Abstract
Background
Response to immune checkpoint inhibitors depends on tumor intrinsic properties and also on host factors in the tumour microenvironment including the presence of immune cells (IC). We hypothesized that nivolumab efficacy varies across different metastatic sites.
Methods
We retrospectively analyzed computed tomography scans of patients with metastatic non-small cell lung carcinoma (NSCLC) receiving nivolumab. RECIST 1.1 criteria were applied to assess the overall response rate (ORR) and organ-specific response rate (OSRR).
Results
We analyzed 52 patients including 44% females, 58% adenocarcinoma and 8% never smokers. Involved organs had target-lesions in the lung (42%), liver (25%), lymph nodes (56%) and soft tissue (13%) and non-target lesions in the bones (23%). ORR and disease control rate (DCR) were 20% and 45%, respectively. Median overall survival, progression-free survival and duration of response were 11.9, 2.3 and 10.3 months. OSRR and organ-specific DCR (OSDCR) were 28% and 90% in lymph nodes, 8% and 54 in the liver, and 9% and 55% in lung metastases. Nine out of 12 patients with bone metastases had progressive lesions. The cumulative incidence probability of organ-specific progression at 6 months was 14% in lymph nodes, 42% in the liver, 36% in lung metastases and 26% in the primary tumor, 29% in soft tissue and 33% in adrenal metastases.
Conclusion
In conclusion, the efficacy of immunotherapy is dependent on the metastatic location. Treatment appears more active in lymph nodes compared to other organ sites such as liver, adrenals and bone. Future strategies may include additional local treatment in case of oligoprogression in these organs in patients with otherwise sustained treatment benefit.
Keywords: NSCLC, Patterns of response, Organ-specific response, Checkpoint inhibitors
Introduction
Immunotherapy with immune checkpoint inhibitors has emerged as a standard treatment option in patients with advanced non-small cell lung cancer (NSCLC), both, in the first-line setting in patients whose tumors have a high programmed death ligand 1 (PD-L1) expression and in the second-line setting [1–5]. For patients progressing after first-line platinum-based chemotherapy, nivolumab, pembrolizumab and atezolizumab are currently approved, based on four randomized phase III trials demonstrating superior response rates and overall survival (OS). Nivolumab represents an active treatment strategy with the potential of long-term disease control irrespective of the PD-L1 expression status [6]. Unfortunately, reliable biomarkers are lacking and, consequently, nivolumab has not been considered to be cost-effective in several medical systems [7, 8]. Although, PD-L1 expression status identifies patients who are more likely to benefit, PD-L1 status is influenced by dynamic changes over time, intratumoral heterogeneity and the availability of various test methods using different thresholds for PD-L1 positivity [9, 10]. Apart from PD-L1 expression, various potentially predictive biomarkers have been proposed among which in particular high tumor mutational burden [11] and consecutive increase of neoantigens holds great promise [12]. However, apart from PD-L1 no other predictive markers for the benefit of checkpoint inhibitor therapy is currently available in daily clinical practice, and prospective validation in large clinical trials is ongoing.
Response to immune checkpoint inhibitors may also depend on the tumor microenvironment (TME), which is composed of different cell types including fibroblasts, endothelial cells and immune cells, possibly affecting the activity of nivolumab according to the location of the metastases which typically differ in immune cell content under physiological condition [13].
Different immune profiles of tumors may play a key role in the efficacy of treatment with checkpoint inhibitors. Immune-inflamed phenotypes, basically characterized by the presence of CD4 and CD8-expressing T cells, correlate with higher response rates to PD1/L1 therapy compared to the immune-desert phenotype with absence of T cells. Apart from CD4 and CD8 T cells, different proinflammatory cytokines, i.e., interleukin-2, in the tumor microenvironment potentially enhance T cell activation [14].
Antigen-presenting cells (APC) and T lymphocytes are the principal mediators of tumor cell-directed immune responses. Cytotoxic T cells attack tumor cells that express tumor-derived peptides bound to major histocompatibility complex (MHC) molecules. T cell priming and activation occurs in tumor-draining lymph nodes with subsequent infiltration of T cells into the tumor through the TME. Data in melanoma patients have demonstrated that the efficacy of checkpoint inhibitors depend on the presence of immune cells in tumor metastases [15]. Lymph nodes are characterized by an abundance of immune cells and a close interaction of specialized antigen-presenting cells with representatives of the adaptive immune system including B and T cells. Recent work suggests that tumour-infiltrating lymphocytes within lymph node metastasis are associated with a better response rate to anti-CTLA4 [16]. Furthermore, the liver is considered to be an inhibitory immune modulatory organ which can dampen immune responses and induce immune tolerance [17]. Melanoma and NSCLC patients with liver metastases have previously been reported to have significantly lower response rates and shortened PFS to anti-PD-1 inhibition compared to patients without liver metastases [18]. In addition, lack of response correlated with a low degree of CD8+ infiltration [18].
In conclusion, varying pre-treatment immune cell infiltration may lead to differential activity of nivolumab depending on the involved organ. We hypothesized that response to immune checkpoint inhibition may vary depending on the organ sites involved. We therefore analyzed organ-specific radiologic response rates (OSRR) to nivolumab in a cohort of NSCLC patients. Knowledge of differing activity of nivolumab in various organs is clinically relevant in guiding radiological surveillance under treatment and may identify patients with oligo–progressive disease amenable to additive local therapy.
Methods
This is a retrospective, single center study to investigate the patterns of response and recurrence to the PD-1 antibody nivolumab (Opdivo®, Bristol-Myers Squibb, New York, USA). Besides overall response (ORR), progression-free survival (PFS) and overall survival (OS), we analyzed organ-specific response (OSRR) and cumulative incidence of organ-specific progression at different organ sites to identify potential differences in activity of nivolumab across different organ sites.
Patient population
We analyzed patients with metastatic NSCLC who had received at least one infusion of nivolumab with the standard dose of 3 mg/kg every 2 weeks. All patients were treated at the Cantonal Hospital St. Gallen (Switzerland) between May 2015 and August 2016. As most patients were treated within an early access program (EAP), they must have fulfilled the following criteria: Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0 or 1, adequate organ function, no prior autoimmune disease and prior platinum-based chemotherapy. Patients were not allowed to receive steroids (above 10 mg of prednisone or equivalent dose) or antibiotics for at least 2 weeks before treatment start. Clinical data were extracted from the electronic patient record system. The study was approved by the local research ethics committee.
Radiological analyses
Serial computed tomography (CT) scans applying Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [19] to assess ORR and OSRR were analyzed. CT was scheduled every 2–3 months until progressive disease (PD) according to RECIST, death or patient refusal, whichever occurred first. All CT examinations were performed on a third-generation dual-source CT system (Siemens Healthineers, Forchheim, Germany). Tube voltage and tube current–time products were set to automatically adapt to patient habitus using the CareDose and CarekV algorithms. Images were reconstructed with a slice thickness of 2 mm and a slice increment of 1.5 mm using a soft tissue convolution filter. All measurements were performed by two experienced radiologists on the axial image data with an electronic caliper tool according to the RECIST 1.1, in up to five lesions in total and up to two lesions per organ for target lesions. For OSRR, a maximum of five target lesions per organ were considered. Non-target lesions (all other lesions including pathological lymph nodes) recorded at baseline were followed as present, absent or unequivocal progression. Lymph nodes were classified as one organ irrespective of lymph node site. The classification of lymph nodes as an organ implied that the longest diameter of the lesion was measured as for all other organ sites as defined by RECIST 1.1. The distinction between pulmonary metastases and primary tumor in situ was based on the determination of two trained radiologists assigned for this project.
Statistical analysis
Median follow-up time was calculated based on reverse Kaplan–Meier method. OS was calculated from the treatment start to death. Patients who were lost to follow-up or no death was experienced were censored at the last date known to be alive. PFS was calculated from the treatment start until tumor progression according to RECIST or death due to any reason, whichever occurred first. Patients who were still alive and having no progression were censored at the last tumor assessment. ORR was defined as the percentage of patients who had complete remission (CR) or partial remission (PR) as the best overall response; organ-specific response rate (OSRR) was defined as the percentage of patients who had CR or PR as the best response of the target lesions in a specific organ assessed according to RECIST 1.1 criteria applied for this particular organ only (with a maximum of five target lesions per organ considered for response assessment). Disease control rate (DCR) was defined as the percentage of patients having at least CR, PR or stable disease (SD) as the best overall response. Organ-specific disease control rate (OSDCR) was defined as the percentage of patients having at least CR, PR or SD as the best organ-specific response. Since CT scans were only scheduled until RECIST PD, death or patient refusal, the early occurrence of RECIST PD or death prevented us from observing organ-specific PD. Therefore, time to organ-specific progression was estimated based on a competing risk approach considering RECIST PD and death as competing risks. Analyses were performed using R 3.3.3.
Results
Baseline characteristics (Table 1)
Table 1.
Patient characteristics
| Age | |
| Median age at start of nivolumab (years) | 66.2 |
| ECOG | |
| 1, N (%) | 10 (19) |
| 2, N (%) | 26 (50) |
| 3, N (%) | 8 (15) |
| Missing, N (%) | 7 (13) |
| Gender | |
| Male, N (%) | 29 (56) |
| Female, N (%) | 23 (44) |
| Histology | |
| Adenocarcinoma, N (%) | 30 (58) |
| Squamous, N (%) | 18 (35) |
| PD-L1 expression | |
| Yes, N (%) | 13 (25) |
| No, N (%) | 11 (21) |
| Missing, N (%) | 28 (54) |
| Tobacco | |
| Current smoker, N (%) | 21 (40) |
| Former smoker, N (%) | 27 (52) |
| Never smoker, N (%) | 4 (8) |
| Median pack years | 40 |
| Prior lines of therapy | |
| 0, N (%) | 2 (4) |
| 1, N (%) | 29 (56) |
| 2, N (%) | 13 (25) |
| 3, N (%) | 6 (12) |
| > 3, N (%) | 2 (4) |
In total, 52 patients were included in our analysis with a median follow-up of 14 months. The majority of patients had one prior line of platinum-based chemotherapy (56%), had adenocarcinoma (58%) and were former (52%) or active smokers (40%) with only a minority of never smokers (8%). Detailed patient and tumor characteristics are summarized in Table 1. Four patients were identified with oncogenic alterations: two had an epidermal growth factor receptor (EGFR) mutation and two an anaplastic lymphoma kinase (ALK) translocation.
Before start of treatment with nivolumab, metastatic target lesions were found at the following metastatic sites: lymph nodes (56%, n = 29), lung (42%, n = 22), liver (25%, n = 13), soft tissue (13%, n = 7), adrenal glands (12%, n = 6), brain (2%, n = 1), pancreas (4%, n = 2) and kidney (4%, n = 2). The primary tumor was in situ in 62% (n = 32) of the patients. Fifteen patients (29%) had previously treated brain metastases considered as non-target lesions. Non-target lesions in the bone were found in 23% (n = 12).
Overall and organ-specific response
Overall response rate and DCR in the entire cohort were 20% and 45%, respectively. The median duration of response was 10.3 months.
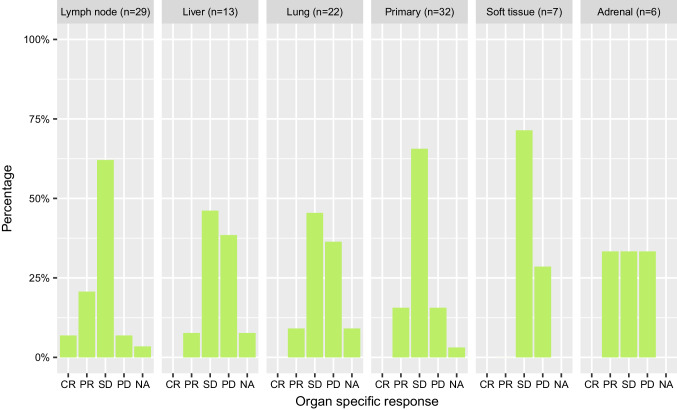
Figure 1 shows the OSRR and ORR stratified by different organs.
Fig. 1.
Overall and organ-specific response in the adrenal, liver, lung, lymph node and soft tissue metastases and primary tumor
LN (n = 29): OSRR was 28% (n = 8), including two patients with CR, and OSDCR was 90% (n = 26). Of 17 patients with lymph node metastases and PD as best overall response (OR), 15 had SD as the best organ-specific response at the lymphatic sites. Only two patients had overall PD and lymph node PD at the same time.
Liver (n = 13): In liver metastases, OSRR was 8% (n = 1) and OSDCR was 54% (n = 7). 38% of patients (n = 5) had primary refractory disease of the liver. Of seven patients who had PD as the best OR, 71% (n = 5) had also PD as organ-specific response (OSR) at the same time. All patients with primary refractory disease of the liver also had PD overall (n = 5).
Lung (n = 22): In lung metastases, OSRR was 9% (n = 2) with an OSDCR of 55% (n = 12). 36% of the patients (n = 8) were primary refractory to nivolumab treatment regarding lung metastases. Of 13 patients who had PD as the best overall response, 46% (n = 6) and 54% (n = 7) had PD and SD as the overall response, respectively. Six out of eight patients, who were primary refractory regarding lung metastases, also had PD as the best overall response.
Primary (n = 32): With regard to patients without prior resection of their primary tumor OSRR was 16%, although OSDCR was over 80% (n = 27). Sixteen percent (n = 5) of the patients were primary refractory to nivolumab regarding the primary tumor. Of 18 patients who had PD as the best overall response, 28% (n = 5), 67% (n = 12) and 6% (n = 1) of the patients had PD, SD and even PR as OSR in the primary tumor, respectively. All patients, who were primary refractory of the primary tumor, also had PD as the best OR (n = 5).
Soft tissue (n = 7): In soft tissue metastases, OSRR was 0%, OSDCR 71% (n = 5) and 29% (n = 2) were primary refractory to nivolumab treatment in soft tissue as well as overall. Only one patient having PD as best OR still had disease control in the soft tissue metastases.
Adrenal (n = 6): In the adrenal glands, OSRR was 33% (n = 2) and OSDCR 67% (n = 4). Two patients were primary refractory to nivolumab treatment in the adrenals as well as being PD overall.
Brain (n = 1): Since only one patient included in our analysis had target lesions in the brain at the start of nivolumab treatment, no detailed organ-specific response assessment was done.
With regard to bone metastasis, no formal response assessment could be done since only non-measurable non-target lesions were included; however, 9 out of 12 patients had progressive lesions at the time of overall tumor progression.
Survival end points
In total, 41 patients out of 52 had progressive disease with a median PFS of 2.3 months. Median OS was 11.9 months. In total, 29 patients died.
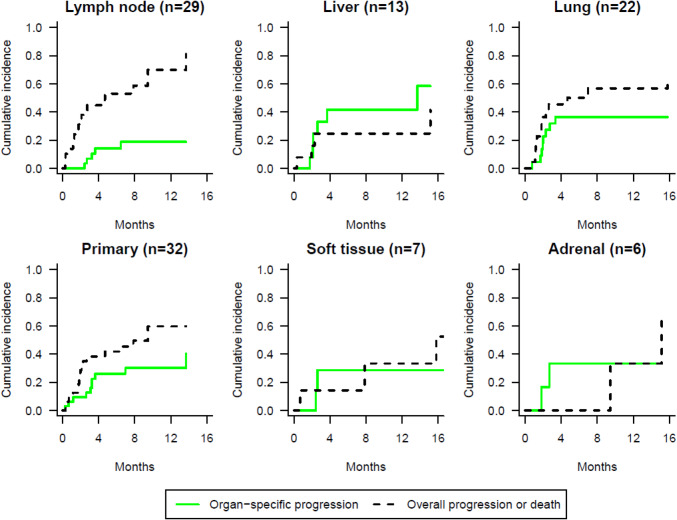
The cumulative incidence probability of organ-specific progression at 6 months was 14% in lymph nodes, 42% in liver, 36% in lung metastases and 26% in the primary tumor, 29% in soft tissue and 33% in adrenal metastases (Fig. 2).
Fig. 2.
Cumulative incidence probability of organ-specific progression using competing risk approach
Discussion
The patterns of response under nivolumab therapy are clinically relevant, as they help to guide methods used for radiological surveillance and may identify situations of oligoprogression. Our knowledge on tumor response patterns to second- or later line nivolumab in metastatic NSCLC is limited. In our cohort of NSCLC patients treated with nivolumab outside of a study protocol at a tertiary center, we observed an ORR of 20%, a median PFS of 2.3 months and a median OS of 11.9 months, which is in line with published data [2–5]. Of note, 61% had progressive disease as the best response. The aim of our study was to evaluate whether tumor response differed across various organs.
Response and disease control rates were higher and the cumulative incidence of probability of organ-specific progression prior to overall progression or death was lower in lymph nodes compared to other organ sites. The majority of patients with lymph node metastases (88%) had ongoing disease control despite overall progression according to RECIST, whereas concordance of overall and organ-specific response was high at most organ sites. Interestingly, in the three responders with PD at data cutoff, disease progression occurred in the primary tumor (N = 1) and bones (n = 2).
Interpretation of response rates in the brain, adrenals and soft tissue was limited due to the low numbers of organ involvement and limited numbers of target lesions at these sites.
Our findings are in line with another NSCLC cohort recently published by Nishino at al., who also reported high OSRR and OSDCR in lymph node metastases, also supporting the concept of better response in organs with postulated high pre-treatment immune cell infiltration [20].
Furthermore, our findings corroborate previous reports indicating worse activity in liver metastases [18] with the liver having inhibitory immunomodulation properties [17], although due to limited numbers of patients with liver metastases in our analysis this observation remains to be proven in a larger patient population. The lungs are chronically exposed to pathogens and pollution and contain a specialized system of alveolar macrophages and dendritic cells. Specialized antigen-presenting cells are able to trigger an adaptive T cell response not only against viral and bacterial infections, but also against cancer [21]. We therefore postulated that immunologically active microenvironment OSRR and OSDCR in lung metastases and the primary tumor would be high. Yet, the response was low in metastatic lung lesions and the primary tumor with an OSRR of only 9% and 16%, respectively; however, OSDCR was intermediate in lung metastases and very good in the primary tumor with an OSDCR of 80%. The reason for this different activity of nivolumab in lung metastasis and primary tumor in the lung remains unclear.
The adrenal gland is thought to have immune-modulating properties [22, 23] and in the cohort of Nishino et al [20] the adrenals showed high RR in the lesion-based assessment. In contrast to these findings, OSRR and OSDCR were low in the adrenals in our cohort, though the small number of patients with adrenal metastasis prohibited a conclusive statement. Further studies are needed to clarify the activity of nivolumab in adrenal metastases compared to other sites.
RECIST criteria [19] were developed to assess radiologic response to cytotoxic therapy. Currently, RECIST criteria remain the standard assessment tool for response evaluation in immunotherapy trials in patients with NSCLC. In melanoma, immune-related response criteria were introduced, which however have not been adopted in NSCLC studies. RECIST 1.1. does not take into account potentially different activity of treatment across different organ sites, which would be clinically important in situations of oligoprogression, e.g., in bone or adrenal metastases, as it may identify a group of patients who could benefit from ongoing nivolumab therapy with controlled disease, e.g., in lymph nodes. In these cases, ablative local therapy such as radiotherapy to the progressing organ site and continuation of nivolumab treatment may be applied. Such a strategy could potentially maximize treatment benefit from immunotherapy in a similar manner to that shown in patients with molecular driver alterations with EGFR as well as ALK-tyrosine kinase inhibitors [24]. In addition, retrospective data [25] as well as a recently presented small randomized prospective phase II trial [26] suggest enhanced antitumor immune responses with the use of radiotherapy due to increased tumor antigen release and improved antigen presentation and T-cell infiltration. Therefore, the administration of stereotactic radiotherapy to a single site of tumor progression would be of particular interest for further study.
Our analysis has several limitations
Clinical limitations: Other factors including PD-L1 expression, mutational burden and CD8 infiltration are associated with response to immunotherapy. However, assessment of PD-L1 expression is not mandated for treatment with nivolumab and therefore was not systematically assessed in our cohort and, therefore, missing in a proportion of patients, particularly in squamous cell carcinoma, where it has not been shown to be predictive for treatment outcomes in a randomized phase III study [3]. Assessment of CD8-infiltration and tumor mutational burden are currently not an approved test to select patients for nivolumab treatment in daily clinical practice and were not performed. Only radiographic assessment was evaluated for response and no information on potential serum predictive biomarkers for response as recently proposed by Huang et al. in melanoma patients was available [27].
Methodological limitations: This was a single-center retrospective analysis. Due to the relatively small sample size, data were analyzed descriptively and need to be confirmed in larger prospective trials. Special attention needs to be drawn to one of the end points, cumulative incidence of organ-specific progression. Due to the fact that CT was no longer analyzed in this retrospective trial, as soon as the patient had RECIST PD that may occur earlier than organ-specific progression, survival analysis with competing risk concept was chosen to calculate the cumulative incidence of organ-specific progression prior to RECIST PD or death, which is slightly different from the cumulative incidence of organ-specific progression.
Recently, studies using whole body PD-1 and PD-L1 positron emission tomography (PET) in NSCLC patients who received 18F-labeled BMS-986192 (18F-PD-L1) and 89zirconium-labeled nivolumab (89Zr-nivo), PET scans were able to identify patients with ≥ 50% PD-L1 expression on tumor cells. The mean standard uptake value (SUV) peak of both tracers was associated with high tumor infiltrating lymphocytes and stromal PD-1 expression [28]. Based on our findings, it would be of interest to explore whether SUV values using these tracers vary in metastases across different organ sites.
Conclusion
Our analysis supports the hypothesis that the efficacy of immunotherapy is dependent on the metastatic location. Treatment appears more active in lymph nodes compared to other organ sites such as the liver, adrenals and bone. Future strategies may include additional local treatment in case of oligoprogression in these organs in patients with otherwise sustained treatment benefit.
Acknowledgements
Institutional grant was received by BMS for data collection and statistical analysis.
Abbreviations
- APC
Antigen-presenting cells
- CR
Complete remission
- CT
Computed
- DCR
Disease control rate
- NSCLC
Non-small cell lung cancer
- ORR
Overall response rate
- OS
Overall survival
- OSDCR
Organ-specific disease control rate
- OSRR
Organ-specific response rate
- PD
Progressive disease
- PD-L1
Programmed death ligand 1
- PFS
Progression-free survival
- PR
Partial remission
- SD
Stable disease
- SUV
Standard uptake value
- TME
Tumor microenvironment
Author contributions
Conception and design: SS, SD, MF. Collection and assembly of data: SS, SD, MK. Data analysis and interpretation. Radiology: LD, SL. Statistics: DK, QL. Overall Interpretation: SS, SD, MF. Manuscript writing: all authors. Final approval of manuscript: all authors.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required. This article does not contain any studies with animals performed by any of the authors. The study was approved by the local Research Ethics Committee (Ethikkommission Ostschweiz-EKOS).
Footnotes
Sabine Schmid and Stefan Diem contributed equally as first authors.
References
- 1.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 2.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2015;87:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 5.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 6.Brahmer J et al (2017) Five-year follow-up from the CA209-003 study of nivolumab in previously treated advanced non-small cell lung cancer: clinical characteristics of long-term survivors (Abstr 003 AACR 2017)
- 7.Aguiar PN, Jr, Perry LA, Penny-Dimri J, et al. The effect of PD-L1 testing on the cost-effectiveness and economic impact of immune checkpoint inhibitors for the second-line treatment of NSCLC. Ann Oncol. 2017;28:2256–2263. doi: 10.1093/annonc/mdx305. [DOI] [PubMed] [Google Scholar]
- 8.Matter-Walstra K, Schwenkglenks M, Aebi S, et al. A cost-effectiveness analysis of nivolumab versus docetaxel for advanced nonsquamous NSCLC including PD-L1 testing. J Thorac Oncol. 2016;11:1846–1855. doi: 10.1016/j.jtho.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 9.Ribas A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov. 2015;5:915–919. doi: 10.1158/2159-8290.CD-15-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ilie M, Long-Mira E, Bence C, et al. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol. 2016;27:147–153. doi: 10.1093/annonc/mdv489. [DOI] [PubMed] [Google Scholar]
- 11.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14:847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 13.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 14.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 15.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diem S, Hasan Ali O, Ackermann CJ et al. Tumor infiltrating lymphocytes in lymph node metastases of stage III melanoma correspond to response and survival in nine patients treated with ipilimumab at the time of stage IV disease. Cancer Immunol Immunother 2017 [DOI] [PMC free article] [PubMed]
- 17.Li F, Tian Z. The liver works as a school to educate regulatory immune cells. Cell Mol Immunol. 2013;10:292–302. doi: 10.1038/cmi.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tumeh PC, Hellmann MD, Hamid O, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. 2017;5:417–424. doi: 10.1158/2326-6066.CIR-16-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Nishino M, Ramaiya NH, Chambers ES, et al. Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. J Immunother Cancer. 2016;4:84. doi: 10.1186/s40425-016-0193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(2015) The lungs at the frontlines of immunity. Nat Immunol 16(1):17. 10.1038/ni.3069 [DOI] [PubMed]
- 22.Bornstein SR, Rutkowski H. The adrenal hormone metabolism in the immune/inflammatory reaction. Endocr Res. 2002;28:719–728. doi: 10.1081/ERC-120016992. [DOI] [PubMed] [Google Scholar]
- 23.Kanczkowski W, Sue M, Zacharowski K, et al. The role of adrenal gland microenvironment in the HPA axis function and dysfunction during sepsis. Mol Cell Endocrinol. 2015;408:241–248. doi: 10.1016/j.mce.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7:1807–1814. doi: 10.1097/JTO.0b013e3182745948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18:895–903. doi: 10.1016/S1470-2045(17)30380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theelen W, Peulen H, Lalezari F, et al. Randomized phase II study of pembrolizumab after stereotactic body radiotherapy (SBRT) versus pembrolizumab alone in patients with advanced non-small cell lung cancer: the PEMBRO-RT study. ASCO 2018. J Clin Oncol. 2018;36(suppl):abstr 9023. doi: 10.1200/JCO.2018.36.15_suppl.9023. [DOI] [Google Scholar]
- 27.Huang AC, Postow MA, Orlowski RJ, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545:60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niemeijer A-L, Smit E, Van Dongen G, et al. Whole body and PD-L1 PET in patients with NSCLC. Annals of Oncology. 2017 doi: 10.1093/annonc/mdx380.008. [DOI] [Google Scholar]