Abstract
The prognostic value of the local immune phenotype in patients with colorectal cancer has been extensively studied. Neoadjuvant radiotherapy and/or chemotherapy may potentially influence these immune responses. In this study, we examined the prognostic role of indoleamine-2,3-Dioxygenase (IDO1) and infiltrating cytotoxic T lymphocytes (CD8+) in locally advanced rectal carcinomas after neoadjuvant treatment. Expression of IDO1 and CD8 was evaluated by immunohistochemistry in 106 archival tumour tissue samples from patients following neoadjuvant chemoradiation and radical resection. The average infiltration of IDO1+ and CD8+ cells was calculated along the tumour invasive front, in the tumour centre and within the neoplastic cells and expressed as total scores. Of the tumour specimens evaluable for immunohistochemistry, 100% showed CD8+ lymphocyte infiltration and 93.4% stained positive for IDO1. Total IDO1 score positively correlated with total CD8 score for all three subsites (p = 0.002, Kendall-tau-b 0.357). A high total CD8 score was positively correlated with lower ypUICC-stages (p = 0.047) and lower ypT-categories (p = 0.032). Total IDO1 expression showed a clear trend towards a lower risk of recurrence (p = 0.078). A high total IDO1 score was an independent prognostic marker for prolonged disease-free survival (HR 0.38, p = 0.046) and a high total CD8 score for favourable overall survival (HR 0.16, p = 0.029). Analysis of the local CD8 and IDO1 expression profile may be a helpful tool in predicting prognosis for patients with locally advanced rectal cancer following neoadjuvant chemoradiation.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02306-y) contains supplementary material, which is available to authorized users.
Keywords: Rectal cancer, Neoadjuvant therapy, IDO1, Tumour-infiltrating lymphocytes, Survival, Prognostic factors
Introduction
Colorectal cancer is the second most common malignancy in developed countries with a lifetime prevalence of 5%. Approximately 30% of these tumours are located in the rectum [1, 2]. The current gold standard for patients with locally advanced rectal cancer (LARC) is a multimodal therapy comprised of neoadjuvant-combined chemoradiation with subsequent low-anterior resection and total mesorectal excision (TME). This increases the success rate of sphincter-sparing operations and substantially reduces local tumour recurrence [3–5]. However, long-term follow-up has so far failed to demonstrate an improvement in disease-free or overall survival (DFS or OS respectively) [6, 7]. Distant metastases rather than local recurrence remain the dominant problem in the modern era of multimodal therapy for LARC. Consequently, more recent approaches have incorporated combination chemotherapy and/or the use of molecularly targeted agents in the adjuvant treatment to improve survival rates in these patients [8].
In the process of implementing a multidisciplinary treatment approach to LARC, patient subgroups based on pathologic examination were identified that have been shown to correlate with the risk of recurrence and overall survival. In colorectal cancer, the tumour–node–metastasis system (TNM) introduced by the American Joint Committee on Cancer/Union Internationale Contre le Cancer (AJCC/UICC-TNM) is the international standard for pathological tumour classification [9]. The TNM system, however, provides only limited prognostic information and does not accurately predict the benefit from certain therapies [10].
With the exception of microsatellite instability, recent molecular analyses of rectal cancers did not provide novel genetic markers [11]. In contrast, the local adaptive immune reaction is a reliable and relevant parameter to predict recurrence and survival. The type, density and location of infiltrating lymphocytes influence the evolution of human colorectal cancers [12]. Based on these findings, an “Immunoscore” was introduced that is more accurate in predicting outcome in patients with colorectal carcinoma than the TNM system [13]. Results from ongoing large-scale studies have substantiated this new scoring system in the routine clinical setting [14, 15].
In the past two decades, tryptophan catabolism has emerged as a powerful mechanism of peripheral immune resistance [16]. This is mainly achieved by the action of indoleamine-2,3-dioxygenase 1 (IDO1) and, to a lesser extent, by tryptophan-2,3-dioxygenase (TDO). As a result, tryptophan is locally depleted, while catabolites (i.e. kynurenine and its derivatives) accumulate. These metabolic changes are considered to support a profoundly immunosuppressive environment by mechanisms that still remain incompletely characterized but which include cell cycle arrest of T-cells [17, 18], reduced T-cell proliferation capacity [19], induced T-cell apoptosis [20, 21] and differentiation of effector T-cells into regulatory T-cells [22, 23]. Therefore, IDO1 has been discussed as one of the potential key players of immune evasion in human cancer [24, 25]. In subsequent studies, IDO1 was found to be expressed in several types of human tumours, including colorectal cancer [26–28]. Interestingly, results in terms of cell types expressing IDO1 (tumour cells vs. stromal cells), the location of IDO1 expression (tumour tissue vs. tumour-draining lymph nodes) and its correlation with patient survival remain completely heterogeneous [29]. In summary, the exact role of IDO1 expression in colorectal cancer remains to be clarified.
The prognostic value of the local immune cell infiltration in patients with colorectal cancer has been extensively studied. In this study, we aimed to investigate a potential prognostic role of IDO1 expression and/or cytotoxic T-cell infiltration in tumour specimens of patients with LARC who had undergone neoadjuvant long-term chemoradiation prior to radical resection.
Materials and methods
Study population
106 consecutive adult patients with histologically proven, locally advanced rectal adenocarcinoma of the middle or lower third (uT3/uT4 uN−/+, uT1 − uT4 uN + cM0) between 2009 and 2015 were identified from a prospectively collected surgical administrative database at the University Hospital Würzburg, Germany.
Tumour classification was performed according to the UICC- and TNM-classification for colorectal adenocarcinoma. All patients underwent physical examination. Local staging was done by rigid endoscopy plus endorectal ultrasound as well as MRI of the pelvis. CT scans of the thorax and abdomen were used to exclude distant metastases. Multimodal therapy consisted of neoadjuvant long-term chemoradiation, low-anterior resection of the rectum with total mesorectal excision and, according to the ypTNM stage, the administration of adjuvant chemotherapy. Baseline characteristics included age, sex, body mass index (BMI), classification of the primary tumour, treatment characteristics covering neoadjuvant treatment protocols and operative procedures as well as 5-year disease-free and overall survival rates. Patient follow-up was carried out according to national guidelines [30].
Immunohistochemistry
According to routine procedures, rectal cancer specimens obtained after tumour resection were fixed in buffered formaldehyde and embedded in paraffin. For the immunohistochemical staining, 4-µm sections from tumour areas were cut, dried for 1 h at 56 °C, deparaffinized in xylol and rehydrated in descending concentrations of alcohol. Antigen retreatment was performed in a steamer for 10 min with citrate buffer pH 6.0. For the CD8+ staining, a semiautomatic staining machine (Tecan, Freedom EVO) was used. All immunohistochemical analyses were performed according to standard protocols using the Advance™ HRP Detection kit (K4068) from Dako and the EnVision™ FLEX DAB + Substrate Chromogen System for visualization. We used a monoclonal anti-CD8+ antibody (Clone C8/144B, Dako, dilution 1:80), a monoclonal anti CD1a antibody (Clone O10, Santa Cruz, dilution 1:100), a monoclonal anti-CD163 antibody (Clone 10D6, Leica Biosystems, dilution 1:200) and a monoclonal anti-CD5 antibody (Clone 4C5, Leica Biosystems, dilution 1:100). For visualization of IDO1 expression, we also used the Advance™ HRP Detection kit (K4068) from Dako and the EnVision™ FLEX DAB + Substrate Chromogen System. The immunohistochemical reactions were performed manually using the purified mouse anti-human IDO1 monoclonal antibody (V1NC3IDO antibody, Mouse IgG2b, kappa, eBiosience, dilution 1:1000), which reacts with IDO1 and does not cross-react with IDO2. Normal placenta was used as a positive control. Negative controls included omission of primary IDO1 antibody on duplicate placenta according to Theaté et al. [31]. Finally, all slides were counterstained with Mayer’s Haematoxylin and automatically cover slipped with tape (Tissue Tek, Diatec). A final validation stage was conducted by a pathologist who performed a visual evaluation of the stained slides under a light microscope (Olympus BX 50) to confirm the usability of the immunostaining. The specimens were evaluated two times by two independent observers (kappa-values observer 1: 0.74–0.89, kappa-values observer 2: 0.67–0.83, interobserver kappa values: 0.71–0.81) to obtain a conclusive judgement regarding the CD8 and IDO1 quantification and to exclude intra- and interobserver variability.
Immunohistochemical evaluation of infiltrating CD8+ cells
Evaluation of immunohistochemical CD8+ staining was performed semi-quantitatively under a light microscope (Olympus BX 50) at three different localizations: the stroma at the invasive tumour front, the stroma in the tumour centre, and lymphocytes within the tumour epithelium (intraepithelial expression). The tumour centre was defined as the stroma within the tumour mass with a clear distance from the invasive tumour front as well as from the luminal border. The invasive tumour front was classified as the area of the deepest tumour invasion into the tissue. Three representative fields were examined at every single subsite (high power fields, 40x objective magnification).
Based on the density of CD8+ cell infiltration, a classification system was established for each area as follows: stroma at the invasive tumour front and stroma in the tumour centre: 1 = no or sporadic (0–10 CD8+-cells/HPF), 2 = moderate (11–30 cells/HPF), 3 = abundant (31–50 cells/HPF), 4 = highly abundant infiltration (> 50 cells/HPF). Intraepithelial: 1 = no or sporadic (0–1 CD8+-cells/HPF), 2 = moderate (2–4 cells/HPF), 3 = abundant (5–10 cells/HPF), 4 = highly abundant infiltration (> 11 cells/HPF). A total CD8 score ranging from 3 to 12 was calculated upon the sum of the single CD8 expression levels of every subsite. Patients were classified into three groups showing a low (3–4), intermediate (5–6) or high (7–12) total CD8 score (according to ref. [32–34]).
Immunohistochemical evaluation of IDO expression
We performed a semi-quantitative evaluation of IDO1 expression at the tumour invasive front and calculated the fraction of infiltrating cells positively stained for IDO1 as percentage of all cells in this compartment. We separately analysed IDO1 expressing neoplastic epithelial cells and stroma cells as previously described [31]: 0 = no IDO1 expression, 1 ≤ 5%, 2 ≥ 5%, 3 ≥ 50% stained IDO1 expressing cells. Consequently, we calculated a total IDO1 score ranging from 0 to 6 for every tumour specimen as the sum of epithelial and stromal IDO1 expression. According to former studies [26], tumours were finally classified into two categories: IDO1-low expression (total IDO1 score 0–2) and IDO1-high expression (total IDO1 score 3–6).
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics 24.0. Cross tabulations were analysed with the Chi-square test for categorical variables or Fischer’s exact test when expected frequencies were less than five. Correlations between different CD8+ and IDO1 scores were determined by nonparametric Kendall Tau-b correlation coefficient. For survival analysis, Kaplan–Meier curves were used to describe overall and disease-free survival. Differences in survival between groups were tested by log-rank tests. Cox proportional hazard regression models were used for univariate and multivariate survival analysis. For both, Kaplan–Meier curves and Cox regression models, we excluded patients with a follow-up less than one year or patients with distant metastasis who did not undergo curative resection. In all analyses, p < 0.05 was considered statistically significant.
Results
Patient characteristics
Between May 2009 and March 2015, a total of 106 adult patients underwent multimodal therapy for the primary diagnosis of locally advanced cancer of the middle or lower rectum at our institution. We excluded all patients with synchronous distant metastases. Approximately two-thirds of the study populations were male (67.9%), median age was 64 years (39–88) and median BMI was 26.2 kg/m2 (17.0–49.5). 97.2% of patients were diagnosed with cT3/T4 tumours and 65.1% with a positive nodal status. Multimodal therapy included neoadjuvant long-term chemoradiation followed by surgery 6–8 weeks thereafter according to national guidelines [30]. Surgery was either performed as low-anterior resection with total mesorectal excision (TME) in 86 cases (81.1%) or as rectum extirpation with TME in 20 cases (18.9%). Pathologic examination revealed a complete tumour regression (ypUICC stage 0) in 14 patients (13.2%) and a 100% R0-resection rate in this cohort. 73 patients (69.5%) received adjuvant chemotherapy. 5-year disease-free survival (DFS) was 63.2% and 5-year overall survival (OS) was 76.8%. Patients’ clinical data and detailed tumour-characteristics are displayed in Supplementary Table S1.
Immunostaining for IDO1 and CD8+ lymphocytes in tissue samples
The presence of IDO1 positive (IDO1+) cells and tumour-infiltrating CD8 positive (CD8+) T-lymphocytes was determined by immunohistochemical evaluation of tissue samples. We excluded all primary tumours with a complete histologic tumour regression after neoadjuvant chemoradiation as there was no residual invasive tumour front for histologic examination. Consequently, we were not able to calculate total scores for IDO1 or CD8 expression for these specimens (as outlined in the methods section). Owing to missing data or inferior staining quality, we finally examined 91 tumour samples (85.8%) for IDO1 expression and 84 specimens (79.2%) for CD8+ expression.
IDO1 and CD8 markers were semi-quantitatively assessed by applying previously documented and adapted scaling systems for IDO1 [26] and CD8 [33, 34] expression and by calculating a total score for each marker. T-lymphocyte infiltration was assessed at three different subsites: the invasive tumour front, the tumour centre and within the tumour epithelium (Fig. 1a, b). IDO1 expression was assessed within the tumour epithelium or within the tumour stroma at the invasive front (Fig. 1c).
Fig. 1.
Immunohistochemical evaluation of infiltration of IDO1+ cells and CD8+ T-cells. a Immunohistochemical evaluation of infiltrating CD8+ T-cells at three different areas: the stroma at the invasive tumour front, the stroma in the tumour centre, and within the tumour epithelium (as indicated by black arrow). b Examples of sections with infiltrating CD8+ T-cells within the tumour epithelium: a no or sporadic, b moderate, c abundant, d highly abundant infiltration of CD8+ T-cells. c Evaluation of IDO1 expression at the invasive tumour front (a). Analysis was performed on either intraepithelial (b as indicated by arrow) and/or stromal (c as indicated by arrow) IDO1 expressing cells
84 out of 84 tumour samples (100%) showed a CD8+ lymphocyte infiltration, 56% of those with a highly abundant T-cell count (total CD8 score 7–12), whereas the remaining showed a weak T-cell infiltration (total CD8 score 3–6). Numeric distribution was nearly equal among the three subsites with a trend towards higher T-cell counts within the tumour stroma (data not shown). 85 out of 91 tumours samples (93.4%) were positive in IDO1 staining. 38.5% of those specimens showed a high IDO1 expression profile (total IDO1 score 3–6). With regard to different subsites, more than 90% of samples showed a positive IDO1 staining within the tumour stroma. Assessment of one-third of specimens revealed that IDO1+ stromal cells mainly consisted of CD163+ histiocytes, CD5+ lymphocytes or granulocytes, but rarely of CD1a + dendritic cells (data not shown). Additionally, 63.8% of tumours showed a positive intraepithelial IDO1 expression.
The varying frequencies of infiltrating CD8+ lymphocytes as well as IDO1-positive cells at all three subsites are summarized in a total score for each marker (as outlined in the methods section) and presented in a cross-tabulation in Table 1. The number of infiltrating CD8+ lymphocytes highly and positively correlated with the number of IDO1+ stroma- or tumour cells (p = 0.002, Kendall-tau-b 0.357). 57.4% of tumours with a high total CD8 score (7–12) also showed a high total IDO1 score (3–6). In contrast, only 18.8% of tumours with a low (3–4) or 19.0% of tumours with a moderate (5–6) total CD8 score did so. These specimens showed a low total IDO1 score (0–2) in 81.3% resp. 81.0% (Table 1). With regard to different tumour subsites at the invasive front, an even stronger association was seen in the tumour stroma: approximately 70% of samples with a high CD8 expression (level 3 or 4) also showed a high IDO1 expression (level 2 or 3) (p = 0.008, Kendall-tau-b 0.409, Supplementary Table S2a). In contrast, one-third to half of samples with a high intraepithelial CD8 expression (level 3 or 4) also showed a high IDO1 expression (level 2 or 3) (p = 0.004, Kendall-tau-b 0.344, Supplementary Table S2b).
Table 1.
Cross-tabulation between total scores of CD8 and IDO1 expression in tumour specimens
| CD8 score (n) | IDO1 score (n, %)a | p value | ||
|---|---|---|---|---|
| Low (0–2) | High (3–6) | Total (n, %) | ||
| Low (3–4) | 13 (81.3) | 3 (18.8) | 16 (100) | 0.002 |
| Intermediate (5–6) | 17 (81.0) | 4 (19.0) | 21 (100) | |
| High (7–12) | 20 (42.6) | 27 (57.4) | 47 (100) | |
| Total (n, %) | 50 (59.5) | 34 (40.5) | 84 (100) | |
aPercentage of tumours with either IDO1 low (0–2) or IDO1 high (3–6) expression within each total CD8 score group [low (3–4), intermediate (5–6) or high (7–12)]
Association of IDO1- and CD8-expression with clinicopathologic variables
The total scores of tumour-infiltrating CD8+ lymphocytes and IDO1 expressing cells were correlated with clinicopathologic parameters (Table 2). Total CD8 expression was significantly correlated with higher age (p = 0.042) and a clear trend towards a lower risk of death (p = 0.054). The total number of CD8+ lymphocytes led to a significant shift towards lower ypT-categories (p = 0.032). In detail, 66.0% of tumour specimens with a high total CD8 score (7–12) were grouped into lower ypT categories (ypT1 and 2) as compared to 31.3% with a low total CD8 score (3–4). With regard to tumour classification (ypUICC), a high total CD8 score was significantly associated with a lower ypUICC stage (p = 0.047). 51.1% of specimens with a high total CD8 score (7–12) were classified into ypUICC stage 1, whereas this was the case in only 31.3% of samples with a low total CD8 score (3–4). There was no association between total CD8 expression and tumour regression grade (p = 0.416) (Table 2).
Table 2.
Correlation of total CD8- and IDO1-scores with clinicopathologic parameters
| Total CD8 score (n = 84) | p value | Total IDO1 score (n = 91) | p value | ||||
|---|---|---|---|---|---|---|---|
| Low (3–4) | Intermediate (5–6) | High (7–12) | Low (0–2) | High (3–6) | |||
| Frequency (n, %) | 16 (19.0) | 21 (25.0) | 47 (56.0) | 56 (61.5) | 35 (38.5) | ||
| Age (years, median) | 59 (42–75) | 62 (39–83) | 67 (42–88) | 0.042a | 61 (39–83) | 68 (45–88) | 0.008a |
| Gender | 0.504c | 0.696b | |||||
| Female | 3 (18.8) | 8 (38.1) | 15 (31.9) | 17 (30.4) | 12 (34.3) | ||
| Male | 13 (81.3) | 13 (61.9) | 32 (68.1) | 39 (69.6) | 23 (65.7) | ||
| ypUICC stage (n, %) | 0.047c | 0.421c | |||||
| 1 | 5 (31.3) | 3 (14.3) | 24 (51.1) | 18 (32.1) | 16 (45.7) | ||
| 2 | 5 (31.3) | 10 (47.6) | 11 (23.4) | 19 (33.9) | 10 (28.6) | ||
| 3 | 6 (37.5) | 8 (38.1) | 12 (25.5) | 19 (33.9) | 9 (25.7) | ||
| Grading (n, %) | 43 | 0.651c | 0.705c | ||||
| G1/G2 | 14 (87.5) | 18 (90.0) | (93.5) | 49 (89.1) | 31 (93.9) | ||
| G3/G4 | 2 (12.5) | 2 (10.0) | 3 (6.5) | 6 (10.9) | 2 (6.1) | ||
| Missing | 0 | 1 | 1 | 1 | 1 | ||
| Regression grade (Dworak, n, %) | 0.416c | 0.902c | |||||
| 0 | 0 (0.0) | 0 (0.0) | 1 (2.4) | 1 (2.0) | 0 (0.0) | ||
| 1 | 2 (13.3) | 8 (40.0) | 7 (17.1) | 10 (19.6) | 7 (21.9) | ||
| 2 | 11 (73.3) | 11 (55.0) | 27 (65.9) | 34 (66.7) | 20 (62.5) | ||
| 3 | 2 (13.3) | 1 (5.0) | 6 (14.6) | 6 (11.8) | 5 (15.6) | ||
| Missing | 1 | 1 | 6 | 5 | 3 | ||
| ypT category (n, %) | 0.032c | 0.204c | |||||
| 1 | 1 (6.3) | 0 (0.0) | 6 (12.8) | 6 (10.7) | 2 (5.7) | ||
| 2 | 4 (25.0) | 6 (28.6) | 25 (53.2) | 18 (32.1) | 19 (54.3) | ||
| 3 | 10 (62.5) | 14 (66.7) | 15 (31.9) | 30 (53.6) | 13 (37.1) | ||
| 4 | 1 (6.3) | 1 (4.8) | 1 (2.8) | 2 (3.6) | 1 (2.9) | ||
| ypN category (n, %) | 0.234c | 0.215b | |||||
| N− | 10 (62.5) | 15 (71.4) | 39 (83.0) | 40 (71.4) | 29 (82.9) | ||
| N+ | 6 (37.5) | 6 (28.6) | 8 (17.0) | 16 (28.6) | 6 (17.1) | ||
| Tumour recurrence (n, %) | 0.338c | 0.078b | |||||
| No | 10 (62.5) | 13 (61.9) | 36 (76.6) | 35 (62.5) | 28 (80.0) | ||
| Yes | 6 (37.5) | 8 (38.1) | 11 (23.4) | 21 (37.5) | 7 (20.0) | ||
| Death (n, %) | 0.054c | 0.514b | |||||
| No | 10 (62.5) | 17 (81.0) | 42 (89.4) | 45 (80.4) | 30 (85.7) | ||
| Yes | 6 (37.5) | 4 (19.0) | 5 (10.6) | 11 (19.6) | 5 (14.3) | ||
aONEWAY-Anova
bChi-squared-test
cFisher’s exact test
Total IDO1 expression showed a positive association with higher age (p = 0.008) and a clear trend towards a lower risk of tumour recurrence (p = 0.078). There was no association observed between total IDO1 expression and ypUICC stages (p = 0.421) (Table 2).
We further analysed the correlation of clinicopathologic variables with the expression of IDO1 and CD8 in different subsites: either intraepithelial (IE) or stromal cells (SC) at the invasive tumour front. Interestingly, we found a stronger association of intraepithelial (IE) CD8 expression compared to the total CD8 score with ypT category (p = 0.029). IE CD8 expression was significantly correlated with lower ypUICC stages (p = 0.006) and a nodal-negative status (0.019) (Supplementary Table S3a). Stromal IDO1 expression was positively correlated with recurrence-free survival (p = 0.05) (Supplementary Table S3b).
Impact of IDO1- and CD8-expression on disease-free and overall survival
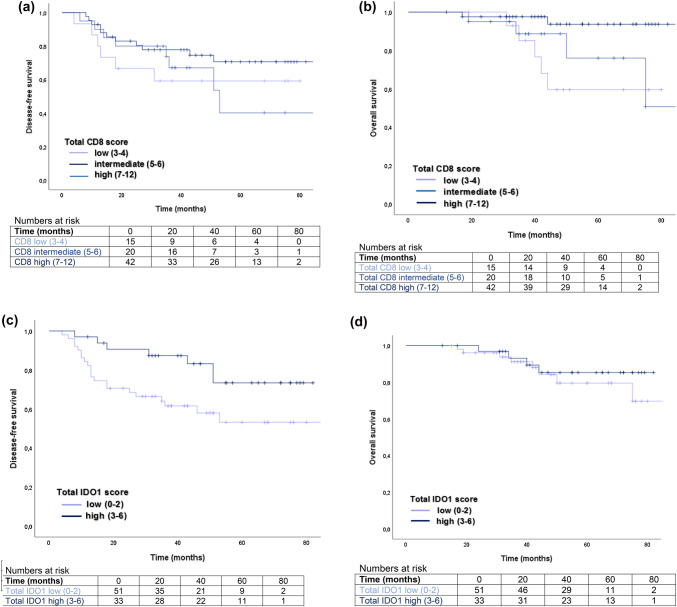
Disease-free (DFS) and overall survival (OS) were compared with the total CD8 or total IDO1 score. Figure 2a, b show Kaplan–Meier plots of DFS and OS of patients with regard to subgroups of total CD8 scores. A high total CD8 score (7–12) showed a statistically significantly improved OS (5-year OS for CD8+ total score 7–12: 93.7% and for CD8+ total score 3–4: 59.6%, log-rank p = 0.023, Fig. 2b and Supplementary Table S4). A high total IDO1 score (3–6) significantly correlated with a prolonged DFS (5-year DFS for IDO1 high: 73.4% and for IDO1 low: 53.1%, log-rank p = 0.033, Fig. 2c and Supplementary Table S4).
Fig. 2.
Impact of the total IDO1 and CD8 score on disease-free and overall survival. a Kaplan–Meier-Plot showing disease-free survival after surgery in patients with low (3–4), intermediate (5–6), and high (7–12) total CD8 score (log rank p = 0.388). b Kaplan–Meier-Plot showing overall survival in patients with low (3–4), intermediate (5–6), and high (7–12) total CD8 score (log rank p = 0.023). c Kaplan–Meier-Plot showing disease-free survival after surgery in patients with low (0–2) and high (3–6) total IDO1 score (log rank p = 0.033). d Kaplan–Meier-Plot showing overall survival in patients with low (0–2) and high (3–6) total IDO1 score (log rank p = 0.478)
Prognostic importance of infiltrating CD8+ lymphocytes and IDO1-expressing cells
We investigated the prognostic significance of the total IDO1 and CD8 score as well as various clinicopathologic variables using Cox proportional hazard models. Total CD8 score (HR 0.14, 95%CI 0.03–0.71, p = 0.018) and ypN category (HR 3.33, 95%CI 1.01–11.02, p = 0.049) constituted prognostic factors for OS in univariate analysis. When analysed in a multivariate model adjusted for those two variables, the total CD8 score remained an independent prognostic factor for OS (HR 0.16, 95%CI 0.03–0.83, p = 0.029) (Table 3). Total IDO1 score (HR 0.34, 95%CI 0.13–0.84, p = 0.02), ypN category (HR 2.66, 95%CI 1.19–5.97, p = 0.017) and ypT category (HR 2.34, 95%CI 1.01–5.45, p = 0.048) were prognostic factors for DFS in univariate analysis. Total IDO1 score remained an independent prognostic variable for DFS in multivariate analysis adjusted for IDO1, ypN and ypT category (p = 0.046) (Table 4).
Table 3.
Uni- and multivariate analysis for overall survival in locally advanced rectal cancer
| Univariate analysis | Multivariate analysisa | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Total CD8 score | ||||||
| Low (3–4) | 1 | 1 | ||||
| Medium (5–6) | 0.691 | 0.185–2.580 | 0.583 | 0.689 | 0.187–2.611 | 0.594 |
| High (7–12) | 0.137 | 0.027–0.708 | 0.018 | 0.158 | 0.030–0.830 | 0.029 |
| Total IDO1 score | ||||||
| Low (0–2) | 1 | |||||
| High (3–6) | 0.420 | 0.111–1.591 | 0.202 | |||
| ypN category | ||||||
| N− | 1 | 1 | ||||
| N+ | 3.331 | 1.007–11.019 | 0.049 | 2.584 | 0.769–8.679 | 0.125 |
| ypT category | ||||||
| Low (1/2) | 1 | |||||
| High (3/4) | 4.608 | 0.991–21.434 | 0.051 | |||
| Age (years) | ||||||
| < 65 | 1 | |||||
| > 65 | 0.837 | 0.254–2.755 | 0.770 | |||
1Multivariate analysis adjusted for total CD8 score and ypN stage
Table 4.
Uni-and multivariate analysis for disease-free survival in locally advanced rectal cancer
| Univariate analysis | Multivariate analysisa | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Total CD8 score | ||||||
| Low (3–4) | 1 | |||||
| Medium (5–6) | 0.928 | 0.321–2.680 | 0.890 | |||
| High (7–12) | 0.556 | 0.205–1.506 | 0.249 | |||
| Total IDO1 score | ||||||
| Low (0–2) | 1 | 1 | ||||
| High (3–6) | 0.335 | 0.133–0.843 | 0.020 | 0.384 | 0.150–0.983 | 0.046 |
| ypN category | ||||||
| N− | 1 | 1 | ||||
| N+ | 2.663 | 1.188–5.970 | 0.017 | 2.089 | 0.880–4.961 | 0.095 |
| ypT category | ||||||
| Low (1/2) | 1 | 1 | ||||
| High (3/4) | 2.344 | 1.009–5.446 | 0.048 | 1.618 | 0.652–4.017 | 0.299 |
| Age (years) | ||||||
| < 65 | 1 | |||||
| > 65 | 0.524 | 0.231–1.188 | 0.122 | |||
aMultivariate analysis adjusted for total IDO1 score, ypN stage and ypT stage
Discussion
Standard treatment algorithms for patients with colorectal cancer are well defined by national and international guidelines [30]. The therapeutic approach for patients diagnosed with carcinomas of the mid and lower rectum is based on clinical findings (cTNM) and on the histopathological AJCC/UICC-TNM scoring system [9]. The pathological tumour classification system ((y)pUICC stage I–IV) further provides important information about patients’ prognosis, but it does not capture the biological complexity of the tumour microenvironment and the contribution of anti-tumour immune responses. There is clear evidence that density, phenotype and location of tumour-infiltrating lymphocytes (TILs) within primary colorectal carcinomas provide an accurate prediction of clinical outcome [12, 35]. Quantification of the density, localization and type of TILs, termed ‘Immunoscore’, proved to even surpass the gold standard TNM-system in predicting disease-free and overall survival [14, 36, 37]. Furthermore, the accuracy of the ‘Immunoscore’ can be improved by delineating the location of TILs into two areas within the primary tumour: the centre and the invasive margin [12]. However, regulation of lymphocyte infiltration within the tumour microenvironment remains complex [38]. IDO1, a tryptophan-degrading enzyme, appears to have a profound influence on local innate and adaptive immune responses. IDO1 was found to be either expressed by tumour or stromal cells (mainly antigen-presenting cells) [39, 40]. Local tryptophan depletion and/or accumulation of metabolites interfere with T-cell function, proliferation or differentiation. This suggests that IDO1 orchestrates an immunosuppressive environment, presumably by controlling exaggerated T-cell responses [16].
In this study, we evaluated the density and location of tumour-infiltrating CD8+ T-lymphocytes as well as IDO1 expression in surgically resected tumours of patients with LARC after neoadjuvant chemoradiation. Earlier studies that focused on the local immune profile of colorectal cancer patients predominantly excluded patients with rectal carcinoma after neoadjuvant chemoradiation [12, 33, 41]. Concerns about unpredictable or even reducing effects on T-cell infiltration or profound histologic changes in the architecture of the primary tumour were raised as main opposing arguments to include pre-treated tumour specimens. The latter might hold true for methods using quantitative automated immunohistochemistry scores [37]. However, in this study, we instead employed a semi-quantitative analysis of tumour sections. The specimens were evaluated twice by two independent observers to obtain a conclusive judgement regarding CD8 and IDO1 quantification and to exclude intra- and interobserver variability.
Using an immunohistochemical approach and applying established quantification methods for CD8+ lymphocytes as well as IDO1 expression, we detected a positive correlation of CD8+ lymphocyte numbers and the extent of IDO1 expression in LARC. Both CD8+ lymphocyte numbers and IDO1 expression were associated with patient survival and turned out to be independent prognostic markers in multivariate analysis.
By applying this method, we identified 15 out of 106 patients with a complete histological tumour response (ypT0) and excluded those from immunohistochemical analysis. All examined specimens showed infiltration with CD8 positive T-lymphocytes; 56% with a high total CD8 score. Cell death induced by chemotherapy has long been assumed to result in apoptosis. Consequently, the default response should be immunologic tolerance rather than immune activation. However, chemotherapy-associated cell death was recently shown to be capable of inducing a sustained tumour antigen-specific immune response. This was dependent on the chemotherapeutic agent used [42, 43]. Interestingly, these findings can be partially extended to radiotherapy-induced cell death too. Radiation was found to affect the local immune response by either upregulation of stimulatory molecules on the tumour cell surface or by shaping the local immune cell infiltrate [44]. Summarizing these observations, distinct chemo- and/or radiotherapeutic regimens seem to promote an immunogenic process of cancer cell death leading to local and/or systemic anti-tumour immune responses. In this context, our results represent a clinically relevant finding: tumour specimens with extensive infiltration of CD8 positive lymphocytes after neoadjuvant chemoradiation were more often classified into lower tumour categories with regard to tumour size (ypT1 or ypT2) and nodal status (ypN-). Furthermore, intense CD8 lymphocyte infiltration resulted in a significant shift towards lower ypUICC stages, a well-known prognostic parameter for overall survival. In this context, patients with a high total CD8 score had significantly prolonged 5-year survival rates. We observed a prognostic discrepancy regarding intratumoural subsites when comparing intraepithelial CD8 lymphocyte infiltration to that in the tumour stroma with a better prognosis for intraepithelial T cells. The biological explanation of why intraepithelial CD8 T cells would have a greater impact on prognosis than the ones located in the tumour stroma is likely due to their direct anti-tumour effects. The CD8 T cells infiltrating the tumour epithelium, being in the immediate proximity, are most likely specifically directed against the tumour, and could, therefore, be considered more effective. The hypothesis that the sublocalisation of infiltrating lymphocytes bears prognostic importance has gained momentum from earlier studies [12, 41]. An intra-epithelial location of cytotoxic T cells could possibly mean a more effective protection in that respect.
The immunosuppressive effect of IDO1 was first described in a mouse model of fetal protection against maternal immune rejection [45]. Treatment of pregnant mice with an IDO1 inhibitor (1-methyl-tryptophan) resulted in T-cell-mediated rejection of allogeneic embryos [45]. Paradoxically, IDO1 seems to be dispensable for the maintenance of self-tolerance under basal conditions [46]. However, the concept of IDO1-mediated immune regulation was consequently extended to malignant disease [47]. Over the past years, IDO1 expression has been partly associated with a more aggressive disease and poorer prognosis in several human carcinomas [29]. Data remain scarce and results heterogeneous in colorectal cancer. Inconsistencies appear when studies are compared with regard to the expression profiles of IDO1 (constitutive vs. inducible by inflammatory stimuli), the nature of IDO1 expressing cells (tumour cells vs. antigen-presenting cells), the density of IDO1 expression and its localization within different tumour compartments (tumour centre vs. invasive tumour front). Furthermore, its prognostic value or impact on DFS and OS, respectively, are not consistently reported [26–28]. Possible confounders for these divergent results have been extensively studied and discussed [31, 39, 40, 48–50].
Using a defined study population, more than 90% of pre-treated rectum carcinoma samples showed a positive staining for IDO1. Of those, 90% stained positive for IDO1 within the tumour stroma. Interestingly, tumours with a high total IDO1 score also showed a high total CD8 score, a finding which is so far divergent to former observations in patients with colorectal cancer [26, 27]. We hypothesize that these findings imply a state of immune activation rather than immune tolerance in terms of a local inflammatory milieu following neoadjuvant chemoradiation. In animal models, IDO1 was shown to be upregulated in tumours in response to local radiation therapy. Interestingly, pharmacological blockade of IDO1-acitivity did not result in reduced tumour growth or enhanced local anti-tumour immune responses [51]. Part of the IDO1 expression of neoplastic or stromal cells might result from an ongoing immune response involving CD8+ lymphocytes producing IFN-y, the main inductor of IDO1 expression [25]. Data from a mouse melanoma model clearly show that IDO1 expression is strictly dependent on the presence of intratumoural CD8+ lymphocytes producing IFN-y [52]. Moreover, IDO1 belongs to a group of genes whose expression in tumours prior to immunotherapy is of predictive value for a better clinical response, along with T-cell-specific genes and other IFN-y-induced genes [53]. In this study, comparable to our results, the key predictive factor is most likely the presence of tumour-infiltrating CD8+ lymphocytes and IDO1 is secondarily induced in response to IFN-y produced by those lymphocytes. We are well aware of the fact that immunohistochemistry studies do not allow functional conclusions on the local interplay of IDO1 expression and lymphocyte infiltration. However, our results clearly support a prognostic value of both IDO1 and CD8 scores. The total IDO1 score turned out to be an independent prognostic marker for DFS, whereas the total CD8 score showed prognostic relevance in terms of OS.
Multimodal treatment of LARC has dramatically reduced local recurrence rates but has not yet been proved to improve overall survival [6]. It has, however, become evident that the individual response of rectal carcinomas to neoadjuvant therapies varies considerably. Pathological staging such as the ypTNM classification is widely used in clinical practice, but the delivery of adjuvant therapy to theoretically decrease the risk of distant metastasis is still controversial [54]. Applying both histological criteria and defined immune phenotypes might help to better stratify adjuvant therapeutic approaches according to different immune profiles in the long run. Andersen and colleagues have shown that IDO1 is spontaneously recognized by specific CD8 T cells in humans [55]. Indeed, those cells appear to act as specific cytotoxic T lymphocytes that can recognize and kill IDO1 expressing tumour cells [56]. These observations have prompted efforts to explore IDO1 peptides as anti-cancer vaccines, as examined in a recent clinical trial where early evidence was obtained of long-lasting disease stabilization in metastatic lung cancer patients [57, 58]. LARC patients with a strong IDO1 expression profile after neoadjuvant chemoradiation might benefit from an additive IDO1 targeted immunotherapeutic approach such as vaccination with IDO1-derived peptides. Degradation of the local immunosuppressive environment, i.e. inhibition of IDO1 activity, might result in intensified antigen-specific immune responses in these patients. IDO1 inhibitors, when used as monotherapy, showed disappointing results in preclinical trials [59]. When IDO1 inhibitors were used in combinatorial immunotherapeutic or chemotherapeutic regimens, they seem to act as immunometabolic adjuvants which can modulate inflammatory processes [60]. Surprisingly, recent results from the ECHO-301 trial, the first large phase 3 trial, showed that epacadostat, an IDO1-selective enzyme inhibitor, failed to improve disease-free survival in addition to anti-PD1 immune checkpoint inhibitor therapy in metastatic melanoma patients [61]. Based on the negative results of the ECHO-301 trial in melanoma, as well as in the pancreatic cancer cohort of ECHO-203, several trials of the IDO inhibitor epacadostat were stopped. As a consequence, other trials of different IDO inhibitors have been scaled back or even halted [62]. Although researchers offered reasons for the disappointing trial results, the role of IDO1 as key player in tumour immune escape becomes even more questionable. A generally immunomodulatory role of IDO1 in cancer remains challenged by three important facts: (1) IDO1 expression can be associated with favourable outcome in cancer patients, as in our defined patient cohort, (2) IDO knock-out mice do not show overt signs of autoimmunity [49] and (3) failure of a selective IDO1-blocker in a phase 3 trial. So far, IDO1 biology remains incompletely understood.
In the long run, we need to further improve our understanding about the functional role of IDO1 within the tumour microenvironment of patients with LARC who had undergone preoperative chemoradiation. Consequently, we might be able to answer the question if IDO1 is a friend or foe of tumour-infiltrating CD8+ lymphocytes and to define its exact role in the complex orchestra of tumour-driven immune evasion in these patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Sabine Roth for her tireless efforts and qualified support in the set-up of IDO1 immunohistochemistry. We thank Mohammed Hankir for proofreading the manuscript.
Abbreviations
- AJCC/UICC
American Joint Committee on Cancer/Union Internationale Contre le Cancer
- CD8
Cytotoxic T lymphocyte
- DFS
Disease-free survival
- HR
Hazard ratio
- IDO1
Indoleamine-2,3-Dioxygenase 1
- IE
Intraepithelial
- LARC
Locally advanced rectal cancer
- OS
Overall survival
- ST
Stromal
- TDO
Tryptophan-2,3-dioxygenase
- TME
Total mesorectal excision
- TNM
Tumour-node-metastasis
Author contributions
Julia Schollbach: performed immunohistochemistry, manuscript writing. Stefan Kircher: performed immunohistochemistry. Armin Wiegering: study design, manuscript writing. Florian Seyfried: study design, manuscript writing. Ingo Klein: study design. Andreas Rosenwald: study design. Christoph-Thomas Germer: manuscript writing. Stefan Löb: study design, manuscript writing.
Funding
No relevant funding.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Patient and treatment characteristics were retrospectively collected from patient records and the Würzburg comprehensive cancer registry. All procedures performed were in accordance with the standards of the institutional ethical committee and with the 1964 Helsinki declaration and its later amendments. Approval of the study was obtained from the Ethics Committee, Medical Faculty, University of Würzburg, Germany (reference number 34/16). All patients provided informed written consent to the use of their anonymized data in scientific studies.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence and Mortality Rates and Trends–An Update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 3.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1(8496):1479–1482. doi: 10.1016/S0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 4.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R, German Rectal Cancer Study G Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 5.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, Leer JW, van de Velde CJ. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. Dutch Colorectal Cancer N Engl J Med. 2001;345(9):638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 6.Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H, Wittekind C, Beissbarth T, Rodel C. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 7.van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van de Velde CJ, Dutch Colorectal Cancer G Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12(6):575–582. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 8.Smith JJ, Garcia-Aguilar J. Advances and challenges in treatment of locally advanced rectal cancer. J Clin Oncol. 2015;33(16):1797–1808. doi: 10.1200/JCO.2014.60.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 10.Nagtegaal ID, Quirke P, Schmoll HJ. Has the new TNM classification for colorectal cancer improved care? Nat Rev Clin Oncol. 2011;9(2):119–123. doi: 10.1038/nrclinonc.2011.157. [DOI] [PubMed] [Google Scholar]
- 11.Benson AB, 3rd, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, Engstrom PF, Enzinger PC, Fakih MG, Fuchs CS, Grem JL, Hunt S, Leong LA, Lin E, Martin MG, May KS, Mulcahy MF, Murphy K, Rohren E, Ryan DP, Saltz L, Sharma S, Shibata D, Skibber JM, Small W, Jr, Sofocleous CT, Venook AP, Willett CG, Freedman-Cass DA, Gregory KM. Rectal cancer. J Natl Compr Canc Netw. 2012;10(12):1528–1564. doi: 10.6004/jnccn.2012.0158. [DOI] [PubMed] [Google Scholar]
- 12.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 13.Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pages F, Galon J. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29(6):610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 14.Galon J, Pages F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G, Tatangelo F, Britten CM, Kreiter S, Chouchane L, Delrio P, Arndt H, Asslaber M, Maio M, Masucci GV, Mihm M, Vidal-Vanaclocha F, Allison JP, Gnjatic S, Hakansson L, Huber C, Singh-Jasuja H, Ottensmeier C, Zwierzina H, Laghi L, Grizzi F, Ohashi PS, Shaw PA, Clarke BA, Wouters BG, Kawakami Y, Hazama S, Okuno K, Wang E, O’Donnell-Tormey J, Lagorce C, Pawelec G, Nishimura MI, Hawkins R, Lapointe R, Lundqvist A, Khleif SN, Ogino S, Gibbs P, Waring P, Sato N, Torigoe T, Itoh K, Patel PS, Shukla SN, Palmqvist R, Nagtegaal ID, Wang Y, D’Arrigo C, Kopetz S, Sinicrope FA, Trinchieri G, Gajewski TF, Ascierto PA, Fox BA. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pages F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, Nagtegaal ID, Vink-Borger E, Hartmann A, Geppert C, Kolwelter J, Merkel S, Grutzmann R, Van den Eynde M, Jouret-Mourin A, Kartheuser A, Leonard D, Remue C, Wang JY, Bavi P, Roehrl MHA, Ohashi PS, Nguyen LT, Han S, MacGregor HL, Hafezi-Bakhtiari S, Wouters BG, Masucci GV, Andersson EK, Zavadova E, Vocka M, Spacek J, Petruzelka L, Konopasek B, Dundr P, Skalova H, Nemejcova K, Botti G, Tatangelo F, Delrio P, Ciliberto G, Maio M, Laghi L, Grizzi F, Fredriksen T, Buttard B, Angelova M, Vasaturo A, Maby P, Church SE, Angell HK, Lafontaine L, Bruni D, El Sissy C, Haicheur N, Kirilovsky A, Berger A, Lagorce C, Meyers JP, Paustian C, Feng Z, Ballesteros-Merino C, Dijkstra J, van de Water C, van Lent-van Vliet S, Knijn N, Musina AM, Scripcariu DV, Popivanova B, Xu M, Fujita T, Hazama S, Suzuki N, Nagano H, Okuno K, Torigoe T, Sato N, Furuhata T, Takemasa I, Itoh K, Patel PS, Vora HH, Shah B, Patel JB, Rajvik KN, Pandya SJ, Shukla SN, Wang Y, Zhang G, Kawakami Y, Marincola FM, Ascierto PA, Sargent DJ, Fox BA, Galon J. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391(10135):2128–2139. doi: 10.1016/S0140-6736(18)30789-X. [DOI] [PubMed] [Google Scholar]
- 16.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34(3):137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189(9):1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22(5):633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Metz R, Rust S, Duhadaway JB, Mautino MR, Munn DH, Vahanian NN, Link CJ, Prendergast GC. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: a novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology. 2012;1(9):1460–1468. doi: 10.4161/onci.21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9(10):1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 21.Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, Opelz G. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196(4):447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176(11):6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 23.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185(6):3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 25.van Baren N, Van den Eynde BJ. Tumoral immune resistance mediated by enzymes that degrade tryptophan. Cancer Immunol Res. 2015;3(9):978–985. doi: 10.1158/2326-6066.CIR-15-0095. [DOI] [PubMed] [Google Scholar]
- 26.Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, Werner ER, Werner-Felmayer G, Weiss HG, Gobel G, Margreiter R, Konigsrainer A, Fuchs D, Amberger A. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12(4):1144–1151. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- 27.Ferdinande L, Decaestecker C, Verset L, Mathieu A, Moles Lopez X, Negulescu AM, Van Maerken T, Salmon I, Cuvelier CA, Demetter P. Clinicopathological significance of indoleamine 2,3-dioxygenase 1 expression in colorectal cancer. Br J Cancer. 2012;106(1):141–147. doi: 10.1038/bjc.2011.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao YF, Peng RQ, Li J, Ding Y, Zhang X, Wu XJ, Pan ZZ, Wan DS, Zeng YX, Zhang XS. The paradoxical patterns of expression of indoleamine 2,3-dioxygenase in colon cancer. J Transl Med. 2009;7:71. doi: 10.1186/1479-5876-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Godin-Ethier J, Hanafi LA, Piccirillo CA, Lapointe R. Indoleamine 2,3-dioxygenase expression in human cancers: clinical and immunologic perspectives. Clin Cancer Res. 2011;17(22):6985–6991. doi: 10.1158/1078-0432.CCR-11-1331. [DOI] [PubMed] [Google Scholar]
- 30.Schmiegel W, Buchberger B, Follmann M, Graeven U, Heinemann V, Langer T, Nothacker M, Porschen R, Rodel C, Rosch T, Schmitt W, Wesselmann S, Pox C. S3-Leitlinie Kolorektales Karzinom. Z Gastroenterol. 2017;55(12):1344–1498. doi: 10.1055/s-0043-121106. [DOI] [PubMed] [Google Scholar]
- 31.Theate I, van Baren N, Pilotte L, Moulin P, Larrieu P, Renauld JC, Herve C, Gutierrez-Roelens I, Marbaix E, Sempoux C, Van den Eynde BJ. Extensive profiling of the expression of the indoleamine 2,3-dioxygenase 1 protein in normal and tumoral human tissues. Cancer Immunol Res. 2015;3(2):161–172. doi: 10.1158/2326-6066.CIR-14-0137. [DOI] [PubMed] [Google Scholar]
- 32.Dahlin AM, Henriksson ML, Van Guelpen B, Stenling R, Oberg A, Rutegard J, Palmqvist R. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod Pathol. 2011;24(5):671–682. doi: 10.1038/modpathol.2010.234. [DOI] [PubMed] [Google Scholar]
- 33.Ling A, Edin S, Wikberg ML, Oberg A, Palmqvist R. The intratumoural subsite and relation of CD8(+) and FOXP3(+) T lymphocytes in colorectal cancer provide important prognostic clues. Br J Cancer. 2014;110(10):2551–2559. doi: 10.1038/bjc.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogino S, Nosho K, Irahara N, Meyerhardt JA, Baba Y, Shima K, Glickman JN, Ferrone CR, Mino-Kenudson M, Tanaka N, Dranoff G, Giovannucci EL, Fuchs CS. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res. 2009;15(20):6412–6420. doi: 10.1158/1078-0432.CCR-09-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 36.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, Nagtegaal ID, Palmqvist R, Masucci GV, Botti G, Tatangelo F, Delrio P, Maio M, Laghi L, Grizzi F, Asslaber M, D’Arrigo C, Vidal-Vanaclocha F, Zavadova E, Chouchane L, Ohashi PS, Hafezi-Bakhtiari S, Wouters BG, Roehrl M, Nguyen L, Kawakami Y, Hazama S, Okuno K, Ogino S, Gibbs P, Waring P, Sato N, Torigoe T, Itoh K, Patel PS, Shukla SN, Wang Y, Kopetz S, Sinicrope FA, Scripcariu V, Ascierto PA, Marincola FM, Fox BA, Pages F. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232(2):199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galon J, Pages F, Marincola FM, Thurin M, Trinchieri G, Fox BA, Gajewski TF, Ascierto PA. The immune score as a new possible approach for the classification of cancer. J Transl Med. 2012;10:1. doi: 10.1186/1479-5876-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Church SE, Galon J. Regulation of CTL Infiltration Within the Tumor Microenvironment. Adv Exp Med Biol. 2017;1036:33–49. doi: 10.1007/978-3-319-67577-0_3. [DOI] [PubMed] [Google Scholar]
- 39.Lob S, Konigsrainer A, Schafer R, Rammensee HG, Opelz G, Terness P. Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood. 2008;111(4):2152–2154. doi: 10.1182/blood-2007-10-116111. [DOI] [PubMed] [Google Scholar]
- 40.Lob S, Konigsrainer A, Zieker D, Brucher BL, Rammensee HG, Opelz G, Terness P. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother. 2009;58(1):153–157. doi: 10.1007/s00262-008-0513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8 + T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58(16):3491–3494. [PubMed] [Google Scholar]
- 42.Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14(2):135–146. doi: 10.1038/nrc3670. [DOI] [PubMed] [Google Scholar]
- 43.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell. 2015;28(6):690–714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 44.Frey B, Ruckert M, Deloch L, Ruhle PF, Derer A, Fietkau R, Gaipl US. Immunomodulation by ionizing radiation-impact for design of radio-immunotherapies and for treatment of inflammatory diseases. Immunol Rev. 2017;280(1):231–248. doi: 10.1111/imr.12572. [DOI] [PubMed] [Google Scholar]
- 45.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 46.Ravishankar B, Liu H, Shinde R, Chandler P, Baban B, Tanaka M, Munn DH, Mellor AL, Karlsson MC, McGaha TL. Tolerance to apoptotic cells is regulated by indoleamine 2,3-dioxygenase. Proc Natl Acad Sci U S A. 2012;109(10):3909–3914. doi: 10.1073/pnas.1117736109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9(10):1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 48.Baban B, Chandler P, McCool D, Marshall B, Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J Reprod Immunol. 2004;61(2):67–77. doi: 10.1016/j.jri.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 49.de Faudeur G, de Trez C, Muraille E, Leo O. Normal development and function of dendritic cells in mice lacking IDO-1 expression. Immunol Lett. 2008;118(1):21–29. doi: 10.1016/j.imlet.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Lob S, Konigsrainer A, Rammensee HG, Opelz G, Terness P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev Cancer. 2009;9(6):445–452. doi: 10.1038/nrc2639. [DOI] [PubMed] [Google Scholar]
- 51.Monjazeb AM, Kent MS, Grossenbacher SK, Mall C, Zamora AE, Mirsoian A, Chen M, Kol A, Shiao SL, Reddy A, Perks JR, W TNC, Sparger EE, Canter RJ, Sckisel GD, Murphy WJ. Blocking indolamine-2,3-dioxygenase rebound immune suppression boosts antitumor effects of radio-immunotherapy in murine models and spontaneous canine malignancies. Clin Cancer Res. 2016;22(17):4328–4340. doi: 10.1158/1078-0432.CCR-15-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5(200):200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer J. 2010;16(4):399–403. doi: 10.1097/PPO.0b013e3181eacbd8. [DOI] [PubMed] [Google Scholar]
- 54.Netter J, Douard R, Durdux C, Landi B, Berger A, Taieb J. Advances in management of adjuvant chemotherapy in rectal cancer: Consequences for clinical practice. Clin Res Hepatol Gastroenterol. 2016;40(5):546–552. doi: 10.1016/j.clinre.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 55.Sorensen RB, Berge-Hansen L, Junker N, Hansen CA, Hadrup SR, Schumacher TN, Svane IM, Becker JC, thor Straten P, Andersen MH. The immune system strikes back: cellular immune responses against indoleamine 2,3-dioxygenase. PLoS One. 2009;4(9):e6910. doi: 10.1371/journal.pone.0006910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sorensen RB, Hadrup SR, Svane IM, Hjortso MC, Thor Straten P, Andersen MH. Indoleamine 2,3-dioxygenase specific, cytotoxic T cells as immune regulators. Blood. 2011;117(7):2200–2210. doi: 10.1182/blood-2010-06-288498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andersen MH, Svane IM. Indoleamine 2,3-dioxygenase vaccination. Oncoimmunology. 2015;4(1):e983770. doi: 10.4161/2162402X.2014.983770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iversen TZ, Engell-Noerregaard L, Ellebaek E, Andersen R, Larsen SK, Bjoern J, Zeyher C, Gouttefangeas C, Thomsen BM, Holm B, Thor Straten P, Mellemgaard A, Andersen MH, Svane IM. Long-lasting disease stabilization in the absence of toxicity in metastatic lung cancer patients vaccinated with an epitope derived from indoleamine 2,3 dioxygenase. Clin Cancer Res. 2014;20(1):221–232. doi: 10.1158/1078-0432.CCR-13-1560. [DOI] [PubMed] [Google Scholar]
- 59.Vacchelli E, Aranda F, Eggermont A, Sautes-Fridman C, Tartour E, Kennedy EP, Platten M, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology. 2014;3(10):e957994. doi: 10.4161/21624011.2014.957994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prendergast GC, Mondal A, Dey S, Laury-Kleintop LD, Muller AJ. Inflammatory Reprogramming with IDO1 Inhibitors: Turning Immunologically Unresponsive ‘Cold’ Tumors ‘Hot’. Trends Cancer. 2018;4(1):38–58. doi: 10.1016/j.trecan.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muller AJ, Manfredi MG, Zakharia Y, Prendergast GC. Inhibiting IDO pathways to treat cancer: lessons from the ECHO-301 trial and beyond. Semin Immunopathol. 2018 doi: 10.1007/s00281-018-0702-0. [DOI] [PubMed] [Google Scholar]
- 62.Companies Scaling Back IDO1 Inhibitor Trials Cancer Discov. 2018;8(7):OF5. doi: 10.1158/2159-8290.CD-ND2018-007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.