Abstract
Vitamin D3 (25-OH-D3) deficiency impairs rituximab-dependent cellular cytotoxicity and the outcome of patients with diffuse large B-cell and follicular lymphomas (DLBCL). Since the optimum 25-OH-D3 serum levels for NK cell-mediated antibody-dependent cellular cytotoxicity (ADCC) are unknown, we determined the 25-OH-D3 serum levels associated with maximum NK cell-mediated ADCC. CD20 antibody-loaded CD20+ B-cell lymphoma cell lines were cultured with NK cells and ADCC activity was determined by lactate dehydrogenase release assays. Using a newly developed formula, pre-defined 25-OH-D3 serum levels were achieved with high individual precision over a wide range. NK cells from 20 healthy individuals killed antibody-treated CD20+ lymphoma cells in a concentration- and E:T ratio-dependent manner with obinutuzumab displaying a stronger ADCC activity than rituximab. Maximum NK-cell activity and ADCC were observed at 65 ng/ml 25-OH-D3, the middle of the normal range (30–100 ng/ml). 25-OH-D3 serum levels around this range should be the target in interventional trials aiming at improving NK cell-mediated ADCC by 25-OH-D3 substitution. Lower levels do not provide significant ADCC improvements in individuals with 25-OH-D3 deficiency or insufficiency and might result in the failure of interventions with 25-OH-D3.
Keywords: Lymphoma, NK cells, Rituximab, Obinutuzumab, ADCC, Vitamin D3
Introduction
The addition of rituximab to CHOP chemotherapy has significantly improved the outcome of patients with DLBCL [1–3]. Rituximab is a chimeric antibody with a human Fc part and a murine antigen-binding site directed against the human CD20 antigen. Different mechanisms of actions of rituximab have been described [4, 5]. The major mechanism of action of rituximab is ADCC, with complement dependent cytotoxicity (CDC) and direct induction of apoptosis playing a less important role [6–9]. Different types of effector cells are involved in rituximab-mediated ADCC. Besides neutrophils [10] and M2 macrophages [11–13], NK cells are the most important effector cells responsible for ADCC [14, 15].
NK cell-mediated ADCC activity is determined by activating and inhibitory signals. Cytokines such as GM-CSF and IL-2 stimulate NK cells, [14, 16] and the efficacy of rituximab has been shown to depend also on single-nucleotide polymorphisms of the low affinity Fc-gamma receptor type III (FcγRIIIa/CD16) gene which is responsible for binding the Fc part of IgG molecules to the NK cell, with the 158-valine and 158-phenylalanine variants in DLBCL patients having a different outcome [17, 18]. An association of ADCC activity and 25-OH-D3 has been described for macrophages [19, 20], but these studies assessed 25-OH-D3 levels in vitro only. In a clinical study, Hossein-Nezhad et al. [21] described differences in the gene expression profile of white blood cells before and after the substitution of 25-OH-D3, however, not specifically for NK cells.
25-OH-D3 is well known for its utmost importance in bone metabolism and, therefore, deficiency was defined as serum level < 10 ng/ml where osteomalacia may occur. As 25-OH-D3 has additional functions in the immune system and in many pathologic states such as cancer, multiple sclerosis and vascular disease some authors advocate that a level of 10–30 ng/ml is insufficient for a healthy human being [22] and levels above 30 ng/ml should be goal to achieve. This definition is used throughout this manuscript.
In a retrospective analysis of the RICOVER-60 trial (NCT00052936) we had shown that low 25-OH-D3 serum levels were associated with a worse outcome of elderly patients with DLBCL [23] and this finding was confirmed in patients with follicular lymphoma [24]. We also demonstrated that the rituximab-dependent NK cell-mediated ADCC can be improved in healthy individuals by increasing their 25-OH-D3 levels by cholecalciferol substitution. Since the 25-OH-D3 serum level associated with optimum NK cell-mediated ADCC against B-lymphoma cells is unknown, the aim of the current study was to determine this serum level and to test whether other antibodies used for the treatment of human malignancies that are not directed against CD20 (e. g. trastuzumab for HER2-positive breast cancer [25]) have a similar 25-OH-D3 dependency of their NK-ADCC.
Materials and methods
25-OH-D3 substitution in vivo
NK-cell activity was analyzed at four pre-defined serum levels of 25-OH-D3: (1) before any substitution with serum levels ranging between deficiency (< 10 ng/ml) and insufficiency (10 to < 30 ng/ml); (2) after substitution in vivo to a target level of 30 ng/ml (lower normal range), (3) 65 ng/ml (mid normal range) and (4) 90 ng/ml (high normal range). DEKRISTOL™ capsules (Mibe GmbH Arzneimittel, Brehna, Germany) containing 20,000 IU cholecalciferol per capsule were given for substitution. To calculate the required total dose for a given 25-OH-D3 target serum level, a formula suggested by van Groningen et al. [26] was initially used. However, this formula underestimated the doses needed. Thus, the formula was modified successively until the individually calculated cholecalciferol doses achieved the pre-defined 25-OH-D3 serum levels within a narrow range. The modified formula is described in the “Results” section.
Antibodies and cell lines
To investigate ADCC mediated by NK cells, three different antibodies with two different target molecules were investigated: rituximab (MabThera™, Roche, Basel, Switzerland) and obinutuzumab (Gazyvaro™, Roche), both directed against the CD20 antigen expressed on normal and malignant B cells and trastuzumab (Herceptin™, Roche) which binds to HER2/neu overexpressing breast cancer cells. Antibody stocks were stored at 1 mg/ml at 4–8 °C. For the NK-cell assays antibodies were used at the indicated concentrations and incubated (37 °C) for 20 min with the corresponding target cells. Targets for the rituximab- and obinutuzumab-mediated ADCC were the CD20+ Burkitt’s lymphoma cell line DAUDI (DSMZ, Braunschweig, Germany). As targets for the trastuzumab-dependent ADCC, HER2/neu+ cells of the breast cancer cell line ZR-75-1 (CLS Cell lines Service GmbH, Eppelheim, Germany) were used. The specific binding of the antibodies to the respective targets was confirmed by flow cytometry. DAUDI cells were cultured in RPMI 1640 (PAN-Biotech GmbH, Aidenbach, Germany) supplemented by 20% fetal calf serum (Sigma–Aldrich Chemie GmbH, Munich, Germany), 2 mM L-glutamine (Sigma–Aldrich Chemie GmbH), and antibiotics. ZR-75-1 was cultured in RPMI 1640 supplemented by 10% fetal calf serum, 2 mM l-glutamine, 1 mM Na-pyruvate, 25 mM Hepes (both by PAN-Biotech GmbH), and antibiotics.
Isolation of NK cells
PBMCs were isolated by density gradient centrifugation from the 50 ml EDTA blood donation of the respective donor. Thereafter NK cells were isolated from PBMCs by magnetic depletion of all non-NK cells using the CD56+/CD16+ human NK-Cell Isolation Kit (Miltenyi Biotech GmbH, Bergisch Gladbach, Germany) according to the manufacturer`s instructions. NK cells were isolated immediately before the ADCC assay without additional activation (e. g. by IL-2). The viability of the NK cells after isolation averaged 99% and the share of the CD16+ fraction was between 90% and 98%, as assessed by flow cytometry using the corresponding antibodies (Miltenyi). The yield of CD16+ NK cells was between 3 × 106 cells and 1 × 107 cells. Cell counting was made according to Neubauer. A FACSCalibur (BD Biosciences, Heidelberg, Germany) was used for flow cytometry analysis. Starting with a forward scatter (FSC) versus sideward scatter scan (SSC) to gate the lymphocyte population and followed by analysis of the corresponding fluorescence using CellQuest software (BD Biosciences). 5000 or 10,000 lymphocytes were examined per run.
ADCC assay
NK-cell activity (against antibody-naïve target cells) and NK cell-mediated ADCC (against antibody-treated target cells) was assessed by LDH release from target cells. NK effector cells were co-incubated (96-well U-bottom, 4 h, 37 °C) with 4000 corresponding target cells using E:T ratios of 5:1 and 2.5:1. ADCC medium used to suspend all cells consisted of pyruvate-free X-Vivo 15 (Lonza, Verviers, Belgium) supplemented by 2% human serum albumin (CSL Behring GmbH, Marburg, Germany) and was not pretested. Supernatants were analyzed for their LDH content by ELISA using the Cytotoxicity Detection Kit Plus (Roche/Sigma–Aldrich Chemie GmbH) according to the manufacturer’s instructions. ELISA plates were quantified using a Wallac Victor 2 (PerkinElmer LAS, Rodgau, Germany). NK-cell activity was calculated relatively to the detergence-induced maximum LDH release corrected by the spontaneous release from the corresponding target cells. Spontaneous LDH release averaged less than 5%. At values above 10%, the assay was discarded and repeated. Maximal and spontaneous lyses of the respective target cells were used as internal (inter-assay) controls.
Statistical analysis
NK-cell activity and ADCC assays were run with all samples in triplicates. The difference of the NK-cell activity against untreated targets (0 ng/ml of the corresponding antibody) and the antibody-treated targets was determined by F test followed by a two-sided Student’s T test. In addition, ANOVA was used to prove that the extent of the ADCC depended on the antibody concentration. ADCC activity at different 25-OH-D3 serum levels was compared using the Wilcoxon signed-rank test.
Results
Twenty (ten male, ten female) volunteers with no apparent health problems and a median age of 61.5 (range 30–86) years were included in this study (Table 1). Written informed consent was obtained from all individual participants included in the study.
Table 1.
Sex, age and 25-OH-D3 serum levels of the test persons included in this study
| ID | Sex | Age (years) | Before substitution | After substitution | ||
|---|---|---|---|---|---|---|
| Deficiency/insufficiency (ng/ml) | Lower normal range (ng/ml) | Mid normal range (ng/ml) | High normal range (ng/ml) | |||
| 1 | m | 50 | – | 35.1a | 70.1 | 123.0 |
| 4 | f | 46 | – | 32.2a | 71.5 | 110.0 |
| 50 | m | 33 | 11.4 | 34.6 | 57.2 | 103.0 |
| 55 | m | 32 | 14.3 | 33.3 | 67.4 | 93.6 |
| 56 | m | 59 | 4.0 | 32.5 | 71.7 | – |
| 57 | f | 30 | – | 31.5a | 61.2 | 90.5 |
| 61 | f | 40 | – | 36.9a | 68.4 | 102 |
| 66 | f | 64 | 8.7 | 27.7 | 68.2 | 86.4 |
| 67 | m | 70 | 9.0 | 33.8 | 65.5 | 89.6 |
| 70 | m | 78 | 5.9 | 24.5 | 64.3 | 110.0 |
| 71 | f | 71 | 23 | 33.9 | 68.2 | 115.0 |
| 72 | m | 57 | 15.7 | 45.8 | 62.6 | 89.0 |
| 73 | f | 78 | 4.6 | 28.1 | 68.8 | 83.4 |
| 74 | m | 79 | 6.1 | 31.3 | 72.8 | 94.9 |
| 75 | f | 79 | 9.7 | 31.7 | 68.5 | 72.1 |
| 87 | m | 56 | – | 30.5a | 69.4 | 81.0 |
| 91 | f | 83 | 9.8 | 27.6 | 63.6 | 88.8 |
| 92 | m | 86 | 10.3 | 27.3 | 58.2 | 74.4 |
| 93 | f | 80 | 5.9 | 25.8 | 63.7 | – |
| 99 | f | 42 | 8.8 | 38.8 | 61.5 | 86.8 |
| Mean | f and m | 60.7 | 9.8 | 32.1 | 66.1 | 94.1 |
| SD | 18.3 | 4.9 | 4.9 | 4.4 | 13.9 | |
| Mean | f | 61.3 | 10.1 | 31.4 | 66.4 | 92.8 |
| SD | 18.9 | 6.0 | 4.2 | 3.5 | 13.6 | |
| Mean | m | 60 | 9.6 | 32.9 | 65.9 | 95.4 |
| SD | 17.6 | 4.2 | 5.6 | 5.4 | 14.8 | |
f Female, m male
aDue to the season or self-initiated prophylactic substitution these test persons did not receive Dekristol to reach the lower normal range
NK cell-mediated ADCC before vitamin D3 substitution
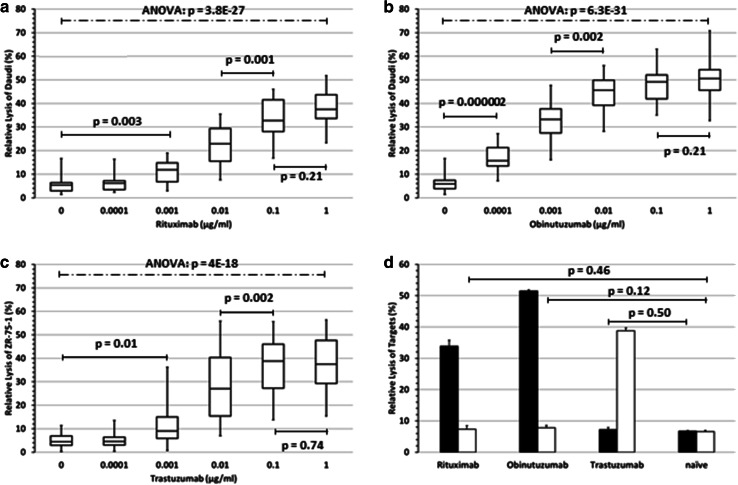
Fifteen of the 20 test volunteers had base-line 25-OH-D3 serum levels below the lower normal level (< 30 ng/ml) with values ranging from 4 to 23 ng/ml (Table 1), of whom five had 25-OH-D3 insufficiency (10–30 ng/ml), and ten 25-OH-D3 deficiency (< 10 ng/ml). Target cells were loaded with the corresponding antibody at increasing concentrations. To analyze the ADCC against lymphoma, CD20+ DAUDI lymphoma cells were used as targets and labeled with the anti-CD20 antibodies rituximab and obinutuzumab, respectively. DAUDI cells lack MHC-I molecules, and thus preclude unspecific inhibitory effects on NK cells. ADCC against the HER2/neu-expressing breast cancer cells was tested with ZR-75-1 cells labeled with the anti-HER2/neu antibody trastuzumab. The results of the 15 subjects with 25-OH-D3 deficiency or insufficiency before substitution are shown in Fig. 1a–c. Rituximab-dependent ADCC against DAUDI cells mediated by NK cells depended on the antibody concentration used to load the target cells and required at least 0.001 µg/ml antibody to become significantly superior compared to the NK-cell activity against antibody-naïve DAUDI cells. A maximum effect was observed at 0.1 µg/ml rituximab (Fig. 1a). Obinutuzumab, a second generation anti-CD20 antibody with a glyco-engineered Fc region for optimized ADCC, induced a significantly stronger ADCC compared to NK-cell activity against antibody-naïve targets starting at concentrations as low as 0.0001 µg/ml with a maximum at 0.01 µg/ml (Fig. 1b). NK-cell activity against untreated or ADCC against trastuzumab-treated ZR-75-1 cells is shown in Fig. 1c. Trastuzumab-mediated ADCC of NK cells showed the highest individual variability. An ANOVA analyzing the entire range of antibody concentrations confirmed with very high significance (Rituximab: p = 3.8 × 10−27; Obinutuzumab: p = 6.3 × 10−31; Trastuzumab: 4 × 10−18) that the increasing means of ADCC activity were not accidental but dependent on the antibody concentration (Fig. 1a–c).
Fig. 1.
NK-cell activity and NK cell-mediated ADCC before vitamin D3 substitution NK-cell activity (0 µg/ml antibody) and NK cell-mediated ADCC of all 15 subjects with a deficiency or insufficiency of vitamin D3 against CD20+ DAUDI cells treated with rituximab (a) or obinutuzumab (b) and ZR-75-1 breast cancer cells treated with trastuzumab, respectively (c). The E:T ratio was 5:1. Boxplots indicate lower quartile, median, and the upper quartile. The lower whisker depicts the lowest and the upper whisker the highest lysis rate. Statistics: p values below or above the solid lines were determined by two-sided T test and p values over the broken lines by ANOVA. d In contrast to both anti-CD20 antibodies rituximab and obinutuzumab, the ADCC induced by the HER2/neu-specific antibody trastuzumab against CD20+ DAUDI cells (black columns) was not increased compared to the NK-cell activity against naïve DAUDI cells (trastuzumab vs. naïve, p = 0.50). Conversely, rituximab and obinutuzumab dependent ADCC against the HER2/neu+ cell line ZR-75-1 (white columns) was not increased compared to the NK-cell activity (without antibodies) against ZR-75-1 breast carcinoma cells (rituximab vs. naïve, p = 0.46; obinutuzumab vs. naïve, p = 0.12). NK cells used in this ADCC were derived from subject ID #75 before substitution and used at an E:T ratio of 5:1
To exclude an ADCC induced by nonspecific binding of the antibodies to the “false” targets, the HER2/neu+ cell line ZR-75-1 was labeled with both CD20-specific antibodies rituximab and obinutuzumab and used as target. Conversely, CD20+ DAUDI cells were treated with the HER2/neu-specific antibody trastuzumab. The NK cells of subject ID #75 (9.7 ng/ml 25-OH-D3) were tested as effector cells in the ADCC. The result of this ADCC is shown in Fig. 1d and confirms that none of the antibodies used in this study was cross-reactive thus excluding unspecific ADCC. Moreover, specific binding of the antibodies to the respective targets was confirmed by flow cytometry (data not shown).
Development of a rapid and precise 25-OH-D3 substitution protocol
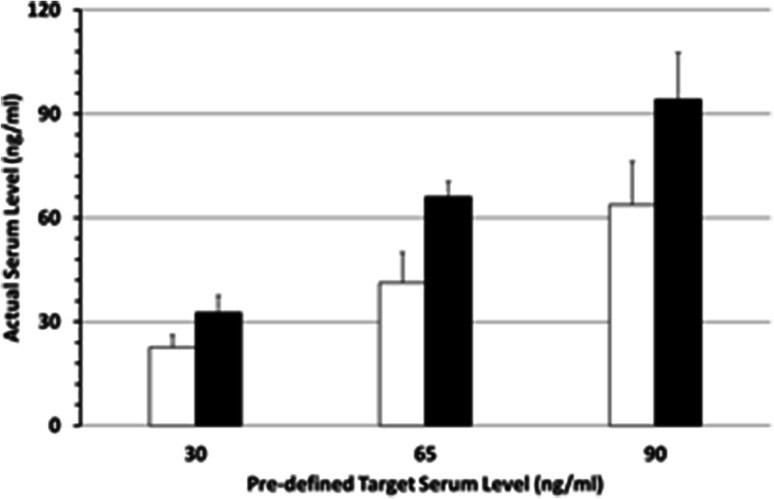
To determine the 25-OH-D3/ADCC relationship, we investigated the NK-cell activity and NK cell-mediated ADCC at different 25-OH-D3 serum levels in each individual. We modified a formula suggested by van Groningen et al. [26] to achieve the desired 25-OH-D3 serum levels within a short period and calculated the required vitamin D3 dose as: cholecalciferol (I.U.) = Δ 25-OH-D3 (ng/ml) × body weight (kg) × 200 (with “Δ 25-OH-D3” meaning the difference between the actual and the pre-defined 25-OH-D3 serum level). Applying this formula, the pre-defined serum level was achieved with a narrow margin in nearly all individuals (Fig. 2). Overdosing was rare and occurred only at the 90 ng/ml target level. The maximum level observed was 123 ng/ml (Table 1); it was not associated with any symptoms and returned to normal ranges within one week. Hypercalcemia never occurred and serum values of phosphate remained below the limit. Moreover, the serum levels achieved using this formula were independent of sex, age and dose increments of vitamin D3 levels, i.e. the difference between the starting 25-OH-D3 serum level and the pre-defined target level. As in the reference publication [26], the maximum daily vitamin D3 intake was restricted to 200,000 I.U.; this resulted in a vitamin D3 substitution period of a minimum of two to a maximum of 4 days. The minimum interval between the last day of 25-OH-D3 intake and the next ADCC assay was five days.
Fig. 2.
Vitamin D3 serum levels after substitution shown are the targeted and the actually achieved 25-OH-D3 serum levels using the primary van Groningen formula (white columns) and our new established formula (black columns) 4–8 days after taking the last tablet
NK-cell activity and ADCC after 25-OH-D3 substitution
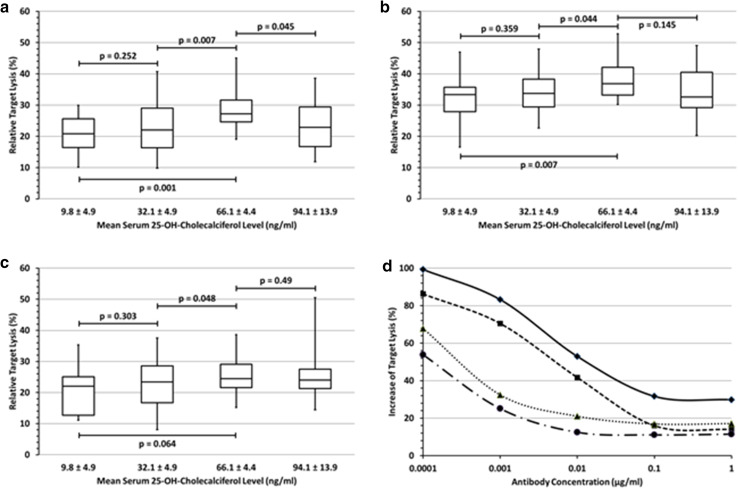
NK-cell activity (i.e. NK cell-mediated killing of target cells in the absence of antibody) and NK cell-mediated ADCC against DAUDI cells increased significantly with increasing 25-OH-D3 serum levels of the NK-cell donor. As shown in Fig. 3a, rituximab-dependent ADCC against the lymphoma cell line DAUDI increased significantly with rising 25-OH-D3 serum levels compared to base-line deficiency and insufficiency levels at 65 ng/ml. Substitution to serum levels in the lower normal range (ca. 30 ng/ml) did not result in a significantly improved ADCC compared to base line. However, on the other hand, serum levels exceeding 65 ng/ml and close to the upper normal limit of 100 ng/ml resulted in a decreased ADCC, indicating that the optimal 25-OH-D3 serum level of a donor is at the middle of the normal range (30–100 ng/ml) at around 65 ng/ml (Fig. 3a). Using obinutuzumab-labeled DAUDI cells as targets, an increase of ADCC activity with increasing 25-OH-D3 serum levels was observed (Fig. 3b) which was similar to the one observed with rituximab, but lysis rates with obinutuzumab were higher and the effect of 25-OH-D3 on the obinutuzumab-induced ADCC was less pronounced. Similar to rituximab, the optimum lysis rate was observed at 65 ng/ml 25-OH-D3. However, the decline after further substitution to ca. 90 ng/ml (in contrast to the observation with rituximab) was not significant. Results in Fig. 3a–c are shown for an antibody concentration of 0.1 µg/ml, which is within the range of rituximab concentrations achieved in lymphoma patients treated with this antibody. Effects of 25-OH-D3 were virtually identical at all antibody concentrations (0.0001–1 µg/ml) tested. ADCC activity against trastuzumab-treated ZR-75-1 breast cancer cells showed a similar 25-OH-D3 dependent trend, but in contrast to the CD20 antibodies rituximab and obinutuzumab, differences in trastuzumab-mediated ADCC were not significant (Fig. 3c).
Fig. 3.
Effect of vitamin D3 levels on NK cell-mediated ADCC a–c summarize the ADCC-mediated lysis rates of all individuals at four actually achieved 25-OH-D3 serum levels at an E:T ratio of 2.5:1 and 0.1 µg/ml of rituximab-loaded DAUDI cells (a), obinutuzumab-loaded DAUDI cells (b) and trastuzumab-loaded ZR-75-1 breast cancer cells (c). 25-OH-D3 substitution restricted to the lower normal range (32.1 ng/ml) did not affect the NK cell-mediated ADCC. After continued substitution only rituximab- (p = 0.001) and obinutuzumab-mediated ADCC (p = 0.007) increased significantly at a 25-OH-D3 serum level of 66.1 ng/ml compared to base line, while the increase of trastuzumab-mediated ADCC was not significant (p = 0.064). Further 25-OH-D3 substitution to 94.1 ng/ml resulted in a weak, but significant decline of the rituximab-mediated ADCC (p = 0.045) compared to the maximum, while this decline was minimal for obinutuzumab (p = 0.145) and remained unchanged for trastuzumab (p = 0.49). d Relative 25-OH-D3 induced increase of target cell lysis at different antibody concentrations and E:T ratios after the substitution of 25-OH-D3 serum levels to 66.1 ng/ml. The curves show the decreasing impact of 25-OH-D3 with increasing antibody concentrations and higher E:T ratios and the smaller effect on obinutuzumab-ADCC compared to rituximab-ADCC. Signs and symbols: Check/closed line: rituximab (E:T = 2.5:1); quadrate/dashed line: rituximab (E:T = 5:1); triangle/dotted line: obinutuzumab (E:T = 2.5:1); circle/chain line: obinutuzumab (E:T = 5:1)
Quantification of the 25-OH-D3 effect on ADCC
We also quantified ADCC before and after 25-OH-D3 substitution. Due to the lack of significant 25-OH-D3 effects on trastuzumab-mediated ADCC, we restricted the quantitative analysis to the anti-CD20 antibodies rituximab and obinutuzumab. The results are shown in Fig. 3d and can be summarized as follows: (1) the effect of 25-OH-D3 on the NK cell-mediated ADCC was more pronounced with rituximab compared to obinutuzumab; (2) the 25-OH-D3 effect on ADCC was stronger at lower antibody concentrations. (3) Choosing only two rather low E:T ratios, it was not possible to make a statement about the effect of vitamin D3 at higher E:T ratios. Anyway, at all antibody concentrations of rituximab and obinutuzumab used to label the target cells, vitamin D3 showed a stronger effect on the NK cell-mediated ADCC when effector and target cells were applied in the lowest ratio of 2.5:1 compared to 5:1. The optimum effect of 25-OH-D3 with a doubling of the lysis rate compared to base line values was observed at 65 ng/ml 25-OH-D3 at a rituximab concentration of 0.0001 µg/ml and at an E:T ratio of 2.5:1 (p < 0.008). Yet, still at the highest antibody concentration (1 µg/ml) and E:T ratio (5:1), 25-OH-D3 substitution resulted in a 12% higher lysis rate with obinutuzumab (p < 0.03) and an even 18% higher lysis rate with rituximab (p < 0.01) compared to base line values.
Sex-specific 25-OH-D3 effects on NK cell-mediated ADCC
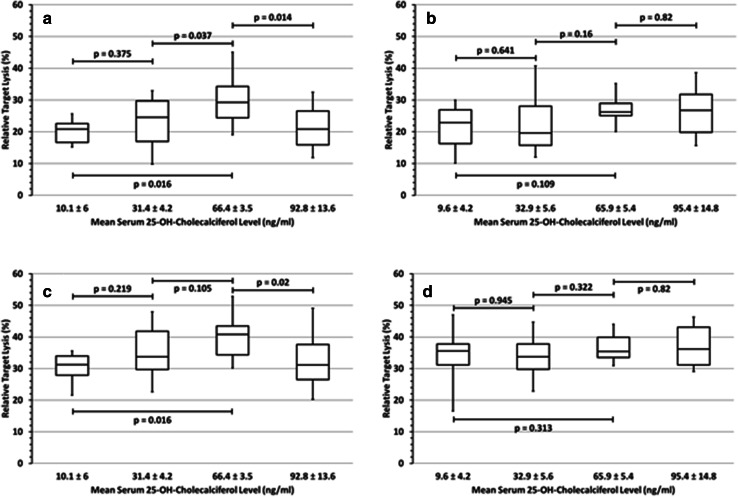
In our previous study [23], we had demonstrated that 25-OH-D3 deficiency impairs the clinical outcome of lymphoma patients, and this was more pronounced in female compared to male patients (Supplementary Fig. 2 [23]). We, therefore, compared rituximab- and obinutuzumab-mediated ADCC between males and females before and after 25-OH-D3 substitution. Indeed, the effects of 25-OH-D3 on NK-cell activity and NK cell-mediated ADCC were significant when female NK-cell donors (Fig. 4a/c) were analyzed separately and, in contrast, less pronounced in males and—due to the limited number of individuals studied—was not significant (Fig. 4b/d). This sex-specific difference of ADCC was observed with both antibodies rituximab (Fig. 4a/b) and obinutuzumab (Fig. 4c/d) at all antibody concentrations and E:T ratios investigated.
Fig. 4.
Sex-specific effects of vitamin D3 on NK cell-mediated ADCC In contrast to female volunteers, where 25-OH-D3 substitution resulted in a significant increase of rituximab-dependent ADCC activity (a), there was only a trend in males (b). The same was observed for obinutuzumab-mediated ADCC in females (c) and males (d). Further substitution to 92.8 ng/ml led to a significantly decreased ADCC in females compared to the maximum (p ≤ 0.02), irrespective of the antibody—antibody concentrations 0.1 µg/ml; E:T ratio 2.5:1
Discussion
After demonstrating the effect of 25-OH-D3 on antibody-dependent cellular phagocytosis (ADCP) mediated by macrophages in a previous study [20], we focused our investigations on NK cells because of the limited number of effector cells that can be obtained from one donor in a single blood sampling. For this reason, neutrophils that also mediate ADCC were not included in the current study. NK cell-mediated ADCC is the major mechanism of action of both rituximab and obinutuzumab and improved significantly in 25-OH-D3 deficient and insufficient individuals after 25-OH-D3 substitution. The results of this study do not only explain the findings of a worse outcome of patients with low 25-OH-D3 levels in both DLBCL [23] and follicular lymphoma [24], they also help to design interventional trials aiming at improving the outcome of CD20 antibody-treated patients by 25-OH-D3 substitution. For such trials knowledge of the optimum serum level and schedules of the 25-OH-D3 substitution is a prerequisite. The formula published by van Groningen et al. to calculate the substitution dose proved to be unsuitable to achieve the predefined serum levels within less than a week, the usual time of the so-called prephase treatment with prednisone before the start of immunochemotherapy for DLBCL [23]. We adjusted the formula which now enables a safe and precise achievement of optimum serum target levels by the time of the first application of rituximab, which is usually urgent, at least in fast-growing cases of DLBCL. Consequently, we changed the substitution formula in our interventional OPTIMAL > 60 trial (NCT01478542/amendment#3) in elderly patients with DLBCL, where patients now will be substituted to mid-normal 25-OH-D3 target levels.
The measurement of the release of an intracellular enzyme, such as the lactate dehydrogenase (LDH), has been found to be almost as sensitive but substantially easier to handle compared to the Cr-release assay [27]. By analyzing 25-OH-D3 serum levels of 30 ng/ml, 65 ng/ml and 90 ng/ml for NK-cell activity and both rituximab- and obinutuzumab-mediated NK-ADCC, a consistent optimum was observed at serum levels around 65 ng/ml which is in the middle of the normal range. This is remarkable because the normal range was originally defined based on parameters of bone metabolism [28].
Our current study demonstrates a more pronounced effect of 25-OH-D3 in females, explaining our clinical observation in the RICOVER-60 study [23] where only female patients with a low 25-OH-D3 serum level (< 8 ng/ml) fared significantly worse than females with higher 25-OH-D3 levels, while the difference in male patient did not reach significance. Since all female patients in the RICOVER-60 trial [23] and the majority of the volunteers in this study were post-menopausal, a negative effect of testosterone is the most likely mechanism for this sex-specific effect of vitamin D3. In support of this hypothesis Olmoz-Ortiz could show that testosterone inhibits the conversion to active 1,25-OH vitamin D3 by down regulation of CYP27B1 and increases the metabolism to inactive vitamin D3 by CYP24A1 [29]. Whether and to which degree differences in hepatic enzymatic activities between elderly women and men play a role, can only be speculated on [30].
Possible mechanisms or signaling pathways of vitamin D3 to affect NK cell-mediated ADCC were not investigated in this study. Nevertheless, CD16 expression could be examined in the context of quality control after isolation of the NK cells. In contrast to the considerably fluctuating CD16 expression between the single subjects, there was no significant different mean fluorescence intensity of CD16 on NK cells of a single subject at the four different 25-OH serum levels as assessed by flow cytometry.
The influence of 25-OH-D3 was dependent on experimental conditions. Thus, the increase in lysis rates after the substitution of 25-OH serum levels to 65 ng/ml using the ADCC-optimized antibody obinutuzumab was lower compared to rituximab. Similarly, an increasing effect of vitamin D3 on NK cell-mediated ADCC was also observed with decreasing antibody concentrations, irrespective of whether rituximab or obinutuzumab was used. Regarding the E:T ratio, the influence of vitamin D3 on ADCC after substitution to 65 ng/ml was stronger at an E:T ratio of 2.5:1 than at 5:1. However, since both E:T ratios were rather low, it was not possible to predict the effect of vitamin D3 at high E:T ratios (10:1 and higher). NK cells, such as immune cells in general, have a limited maximal activity. The scope for improvement in NK cell-mediated ADCC by vitamin D3 was more obvious at “suboptimal” conditions such as non ADCC-optimized antibody and low antibody concentration. However, in consideration of the conditions in vivo with peripheral concentrations of therapeutic antibodies hardly exceeding 0.1 µg/ml and a NK-cell/tumor-cell ratio (E:T ratio) hardly above 1:1 the effect of vitamin D3 on NK cell-mediated ADCC has a vitally high impact, and thus can decisively improve the clinical outcome of immunotherapies based on these antibodies.
In contrast to the consistent 25-OH-D3 effects on ADCC mediated by the CD20 antibodies rituximab and obinutuzumab, this was not observed to a similar degree with trastuzumab, where 25-OH-D3 effects showed a similar trend, but did not reach significance. This is most likely due the fact that the NK cell-dependent ADCC is not the major mechanism of action of this antibody [31, 32]. In addition, the trastuzumab-mediated ADCC showed a high inter-individual and intra-individual variability, making the demonstration of statistically significant 25-OH-D3 effects even more challenging.
In summary, additional to providing a reliable formula for a rapid and precise 25-OH-D3 substitution to pre-defined serum levels, our study shows for the first time an improved rituximab- and obinutuzumab-induced NK cell-mediated ADCC after 25-OH-D3 substitution in vivo. Since the maximum ADCC was consistently observed at serum levels around 65 ng/ml and declined with further substitution, this 25-OH-D3 serum level should be the target in interventional trials. That most interventional trials with vitamin D3 have failed to date [33–35] might also be due to the fact that in these studies the serum levels after substitution were either not controlled or did not aim at and did not achieve 25-OH-D3 serum levels at the mid-normal range. Unfortunately, this also holds true for many recently completed or ongoing trials of vitamin D in cancer patients (for details see http://www.clinicaltrials.gov). A planned interim analysis of our OPTIMAL > 60 study revealed, that the majority of patients did not achieve the pre-defined optimum 25-OH-D3 serum levels. However, in this study so far the vitamin D3 dose to be substituted was calculated according to the conventional van Groningen formula (study amendment#2), which made it necessary to change to our modified formula (amendment#3). This may give a definite answer to the question whether low 25-OH-D3 levels are just a marker for poor prognosis or whether vitamin D3 deficiency and insufficiency are druggable targets with the potential of improving the outcome of the respective patients. A recent study [36] gives an initial indication that adequate vitamin D3 substitution may actually improve the clinical outcome in lymphoma patients receiving immunochemotherapy.
Acknowledgements
The authors thank all volunteers who have participated in this study.
Abbreviations
- ADCC
Antibody-depending cellular cytotoxicity
- CHOP
Cyclophosphamide, hydroxydaunorubicin, oncovin™, predniso(lo)n
- CYP24A1
Cytochrome P450/family 24/subfamily A/member 1
- CYP27B1
Cytochrome P450/family 27/subfamily B/member 1
- DLBCL
Diffuse large B-cell lymphoma
- Fc region
Fragment crystallizable region
- RICOVER-60
Rituximab + CHOP in patients over 60
- 25-OH-D3
25-OH-cholecalciferol vitamin D3
Author contributions
FN conducted the experiments and wrote the manuscript. FA acquired the samples, performed the experiments and did the statistical analysis. CS performed the experiments. MP designed the study, wrote and revised the (primary) manuscript. JTB designed the study and revised the manuscript. All authors were involved in the interpretation of data and drafting the manuscript for important intellectual content. All authors agree to be accountable for the integrity of the data. During the preparation of this paper Michael Pfreundschuh sadly passed away.
Funding
This study was supported by the Eva Mayr-Stihl foundation and by the Förderverein Krebsforschung Saar-Pfalz-Mosel, both charity organizations.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This study had been approved by the local ethical review board (Ethikkommission der Ärztekammer des Saarlandes—No. 178/17). All procedures performed in this study were in accordance with the ethical standards of this institutional committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included in the study.
Cell lines authenticity
The Burkitt lymphoma cell line DAUDI was acquired on March 18, 2015 from the “Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures” (Braunschweig, Germany, Invoice 01502841-1) and the breast cancer cell line ZR-75-1 was acquired on May 07, 2014 from “CLS-Cell Lines Service Ltd.” (Eppelheim, Germany, Invoice R140381). Both providers guarantee the authenticity of the respective cell line. Thus, at the time of the study, the two cell lines were not older than 2 years and were used only in our laboratory. During the study, the authenticity of the lines was ensured by the fact that the antibodies used to induce the ADCC always functioned cell-line-specifically. Moreover, these cell lines are also not included in the list of confirmed misidentified and cross-contaminated cell lines.
Footnotes
Michael Pfreundschuh: Deceased.
References
- 1.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;24(4):235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 2.Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, Ma D, Gill D, Walewski J, Zinzani PL, Stahel R, Kvaloy S, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7(5):379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 3.Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, Reiser M, Nickenig C, Clemens M, Peter N, Bokemeyer C, Eimermacher H, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20 + B-cell lymphomas: a randomised controlled trial (RICOVER-60) Lancet Oncol. 2008;9(2):105–116. doi: 10.1016/S1470-2045(08)70002-0. [DOI] [PubMed] [Google Scholar]
- 4.Smith MR. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 2003;22(47):7359–7368. doi: 10.1038/sj.onc.1206939. [DOI] [PubMed] [Google Scholar]
- 5.Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47(2):115–123. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Kolk LE, Grillo-Lopez AJ, Baars JW, Hack CE, van Oers MH. Complement activation plays a key role in the side-effects of rituximab treatment. Br J Haematol. 2001;115(4):807–811. doi: 10.1046/j.1365-2141.2001.03166.x. [DOI] [PubMed] [Google Scholar]
- 7.Di Gaetano N, Cittera E, Nota R, Vecchi A, Grieco V, Scanziani E, Botto M, Introna M, Golay J. Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol. 2003;171(3):1581–7. doi: 10.4049/jimmunol.171.3.1581. [DOI] [PubMed] [Google Scholar]
- 8.Golay J, Cittera E, Di Gaetano N, Manganini M, Mosca M, Nebuloni M, van Rooijen N, Vago L, Introna M. The role of complement in the therapeutic activity of rituximab in a murine B lymphoma model homing in lymph nodes. Haematologica. 2006;91(2):176–183. [PubMed] [Google Scholar]
- 9.Zhou X, Hu W, Qin X. The role of complement in the mechanism of action of rituximab for B-cell lymphoma: implications for therapy. Oncologist. 2008;13(9):954–966. doi: 10.1634/theoncologist.2008-0089. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez-Ilizaliturri FJ, Jupudy V, Ostberg J, Oflazoglu E, Huberman A, Repasky E, Czuczman MS. Neutrophils contribute to the biological antitumor activity of rituximab in a non-Hodgkin’s lymphoma severe combined immunodeficiency mouse model. Clin Cancer Res. 2003;9(16 Pt 1):5866–5873. [PubMed] [Google Scholar]
- 11.Lefebvre ML, Krause SW, Salcedo M, Nardin A. Ex vivo-activated human macrophages kill chronic lymphocytic leukemia cells in the presence of rituximab: mechanism of antibody-dependent cellular cytotoxicity and impact of human serum. J Immunother. 2006;29(4):388–397. doi: 10.1097/01.cji.0000203081.43235.d7. [DOI] [PubMed] [Google Scholar]
- 12.Dall’Ozzo S, Tartas S, Paintaud G, Cartron G, Colombat P, Bardos P, Watier H, Thibault G. Rituximab-dependent cytotoxicity by natural killer cells: influence of FCGR3A polymorphism on the concentration-effect relationship. Cancer Res. 2004;64(13):4664–4669. doi: 10.1158/0008-5472.CAN-03-2862. [DOI] [PubMed] [Google Scholar]
- 13.Leidi M, Gotti E, Bologna L, Miranda E, Rimoldi M, Sica A, Roncalli M, Palumbo GA, Introna M, Golay J. M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than m1 cells in vitro. J Immunol. 2009;182(7):4415–4422. doi: 10.4049/jimmunol.0713732. [DOI] [PubMed] [Google Scholar]
- 14.Seidel UJ, Schlegel P, Lang P. Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies. Front Immunol. 2013;4:76. doi: 10.3389/fimmu.2013.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol. 2015;6:368. doi: 10.3389/fimmu.2015.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golay J, Manganini M, Facchinetti V, Gramigna R, Broady R, Borleri G, Rambaldi A, Introna M. Rituximab-mediated antibody-dependent cellular cytotoxicity against neoplastic B cells is stimulated strongly by interleukin-2. Haematologica. 2003;88(9):1002–1012. [PubMed] [Google Scholar]
- 17.Ahlgrimm M, Pfreundschuh M, Kreuz M, Regitz E, Preuss KD, Bittenbring J. The impact of Fc-gamma receptor polymorphisms in elderly patients with diffuse large B-cell lymphoma treated with CHOP with or without rituximab. Blood. 2011;118(17):4657–4662. doi: 10.1182/blood-2011-04-346411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishima Y, Terui Y, Mishima Y, Kuniyoshi R, Matsusaka S, Mikuniya M, Kojima K, Hatake K. High reproducible ADCC analysis revealed a competitive relation between ADCC and CDC and differences between FcgammaRllla polymorphism. Int Immunol. 2012;24(8):477–483. doi: 10.1093/intimm/dxs048. [DOI] [PubMed] [Google Scholar]
- 19.Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, Lee ZW, Lee SH, Kim JM, Jo EK. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6(3):231–243. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Bruns H, Buttner M, Fabri M, Mougiakakos D, Bittenbring JT, Hoffmann MH, Beier F, Pasemann S, Jitschin R, Hofmann AD, Neumann F, Daniel C, et al. Vitamin D-dependent induction of cathelicidin in human macrophages results in cytotoxicity against high-grade B cell lymphoma. Sci Transl Med. 2015;7(282):282ra47. doi: 10.1126/scitranslmed.aaa3230. [DOI] [PubMed] [Google Scholar]
- 21.Hossein-nezhad A, Spira A, Holick MF. Influence of vitamin D status and vitamin D3 supplementation on genome wide expression of white blood cells: a randomized double-blind clinical trial. PLoS One. 2013;8(3):e58725. doi: 10.1371/journal.pone.0058725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364(3):248–254. doi: 10.1056/NEJMcp1009570. [DOI] [PubMed] [Google Scholar]
- 23.Bittenbring JT, Neumann F, Altmann B, Achenbach M, Reichrath J, Ziepert M, Geisel J, Regitz E, Held G, Pfreundschuh M. Vitamin D deficiency impairs rituximab-mediated cellular cytotoxicity and outcome of patients with diffuse large B-cell lymphoma treated with but not without rituximab. J Clin Oncol. 2014;32(29):3242–3248. doi: 10.1200/JCO.2013.53.4537. [DOI] [PubMed] [Google Scholar]
- 24.Kelly JL, Salles G, Goldman B, Fisher RI, Brice P, Press O, Casasnovas O, Maloney DG, Soubeyran P, Rimsza L, Haioun C, Xerri L, et al. Low serum vitamin D levels are associated with inferior survival in follicular lymphoma: a prospective evaluation in SWOG and LYSA studies. J Clin Oncol. 2015;33(13):1482–1490. doi: 10.1200/JCO.2014.57.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;20(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 26.van Groningen L, Opdenoordt S, van Sorge A, Telting D, Giesen A, de Boer H. Cholecalciferol loading dose guideline for vitamin D-deficient adults. Eur J Endocrinol. 2010;162(4):805–811. doi: 10.1530/EJE-09-0932. [DOI] [PubMed] [Google Scholar]
- 27.Korzeniewski C, Callewaert DM. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983;64(3):313–320. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- 28.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16(7):713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 29.Olmos-Ortiz A, Garcia-Quiroz J, Lopez-Marure R, Gonzalez-Curiel I, Rivas-Santiago B, Olivares A, Avila E, Barrera D, Halhali A, Caldino F, Larrea F, Diaz L. Evidence of sexual dimorphism in placental vitamin D metabolism: testosterone inhibits calcitriol-dependent cathelicidin expression. J Steroid Biochem Mol Biol. 2016;163:173–182. doi: 10.1016/j.jsbmb.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol. 2004;44:499–523. doi: 10.1146/annurev.pharmtox.44.101802.121453. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki E, Toi M. Improving the efficacy of trastuzumab in breast cancer. Cancer Sci. 2007;98(6):767–771. doi: 10.1111/j.1349-7006.2007.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haen SP, Schmiedel BJ, Rothfelder K, Schmied BJ, Dang TM, Mirza N, Mohle R, Kanz L, Vogel W, Salih HR. Prognostic relevance of HER2/neu in acute lymphoblastic leukemia and induction of NK cell reactivity against primary ALL blasts by trastuzumab. Oncotarget. 2016;7(11):13013–13030. doi: 10.18632/oncotarget.7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caballero-Velazquez T, Montero I, Sanchez-Guijo F, Parody R, Saldana R, Valcarcel D, Lopez-Godino O, Ferra IC, Cuesta M, Carrillo-Vico A, Sanchez-Abarca LI, Lopez-Corral L, et al. Immunomodulatory Effect of Vitamin D after Allogeneic Stem Cell Transplantation: Results of a Prospective Multicenter Clinical Trial. Clin Cancer Res. 2016;22(23):5673–5681. doi: 10.1158/1078-0432.CCR-16-0238. [DOI] [PubMed] [Google Scholar]
- 34.Manaseki-Holland S, Maroof Z, Bruce J, Mughal MZ, Masher MI, Bhutta ZA, Walraven G, Chandramohan D. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet. 2012;379(9824):1419–1427. doi: 10.1016/S0140-6736(11)61650-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murdoch DR, Slow S, Chambers ST, Jennings LC, Stewart AW, Priest PC, Florkowski CM, Livesey JH, Camargo CA, Scragg R. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308(13):1333–1339. doi: 10.1001/jama.2012.12505. [DOI] [PubMed] [Google Scholar]
- 36.Hohaus S, Tisi MC, Bellesi S, Maiolo E, Alma E, Tartaglia G, Corrente F, Cuccaro A, D’Alo’ F, Basile U, Larocca LM, De S. Vitamin D deficiency and supplementation in patients with aggressive B-cell lymphomas treated with immunochemotherapy. Cancer Med. 2018;7(1):270–281. doi: 10.1002/cam4.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]