Abstract
Immuno-therapy has begun to revolutionize cancer treatment. However, despite the significant progress achieved in regard to the duration of clinical benefits, a substantial number of patients do not respond to these therapies. To improve the outcome of patients receiving immuno-therapy, there is a need for novel biomarkers that can predict and monitor treatment. Tumor microenvironment alterations, more specifically the state of chronic inflammation and desmoplasia (tumor fibrosis), are important factors to consider in this context. Here, we discuss the potential for quantification of altered tissue turnover in a liquid biopsy as a proposed precision medicine tool to assess chronic inflammation and desmoplasia in the immuno-oncology (IO) setting. We highlight the need for novel non-invasive biomarkers in IO and the importance of addressing tumor microenvironment alterations. We focus on desmoplasia and extracellular matrix (ECM) remodeling, and how the composition of the ECM defines T-cell permissiveness in the tumor microenvironment and opens up the possibility for associated liquid biopsy biomarkers. Moreover, we address the importance of the assessment of chronic inflammation, primarily macrophage activity, in a liquid biopsy.
Keywords: Immuno-oncology, Desmoplasia, Inflammation, Extracellular matrix (ECM), Liquid biopsy, Prediction
Introduction
While chemo- and targeted therapies have prevailed in cancer treatment for several decades, immuno-therapy has begun to revolutionize the field, and cancer patients may benefit from durable long-term responses. However, despite the substantially extended clinical benefit experienced by some patients, a large percentage of patients included in clinical trials do not respond to this type of treatment. This is the case, even though precision medicine serves to guide selection of an appropriate patient population with a higher likelihood of success [1].
The limitations to patient selection for immuno-oncology (IO) therapies were indicated by reported response rates ranging from 23 to 67% in clinical trials with non-small cell lung cancer (NSCLC) patients, where anti-PD-1 or anti-PD-L1 antibodies were used with a PD-L1 companion diagnostic (CDx) as a precision medicine tool [2–4]. One limiting factor for the ability to use PD-L1 expression to predict treatment response is that PD-L1 is currently based on immunohistochemistry, of which clinical accuracy is often compromised by tumor heterogeneity and dynamic changes [5, 6].
The development of reliable CDx tests for immuno-therapies and IO has been complicated further by the fact that treatment response is not driven by specific driver mutations (as most currently available CDx for targeted therapies), but rather relies on complex interactions between tumor cells and the immune system [7]. This entails a need for biomarkers reflecting tumor microenvironment alterations and requires both protein and cell-based assays. Interestingly, serum levels of angiopoietin 2 (ANGPT2), a protein related to angiogenesis in immune regulation, have recently been tested as a predictive and prognostic biomarker for immuno-therapy in patients with advanced melanoma; here, high pre-treatment ANGPT2 concentrations and early increases in serum ANGPT2 were associated with reduced response rates [8]. Several other potential blood-based biomarkers (liquid biopsies) are being investigated. Most of these focus on circulating tumor cells (CTC) and immune cells, soluble checkpoint molecules, free nucleic acids, and exosomes. Examples of liquid biopsy biomarkers in development are shown in Table 1. Combined, these examples emphasize the precedence that measurement of biomarkers in a liquid biopsy may provide a novel assessment of alterations in the dynamic tumor microenvironment relevant for the IO setting.
Table 1.
Examples of the immuno-oncology relevant liquid biopsy biomarkers currently being investigated
| Biomarker | Description | Suggested indication (example) |
|---|---|---|
| PD-L1 | Checkpoint molecule on CTCs | PD-L1(+) CTCs at baseline were associated with poor outcome in NSCLC patients receiving checkpoint inhibitor therapy. Patients with PD-L1(−) CTCs at 6 month follow-up all obtained clinical benefit, whereas patients with PD-L1(+) CTCs all progressed [92] |
| PD-1 | Checkpoint molecule on T-cells | Only PD-1(+), not PD-1(−), circulating T-cells effectively targeted melanoma patient-specific neoantigens (recognized autologous tumors) [93] |
| sPD-L1 | Secreted soluble splice-variants of PD-L1 | High pre-treatment levels of sPD-L1 were associated with increased likelihood of progressive disease in melanoma patients treated with checkpoint inhibitors. Changes in sPD-L1 early after treatment were not able to distinguish responders from non-responders [94] |
| ANGPT2 | Expressed at sites of vascular remodeling | High pre-treatment levels of ANGPT2 and early increases associates with reduced response rate in melanoma patients receiving checkpoint inhibitors [8] |
| ctDNA | Cell-free circulating DNA released from the tumor | ctDNA (e.g., B-RAF) was detected in plasma of metastatic melanoma patients prior to checkpoint inhibitor treatment. Lower baseline levels were associated with response to treatment. Levels did not decrease as a function of response [95] |
| miRNA | Small single-stranded RNA. Regulate transcription levels of target genes | High levels of specific miRNA molecules (miR-6826 and miR-6875) were associated with poor prognosis in mCRC patients receiving cancer vaccine (cocktail of five therapeutic epitope peptides) [96] |
| Exosomes | Cell derived vesicles released from tumor and immune cells | Exosomes derived from dendritic cells were isolated from patients with metastatic melanoma treated with checkpoint inhibitor. A significant increase of CD86+ exosomes occurred compared to baseline [97] |
ANGPT2 angiopoietin 2, B-RAF proto-oncogene B-raf, CTC circulating tumor cell, ctDNA circulating tumor DNA, mCRC metastatic colorectal cancer, miRNA microRNA, NSCLC non-small cell lung cancer
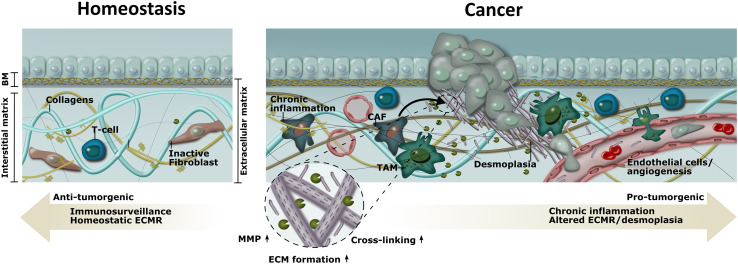
Important influencers on the dynamic changes in the tumor microenvironment are the states of chronic inflammation and desmoplasia (tumor fibrosis). As shown in Fig. 1, in the homeostatic state of a (healthy) tissue, anti-tumorigenic immuno-surveillance and balanced tissue turnover are predominant. In contrast, pro-tumorigenic chronic inflammation and desmoplasia pre-dominate in the tumor microenvironment. The dominant leukocyte population found in the tumor microenvironment is tumor-associated macrophages (TAMs), which are key mediators maintaining the chronic inflammatory process and avoidance of immune destruction. Cancer-associated fibroblasts (CAFs) also accumulate in the tumor and contribute to a dense and fibrous connective tissue consequent to overproduction and remodeling (cross-linking and degradation) of the extracellular matrix (ECM) leading to desmoplasia.
Fig. 1.
Tumor microenvironment, and the normal homeostatic microenvironment, at a glance, with focus on chronic inflammation and desmoplasia (tumor fibrosis) in relation to tumor progression. The states of chronic inflammation and desmoplasia are important influencers on dynamic changes in the tumor microenvironment that directly affect tumorgenesis. In the homeostatic state, anti-tumorigenic immuno-surveillance and homeostatic ECM remodeling (ECMR) are predominant. In contrast, pro-tumorigenic chronic inflammation and desmoplasia pre-dominate in the tumor microenvironment. Desmoplasia, which is defined by a dense and fibrous connective tissue mediated by activated cancer-associated fibroblasts (CAFs), is the result of overproduction and remodeling (cross-linking, degradation) of the ECM. The dominant leukocyte population found in the tumor microenvironment is tumor-associated macrophages (TAMs), which are key mediators maintaining the chronic inflammatory process and avoidance of immune destruction—an essential part of tumorgenesis. Moreover, TAMs contribute to tumorgenesis by participating in ECM remodeling/degradation induced by the secretion of matrix metalloproteases (MMPs) and by inhibiting anti-tumor immune surveillance through release of various immune-mediators
Interestingly, an emerging concept even suggests that cancer is promoted by a sequence of events that include chronic inflammation and desmoplasia with associated changes in the tumor microenvironment that both supports the transition of normal cells to cancer cells, as well as the ongoing proliferation, differentiation, and invasion of cancer cells leading to tumor growth [9]. Chronic inflammation is increasingly recognized as a driving factor in a variety of cancers and in general, the longer the inflammation persists, the higher the risk of cancer [10]. Desmoplasia, which is defined by a dense and fibrous connective tissue, results from overproduction and remodeling of ECM, and is often associated with invasive cancer [11]. The ECM is a three-dimensional protein-structure that encapsulates cells and provides support for tissues and regulates tissue homeostasis. The ECM can be divided into the interstitial matrix and the basement membrane (BM). The interstitial matrix primarily surrounds stromal cells and forms the connective tissue. The BM is a specialized layer of ECM that divides epithelial and endothelial cells from the underlying stroma. It is well established that activated CAFs accumulate in the tumor tissue during cancer and drive an increased deposition, and post-translational modifications (PTMs), of the ECM [11]. This ultimately generates a microenvironment with increased ECM density and stiffness (desmoplasia). For example, it has been shown that whereas the expression of type I collagen and type III collagen (the two major components of the interstitial ECM) was relatively weak in benign tissue, it was increased in the malignant counterpart [12]. In line with this, lessons learned from fibrotic diseases indicate that interstitial ECM remodeling is associated with disease progression. A study of idiopathic pulmonary fibrosis (IPF) using non-invasive biomarkers of ECM remodeling was able to identify patients with IPF and also associate the rate of change in these ECM protein fragments with disease worsening and poor survival [13]. This example highlights the biomarker potential of quantifying ECM remodeling (non-invasively) in diseases with ECM remodeling as a common denominator (fibrosis and cancer), as previously described by Karsdal et al. [14]. Here, we focus on the importance and relevance of quantifying desmoplasia and chronic inflammation in the tumor microenvironment, in a liquid biopsy, in relation to the IO setting.
The tumor microenvironment
The tumor microenvironment plays an essential role in cancer development and progression, thereby contributing, directly or indirectly, to the acquisition of the hallmarks of cancer [15]. The tumor microenvironment consists of cancer cells and the surrounding stroma comprising various (non-malignant) cell types [16]. All the various cells interact with the ECM and other extracellular molecules such as secreted proteins, growth factors, and different kinds of enzymes.
Emerging evidence suggests that the altered tumor tissue architecture, i.e., the composition and quality of the tumor microenvironment (stroma)—is a significant part of the core of carcinogenesis. For instance, findings indicate that when given the right signals from the tumor microenvironment, cancer cells (tumors) can enter/maintain a quiescent state, as observed after normal embryogenesis and development, also known as cancer dormancy [17]. In line with this, many in situ cancers never progress into an invasive phenotype, an observation referred to as “cancer without disease” [18].
Lessons learned from breast cancer indicate that the stroma rather than the epithelium is the actual target of a carcinogenic stimulus. For instance, it has been shown that when transplanting mammary epithelial cells into rodents exposed to either a carcinogen or vehicle, the mammary epithelial cells transformed into a neoplastic phenotype only when the rodents (the stroma) were exposed to the carcinogen [19]. Furthermore, when transplanting mammary epithelial cells into the mammary glands of irradiated mice, tumors developed faster and grew larger, compared to non-irradiated mice, suggesting that the radiation has effects on the stroma and can facilitate the neoplastic transformation [20]. It has also been shown that mammary epithelial cells exposed to carcinogenic stimuli were able to form phenotypically normal acini when introduced into the stroma of healthy rats [21]. Weaver et al. reached similar conclusions through the HMT-3522 breast cancer series, showing that it is possible to repress the tumorigenic phenotype of breast cancer by modulating the ECM and its receptors [22]. Taken together, these examples suggest that cancer is a dynamic and adaptive phenomenon taking place at the tissue level, emphasizing i) that cancer progression requires a tumor-growth permissive microenvironment and ii) that the microenvironment/stroma may play an important role in restraining cancer, as has also been excellently reviewed by Bissell and Hines [23]. The composition of the ECM is increasingly recognized as a major component in this context.
ECM remodeling and desmoplasia—an important component in cancer
Under normal conditions, damage to healthy tissue activates mechanisms that repair the affected tissue. If the damaging insult is chronic, this can lead to the onset of tissue fibrosis through persistent activation of fibroblasts [24]. Thus, whereas the ECM is maintained in a delicate equilibrium between protein formation, degradation, and post-translational modifications (PTMs) in healthy tissue, this balance is altered as part of chronic insult (e.g., tumorgenesis) in the tissue. Ultimately, when the ECM fails to maintain the homeostatic state, there is a loss of epithelial polarity leading to uncontrolled cell growth associated with malignancy [25–27]. Although fibroblasts are the primary cells producing ECM, if given the right signals, all cell types are able to synthesize and secrete ECM molecules and hereby contribute to desmoplasia [28].
In addition to increased ECM protein expression, altered ECM degradation is present consequent to the change in overall proteolytic activity found in cancer [29]. The matrix metalloproteases (MMPs) are the primary proteases involved in degradation of the ECM [30]. The MMPs are secreted from either the cancer cells or cells of the tumor microenvironment such as TAMs and may contribute further to the complexity of desmoplasia by affecting ECM homeostasis. As such, MMPs have both tumor promoting and tumor suppressing roles [31]. Most likely, the individual role as a tumor-suppressor or tumor-promoter is dependent on the total picture of proteases, their substrates, and the degradation products. Moreover, the presence of protease inhibitors is important, and whereas the total protease inhibitor concentration exceeds the concentration of MMPs in healthy tissue, the balance is shifted in cancer [32, 33].
The stiffness of the ECM is altered in the tumor tissue, as well. This is due to the presence of lysyl oxidase (LOX) that cross-links collagens and elastin which again promotes migration, invasion, and metastasis of cancer cells [34, 35]. Although the precise mechanism by which LOX promotes cancer invasions and metastasis still remains unclear, it has been shown to involve integrin signaling and the focal adhesion kinase (FAK)/SRC-signaling pathway [36].
Desmoplasia can be observed in most solid tumors but has been highly associated with pancreatic ductal adenocarcinomas (PDAC) which are some of the most stroma-rich cancers [37]. Desmoplasia has been found in both primary tumors and metastatic sites in tissues isolated from PDAC patients, and patients with most desmoplasia (defined by the type I collagen content) had poorer overall survival, suggesting that desmoplasia is a prognostic factor supporting tumorgenesis [38]. However, desmoplasia is also involved in restraining tumorgenesis. Rhim et al. have shown that when deleting the sonic hedgehog (SHH) protein in a PDAC mouse model, the stromal content was reduced compared to control mice [39]. Surprisingly, however, the mice with deleted SHH had much more aggressive tumors. Similar findings were obtained by Özdemir et al, showing that the depletion of cancer-associated fibroblasts (CAFs) in mice led to much more aggressive tumors [40]. These findings highlight that the ECM architecture and composition in the tumor microenvironment possesses complex and opposing roles in tumorgenesis and highlights the need for further understanding the desmoplastic reaction.
Assessment of desmoplasia and excessive ECM formation in a liquid biopsy
Consequent to the importance of desmoplasia, tools to measure and quantify the desmoplastic reaction are needed. The hallmark of desmoplasia is excessive ECM formation, and different technologies exist for assessing ECM formation. So far, the most widely used way of measuring desmoplasia in the tumor has been by use of tumor tissue biopsies followed by immunohistochemical staining of ECM components, mainly the interstitial ECM proteins of type I and type III collagen. An intrinsic problem with this approach is related to the invasive nature of a biopsy that may cause complications for patients in addition to the problems associated with sampling error, observer variability, and tumor heterogeneity [5, 6].
It is well established that non-invasive biomarkers are preferred over biomarkers assessed by invasive techniques, such as biopsies [41]. Consequently, there has been increasing attention in measuring ECM-associated biomarkers in a liquid biopsy (blood) [42]. While type I collagen is the most abundant interstitial ECM protein, it is also highly associated with bone-turnover and bone-metastasis. In contrast, type III collagen is more related to soft tissue ECM, and type III collagen formation has also been described to dominate in the early phases of the wound healing response and hence possibly more associated with desmoplasia/fibrosis [42].
Type III collagen formation biomarkers such as PIIINP and Pro-C3 can be used to assess excessive ECM (type III collagen) formation in a liquid biopsy. Both assays target the pro-peptide that is released during collagen formation and maturation and hereby intrinsically reflect excessive ECM formation, which is the major component of desmoplasia. PIIINP is a widely used marker for liver fibrosis and has been found to have prognostic potential when assessed in a liquid biopsy from ovarian and colorectal cancer patients, both cancer types that are characterized by strong desmoplastic reactions [43, 44]. Pro-C3 was also originally developed for fibrotic disorders, and has been shown to reflect stage and progression of disease [45, 46]. The Pro-C3 assay differs from PIIINP in that it targets the actual cleavage site of the type III collagen pro-peptide [47]. As removal of the pro-peptide is sometimes incomplete, Pro-C3 may be more indicative of true type III collagen formation, in contrast to PIIINP that may reflect both formation and degradation.
Pro-C3 was recently shown to be related to severity of disease and has the ability to predict outcome in the oncology setting. A study of colorectal cancer patients showed that Pro-C3 was able to differentiate between colorectal cancer patients, subjects with adenomas and controls, and that Pro-C3 was significantly elevated in metastatic (stage IV) patients when compared to earlier stages (stages I–III) [48]. The latter findings may be explained by a resemblance between the collagen deposition process observed during the development of fibrosis and development of a pre-metastatic niche [24]. The association between Pro-C3 and outcome was found in a clinical trial of second-line hormone receptor-positive metastatic breast cancer, where elevated levels of Pro-C3 were associated with shorter time to progression and overall survival [49].
Altogether, these results suggest that type III collagen pro-peptides assessed in a liquid biopsy reflect desmoplasia (excessive ECM formation) and can be used to assess tumor activity/invasiveness as well as to identify patients with poor prognosis and response to treatment. We hypothesize that such liquid biopsies may be applied as a precision medicine tool and CDx for predicting and monitoring efficacy of intervention. This may be highly relevant for IO where the ECM composition is emerging as a key component, as discussed in more detail in the following section.
The composition of the ECM in defining T-cell permissiveness in the tumor microenvironment
Analysis of the tumor microenvironment in patients with a variety of solid tumors has revealed that patients can be divided into phenotypes based on T-cell infiltration: tumors with T-cell infiltration and tumors without T-cell infiltration [50]. Interestingly, it has been shown that the architecture of the ECM defines both the preferential localization and migration of T-cells into the stroma of human tumors. IO therapies (such as anti-PD1/PD-L1 T-cell checkpoint inhibitors) are being studied in multiple cancer types and with a medical need for selecting the right patients to treat. One striking characteristic of efficient IO therapies such as checkpoint inhibitors is that the T-cells are actually recruited to the tumor [51]. That is, if T-cells are not homing to the tumor, treatment will have a poor effect. Therefore, it is important to identify patients with a T-cell permissive (eligible for T-cell trafficking) tumor microenvironment.
Evidence from lung cancer shows that T-cells preferably accumulate in the tumor stromal regions characterized by a relatively loose network of collagen and fibronectin, whereas tumor stromal regions exhibiting a dense ECM network were nearly devoid of T-cells [52]. To investigate further whether a dense ECM surrounding the tumor was responsible for the poor ability of T-cells to reach the cancer cells, human lung tumor slices were treated with collagenase. This treatment resulted in a marked decrease in stromal collagen content and while no alterations in tumor architecture were detected as such, a twofold increase in the number of T-cells was observed in stromal regions immediately adjacent to the cancer cells.
The ECM architecture in relation to permissiveness/resistance to infiltration by adoptively transferred T-cells has also been investigated [53]. Lung cancer metastases with a T-cell permissive environment were compared to lung cancer metastases with a T-cell resistant environment in relation to the ECM content (presence of collagen, laminin and fibronectin). Here, the ECM content in the T-cell permissive tumors was similar to the normal lung tissue, whereas the ECM content was much lower in T-cell resistant tumors. This indicates that when the ECM becomes too compromised, it affects recruitment of T-cells to the tumor as well. Importantly, this study differs from the above-mentioned study, in that they do not see any particular dense ECM as compared to normal lung tissue and highlights that a certain density of the ECM may be preferable for T-cell infiltration. In line with this, Peranzoni et al. have suggested that the ability of T-cells to mount an anti-tumor response is dependent on the structure of the ECM, more precisely on the balance between pro-migratory fiber networks and unfavorable migration zones composed of dense ECM structures (desmoplasia) [54]. Taken together, these findings indicate that the presence and composition of the ECM in tumors clearly affect the migratory behavior of T-cells as well as their ability to reach and attack the cancer cells, with a dense (desmoplastic) ECM preventing the T-cells from reaching the tumor cells.
As the desmoplastic reaction seems to be associated with T-cell permissiveness, patients with a high level of desmoplasia would be benefitting the least from checkpoint inhibitors. Hence, it is important to identify such patients and it could be relevant to evaluate levels of type III collagen for predicting (lack of) response to IO therapies by reflecting a desmoplastic and non-T-cell inflamed tumor microenvironment.
Modifying the ECM to improve T-cell infiltration into the tumor and possibility for associated biomarkers
Several ECM modifying drugs are currently being investigated as possible interventions for modulating the tumor microenvironment to increase the T-cell permissiveness. As an example, it has been shown in mice with PDAC and hyper-activated FAK, that when treated with a FAK-inhibitor, the mice were not only presented with reduced desmoplasia (as seen by both decreased collagen deposition and reduced numbers of activated fibroblasts), but also became responsive to anti-PD-1 therapy [55]. Moreover, FAK activity was also elevated in human PDAC tissues and correlated with high levels of desmoplasia and poor T-cell infiltration. These findings suggest that FAK inhibition increases T-cell permissiveness in PDAC by overcoming the desmoplastic (and immuno-suppressive) tumor microenvironment and hereby renders tumors responsive to checkpoint inhibitors. Interestingly, it has also been shown that T-cells which were engineered to express heparanase had improved ability to degrade the ECM and also possessed greater tumor infiltration potential and anti-tumor activity in a xenograft model [56]. Thus, by engineering T-cells to modify the ECM and overcome the desmoplastic barrier, T-cell infiltration was obtained. Interestingly, ECM modifying drugs may not only act by overcoming the desmoplastic barrier but may also induce various immune modulations in the tumor microenvironment that affects the response to checkpoint inhibitors and other immuno-therapeutic compounds. For instance, it has been shown in syngenic mouse models with colon and melanoma cancer that the treatment with lenvatinib (an anti-VEGFR/FGFR dual inhibitor) in combination with checkpoint inhibitor therapy had a significant inhibitory effect on tumor growth compared to the individual treatments alone, and this associated with reductions in TAMs, up-regulation of IFN signaling related genes, and an increased ratio of memory T-cells in the tumor [57].
Common for most ECM modifying drugs is that patients with a high level of desmoplasia would benefit the most from such treatments. Hence, it is important to identify these patients. Biomarkers intrinsically measuring excessive ECM formation (desmoplasia) could fulfill the criteria for identifying patients most likely to have desmoplastic tumors and hence those benefiting the most from ECM modifying drugs in combination with immuno-therapy as well as other therapies. By combining the existing CDx biomarkers for targeted therapy (e.g., based on target gene profiling) with, e.g., PD-L1 for checkpoint inhibitors and biomarkers of desmoplasia, it might be possible to identify patient-subgroups that will benefit the most from various combinatorial therapy approaches. This concept is illustrated graphically in Fig. 2.
Fig. 2.
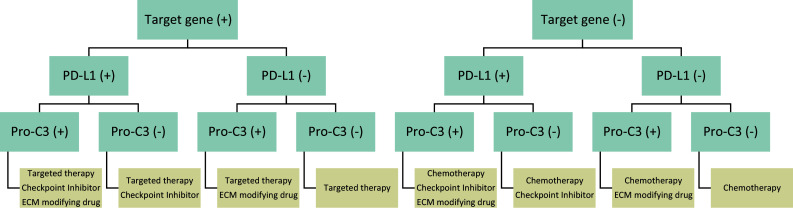
Proposed decision-tree for patient-subgrouping based on multiple biomarker assessments for combinatorial treatment approaches. Given the fact that cancer progression is driven by complex interactions between tumor cells, the stroma and the immune system, combinatorial therapy approaches may be needed. This illustration shows how performing companion diagnostic tests of given available treatments simultaneously helps to identify the patient-subgroups that have the highest likelihood of benefitting from each available therapy combination. In the depicted scenario, patients are initially divided according to eligibility of targeted therapy based on gene testing. Patients are then sub-divided according to the need for combining treatments with immuno-therapy (checkpoint inhibitors), based on a test for the expression of PD-L1. Finally, patients are tested for Pro-C3 to assess ongoing desmoplasia and identify patients with the potential need for modifications to the extracellular matrix (ECM), hereby reducing desmoplasia and increase likelihood of response to the given interventions
ECM degradation fragments measured in a liquid biopsy as novel biomarkers for immuno-oncology
Interestingly, it has been shown that specific protease-derived ECM fragments from proteins, such as type I collagen, type IV collagen, elastin, fibronectin, laminin and nidogen (amongst others), promote recruitment of immune cells through chemotactic properties [58]. This indicates that ECM degradation products may also reflect a specific inflammatory phenotype, and hence have the capacity to identify which patients will benefit the most from immuno-therapeutic modalities. We have previously seen that specific ECM protein degradation fragments are elevated in a liquid biopsy from patients with various solid tumors [59–62] and are associated with increased risk of developing cancer [63] as well as poor outcome [49]. However, whether these ECM degradation fragments have similar chemotactic properties as the above-mentioned example or whether they are associated with an inflammatory/T-cell permissive phenotype in cancer remains to be established. Supporting their association with an inflammatory phenotype are the numerous findings of the same markers elevated in diseases associated with chronic inflammation and fibrosis [64–72].
The above-mentioned ECM degradation fragments are primarily generated by specific MMPs. Possibly, different protease profiles may dominate individual tumors or tumor types, and in combination with different signature proteins from the diseased tissue, this may provide optimal specificity and sensitivity for future protein-based liquid biopsy biomarker development [73]. Moreover, combining specific post-translational modifications, such as cross-linking or citrullination, to the proteolytic cleavage site has the potential to increase the specificity for a pathological event further [74] and hereby reflect more specifically a given inflammatory phenotype. Interestingly, a paper was recently published showing that the T-cell specific protease granzyme B (GrzB) promoted T-cell transmigration from the blood to the tissue via remodeling (degradation) of the vascular basement membrane [75]. In this study, it was shown that GrzB contributed to T-cell extravasation and homing in vivo by degradation of specific ECM proteins, including type IV collagen, laminin, and nidogen-2. From this, a hypothesis could be formulated that measuring GrzB degraded ECM in a liquid biopsy would predict which patients had ongoing T-cell recruitment to their tumor (hence a T-cell permissive tumor microenvironment), and, therefore, would be most likely to benefit from immuno-therapies.
Tumors as chronically inflamed tissues and the importance of TAMs
The first observation that cancer seemed to develop at sites of previous chronic inflammation was proposed in 1863 by Rudolf Virchow [76]. The chronic inflammation is mediated both by the presence of innate and adaptive immune cells [50] and avoiding immune destruction is an essential part of tumorgenesis [77]. This can be accomplished by direct changes in the tumor cells themselves (e.g., by loss of tumor antigens), and/or by induction of an immuno-suppressive environment [78, 79]. Recently, it has become evident that both cancer cells and other cell types of the tumor microenvironment are able to modulate the immune cells and hereby contribute to tumor progression [80].
Tumor-associated macrophages (TAMs) are the dominant leukocyte population found in the tumor microenvironment where they are key mediators maintaining the chronic inflammatory process in the tumor [81]. Furthermore, TAMs and other myeloid cells are universally found in the tumor microenvironment and can contribute to immune evasion [50]. TAMs contribute to tumorgenesis by participating in ECM remodeling/degradation induced by the secretion of MMPs and by inhibiting the anti-tumor immune surveillance through release of various immune-mediators.
Due to the overall pro-tumorigenic effect of TAMs, promising anti-TAM therapies are emerging for the treatment of cancer. Cytokines such as the granulocyte maturation colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF), and their receptors, which both contribute to the maturation of monocytes into TAMs, have been suggested as promising targets. For instance, by treating cancer patients with various solid tumors with an antibody against the CSF-1 receptor (RG7155). In all analyzed patients the treatment induced a significant reduction of TAMs in the on-treatment tumor biopsies compared to matched biopsies taken prior to treatment [82]. Moreover, in patients with diffuse-type giant cell tumor (Dt-GCT), a neoplastic disorder characterized by CSF-1 overexpression, RG7155 treatment provided significant clinical benefits [82]. This highlights both that TAMs are attractive targets in cancer and that quantification of macrophage activity in patients is an important parameter.
Assessment of chronic inflammation and macrophage activity in a liquid biopsy
Consequent to the important role of TAMs in relation to chronic inflammation in the tumor microenvironment and anti-TAM modalities, the quantification of macrophage activity in patients becomes important. Vimentin is secreted by activated macrophages [83] making it a target for MMP degradation and modification by the peptidylarginine deiminase (PAD) enzymes that are responsible for the citrullination process associated with chronic inflammation [84]. In perfect alignment with these observations, a recent study showed that MMP-degraded and citrullinated vimentin (VICM) was detectable in supernatant from activated macrophages [85].
The VICM biomarker assay was developed by Vassiliadis et al. [86], and initially evaluated in liver fibrosis-related pathology where chronic inflammation is a hallmark of disease. VICM has also been found significantly elevated in serum from patients with RA, AS, and CD—all diseases that share the involvement of macrophages and where citrullinated vimentin is part of the pathogenesis [64, 87, 88]. VICM has been shown to be highly associated with lung cancer and was found elevated in all stages of the disease, and especially high in the non-small cell lung cancer (NSCLC) subtype compared to small cell lung cancer (SCLC) and other cancer types investigated [61]. In line with this, vimentin has been shown to be applicable for clinical pathology in pulmonary sarcomatoid carcinoma, a subtype of NSCLC [89]. In addition, the increasing evidence that tobacco smoke exposure induces inflammatory and mutagenic effects in the lungs that promote a pro-cancer immune response prompts us to consider the observed distinctively high level of VICM in NSCLC as an indication that VICM reflects inflammation [90]. Recent findings showing that VICM was released from ex vivo culture of human colorectal cancer tissue suggest that immune dysregulation plays an important role in the pathogenesis of this malignant disease as well [91].
Recently, in a large prospective study of nearly 6000 post-menopausal women, VICM was found to be elevated prior to the diagnosis of cancer and predicted an increased risk of being diagnosed with cancer within the first year from baseline [63]. In line with this is the previously presented concept suggesting that inflammatory conditions and altered tissue turnover are present before a malignant change occur [9].
Taken together, VICM may reflect an inflammatory phenotype and altered macrophage activity. Therefore, we hypothesize that VICM, as a liquid biopsy, may be applied as a precision medicine tool and CDx for predicting and monitoring efficacy of intervention and pharmacodynamics of novel anti-TAM therapies, in contrast to relying solely on (repeated) tumor tissue biopsies as in the examples above. In support of this is data from RA patients showing that efficacy of an anti-GM-CSF therapy is reflected by VICM measured in serum [85].
Summary/conclusion
There has been an increased recognition of the importance of the tumor microenvironment for understanding carcinogenesis. Especially in relation to the IO setting, the tumor microenvironment is an intrinsic part of the success of current and future immuno-therapeutic anti-tumor modalities. However, we are only beginning to understand how the different aspects of the tumor microenvironment, such as inflammation and desmoplasia, impact disease progression (prognosis) and response to the immuno-therapeutic compounds. To increase this understanding, there is a need for novel tools to study various aspects of the tumor microenvironment.
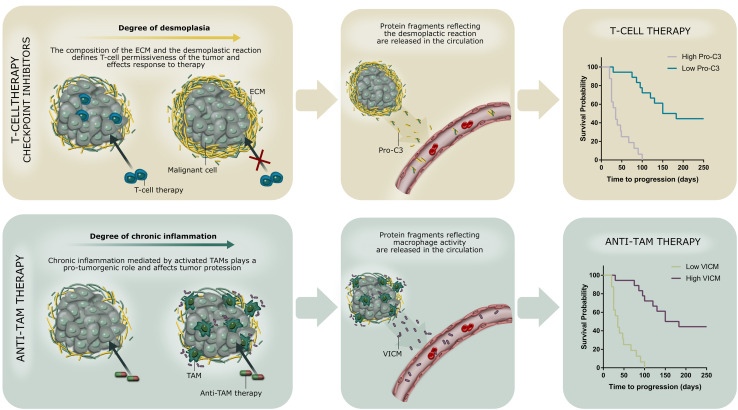
In this review, we have highlighted the importance of chronic inflammation and desmoplasia in cancer and discussed how novel protein-based liquid biopsy biomarkers may be used in the IO setting (Fig. 3). The Pro-C3 and VICM liquid biopsy biomarkers presented here may be applied to interrogate the stromal component of the tumor (desmoplasia and chronic inflammation, respectively) both individually and combined. The clinical potential of quantifying chronic inflammation and desmoplasia in relation to the IO setting stems from the indicated capability to detect immuno-suppressiveness as well as T-cell permissiveness of the tumor microenvironment; two recognized causative factors for tumor progression [10, 52]. Both chronic inflammation, e.g., recruitment and activation of TAMs, and desmoplasia, e.g., altered ECM remodeling and recruitment and activation of CAFs, ultimately lead to significant alterations in the composition and quality of the tumor tissue, including the ECM, and these alterations are linked to a pro-cancerous niche supporting tumorgenesis. Future studies will determine the actual use of biomarkers for interrogating the stromal component of the tumor and for enhancing the mechanistic understanding of desmoplasia and immune system function. Moreover, the association with response to anti-tumor IO therapies needs further attention.
Fig. 3.
Quantification of altered tissue turnover in a liquid biopsy—a proposed precision medicine tool to assess desmoplasia (top) and chronic inflammation (bottom) associated with response to immuno-therapeutic anti-tumor modalities. Biomarkers reflecting desmoplasia and activity of tumor-associated macrophages (TAMs), here exemplified by Pro-C3 (desmoplasia) and VICM (TAM activity), may be applied to the immuno-oncology setting and help predict (and monitor) response to checkpoint inhibitors/T-cell therapy and anti-TAM therapy. ECM extracellular matrix, TAM tumor-associated macrophage, Pro-C3 protein-based liquid biopsy biomarker of type III collagen formation (desmoplasia), VICM protein-based liquid biopsy biomarker of citrullinated and MMP-degraded vimentin (macrophage activity)
Abbreviations
- ANGPT2
Angiopoietin 2
- AS
Ankylosing spondylitis
- BM
Basement membrane
- B-RAF
Proto-oncogene B-Raf
- CAF
Cancer-associated fibroblast
- CD
Crohn’s disease
- CDx
Companion diagnostic
- CTC
Circulating tumor cell
- ctDNA
Circulating tumor DNA
- Dt-GCT
Diffuse-type giant cell tumor
- ECM
Extracellular matrix
- FAK
Focal adhesion kinase
- FGFR
Fibroblast growth factor receptor
- GrzB
Granzyme B
- IO
Immuno-oncology
- IPF
Idiopathic pulmonary fibrosis
- LOX
Lysyl oxidase
- mCRC
Metastatic colorectal cancer
- miRNA
MicroRNA
- MMP
Matrix metalloprotease
- NSCLC
Non-small cell lung cancer
- PAD
Peptidylarginine deiminase
- PDAC
Pancreas ductal adenocarcinoma
- PRO-C3
Pro-peptide of type III collagen formation
- PTM
Post-translational modification
- RA
Rheumatoid arthritis
- SCLC
Small cell lung cancer
- SHH
Sonic hedgehog
- SRC
Proto-oncogene tyrosine-protein kinase Src
- TAM
Tumor-associated macrophage
- VEGFR
Vascular endothelial growth factor receptor
- VICM
MMP-degraded and citrullinated vimentin
Author contributions
Conception of the work: NW and MK. Drafting the article: NW, LBT, and CJ. Preparing figures: CLB, NW, and LBT. Critical revision of the article: MK. Final approval: NW, LBT, CLB, CJ, and MAK.
Compliance with ethical standards
Conflict of interest
All authors are employed at Nordic Bioscience involved in discovery and development of serological biomarkers.
References
- 1.Ciardiello F, Arnold D, Casali PG, et al. Delivering precision medicine in oncology today and in future-the promise and challenges of personalised cancer medicine: a position paper by the European Society for Medical Oncology (ESMO) Ann Oncol. 2014;25:1673–1678. doi: 10.1093/annonc/mdu217. [DOI] [PubMed] [Google Scholar]
- 2.Garon EB, Leighl NB, Rizvi NA et al (2014) Safety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer (NSCLC). J Clin Oncol. 32: suppl; abstr 8020. doi:10.1200/jco.2014.32.15_suppl.8020
- 3.Gettinger SN, Shepherd FA, Antonia SJ et al (2014) First-line nivolumab (anti-PD-1; BMS-936558, ONO-4538) monotherapy in advanced NSCLC: Safety, efficacy, and correlation of outcomes with PD-L1 status. J Clin Oncol. 32: suppl; abstr 8024. doi:10.1200/jco.2014.32.15_suppl.8024
- 4.Brahmer JR, Rizvi NA, Lutzky J et al (2014) Clinical activity and biomarkers of MEDI4736, an anti-PD-L1 antibody, in patients with NSCLC. J Clin Oncol. 32:suppl; abstr 8021. doi:10.1200/jco.2014.32.15_suppl.8021
- 5.Elmore JG, Longton GM, Carney PA, et al. Diagnostic concordance among pathologists interpreting breast biopsy specimens. JAMA. 2015;313:1122–1132. doi: 10.1001/jama.2015.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janku F. Tumor heterogeneity in the clinic: is it a real problem? Ther Adv Med Oncol. 2014;6:43–51. doi: 10.1177/1758834013517414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dracopoli NC, Boguski MS. The evolution of oncology companion diagnostics from signal transduction to immuno-oncology. Trends Pharmacol Sci. 2017;38:41–54. doi: 10.1016/j.tips.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Wu X, Giobbie-Hurder A, Liao X, et al. Angiopoietin-2 as a biomarker and target for immune checkpoint therapy. Cancer Immunol Res. 2017;5:17–29. doi: 10.1158/2326-6066.CIR-16-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brücher BL, Jamall IS. Epistemology of the origin of cancer: a new paradigm. BMC Cancer. 2014;14:331. doi: 10.1186/1471-2407-14-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology. 2002;16:217–226. [PubMed] [Google Scholar]
- 11.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 12.Kauppila S, Stenback F, Risteli J, et al. Aberrant type I and type III collagen gene expression in human breast cancer in vivo. J Pathol. 1998;186:262–268. doi: 10.1002/(SICI)1096-9896(1998110)186:3<262::AID-PATH191>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins RG, Simpson JK, Saini G, et al. Longitudinal change in collagen degradation biomarkers in idiopathic pulmonary fibrosis: an analysis from the prospective, multicentre PROFILE study. Lancet Respir Med. 2015;3:462–472. doi: 10.1016/S2213-2600(15)00048-X. [DOI] [PubMed] [Google Scholar]
- 14.Karsdal MA, Nielsen MJ, Sand JM, et al. Extracellular matrix remodeling: the common denominator in connective tissue diseases. Possibilities for evaluation and current understanding of the matrix as more than a passive architecture, but a key player in tissue failure. Assay Drug Dev Technol. 2013;11:70–92. doi: 10.1089/adt.2012.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125:5591–5596. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- 17.Páez D, Labonte MJ, Bohanes P, et al. Cancer dormancy: a model of early dissemination and late cancer recurrence. Clin Cancer Res. 2012;18:645–653. doi: 10.1158/1078-0432.CCR-11-2186. [DOI] [PubMed] [Google Scholar]
- 18.Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427:787. doi: 10.1038/427787a. [DOI] [PubMed] [Google Scholar]
- 19.Maffini MV, Soto AM, Calabro JM, et al. The stroma as a crucial target in rat mammary gland carcinogenesis. J Cell Sci. 2004;117:1495–1502. doi: 10.1242/jcs.01000. [DOI] [PubMed] [Google Scholar]
- 20.Barcellos-Hoff MH, Ravani SA. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 2000;60:1254–1260. [PubMed] [Google Scholar]
- 21.Maffini MV, Calabro JM, Soto AM, Sonnenschein C. Stromal regulation of neoplastic development: age-dependent normalization of neoplastic mammary cells by mammary stroma. Am J Pathol. 2005;167:1405–1410. doi: 10.1016/S0002-9440(10)61227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weaver VM, Petersen OW, Wang F, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bissell MJ, Hines WC. Why don’ t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox TR, Erler JT. Molecular pathways: connecting fibrosis and solid tumor metastasis. Clin Cancer Res. 2014;20:3637–3643. doi: 10.1158/1078-0432.CCR-13-1059. [DOI] [PubMed] [Google Scholar]
- 25.Bissell MJ, Radisky DC, Rizki A, et al. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70:537–546. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fata JE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 2004;6:1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo X, Wu Y, Hathaway HJ, Hartley RS. Microenvironmental control of the breast cancer cell cycle. Anat Rec. 2012;295:553–562. doi: 10.1002/ar.22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. 2015;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Mason SD, Joyce JA. Proteolytic networks in cancer. Trends Cell Biol. 2011;21:228–237. doi: 10.1016/j.tcb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu P, Takai K, Weaver VM, Werb Z (2011) Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 3. doi:10.1101/cshperspect.a005058 [DOI] [PMC free article] [PubMed]
- 31.Martin MD, Matrisian LM. The other side of MMPs: protective roles in tumor progression. Cancer Metastasis Rev. 2007;26:717–724. doi: 10.1007/s10555-007-9089-4. [DOI] [PubMed] [Google Scholar]
- 32.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 33.Zucker S, Cao J. Matrix Metalloproteinases and cancer cell invasion/metastasis. In: Bagley RG, editor. The tumor microenvironment. 1. New York: Springer; 2010. pp. 531–554. [Google Scholar]
- 34.Payne SL, Hendrix MJ, Kirschmann DA. Paradoxical roles for lysyl oxidases in cancer—a prospect. J Cell Biochem. 2007;101:1338–1354. doi: 10.1002/jcb.21371. [DOI] [PubMed] [Google Scholar]
- 35.Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol. 2010;22:697–706. doi: 10.1016/j.ceb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker A-M, Bird D, Lang G, et al. Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene. 2013;32:1863–1868. doi: 10.1038/onc.2012.202. [DOI] [PubMed] [Google Scholar]
- 37.Feig C, Gopinathan A, Neesse A, et al. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whatcott CJ, Diep CH, Jiang P, et al. Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin Cancer Res. 2015;21:3561–3568. doi: 10.1158/1078-0432.CCR-14-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Özdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanash SM, Baik CS, Kallioniemi O. Emerging molecular biomarkers—blood-based strategies to detect and monitor cancer. Nat Rev Clin Oncol. 2011;8:142–150. doi: 10.1038/nrclinonc.2010.220. [DOI] [PubMed] [Google Scholar]
- 42.Risteli L, Koivula MK, Risteli J. Procollagen assays in cancer. Adv Clin Chem. 2014;66:79–100. doi: 10.1016/B978-0-12-801401-1.00003-7. [DOI] [PubMed] [Google Scholar]
- 43.Kauppila A, Puistola U, Risteli J, Risteli L. Amino-terminal propeptide of type III procollagen: a new prognosis indicator in human ovarian cancer. Cancer Res. 1989;49:1885–1889. [PubMed] [Google Scholar]
- 44.Plebani M, Basso D, Roveroni G, et al. N-terminal peptide of type III procollagen: a possible predictor of colorectal carcinoma recurrence. Cancer. 1997;79:1299–1303. doi: 10.1002/(SICI)1097-0142(19970401)79:7<1299::AID-CNCR5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 45.Jansen C, Leeming DJ, Mandorfer M, et al. PRO-C3-levels in patients with HIV/HCV-co-infection reflect fibrosis stage and degree of portal hypertension. PLoS ONE. 2014;9:e108544. doi: 10.1371/journal.pone.0108544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nielsen MJ, Veidal SS, Karsdal MA, et al. Plasma Pro-C3 (N-terminal type III collagen propeptide) predicts fibrosis progression in patients with chronic hepatitis C. Liver Int. 2015;35:429–437. doi: 10.1111/liv.12700. [DOI] [PubMed] [Google Scholar]
- 47.Nielsen MJ, Nedergaard AF, Sun S, et al. The neo-epitope specific PRO-C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am J Transl Res. 2013;5:303–315. [PMC free article] [PubMed] [Google Scholar]
- 48.Kehlet SN, Sanz-Pamplona R, Brix S, et al. Excessive collagen turnover products are released during colorectal cancer progression and elevated in serum from metastatic colorectal cancer patients. Sci Rep. 2016;6:30599. doi: 10.1038/srep30599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leitzel K, Ali SMi, Vasekar MK et al (2016) Serum collagen fragments and outcomes in hormone receptor-positive metastatic breast cancer. J Clin Oncol. 34: suppl; abstr 539. doi:10.1200/JCO.2016.34.15_suppl.539
- 50.Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abastado JP. The next challenge in cancer immunotherapy: controlling T-cell traffic to the tumor. Cancer Res. 2012;72:2159–2161. doi: 10.1158/0008-5472.CAN-11-3538. [DOI] [PubMed] [Google Scholar]
- 52.Salmon H, Franciszkiewicz K, Damotte D, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Investig. 2012;122:899–910. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Q, Goding S, Hagenaars M, et al. Morphological appearance, content of extracellular matrix and vascular density of lung metastases predicts permissiveness to infiltration by adoptively transferred natural killer and T cells. Cancer Immunol Immunother. 2006;55:699–707. doi: 10.1007/s00262-005-0043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peranzoni E, Rivas-Caicedo A, Bougherara H, et al. Positive and negative influence of the matrix architecture on antitumor immune surveillance. Cell Mol Life Sci. 2013;70:4431–4448. doi: 10.1007/s00018-013-1339-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang H, Hegde S, Knolhoff BL, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. 2016;22:851–860. doi: 10.1038/nm.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caruana I, Savoldo B, Hoyos V, et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat Med. 2015;21:524–529. doi: 10.1038/nm.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kato Y (2017) Upregulation of memory T cell population and enhancement of Th1 response by lenvatinib potentiate antitumor activity of PD-1 signaling blockade. In: Proceedings of the 110th annual meeting of the American Association for Cancer Research; 2017 Apr 4–5; AACR, Washington, DC, 2017. 58: Part B. Abstract 4614
- 58.Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol. 2008;40:1101–1110. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kristensen JH, Karsdal MA, Sand JM, et al. Serological assessment of neutrophil elastase activity on elastin during lung ECM remodeling. BMC Pulm Med. 2015;15:53. doi: 10.1186/s12890-015-0048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bager CL, Willumsen N, Leeming DJ, et al. Collagen degradation products measured in serum can separate ovarian and breast cancer patients from healthy controls: a preliminary study. Cancer Biomark. 2015;15:783–788. doi: 10.3233/CBM-150520. [DOI] [PubMed] [Google Scholar]
- 61.Willumsen N, Bager CL, Leeming DJ, et al. Serum biomarkers reflecting specific tumor tissue remodeling processes are valuable diagnostic tools for lung cancer. Cancer Med. 2014;3:1136–1145. doi: 10.1002/cam4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Willumsen N, Bager CL, Leeming DJ, et al. Extracellular matrix specific protein fingerprints measured in serum can separate pancreatic cancer patients from healthy controls. BMC Cancer. 2013;13:554. doi: 10.1186/1471-2407-13-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bager CL, Willumsen N, Kehlet SN, et al. Remodeling of the tumor microenvironment predicts increased risk of cancer in postmenopausal women: the prospective epidemiologic risk factor (PERF I) study. Cancer Epidemiol Biomark Prev. 2016;25:1348–1355. doi: 10.1158/1055-9965.EPI-16-0127. [DOI] [PubMed] [Google Scholar]
- 64.Mortensen JH, Godskesen LE, Jensen MD, et al. Fragments of citrullinated and MMP-degraded vimentin and MMP-degraded type III collagen are novel serological biomarkers to differentiate Crohn’s disease from ulcerative colitis. J Crohns Colitis. 2015;9:863–872. doi: 10.1093/ecco-jcc/jjv123. [DOI] [PubMed] [Google Scholar]
- 65.Leeming D, He Y, Veidal S, et al. A novel marker for assessment of liver matrix remodeling: an enzyme-linked immunosorbent assay (ELISA) detecting a MMP generated type I collagen neo-epitope (C1M) Biomarkers. 2011;16:616–628. doi: 10.3109/1354750X.2011.620628. [DOI] [PubMed] [Google Scholar]
- 66.Barascuk N, Veidal SS, Larsen L, et al. A novel assay for extracellular matrix remodeling associated with liver fibrosis: an enzyme-linked immunosorbent assay (ELISA) for a MMP-9 proteolytically revealed neo-epitope of type III collagen. Clin Biochem. 2010;43:899–904. doi: 10.1016/j.clinbiochem.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 67.Veidal SS, Karsdal MA, Nawrocki A, et al. Assessment of proteolytic degradation of the basement membrane: a fragment of type IV collagen as a biochemical marker for liver fibrosis. Fibrogenesis Tissue Repair. 2011;4:22. doi: 10.1186/1755-1536-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bay-Jensen AC, Leeming DJ, Kleyer A, et al. Ankylosing spondylitis is characterized by an increased turnover of several different metalloproteinase-derived collagen species: a cross-sectional study. Rheumatol Int. 2012;32:3565–3572. doi: 10.1007/s00296-011-2237-8. [DOI] [PubMed] [Google Scholar]
- 69.Bay-Jensen AC, Byrjalsen I, Siebuhr AS, et al. Serological biomarkers of joint tissue turnover predict tocilizumab response at baseline. J Clin Rheumatol. 2014;20:332–335. doi: 10.1097/RHU.0000000000000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siebuhr A, Bay-Jensen AC, Leeming DJ, et al. Serological identification of fast progressors of structural damage with rheumatoid arthritis. Arthritis Res Ther. 2013;15:R86. doi: 10.1186/ar4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Siebuhr AS, Petersen KK, Rendt-Nielsen L, et al. Identification and characterisation of osteoarthritis patients with inflammation derived tissue turnover. Osteoarthr Cartil. 2014;22:44–50. doi: 10.1016/j.joca.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 72.Leeming DJ, Sand JM, Nielsen MJ, et al. Serological investigation of the collagen degradation profile of patients with chronic obstructive pulmonary disease or idiopathic pulmonary fibrosis. Biomark Insights. 2012;7:119–126. doi: 10.4137/BMI.S9415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leeming DJ, Bay-Jensen AC, Vassiliadis E, et al. Post-translational modifications of the extracellular matrix are key events in cancer progression: opportunities for biochemical marker development. Biomarkers. 2011;16:193–205. doi: 10.3109/1354750X.2011.557440. [DOI] [PubMed] [Google Scholar]
- 74.Karsdal MA, Henriksen K, Leeming DJ, et al. Novel combinations of post-translational modification (PTM) neo-epitopes provide tissue-specific biochemical markers-are they the cause or the consequence of the disease? Clin Biochem. 2010;43:793–804. doi: 10.1016/j.clinbiochem.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 75.Prakash MD, Munoz MA, Jain R, et al. Granzyme B promotes cytotoxic lymphocyte transmigration via basement membrane remodeling. Immunity. 2014;41:960–972. doi: 10.1016/j.immuni.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 76.Virchow R. Die krankhaften Geschwülste: Erster band. 1. Berlin: Verlag von August Hirschwald; 1863. Aetiologie der neoplastischen Geschwülste/Pathogenie der neoplastischen Geschwülste; pp. 57–101. [Google Scholar]
- 77.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 78.Finn OJ. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol. 2012;23(suppl. 8):viii6–9. doi: 10.1093/annonc/mds256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grivennikov SI, Karin M. Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev. 2010;20:65–71. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turley SJ, Cremasco V, Astarita JL. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol. 2015;15:669–682. doi: 10.1038/nri3902. [DOI] [PubMed] [Google Scholar]
- 81.Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204–215. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 82.Ries CH, Cannarile MA, Hoves S, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 83.Mor-Vaknin N, Punturieri A, Sitwala K, Markovitz DM. Vimentin is secreted by activated macrophages. Nat Cell Biol. 2003;5:59–63. doi: 10.1038/ncb898. [DOI] [PubMed] [Google Scholar]
- 84.Gudmann NS, Hansen NUB, Jensen ACB, et al. Biological relevance of citrullinations: diagnostic, prognostic and therapeutic options. Autoimmunity. 2015;48:73–79. doi: 10.3109/08916934.2014.962024. [DOI] [PubMed] [Google Scholar]
- 85.Bay-Jensen AC, Guo X, Mortensen JH, Karsdal MA et al (2015) VICM is a novel biomarker of macrophage activity evaluated in a phase IIb clinical trial of mavrilimumab. Arthritis Rheumatol. 67: suppl 10; abstr 1679
- 86.Vassiliadis E, Oliveira CP, Alvares-da-Silva MR, et al. Circulating levels of citrullinated and MMP-degraded vimentin (VICM) in liver fibrosis related pathology. Am J Transl Res. 2012;4:403–414. [PMC free article] [PubMed] [Google Scholar]
- 87.Bay-Jensen AC, Platt A, Byrjalsen I, et al. Effect of tocilizumab combined with methotrexate on circulating biomarkers of synovium, cartilage, and bone in the LITHE study. Semin Arthritis Rheum. 2014;43:470–478. doi: 10.1016/j.semarthrit.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 88.Bay-Jensen AC, Karsdal MA, Vassiliadis E, et al. Circulating citrullinated vimentin fragments reflect disease burden in ankylosing spondylitis and have prognostic capacity for radiographic progression. Arthritis Rheum. 2013;65:972–980. doi: 10.1002/art.37843. [DOI] [PubMed] [Google Scholar]
- 89.Pelosi G, Melotti F, Cavazza A, et al. A modified vimentin histological score helps recognize pulmonary sarcomatoid carcinoma in small biopsy samples. Anticancer Res. 2012;32:1463–1473. [PubMed] [Google Scholar]
- 90.O’Callaghan DS, O’Donnell D, O’Connell F, O’Byrne KJ. The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thorac Oncol. 2010;5:2024–2036. doi: 10.1097/JTO.0b013e3181f387e4. [DOI] [PubMed] [Google Scholar]
- 91.Willumsen N, Bager C, Bay-Jensen A, et al. Unique insight into microenvironmental changes in colorectal cancer: ex vivo assessment of matrix metalloprotease-mediated molecular changes in human colorectal tumor tissue and corresponding non-neoplastic adjacent tissue. Oncol Lett. 2017;13:3774–3780. doi: 10.3892/ol.2017.5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nicolazzo C, Raimondi C, Mancini M, et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor nivolumab. Sci Rep. 2016;6:31726. doi: 10.1038/srep31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gros A, Parkhurst MR, Tran E, et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med. 2016;22:433–438. doi: 10.1038/nm.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou J, Mahoney KM, Giobbie-Hurder A, et al. Soluble PD-L1 as a biomarker in malignant melanoma and checkpoint blockade. Cancer Immunol Res. 2017;5:480–492. doi: 10.1158/2326-6066.CIR-16-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gray ES, Rizos H, Reid AL, et al. Circulating tumor DNA to monitor treatment response and detect acquired resistance in patients with metastatic melanoma. Oncotarget. 2015;6:42008–42018. doi: 10.18632/oncotarget.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kijima T, Hazama S, Tsunedomi R, et al. MicroRNA-6826 and-6875 in plasma are valuable non-invasive biomarkers that predict the efficacy of vaccine treatment against metastatic colorectal cancer. Oncol Rep. 2017;37:23–30. doi: 10.3892/or.2016.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stucci S, Tucci M, Ascierto P, et al. Dendritic cell-derived exosomes (Dex) are potential biomarkers of response to ipilimumab in metastatic melanoma. J Transl Med. 2015;13(suppl 1):P15. doi: 10.1186/1479-5876-13-S1-P15. [DOI] [Google Scholar]