Abstract
Numerous trials using dendritic cell (DC)-based vaccinations for the treatment of cancer are being carried out. However, an improvement of the quality of DC used is highly warranted. We here generated human monocyte-derived dendritic cells using a 3 day protocol and stimulated the cells using a combination of OK432 (Picibanil), TLR7/8 ligand CL097, and reduced amounts of prostaglandin (PG)E2. We analyzed phenotype, migratory, and T-cell stimulatory capacity compared to a cytokine cocktail consisting of IL-1β, IL-6, TNF, and PGE2. The OK432 cocktail stimulated cells had a similar mature phenotype with upregulated co-stimulatory molecules, HLA-DR and CCR7 as the cytokine cocktail-matured cells and a similar cytokine profile except increased amounts of IL-12p70. Chemotaxis towards CCL19 was reduced compared to the cytokine cocktail, but increased compared to OK432 alone. The T-cell stimulatory capacity was similar to the cytokine cocktail stimulated cells. In conclusion, the OK432 cocktail has the advantage of inducing IL-12p70 production without impairing phenotype or T-cell stimulatory capacity of the cells and might, therefore, be an advantageous alternative to be used in DC-based immunotherapy.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2216-y) contains supplementary material, which is available to authorized users.
Keywords: Dendritic cells, Immunotherapy, OK432, Cancer, Pro-inflammatory cytokines
Introduction
Dendritic cells (DC) are powerful antigen presenting cells that are able to induce both immunogenic and tolerogenic immune responses. DC detect pathogen-associated molecular patterns via pattern recognition receptors, and are able to take up and process antigens. Upon stimulation by “danger” signals such as inflammatory cytokines, they up-regulate surface expression of MHC class II and co-stimulatory molecules such as CD86 and CD80, and secrete certain pro-inflammatory cytokines [1]. Soon after the discovery of DC by Steinman and Cohn in 1973 [2], immunotherapeutic uses of these cells against cancer have been in the focus of research [3–7]. While this DC-based immunotherapy has been proven successful in animal models [8–11], it has been disappointing in clinical trials despite being shown to be safe to use [7, 12]. A protocol to feasibly generate large amounts of immature DC (iDC) ex-vivo from blood monocytes stimulated with IL-4 and GM-CSF was developed in 1994 [13] and formed a vital backbone for ex-vivo DC-based cancer immunotherapy. During the past decade, various maturation stimuli have been used. A cytokine cocktail consisting of IL-1β, IL-6, TNF, and PGE2 [14] has been the gold standard for a long time, as these cytokine cocktail-matured DC (ccDC) showed powerful T-cell stimulatory capacity as well as migratory capacity in vitro [11]. However, disappointing results from clinical trials and further investigation of the compounds used in the cocktail showed the shortcomings of this maturation method for cancer immunotherapy. IL-6 and PGE2 have been shown to limit the generation of immunogenic mature DC capable of stimulating T cells against cancer, and thus, new maturation strategies are required [15–20]. For DC to effectively stimulate naïve T cells into immunogenic T cells against cancer, three signals are required: (i) a tumor associated antigen presented on MHC; (ii) co-stimulatory molecules; and (iii) an immunogenic cytokine profile. Particularly, the requirement of IL-12p70 secretion and Th1 stimulatory capability has been discussed as being of utmost importance in DC-based immunotherapy [21, 22].
OK432, manufactured under the name Picibanil, is a streptococcal preparation that has been used for decades as cancer treatment in Japan [23–26]. While its mechanism is still not clearly understood, its immunostimulatory effects on DC have been analyzed. DC co-cultured with OK432 have a mature phenotype as well as an inflammatory cytokine profile [23, 25, 27, 28]. Furthermore, it was shown previously by our group that OK432 matured DC had increased secretion of different cytokines, among them IL-12p70, and that the DC were stimulated in a TLR3-dependent manner [29, 30]. Unfortunately, OK432-treated DC did not migrate towards CC chemokine ligand (CCL) 19 due to lack of CC chemokine receptor (CCR) 7 expression [29, 30].
Recently, a protocol for generating iDC in 3 days rather than 7 days was developed [31] to save time, promote efficient use of GMP grade cytokines, and better simulate an in-vivo situation, where DC migration to lymph nodes and stimulation of T cells take place within a few days [32, 33].
Despite the undesirable effects of PGE2, its addition to maturation cocktails increases the CCR7 expression of the DC significantly [34]. The aim of this study was to explore if a combination of OK432, a TLR7/8 agonist, and reduced amounts of PGE2 would result in immunogenic DC with an advantageous pro-inflammatory cytokine profile. We used the 3-day iDC generation protocol [31] and compared the cells to DC matured with the Jonuleit cytokine cocktail consisting of IL-1β, IL-6, TNF and PGE2 as well as other TLR ligand containing cocktails. Compared to the Jonuleit cocktail, phenotype and T-cell stimulatory capacity were similar, while chemotaxis towards CCL19 was slightly reduced. However, IL-12p70 secretion was increased in OK432 cocktail stimulated cells compared to the Jonuleit cytokine cocktail even if reduced compared to other TLR ligand containing cocktails suggesting the OK432 cocktail to be an interesting candidate for future DC-based immunotherapy trials.
Materials and methods
Monocyte-derived dendritic cell generation
Human monocyte-derived DC were generated from monocytes isolated from Buffy coat preparations from healthy blood donors (Blood Bank, Haukeland University Hospital, Bergen, Norway) as described previously [29] with some modifications. Briefly, peripheral blood mononuclear cells (PBMC) were separated by density gradient centrifugation (Lymphoprep™, Axis-Shield, catalogue number 1114547). PBMC were quantified with a cell counter (CASY™, Roche) and the monocytes were then isolated using plastic adherence in 6 well plates (Nunc™, Thermo Scientific) at a concentration of 1 × 107 PBMC per 3 ml/well at 37 °C and 5% CO2 humidified atmosphere, for 1 h. Non-adherent cells (= monocyte depleted PBMC; NAC) were collected by subsequent washing with PBS, and cryopreserved at a concentration of 2–5 × 107 cells/ml in X-vivo 20 medium (Lonza, catalogue number BE04-448 Q) and 10% DMSO (Sigma-Aldrich, catalogue number D2650) at − 80 °C in a Mr. Frosty freezing container. The remaining monocytes were cultured with IL-4 (20 ng/ml; Immunotools, catalogue number 11340047) and GM-CSF (100 ng/ml; Immunotools, catalogue number 11343128) in RP10 medium [RPMI 1640 (Lonza, catalogue number 12-702F/U1/12) with 10% FCS (PAA, catalogue number A15-151); 100 units/ml penicillin and 100 µg/ml streptomycin (BioWhittaker, catalogue number 17-602E)] for 3 days to generate DC. For the DC used in antigen-specific T-cell induction and IFN-γ secretion assays, CellGro medium (CellGenix GmbH, catalogue number 20801-0500) was used. Cytokines were replenished 24 h before harvesting, and DC were matured using OK432 (Picibanil; 0.1 KE/ml, Chugai Pharmaceutical Co. Ltd, Tokyo, Japan), TLR7/8 ligand (CL097; 1 µg/ml; Invivogen, catalogue number tlrl-c97) and PGE2, 0.5 µg/ml (Sigma-Aldrich, catalogue number P0409). For the controls, the cells were stimulated with either the Jonuleit cocktail [IL-1β, 10 ng/ml; IL-6, 1000 U/ml; TNF 10 ng/ml (all from Immunotools, catalogue numbers 11340012, 11340064, 11343015) and PGE2, 1 µg/ml], 0.1 KE/ml OK432 alone, and 0.1 KE/ml OK432 with CL097 (1 µg/ml), respectively. For some experiments, additional TLR ligand containing stimulation cocktails were utilized: (i) Kalinski cocktail: TNF (50 ng/ml), IL-1β (25 ng/ml), IFN-α (3000 U/ml; Immunotools, catalogue number 11343514), IFN-γ (1000 U/ml), and polyI:C (20 µg/ml; Sigma-Aldrich, catalogue number P0913) [35]; (ii) Lövgren cocktail: TNF (20 ng/ml), IFN-γ (1000 U/ml), R848 (2.5 µg/ml; Invivogen, catalogue number tlrl-R848), and polyI:C (20 µg/ml) [36]; and (iii) Zobywalski cocktail: TNF (10 ng/ml), IL-1β (10 ng/ml), IFN-γ (5000 U/ml), PGE2 (250 ng/ml), R848 (1 µg/ml), and polyI:C (20 ng/ml) [37]. For autologous MLR and the IFN-γ secretion assay, 1 µg/ml tuberculin-purified protein derivate (PPD, Statens Serum Institut, Copenhagen; Denmark) was added 1 h prior to maturation stimulus as recall antigen.
Flow cytometry
Immunostaining was performed by incubating DC samples with titrated amounts of antibodies in darkness at room temperature for 10 min. The antibodies used were: CD14 FITC (18D11), CD1a PE (HI149) (Immunotools), CD38 PerCP-Cy5.5 (HIT2), CD40 PE-Cy7 (5C3), CD86 Alexa Fluor 647 (IT2.2), CD80 Brilliant violet 605 (2D10) and CCR7 Brilliant violet 421 (CD197; G045H7) (Biolegend), CD83 PE-CF594 (HB15e), and HLA-DR Horizon V500 (G46-6) (BD Biosciences). The cells where then washed and immediately analyzed on an LSR Fortessa (BD Biosciences). Further analysis was done using the software FlowJo V10.3 (Tree Star, Ashland, OR). Representative gating strategy is shown in Supplementary Figure 1.
Mixed lymphocyte reaction
Allogeneic or autologous NAC were thawed and allowed to rest for 24 h before being labelled with carboxyfluorescein diacetate succinimidyl ester (CFDA-SE; Invitrogen, catalogue number V12883) according to the manufacturer’s instructions. Two hundred thousand CFDA-SE labelled lymphocytes were then co-cultured with 5 × 104 extensively washed mature DC in X-Vivo 20 media. At the start of the co-culture, 50 U/ml of IL-2 and 10 ng/ml of IL-7 (both Immunotools, catalogue numbers 11340023, 11340072) were added to ensure CD4 and CD8 T-cell survival. After 5 days, the cells were harvested and analyzed on an LSRFortessa (BD Biosciences) flow cytometer.
IFN-γ secretion assay
To analyze the capacity of the generated DC populations to induce antigen-specific T-cell responses, we utilized an IFN-γ secretion assay (Miltenyi Biotec, catalogue number 130-054-202). Cryopreserved autologous NAC (monocyte depleted PBMC) were thawed and allowed to rest for 24 h, before 2.5 × 106 cells were being co-cultured for 7 days with either 5 × 105 PPD-loaded DC stimulated with OK432, CL097, and PGE2, or DC stimulated with the Jonuleit cytokine cocktail in X-Vivo 20 medium supplemented with IL-7 (10 ng/ml) and IL-2 (50 U/ml). In some experiments, the induced NACs were then harvested, washed and allowed to rest for 5 days before proceeding to the IFN-γ secretion assay according to the manual of the manufacturer. As stimulators, PPD-loaded DC stimulated with the Jonuleit cytokine cocktail were used. Unloaded DC served as negative control. 2 × 105 DC were co-cultured with 8 × 105 induced NAC for 16 h. Staphylococcal enterotoxin B from Staphylococcus aureus (1 µg/ml; Sigma-Aldrich, catalogue number 11100-45-1) was added as positive control. The cells were further stained with anti-CD4 FITC (M-T466, Miltenyi Biotec) and anti-CD8-allophycocyanin (RPA-T8, Biolegend) antibodies. 5 µl 7-actinomycin D (7-AAD; ebioscience, catalogue number 00-6993-50) was added just before acquisition on a BD LSR Fortessa and BD Accuri 6, respectively. At least 2 × 105 events were collected in the Lymphocyte gate (see Supplementary Figure 2). FlowJo was used to analyze the data, and % IFN producing cells were calculated according to the following formula:
IFN-γ producing CD8 + T cells were calculated accordingly. The % IFN-γ-producing cells from the negative control (DC without PPD) were subtracted from the sample values.
Chemotaxis
50 000 DC were added to the upper chamber of an 8 µm transwell 96-well plate (Corning Lifescience, Lowell, MA) and left to migrate towards CCL19 (100 ng/ml, Immunotools, catalogue number 11343240) in X-vivo 20 medium for 4 h at 37 °C, 5% CO2 humidified atmosphere. The migrated cells were counted using a CASY™ cell counter.
Cytokine determination
The cell-free supernatant from each DC population was aliquoted and stored at –20 °C. Cytokine and chemokine concentrations were determined using a 25-plex Luminex assay cytokine and chemokine panel (Invitrogen, catalogue number LHC0009) and run on a Luminex 100 System (Luminex Corporation, Austin, TX) according to the manufacturer’s instructions. To quantify IL-12p70 secretion, a sandwich ELISA (BioLegend, catalogue number 431704) was utilized according to the manufacturer’s instructions.
Statistical analyses
All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, CA). Statistical significance between samples was tested using Kruskal–Wallis one-way ANOVA test with Dunn test.
Results
DC treated with a combination of OK432, TLR7/8 ligand CL097, and PGE2 are phenotypically mature with high expression of CCR7
To determine whether the addition of OK432, TLR7/8 ligand CL097, and reduced amounts of PGE2 for 24 h would be sufficient to generate mature DC after only 3 days in culture, the phenotype was analyzed using flow cytometry. The cells showed high expression of maturation marker CD83, co-stimulatory molecules CD80 and CD86, as well as HLA-DR (Fig. 1). As the previous experiments had shown that DC matured with OK432 alone did not have adequate chemotaxis towards CCL19, the expression of its receptor CCR7 was included in the phenotypical analysis. DC matured with the combination of OK432, CL097, and PGE2 showed higher CCR7 expression than ccDC, while the lack of PGE2 resulted in low expression of the chemokine receptor.
Fig. 1.
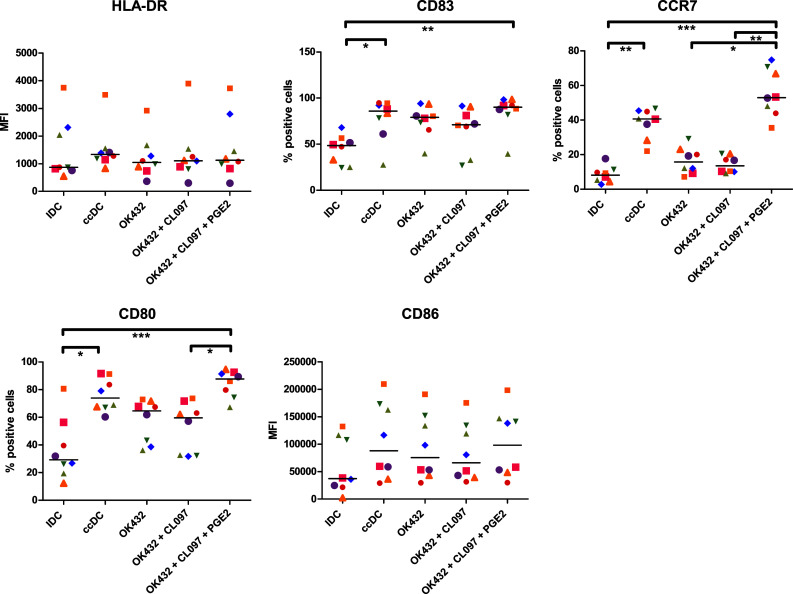
DC matured with a cocktail consisting of OK432, CL097, and PGE2 express high amounts of co-stimulatory molecules and CCR7. HLA-DR was highly expressed on all cell populations. CD83 was increased in all matured DC populations. CCR7 was significantly increased (p < 0.05) in every group matured with PGE2 compared to iDC. CD80 was significantly higher expressed in ccDC and DC treated with OK432, CL097, and PGE2 (p < 0.05) compared to iDC, while the increase of CD86 expression did not reach statistical significance. IDC immature DC, ccDC: DC matured with Jonuleit cytokine cocktail TNF, IL-6, IL-1β, and PGE2; OK432: DC matured with 0.1 KE/ml OK432; OK432 + CL097: DC matured with 0.1 KE/ml OK432 and CL097; OK432 + CL097 + PGE2: DC matured with 0.1 KE/ml OK432, CL097 and 0.5 µg/ml PGE2; MFI: median fluorescence intensity. Each symbol represents a different donor
Migratory capacity depends on PGE2 in maturation stimulus
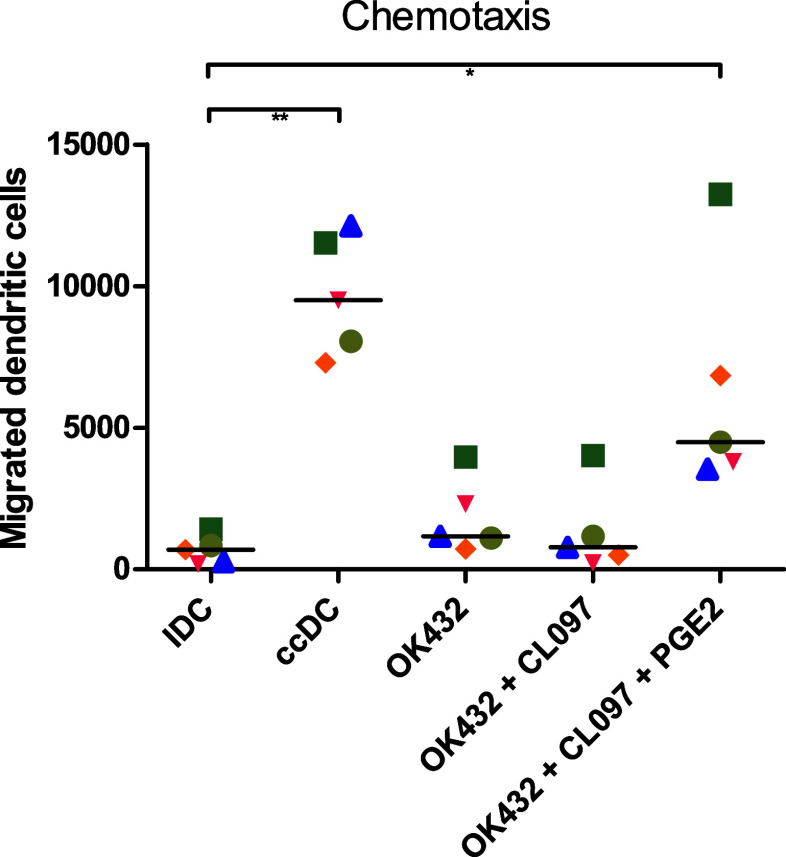
While it was shown previously that OK432 has great potential as maturation stimulus for moDC, these cells had little chemotaxis towards CCL19 due to low CCR7 expression [30]. As the addition of OK432, CL097, and PGE2 resulted in high expression of CCR7, we analyzed their migratory ability in a transwell assay towards CCL19 (Fig. 2). Cytokine cocktail-matured DC migrated best, but the addition of reduced amounts of PGE2 to OK432 and CL097 stimulated DC resulted in significantly increased migration compared to immature DC. Stimulation of the cells with OK432 and CL097 did not improve chemotaxis compared to OK432 alone.
Fig. 2.
DC matured with PGE2 migrate towards CCL19. 50,000 DC were placed in the upper chamber of a transwell plate and incubated for 4 h. Approximately 20% of DC matured with the cytokine cocktail (ccDC) migrated towards CCL19, while approximately 10% of OK432 + CL097 + PGE2 matured DC could be collected in the lower chamber. DC matured without PGE2 did not show considerable chemotaxis towards CCL19. IDC: immature DC; ccDC: DC matured with Jonuleit cytokine cocktail TNFα, IL-6, IL-1β, and PGE2; OK432: DC matured with 0.1 KE/ml OK432; OK432 + CL097: DC matured with 0.1 KE/ml OK432 and CL097; OK432 + CL097 + PGE2: DC matured with 0.1 KE/ml OK432, CL097 and 0.5 µg/ml PGE2. Each symbol represents a different donor
OK432-matured DC have superior cytokine secretion regarding T-cell stimulation compared to the Jonuleit cytokine cocktail
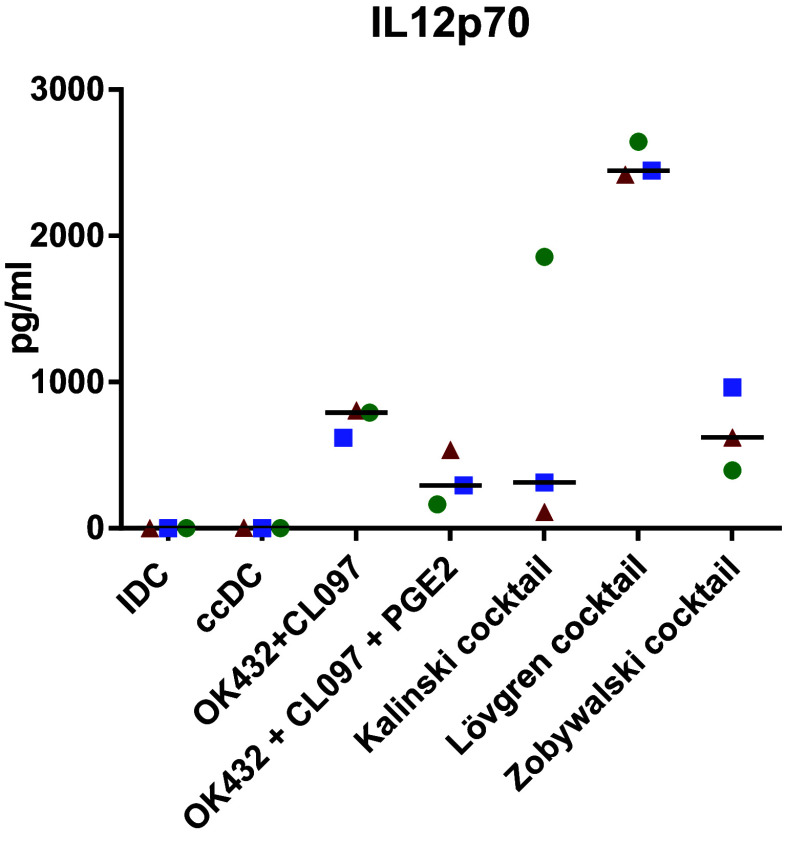
DC can activate T cells with an antigen presented on MHC class II and co-stimulatory molecules, but the cytokine milieu plays a crucial role in determining how the naïve T cells respond to the stimulus [38]. We, therefore, analyzed the cell culture supernatants and determined the presence of 26 different cytokines and chemokines to predict the milieu provided (Fig. 3). All OK432-matured DC had a significant increase in IL-12p70 secretion compared to ccDC and iDC that was further elevated with the addition of CL097, but decreased by PGE2. This pattern was also observed with RANTES/CCL5, MIP-1α/CCL3, and MIP-1β /CCL4. IL-12p40 was highly elevated by the combination of OK432, CL097, and PGE2, but not statistically significant compared to the other matured DC populations. IL-10 secretion was detected at low levels. IL-8 secretion was significantly reduced by the addition of OK432. IL-7 was increased in every matured DC population, but only ccDC reached statistical significance compared to immature DC. IL-17 secretion was increased by OK432 alone, but decreased with the addition of CL097 or PGE2. IL-16, IL-1RA, IFN-γ, IFN-α, MCP-1, IL-2R, and IL-13 secretion did not show obvious differences between sample groups. IP-10, IL-2, IL-5, IL-13, or Eotaxin was secreted at very low levels (data not shown), while IL-4, GM-CSF, IL-1β, IL-6, and TNF were excluded from the analysis as they were used in the culture media. We next analyzed IL-12p70 secretion upon stimulation with various other maturation cocktails described to have beneficial effects on the immunogenicity of DC used for immunotherapy [35–37, 39] (Fig. 4). The Lövgren cocktail clearly excelled at IL-12 production, followed by the Zobywalski cocktail, and OK432 + CL097. Compared to the Kalinski cocktail, which gave varying results depending on the donor, the OK432 cocktail produced similar amounts of IL-12. Surprisingly, the effect of PGE2 on reducing IL-12 secretion did not seem to be dose-dependent (Supplementary Figure 3).
Fig. 3.
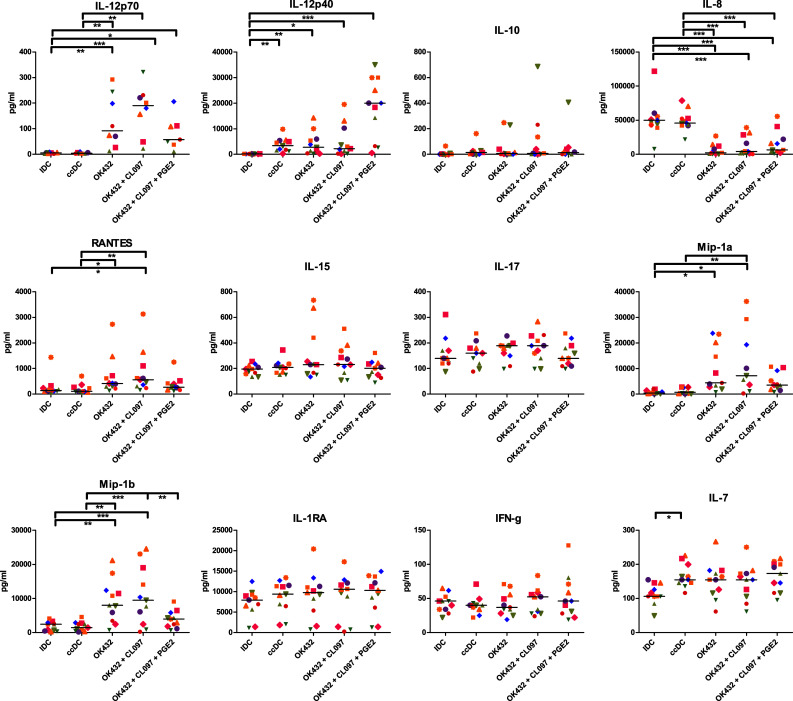
OK432-stimulated DC secrete more cytokines relevant for Th1 cell stimulation. All OK432-matured DC had a significant increase in IL-12p70 secretion compared to ccDC and iDC that was further elevated with the addition of CL097, but decreased by PGE2. The amount of IL-12p70 was reduced by the addition of PGE2, but the cells still produced significantly more IL-12p70 than ccDC (p < 0.05). This pattern was also observed with RANTES/CCL5, MIP-1α/CCL3 and MIP-1β /CCL4. IL-12p40 was highly elevated by the combination of OK432, CL097 and PGE2, albeit not statistically significant compared to other matured DC groups. IDC: immature DC; ccDC: DC matured with Jonuleit cytokine cocktail TNFα, IL-6, IL-1β, and PGE2; OK432: DC matured with 0.1 KE/ml OK432; OK432 + CL097: DC matured with 0.1 KE/ml OK432 and CL097; OK432 + CL097 + PGE2: DC matured with 0.1 KE/ml OK432, CL097, and 0.5 µg/ml PGE2. Each symbol represents a different donor
Fig. 4.
Increased IL-12p70 secretion by DC upon addition of TLR ligand containing maturation cocktails. 3-day DC were matured with different maturation cocktails containing TLR ligands: (i) Kalinski cocktail [35]: TNF, IL-1β, IFN-α, IFN-γ, and polyI:C; (ii) Lövgren cocktail [36]: TNF, IFN-γ, R848, and polyI:C; (iii) Zobywalski cocktail [37]: TNF, IL-1β, IFN-γ, PGE2, R848, and polyI:C. IDC: immature DC; ccDC: DC matured with Jonuleit cytokine cocktail TNFα, IL-6, IL-1β, and PGE2; OK432: DC matured with 0.1 KE/ml OK432; OK432 + CL097: DC matured with 0.1 KE/ml OK432 and CL097; OK432 + CL097 + PGE2: DC matured with 0.1 KE/ml OK432, CL097, and 0.5 µg/ml PGE2. Each symbol represents a different donor
OK432-matured DC are superior at inducing autologous T-cell proliferation
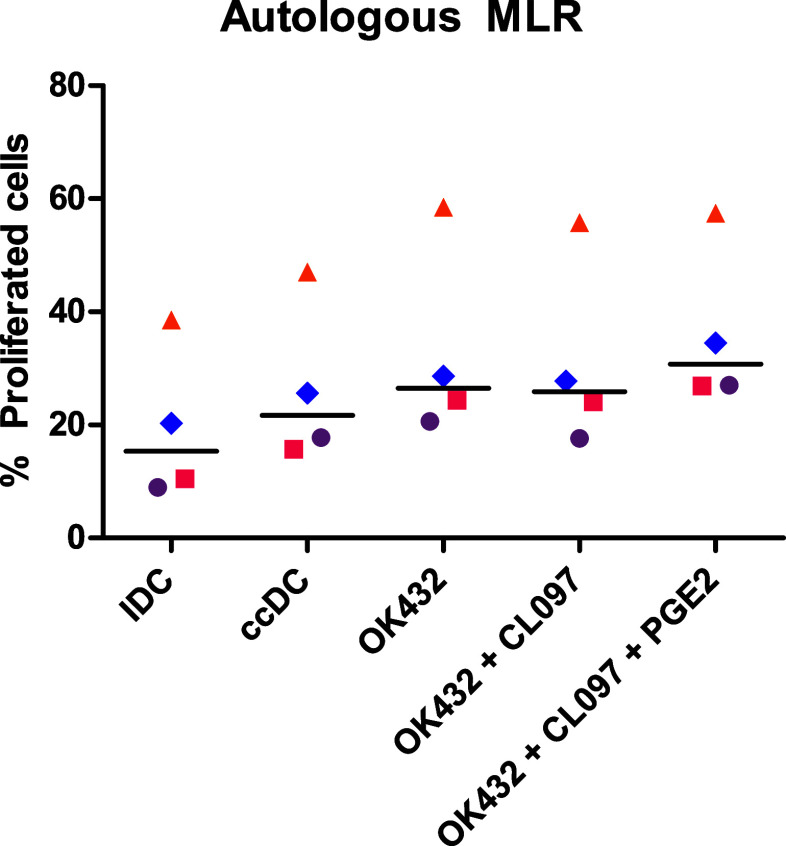
To determine the T-cell stimulatory capacity of the DC populations, allogeneic and autologous MLR were performed. For the autologous MLR, tuberculin was used as recall antigen, as BCG vaccination was compulsory in Norway until 1995. The different DC populations were co-cultured with either autologous (Fig. 5) or allogeneic (data not shown) CFDA-SE-stained NAC, and fluorescence was analyzed after 5 days. OK432-matured DC were superior at stimulating T-cell proliferation compared to ccDC with the addition of PGE2 increasing proliferation. The results suggest that all DC populations were capable of stimulating T cells with all mature DC populations inducing more proliferation than iDC. The addition of OK432 and PGE2 increased the proliferation even further.
Fig. 5.
OK432-matured DC are superior at inducing T-cell proliferation. 2 × 105 CFDA-SE-stained autologous NAC (PBMC depleted of monocytes) were co-cultured with 5 × 104 DC matured with noted stimuli for 5 days. Cells were measured for fluorescence intensity to determine proliferation. OK432 stimulated DC induced more T-cell proliferation than ccDC. IDC: immature DC; ccDC: DC matured with Jonuleit cytokine cocktail TNFα, IL-6, IL-1β, and PGE2; OK432: DC matured with 0.1 KE/ml OK432; OK432 + CL097: DC matured with 0.1 KE/ml OK432 and CL097; OK432 + CL097 + PGE2: DC matured with 0.1 KE/ml OK432, CL097 and 0.5 µg/ml PGE2. Each symbol represents a different donor
OK432-matured DC are able to induce antigen-specific T cells
To further elucidate antigen-specific T-cell induction, PPD-specific CD4+ and CD8+ T cells were quantified with an IFN-γ secretion assay. Both, DC stimulated with OK432, CL097 and PGE2 as well as Jonuleit cytokine cocktail-matured DC were able to induce antigen-specific T cells equally well (Fig. 6).
Fig. 6.
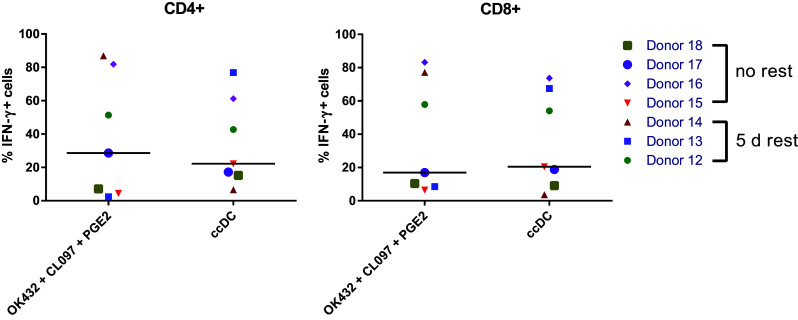
OK432-matured DC are able to induce antigen-specific T cells. Autologous NAC (PBMC depleted of monocytes) were co-cultured with PPD-loaded DC matured with noted stimuli for 7 days, and antigen-specific IFN-gamma secretion by CD4 + and CD8 + cells was analyzed by flow cytometry with ccDC as stimulators (+/− PPD). In some experiments (donors 12–14), the induced NAC were rested for 5 days prior to the IFN-g secretion assay. ccDC: DC matured with Jonuleit cytokine cocktail TNFα, IL-6, IL-1β, and PGE2; OK432 + CL097 + PGE2: DC matured with 0.1 KE/ml OK432, CL097, and 0.5 µg/ml PGE2. Each symbol represents a different donor
Discussion
With cancer recurrence and metastasis being some of the main challenges to overcome, the concept of immunotherapy is believed to hold great potential in preventing postsurgical or radio/chemotherapeutic recurrence as well as being capable of treating inoperable tumors. With several murine studies showing the capability of the immune system to induce tumor regression and long term protection [8–11], the pursuit to find equivalent success in humans remains relentless. The disappointing clinical trials using the gold standard cytokine cocktail (TNF, IL-6, IL-1β, and PGE2) instigated a search for a superior maturation cocktail for DC cancer immunotherapy [6, 7].
The aim of this study was twofold: to reduce the time used to generate moDC and to combine OK432 with a TLR7/8 ligand and PGE2 to stimulate the cells. As controls, we used 3-day moDC stimulated with the Jonuleit cytokine cocktail consisting of TNF, IL-6, IL-1β, and PGE2, OK432 alone, and OK432 in combination with CL097. While ccDC have been shown to stimulate T cells [40], clinical trials with them have been disappointing [7]. This is most likely because of the lack of IL-12p70 secretion that is crucial for Th1 differentiation from naïve T cells [41].
The results of this study indicate that DC matured with a combination of OK432 and CL097 provide an inflammatory milieu and that all OK432 matured sample groups provide a more inflammatory milieu compared to ccDC. Considering that IL-12p70 is very important for differentiation from naïve T cells to Th1 CD4+ helper cells able to stimulate effective CD8+ cytotoxic cells [42, 43], OK432-matured DC show high potential in cancer immunotherapy, even though the cells produced less IL12-p70 compared to DC stimulated with the Lövgren cocktail. However, they produced an increased combination of inflammatory cytokines as well as chemokines attracting lymphocytes and leukocytes [44, 45].
This study shows that DC matured with a combination of OK432, TLR7/8 ligand CL097, and reduced amounts of PGE2 satisfy many of the criteria for an efficient DC vaccine: secretion of IL-12p70, low secretion of IL-10, high expression of co-stimulatory molecules, chemotaxis towards CCL19, and capability of stimulating antigen-specific T cells in an autologous setting. The number of antigen-specific T cells was similar compared to the Jonuleit cytokine cocktail analyzed by IFN-γ secretion and even increased when testing in an autologous MLR using a recall antigen (tuberculin). To be able to determine the suitability of the OK432 cocktail for cancer immunotherapy, these results have to be verified using tumor associated antigens in a future study. Interestingly, despite using only half the amount of PGE2 compared to the Jonuleit cytokine cocktail, the expression of CCR7 was still higher than that of ccDC. However, chemotaxis towards CCL19 was still reduced compared to ccDC meaning that high CCR7 expression alone is not enough for strong chemotaxis. The importance of PGE2 in moDC chemotaxis has been stressed by the investigations done by Luft et al. [46], and in 2008, Yen et al.’s published data suggest the ability of PGE2 to up-regulate metalloproteinase-9 in DC as being vital for their migratory capacity [47]. However, a lower migratory capacity than ccDC should be acceptable as long as the DC are capable of a Th1-oriented stimulus in the lymph nodes [48].
One major difference of OK432-matured DC was the clear reduction of the secretion of IL-8, primarily a neutrophil attractant chemokine and activator [49]. In terms of immunotherapeutic applications, this trait can be viewed as either positive or negative depending on the intended purpose of the generated DC. While some studies focus on the involvement of DC directly on tumors [50], the goal of this study was to find migrating DC capable of stimulating a Th1 response in lymph nodes. For this purpose, a lower amount of secreted IL-8 can be looked upon as positive due to neutrophil inhibition of T-cell proliferation through their expression of PD-L1 [51, 52]. How big of a role IL-8 secreted from mature DC in lymph nodes has on T cells should be further investigated to help future design of DC maturation protocols.
With numerous studies involving OK432 stimulated DC supporting the positive aspects [23, 27, 29, 30], it is evident that OK432-matured DC are potential alternatives to the Jonuleit cytokine cocktail. The increased cytokine secretion induced by CL097 further enhanced desirable traits of OK432-matured DC, and while PGE2 is associated with undesirable effects, it is still the primary inducer of migration towards CCL19. The combination of migratory capacity, IL-12p70 secretion as well as other pro-inflammatory cytokines along with a phenotype capable of strong T-cell induction makes DC stimulated with OK432, in combination with CL097 and PGE2, a favourable candidate fulfilling the current proposed conditions for effective DC cancer immunotherapy [20, 21].
In conclusion, the results from this study show most promising aspects of OK432 cocktail-matured DC. This cocktail consisting of OK432 combined with TLR7/8 agonist and low concentrations of PGE2 should, therefore, be considered a possible base for future maturation cocktails to be used in DC-based cancer immunotherapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- 7-AAD
7-aminoactinomycin D
- ccDC
Jonuleit cytokine cocktail-matured DC
- CCL
CC chemokine ligand
- CCR
CC chemokine receptor
- CFDA-SE
Carboxyfluorescein diacetate succinimidyl ester
- iDC
Immature DC
- NAC
Non-adherent cells (monocyte depleted PBMC)
- PGE2
Prostaglandin E2
- PPD
Tuberculin-purified protein derivate
Author Contributions
SA and RJ conceived of the study. DHY, NS, and KJ acquired and analyzed the data. DHY, RJ, and SA interpreted the data. DHY and SA drafted the manuscript, and all authors revised the manuscript and approved the final version.
Funding
This work was supported by the Broegelmann Foundation, Bergen Research Foundation, Norwegian Research Council, and Faculty of Medicine and Dentistry, University of Bergen, Norway.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study was conducted according to the Declaration of Helsinki. All anonymised biological material was collected at the Blood Bank of the Haukeland University Hospital after written informed consent. This study is a technical and methodological development work that uses anonymised biological material, and no approval from the regional ethical committee was required (https://helseforskning.etikkom.no/reglerogrutiner/soknadsplikt/sokerikkerek?p_dim=34999&_ikbLanguageCode=us).
Informed consent
All blood donors gave written informed consent.
References
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137(5):1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nouri-Shirazi M, Banchereau J, Bell D, Burkeholder S, Kraus ET, Davoust J, et al. Dendritic cells capture killed tumor cells and present their antigens to elicit tumor-specific immune responses. J Immunol. 2000;165(7):3797–3803. doi: 10.4049/jimmunol.165.7.3797. [DOI] [PubMed] [Google Scholar]
- 4.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 5.Gravitz L. A fight for life that united a field. Nature. 2011;478(7368):163–164. doi: 10.1038/478163a. [DOI] [PubMed] [Google Scholar]
- 6.Bloy N, Pol J, Aranda F, Eggermont A, Cremer I, Fridman WH, et al. Trial watch: Dendritic cell-based anticancer therapy. Oncoimmunology. 2014;3(11):e963424. doi: 10.4161/21624011.2014.963424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galluzzi L, Senovilla L, Vacchelli E, Eggermont A, Fridman WH, Galon J, et al. Trial watch: Dendritic cell-based interventions for cancer therapy. Oncoimmunology. 2012;1(7):1111–1134. doi: 10.4161/onci.21494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paglia P, Chiodoni C, Rodolfo M, Colombo MP. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo. J Exp Med. 1996;183(1):317–322. doi: 10.1084/jem.183.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1(12):1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 10.Zitvogel L, Mayordomo JI, Tjandrawan T, DeLeo AB, Clarke MR, Lotze MT, et al. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med. 1996;183(1):87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song W, Levy R. Therapeutic vaccination against murine lymphoma by intratumoral injection of naive dendritic cells. Cancer Res. 2005;65(13):5958–5964. doi: 10.1158/0008-5472.CAN-05-0406. [DOI] [PubMed] [Google Scholar]
- 12.Vacchelli E, Vitale I, Eggermont A, Fridman WH, Fucikova J, Cremer I, et al. Trial watch: dendritic cell-based interventions for cancer therapy. Oncoimmunology. 2013;2(10):e25771. doi: 10.4161/onci.25771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179(4):1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27(12):3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 15.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1(6):510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 16.Kalinski P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML. Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood. 2001;97(11):3466–3469. doi: 10.1182/blood.V97.11.3466. [DOI] [PubMed] [Google Scholar]
- 17.Jongmans W, Tiemessen DM, van Vlodrop IJ, Mulders PF, Oosterwijk E. Th1-polarizing capacity of clinical-grade dendritic cells is triggered by Ribomunyl but is compromised by PGE2: the importance of maturation cocktails. J Immunother. 2005;28(5):480–487. doi: 10.1097/01.cji.0000171290.78495.66. [DOI] [PubMed] [Google Scholar]
- 18.Baratelli F, Lin Y, Zhu L, Yang SC, Heuze-Vourc’h N, Zeng G, et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005;175(3):1483–1490. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- 19.Van Elssen CH, Vanderlocht J, Oth T, Senden-Gijsbers BL, Germeraad WT, Bos GM. Inflammation-restraining effects of prostaglandin E2 on natural killer-dendritic cell (NK-DC) interaction are imprinted during DC maturation. Blood. 2011;118(9):2473–2482. doi: 10.1182/blood-2010-09-307835. [DOI] [PubMed] [Google Scholar]
- 20.Heiro YD, Appel S. Current status and future perspectives of dendritic cell-based cancer immunotherapy. Scand J Immunol. 2013;78(2):167–171. doi: 10.1111/sji.12060. [DOI] [PubMed] [Google Scholar]
- 21.Palucka K, Banchereau J, Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity. 2010;33(4):464–478. doi: 10.1016/j.immuni.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. ImmunolRev. 2007;220:129–150. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- 23.Ryoma Y, Moriya Y, Okamoto M, Kanaya I, Saito M, Sato M. Biological effect of OK-432 (picibanil) and possible application to dendritic cell therapy. Anticancer Res. 2004;24(5C):3295–3301. [PubMed] [Google Scholar]
- 24.Kim SY, Park HC, Yoon C, Yoon HJ, Choi YM, Cho KS. OK-432 and 5-fluorouracil, doxorubicin, and mitomycin C (FAM-P) versus FAM chemotherapy in patients with curatively resected gastric carcinoma: a randomized Phase III trial. Cancer. 1998;83(10):2054–2059. doi: 10.1002/(SICI)1097-0142(19981115)83:10<2054::AID-CNCR2>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 25.Sakamoto J, Teramukai S, Nakazato H, Sato Y, Uchino J, Taguchi T, et al. Efficacy of adjuvant immunochemotherapy with OK-432 for patients with curatively resected gastric cancer: a meta-analysis of centrally randomized controlled clinical trials. J Immunother. 2002;25(5):405–412. doi: 10.1097/00002371-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Okazaki T, Iwatani S, Yanai T, Kobayashi H, Kato Y, Marusasa T, et al. Treatment of lymphangioma in children: our experience of 128 cases. J Pediatr Surg. 2007;42(2):386–389. doi: 10.1016/j.jpedsurg.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Itoh T, Ueda Y, Okugawa K, Fujiwara H, Fuji N, Yamashita T, et al. Streptococcal preparation OK432 promotes functional maturation of human monocyte-derived dendritic cells. Cancer Immunol Immunother. 2003;52(4):207–214. doi: 10.1007/s00262-002-0337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakahara S, Tsunoda T, Baba T, Asabe S, Tahara H. Dendritic cells stimulated with a bacterial product, OK-432, efficiently induce cytotoxic T lymphocytes specific to tumor rejection peptide. Cancer Res. 2003;63(14):4112–4118. [PubMed] [Google Scholar]
- 29.Hovden AO, Karlsen M, Jonsson R, Aarstad HJ, Appel S. Maturation of monocyte derived dendritic cells with OK432 boosts IL-12p70 secretion and conveys strong T-cell responses. BMC Immunol. 2011;12:2. doi: 10.1186/1471-2172-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hovden AO, Karlsen M, Jonsson R, Appel S. The Bacterial Preparation OK432 Induces IL-12p70 Secretion in Human Dendritic Cells in a TLR3 Dependent Manner. PLoS One. 2012;7(2):e31217. doi: 10.1371/journal.pone.0031217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burdek M, Spranger S, Wilde S, Frankenberger B, Schendel DJ, Geiger C. Three-day dendritic cells for vaccine development: antigen uptake, processing and presentation. J Transl Med. 2010;8:90. doi: 10.1186/1479-5876-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427(6970):154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 33.MartIn-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198(4):615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muthuswamy R, Mueller-Berghaus J, Haberkorn U, Reinhart TA, Schadendorf D, Kalinski P. PGE(2) transiently enhances DC expression of CCR7 but inhibits the ability of DCs to produce CCL19 and attract naive T cells. Blood. 2010;116(9):1454–1459. doi: 10.1182/blood-2009-12-258038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, et al. alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64(17):5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 36.Lovgren T, Sarhan D, Truxova I, Choudhary B, Maas R, Melief J, et al. Enhanced stimulation of human tumor-specific T cells by dendritic cells matured in the presence of interferon-gamma and multiple toll-like receptor agonists. Cancer Immunol Immunother CII. 2017;66(10):1333–1344. doi: 10.1007/s00262-017-2029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zobywalski A, Javorovic M, Frankenberger B, Pohla H, Kremmer E, Bigalke I, et al. Generation of clinical grade dendritic cells with capacity to produce biologically active IL-12p70. J Transl Med. 2007;5:18. doi: 10.1186/1479-5876-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luckheeram RV, Zhou R, Verma AD, Xia B. CD4(+)T cells: differentiation and functions. Clin Dev Immunol. 2012;2012:925135. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beck B, Dorfel D, Lichtenegger FS, Geiger C, Lindner L, Merk M, et al. Effects of TLR agonists on maturation and function of 3-day dendritic cells from AML patients in complete remission. J Transl Med. 2011;9:151. doi: 10.1186/1479-5876-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jonuleit H, Giesecke-Tuettenberg A, Tuting T, Thurner-Schuler B, Stuge TB, Paragnik L, et al. A comparison of two types of dendritic cell as adjuvants for the induction of melanoma-specific T-cell responses in humans following intranodal injection. Int J Cancer. 2001;93(2):243–251. doi: 10.1002/ijc.1323. [DOI] [PubMed] [Google Scholar]
- 41.Carreno BM, Becker-Hapak M, Huang A, Chan M, Alyasiry A, Lie WR, et al. IL-12p70-producing patient DC vaccine elicits Tc1-polarized immunity. J Clin Invest. 2013;123(8):3383–3394. doi: 10.1172/JCI68395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 43.Henry E, Desmet CJ, Garze V, Fievez L, Bedoret D, Heirman C, et al. Dendritic cells genetically engineered to express IL-10 induce long-lasting antigen-specific tolerance in experimental asthma. J Immunol. 2008;181(10):7230–7242. doi: 10.4049/jimmunol.181.10.7230. [DOI] [PubMed] [Google Scholar]
- 44.Maghazachi AA, Al-Aoukaty A, Schall TJ. CC chemokines induce the generation of killer cells from CD56+ cells. Eur J Immunol. 1996;26(2):315–319. doi: 10.1002/eji.1830260207. [DOI] [PubMed] [Google Scholar]
- 45.Lebre MC, Burwell T, Vieira PL, Lora J, Coyle AJ, Kapsenberg ML, et al. Differential expression of inflammatory chemokines by Th1- and Th2-cell promoting dendritic cells: a role for different mature dendritic cell populations in attracting appropriate effector cells to peripheral sites of inflammation. Immunol Cell Biol. 2005;83(5):525–535. doi: 10.1111/j.1440-1711.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 46.Luft T, Jefford M, Luetjens P, Toy T, Hochrein H, Masterman KA, et al. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: prostaglandin E(2) regulates the migratory capacity of specific DC subsets. Blood. 2002;100(4):1362–1372. doi: 10.1182/blood-2001-12-0360. [DOI] [PubMed] [Google Scholar]
- 47.Yen JH, Khayrullina T, Ganea D. PGE2-induced metalloproteinase-9 is essential for dendritic cell migration. Blood. 2008;111(1):260–270. doi: 10.1182/blood-2007-05-090613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ten Brinke A, Karsten ML, Dieker MC, Zwaginga JJ, van Ham SM. The clinical grade maturation cocktail monophosphoryl lipid A plus IFNgamma generates monocyte-derived dendritic cells with the capacity to migrate and induce Th1 polarization. Vaccine. 2007;25(41):7145–7152. doi: 10.1016/j.vaccine.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 49.Hammond ME, Lapointe GR, Feucht PH, Hilt S, Gallegos CA, Gordon CA, et al. IL-8 induces neutrophil chemotaxis predominantly via type I IL-8 receptors. J Immunol. 1995;155(3):1428–1433. [PubMed] [Google Scholar]
- 50.Hill KS, Errington F, Steele LP, Merrick A, Morgan R, Selby PJ, et al. OK432-activated human dendritic cells kill tumor cells via CD40/CD40 ligand interactions. J Immunol. 2008;181(5):3108–3115. doi: 10.4049/jimmunol.181.5.3108. [DOI] [PubMed] [Google Scholar]
- 51.de Kleijn S, Langereis JD, Leentjens J, Kox M, Netea MG, Koenderman L, et al. IFN-gamma-stimulated neutrophils suppress lymphocyte proliferation through expression of PD-L1. PLoS One. 2013;8(8):e72249. doi: 10.1371/journal.pone.0072249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pillay J, Tak T, Kamp VM, Koenderman L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cell Mol Life Sci. 2013;70(20):3813–3827. doi: 10.1007/s00018-013-1286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.