and editing. Jon Heath and Olga Ioffe carried out the pathologic analysis of samples.
Funding
Dan Zandberg K12 CA126849. Source: National Cancer Institute.
Compliance with ethical standards
Conflict of interest
Dr. Dan Zandberg receives research support for his role as a site principal investigator for clinical trials from Merck, Macrogenics, Bristol Myers Squibb “BMS”, AstraZeneca, and MedImmune. The other authors declare that they have no conflict of interest.
Ethical approval and ethical standards
This study was reviewed and approved by the University of Maryland, Baltimore IRB.
Informed consent
Informed consent was not required for this study.
References
- 1.Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957–966. doi: 10.1016/j.jaad.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 2.Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease-specific death: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:419–428. doi: 10.1001/jamadermatol.2015.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindelof B, Sigurgeirsson B, Gabel H, Stern RS. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513–519. [PubMed] [Google Scholar]
- 4.Bejar C, Maubec E. Therapy of advanced squamous cell carcinoma of the skin. Curr Treat Options Oncol. 2014;15:302–320. doi: 10.1007/s11864-014-0280-x. [DOI] [PubMed] [Google Scholar]
- 5.Maubec E, Petrow P, Scheer-Senyarich I, et al. Phase II study of cetuximab as first-line single-drug therapy in patients with unresectable squamous cell carcinoma of the skin. J Clin Oncol. 2011;29:3419–3426. doi: 10.1200/JCO.2010.34.1735. [DOI] [PubMed] [Google Scholar]
- 6.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 7.Zandberg DP, Strome SE. The role of the PD-L1:PD-1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2014;50:627–632. doi: 10.1016/j.oraloncology.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inman BA, Sebo TJ, Frigola X, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109:1499–1505. doi: 10.1002/cncr.22588. [DOI] [PubMed] [Google Scholar]
- 10.Wilke CM, Wei S, Wang L, et al. Dual biological effects of the cytokines interleukin-10 and interferon-gamma. Cancer Immunol Immunother. 2011;60:1529–1541. doi: 10.1007/s00262-011-1104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 12.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badoual C, Hans S, Merillon N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–138. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Kang FB, Shan BE. B7-H3-mediated tumor immunology: friend or foe? Int J Cancer. 2014;134:2764–2771. doi: 10.1002/ijc.28474. [DOI] [PubMed] [Google Scholar]
- 18.Fauci JM, Straughn JM, Jr, Ferrone S, Buchsbaum DJ. A review of B7-H3 and B7-H4 immune molecules and their role in ovarian cancer. Gynecol Oncol. 2012;127:420–425. doi: 10.1016/j.ygyno.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Powderly JCG, Flaherty K, Szmulewitz RZ, Ribas A, Weber J, Loo D, Baughman J, Chen F, Moore P, Bonvini E, Vasselli J, Wiggington J, Cohen RB, Burris H, Chmielowski B (2015) Interim results of an ongoing phase I, dose escalation study of MGA271 (Enoblituzumab), an Fc-optimized Humanized Anti-B7-H3 Monoclonal Antibody, in Patients with Advanced Solid Cancer. SITC Annual Meeting November 2015. J Immunother Cancer 3(Suppl 2) (Oral Abstract)
- 20.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaper K, Kother B, Hesse K, et al. The pattern and clinicopathological correlates of PD-L1 expression in cutaneous squamous cell carcinoma. Br J Dermatol. 2017;176:1354–1356. doi: 10.1111/bjd.14955. [DOI] [PubMed] [Google Scholar]
- 23.Slater NA, Googe PB. PD-L1 expression in cutaneous squamous cell carcinoma correlates with risk of metastasis. J Cutan Pathol. 2016;43:663–670. doi: 10.1111/cup.12728. [DOI] [PubMed] [Google Scholar]
- 24.Nuno-Gonzalez A, Vicente-Martin FJ, Pinedo-Moraleda F, Lopez-Estebaranz JL. High-risk cutaneous squamous cell carcinoma. Actas Dermosifiliogr. 2012;103:567–578. doi: 10.1016/j.ad.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Chow LQ, Haddad R, Gupta S, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34:3838–3845. doi: 10.1200/JCO.2016.68.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scilla KAHJ., Ioffe OB, Cellini AL, Edelman MJ, Zandberg DP, Riedel D, Feliciano JL. (2016) PD-1, PD-L1, and B7-H3 expression in Human Immunodeficiency Virus (HIV) infected patients with aerodigestive (AD) cancers (CA). ASCO Annual Meeting 2016. J Clin Oncol 34 (suppl; abstr 11607; Poster Presentation) vol 34, No 15_suppl (May 20 Supplement), 2016: 11607 2016
- 27.Yanik EL, Kaunitz GJ, Cottrell TR, et al. Association of HIV status with local immune response to anal squamous cell carcinoma: implications for immunotherapy. JAMA Oncol. 2017;3:974–978. doi: 10.1001/jamaoncol.2017.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W, Kashiwamura S, Ueda H, et al. Protection of CD8 + T cells from activation-induced cell death by IL-18. J Leukoc Biol. 2007;82:142–151. doi: 10.1189/jlb.0706431. [DOI] [PubMed] [Google Scholar]
- 29.Krynitz B, Lundh Rozell B, Lindelof B. Differences in the peritumoural inflammatory skin infiltrate between squamous cell carcinomas in organ transplant recipients and immunocompetent patients. Acta Derm Venereol. 2010;90:379–385. doi: 10.2340/00015555-0876. [DOI] [PubMed] [Google Scholar]
- 30.Loos M, Hedderich DM, Ottenhausen M, et al. Expression of the costimulatory molecule B7-H3 is associated with prolonged survival in human pancreatic cancer. BMC Cancer. 2009;9:463. doi: 10.1186/1471-2407-9-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun J, Chen LJ, Zhang GB, et al. Clinical significance and regulation of the costimulatory molecule B7-H3 in human colorectal carcinoma. Cancer Immunol Immunother. 2010;59:1163–1171. doi: 10.1007/s00262-010-0841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamato I, Sho M, Nomi T, et al. Clinical importance of B7-H3 expression in human pancreatic cancer. Br J Cancer. 2009;101:1709–1716. doi: 10.1038/sj.bjc.6605375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunner A, Hinterholzer S, Riss P, et al. Immunoexpression of B7-H3 in endometrial cancer: relation to tumor T-cell infiltration and prognosis. Gynecol Oncol. 2012;124:105–111. doi: 10.1016/j.ygyno.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Zhang Q, Chen W, et al. B7-H3 is overexpressed in patients suffering osteosarcoma and associated with tumor aggressiveness and metastasis. PLoS One. 2013;8:e70689. doi: 10.1371/journal.pone.0070689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun TW, Gao Q, Qiu SJ, et al. B7-H3 is expressed in human hepatocellular carcinoma and is associated with tumor aggressiveness and postoperative recurrence. Cancer Immunol Immunother. 2012;61:2171–2182. doi: 10.1007/s00262-012-1278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brin L, Zubair AS, Brewer JD. Optimal management of skin cancer in immunosuppressed patients. Am J Clin Dermatol. 2014;15:339–356. doi: 10.1007/s40257-014-0085-5. [DOI] [PubMed] [Google Scholar]
- 37.Ong M, Ibrahim AM, Bourassa-Blanchette S, et al. Antitumor activity of nivolumab on hemodialysis after renal allograft rejection. J Immunother Cancer. 2016;4:64. doi: 10.1186/s40425-016-0171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipson EJ, Bagnasco SM, Moore J, Jr, et al. Tumor regression and allograft rejection after administration of Anti-PD-1. N Engl J Med. 2016;374:896–898. doi: 10.1056/NEJMc1509268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spain L, Higgins R, Gopalakrishnan K, et al. Acute renal allograft rejection after immune checkpoint inhibitor therapy for metastatic melanoma. Ann Oncol. 2016;27:1135–1137. doi: 10.1093/annonc/mdw130. [DOI] [PubMed] [Google Scholar]
- 40.Riella LV, Paterson AM, Sharpe AH, Chandraker A. Role of the PD-1 pathway in the immune response. Am J Transplant. 2012;12:2575–2587. doi: 10.1111/j.1600-6143.2012.04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pickering CR, Zhou JH, Lee JJ, et al. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin Cancer Res. 2014;20:6582–6592. doi: 10.1158/1078-0432.CCR-14-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borradori L, Sutton B, Shayesteh P, Daniels GA. Rescue therapy with anti-programmed cell death protein 1 inhibitors (PD-1) of advanced cutaneous squamous cell carcinoma and basosquamous carcinoma: preliminary experience in 5 cases. Br J Dermatol. 2016;175:1382–1386. doi: 10.1111/bjd.14642. [DOI] [PubMed] [Google Scholar]
- 46.Winkler JK, Schneiderbauer R, Bender C, et al. Anti-PD-1 therapy in nonmelanoma skin cancer. Br J Dermatol. 2017;176:498–502. doi: 10.1111/bjd.14664. [DOI] [PubMed] [Google Scholar]
- 47.Chang AL, Kim J, Luciano R, et al. A case report of unresectable cutaneous squamous cell carcinoma responsive to pembrolizumab, a programmed cell death protein 1 inhibitor. JAMA Dermatol. 2016;152:106–108. doi: 10.1001/jamadermatol.2015.2705. [DOI] [PubMed] [Google Scholar]
- 48.Papadopoulos KP, Owonikoko TK, Johnson ML, Brana I, Gil-Martin M, Perez RP, Moreno V, Salama A, Calvo E, Yee NS, Safran H, González-Martín A, Aljumaily R, Mahadevan D, Mohan KK, Qin R, Stankevich E, Lowy I, Fury MG, Homsi J. REGN2810: a fully human anti-PD-1 monoclonal antibody, for patients with unresectable locally advanced or metastatic cutaneous squamous cell carcinoma (CSCC)—initial safety and efficacy from expansion cohorts (ECs) of phase I study. ASCO Annual Meeting 2017. J Clin Oncol. 2017;35(suppl):9503. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.