Abstract
Background
Mammary and extra-mammary Paget disease is a rare form of intra-epithelial glandular neoplasm which is characteristically recurrent and necessitates multiple excisions that have an important impact on morbidity. Local immuno-modulating treatments have been applied with promising results, but the local immune markers of Paget disease have not been studied.
Aim of the study
To investigate the local immune micro-environment of Paget disease.
Materials and methods
Sixty-four specimens from 41 patients, including cases with multiple recurrences and underlying primary neoplasm, have been studied for their expression of CD3, PD-L1 and CTLA-4.
Results
Nineteen cases were mammary; 22 were extra-mammary and involved the vulva, the anus, the inguinal region and the lower extremity. PD-L1 was not expressed by any neoplastic lesion or the associated lymphocytes. CTLA-4 expression was found in nine cases. Higher stromal CD3 expression and moderate levels of intra-epithelial CD3 expression were present in most cases. Biopsies, subsequent excision specimens and recurrences showed the same immunohistochemical profile of CD3 and PD-L1, although there were different levels of CTLA-4 in a few cases. The underlying lesions in mammary Paget disease showed the same immunohistochemical profile as the intra-epithelial neoplastic cells. The expression of the markers did not correlate with age, sex, localization or recurrence.
Conclusion
Paget disease is characterized by an intense lymphocytic response, devoid of the immune-suppressive impact of the PD-L1 pathway, but with occasional CTLA-4 expression.
Keywords: Immunotherapy, Intra-epithelial, Prognosis, Vulva, Breast
Introduction
Mammary Paget disease (MPD) and extra-mammary Paget disease (EMPD) are rare neoplastic conditions, which are characterized by an intra-epithelial accumulation of neoplastic cells that show glandular differentiation [1]. MPD affects the nipple/areola complex and may spread to the surrounding skin, while EMPD occurs most commonly in the anogenital region, but can arise in any area of the skin or mucosa [1]. MPD accounts for 1–3% of primary breast tumors [2] and EMPD for 1% of primary vulval neoplasms [3]. EMPD arising in other sites is even more rare.
Unlike MPD, which arises from in situ or invasive carcinoma of the underlying breast tissue in over 90% of the cases [2], EMPD is associated with an underlying neoplasm in a much smaller proportion of cases. The incidence of such an association is 5–30% [4]. Thus, EMPD can be divided into primary and secondary variants, with the latter representing the intra-epithelial spread of an underlying carcinoma that is usually from the urogenital or gastrointestinal tract [5]. Primary EMPD is a form of intra-epithelial adenocarcinoma of uncertain histogenesis, for which cutaneous adnexa, clear cells of Toker, pluripotent stem cells and anogenital mammary-like glands have been proposed as possible sites of origin [4].
The distinction between primary and secondary forms is very important for the treatment of the patients. Patients with Paget disease are mostly treated with surgical excision and with treatment of the underlying primary neoplasm, if any. However, many patients will undergo multiple resections due to frequent local recurrences, which can have potential important implications for morbidity. There has been no reliable evidence to inform decisions about different interventions, especially for extra-mammary Paget disease [6]. Imiquimod, an immunomodulator, was evaluated as a potential local treatment in vulvar Paget disease and the results showed that it is a safe and feasible treatment with a significant response rate [7]. Imiquimod activates toll-like receptor 7 (TLR7), resulting in cytokine release and activated CD8+ T cells. Thus, it acts as an anti-tumor immune response-modifying agent [7]. Furthermore, it has been proposed that EMPD is characterized by an immune-suppressive micro-environment, which is possibly driven by the RANK/RANKL pathway and as such, targeted therapy with a RANKL inhibitor (denosumab) could be also promising [8, 9]. RANKL released by Paget cells stimulates M2 macrophages and epidermal Langerhans cells to produce chemokines, which will recruit T helper 2 cells and regulatory T cells [9].
Recently, immunotherapy has attracted significant interest as a potential treatment option for cancer. There have been very promising results from the use of molecules used to block the major immune-checkpoints, CTLA4 and PD-L1, which boost the anti-tumoral activity of T cells. CTLA4 is expressed by T cells inhibiting their activation, but it can also be found in tumor cells, which is usually associated with worse prognosis; PD-L1 is expressed by tumor cells as it offers protection by inhibiting the PD-1 expressing T cells [10].
There is a lack of studies that have investigated the possible role of these factors in the immune micro-environment of Paget disease. Thus, the aim of this study is to investigate the expression of the major factors in the immunotherapy field, PD-L1 and CTLA4, as the major immune-checkpoints and CD3 as a T-cell marker. This aims to better define the immune micro-environment of Paget disease and delineate a possible role of future immune-modulating treatments.
Materials and methods
Study group
This is a retrospective study of patients histologically diagnosed with a Paget disease at the University Hospital of Saint-Etienne (IRBN132018/CHUSTE). Clinical data were retrieved from electronic files. Age, sex, lesion localization, treatment and follow-up were recoded. All available slides from biopsies, subsequent excisions and recurrences were examined. The diagnosis was based on the typical morphology of an intra-epithelial accumulation of large epithelioid cells with abundant pale, clear or basophilic cytoplasm and large vesicular nuclei with prominent nucleoli (Fig. 1). The diagnosis was immunohistochemically confirmed in all cases by the expression of epithelial markers (CK7) and the absence of melanocytic markers (S100, HMB45 and Melan A).
Fig. 1.
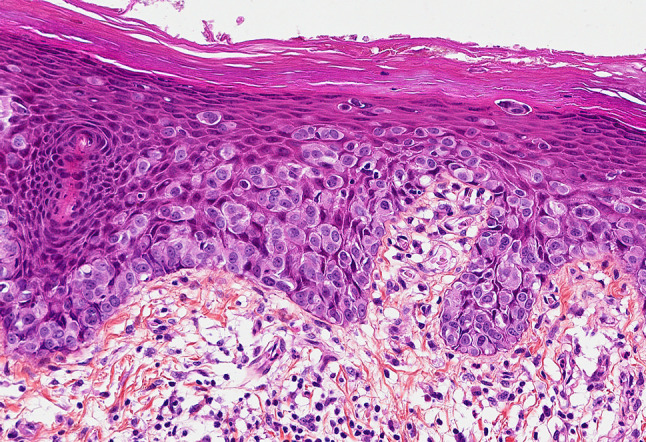
A vulvar Paget disease with large intra-epithelial neoplastic cells (HES ×200)
Immunohistochemical evaluation
Immunohistochemical study was performed in 64 specimens from 41 different patients. They included biopsies, subsequent excisions and excisions from recurrences to compare the immunohistochemical profile in subsequent lesions (initial vs recurrence) and in different materials (biopsies vs. excisions). Underlying tumors, invasive or in situ, were also immunohistochemically studied when the specimen was available (n = 15). When more than one tumor tissue block was available per excision, a representative section was chosen for immunohistochemical analysis based on the most abundant tumor cells and lymphocytic reaction section. Immunohistochemical analysis was performed using 4-µm-thick full sections in an automated staining system (OMNIS, Dako-Agilent). Positive immunoreactions were visualized using 3,3′-diaminobenzidine as the chromogenic substrate. The primary antibodies included CD3 (Dako F7,2,38, 1/100 lymph nodes were used as a positive control), PD-L1 (Dako Agilent, 22C3, 1/50 placenta was used as a positive control Fig. 2) and CTLA-4 (Origene, polyclonal, 1/50 squamous cell carcinoma [10] was used as a positive control).
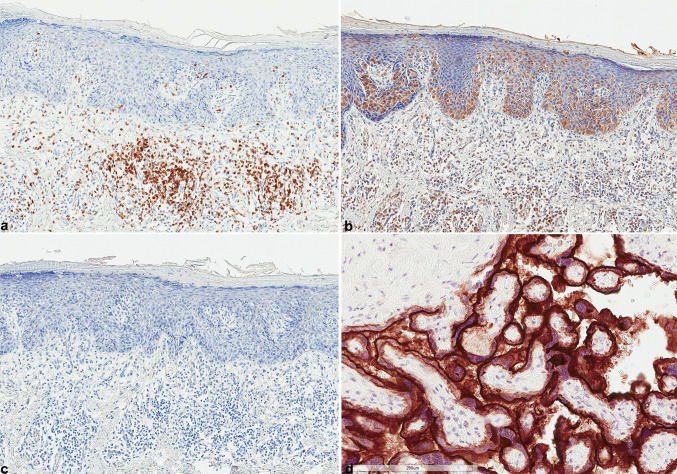
Fig. 2.
a Intense sub-epithelial CD3 expression (DAB ×100). b CTLA-4 expression by neoplastic cells and underlying lymphocytes (DAB ×100). c PD-L1 is not expressed by Paget disease (DAB ×100). d Placenta was used as positive control of PD-L1 immunohistochemistry showing constantly strong expression (DAB ×200). HES hematoxylin, eosin, saffran, DAB 3′,3-diaminobenzidine
A semi-quantitative system was applied to evaluate CD3 expression [10]. The two compartments, intra-epithelial and sub-epithelial, were separately scored as 0: no CD3 positive cells, 1: few CD3 positive cells, 2: moderate number and 3: abundant cells. Any positivity either of neoplastic cells or of lymphocytes was recorded for CTLA-4 and PD-L1. A cut-off value of 1% was used.
Statistical analysis
Data were analyzed using the StatView software (Abacus Concepts, Berckley CA, USA). We used the Fisher’s exact test to explore any relationship between the two groups for categorical data, while factorial analysis of variances (ANOVA) was used to consider the effect of at least one factor on a continuous parameter studied. For all analyses, the statistical significance was set at a p value of < 0.05.
Results
Patient’s characteristics
The patients’ characteristics are shown in Table 1. Most patients were female (n = 36, 87.8%). The age of the patients ranged from 35 to 92 years with a median of 70 years. Nineteen cases were mammary and most of them (n = 17, 89.5%) were associated with an underlying invasive (n = 5) or in situ ductal carcinoma (n = 12). Twenty-two cases were extra-mammary and involved the vulva (n = 13), the anus (n = 3), the inguinal region (n = 3) and the lower extremity (n = 1). None of the extra-mammary cases revealed a synchronous underlying neoplasm of the anogenital region. Four cases, which were all vulvar, harbored micro-invasive foci, while lymphovascular invasion was found in six cases.
Table 1.
Demographics
| n, % | |
|---|---|
| Age (years) | |
| Range | 35–92 |
| Mean, median | 69.3, 70 |
| Sex | |
| Female | 36, 87.8 |
| Male | 5, 12.2 |
| Localization | |
| Mammary | 19, 46.3 |
| Extra-mammary | 22, 53.7 |
| Extra-mammary localization | |
| Vulva | 13, 31.7 |
| Anus | 3, 7.3 |
| Inguinal region | 5, 12.2 |
| Lower extremity | 1, 2.4 |
| Treatment | |
| Surgical | 33, 80.5 |
| Local treatment | 8, 19.5 |
| Underlying lesion | |
| Invasive ductal carcinoma | 5, 12.2 |
| In situ ductal carcinoma | 12, 29.3 |
| None | 24, 58.5 |
| Follow-up (months)a | |
| Range | 4–148 |
| Mean | 48.9 |
| Median | 42 |
| Recurrencea | |
| Yes | 5, 17.9 |
| No | 23, 82.1 |
| PD-L1 | |
| Yes | 0 |
| No | 41 |
| CTLA-4 | |
| Yes | 9, 22 |
| No | 32, 78 |
| CD3 intra-epithelial | |
| 0–1 | 40, 97.6 |
| 2–3 | 1, 2.4 |
| CD3 sub-epithelial | |
| 0–1 | 14, 34.1 |
| 2–3 | 27, 65.9 |
a Follow-up was not available for 13 patients
Most patients were treated by surgical excision (n = 33). During the follow-up (range of 4–148 months, median 42 months) that was available for 28 patients, five of these cases, which were all extra-mammary and all treated surgically in the first place, had one (n = 2) or multiple (n = 3) recurrences that were also treated surgically. One patient with vulvar PD had multiple local recurrences and developed metastatic disease in the uterus and in an inguinal lymph node 8 years later. Another patient developed a regional lymph node metastasis 4 years after the initial diagnosis.
Immunohistochemical results
PD-L1 was not expressed by any neoplastic lesion or the associated lymphocytes. Control tissue was positive in all cases. CTLA-4 expression was found in nine cases. This expression involved the lymphocytes in three cases and the neoplastic cells in six cases. CTLA-4 was focal and found in only a few cells in all, but one case where a diffuse expression was seen. CD3 expression was intense (score of 2–3) in most cases (n = 27) in the sub-epithelial compartment (Fig. 2). CD3 expression by intra-epithelial lymphocytes was present in 28 cases (68.2%), with a score of 1 (few cells) for most of them.
In 15 cases, both biopsies and subsequent excision were available and all showed the same immunohistochemical profile of CD3 and PD-L1. In eight cases, the excision of recurrences was studied which also showed the same immunohistochemical profile as the initial lesion. For the CTLA-4 positive cases, three had more than one specimen to examine; two were positive in the excision specimen, but not the biopsy and one was positive in one of the recurrences. As for the underlying lesions of MPD, they all showed the same immunohistochemical profile as the intra-epithelial neoplastic cells.
As PD-L1 was consistently negative, no statistical analysis was performed for this marker. For CD3, the score from the first lesion at the excision specimen or from the biopsy when the excision was not available was used for further statistical analysis. CTLA-4 was considered positive for further analysis when at least one specimen harbored positive cells. CTLA-4 and CD3 expression did not correlate with any of the clinical factors studied, including age, sex, localization and recurrence. Furthermore, there was no relationship between the two markers (Table 2).
Table 2.
Correlation between immunohistochemical factors and clinical features
| Sex | p | Recurrence | p | Localization | p | Age | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Female n = 36 |
Male n = 5 |
Yes n = 5 |
No n = 23 |
Extra-mammary n = 22 |
Mammary n = 19 |
Mean (years) | |||||
| CTLA-4 | |||||||||||
| Yes (n = 9) | 9 | 0 | 0.5 | 4 | 5 | 0.9 | 5 | 4 | 0.9 | 66.6 | 0.5 |
| No (n = 32) | 27 | 5 | 1 | 18 | 17 | 15 | 70 | ||||
| CD3 intra-epithelial | |||||||||||
| 2–3 (n = 1) | 1 | 0 | 0.9 | 0 | 0 | 1 | 0 | 0.9 | 77 | 0.5 | |
| 0–1 (n = 40) | 35 | 5 | 5 | 23 | 21 | 19 | 69.1 | ||||
| CD3 sub-epithelial | |||||||||||
| 2–3 (n = 27) | 23 | 4 | 1 | 15 | 0.9 | 14 | 13 | 70 | 0.6 | ||
| 0–1 (n = 14) | 13 | 1 | 0.6 | 4 | 8 | 8 | 6 | 0.9 | 68 | ||
Discussion
In the current study, we show that Paget disease is almost always accompanied by an important reaction of sub-epithelial T lymphocytes and few intra-epithelial T lymphocytes. PD-L1 is not expressed in either the tumor cells or lymphocytes in this neoplastic disease, while only a few cases had CTLA-4 positive neoplastic cells or lymphocytes. These immunohistochemical findings are not associated with other clinicopathological features.
Paget disease is rarely life-threatening as in most cases it remains an intra-epithelial neoplasm although it carries significant morbidity. In a very rare setting, Paget disease can actually metastasize [11] and this was the case for two of the patients in this series, who developed metastatic Paget disease in lymph nodes and the uterus many years after the initial diagnosis and after multiple local recurrences.
Mammary Paget disease associated with in situ or invasive carcinoma is generally treated by excision of the nipple/areola complex, but surgery is ultimately based on the complete excision of the associated intramammary disease. As there is a high prevalence of multifocal disease in these patients, they will often undergo mastectomy rather than breast-conserving surgery [1]. The optimal management of Paget’s disease of the vulva remains unclear [12]. The treatment modalities most frequently include surgery such as radical vulvectomy, wide local excision or skinning vulvectomy. In a few cases, topical therapy, such as imiquimod or laser ablation, can be used [12]. The limitations for surgical excision include the often multifocal nature of the disease, the extension of the lesions over the clinically apparent borders resulting in positive margins and the complicated anatomy of the vulva [12]. In addition, EMPD is well-known for its high recurrence rate requiring multiple excisions that carries significant morbidity. There are no well-designed, randomized or not, studies that have compared the various treatment modalities [6]. Few retrospective studies exist, with the results showing that the most common treatment modality is surgery with the majority of women undergoing wide local excision, while the recurrence rate is not associated with the type of treatment or even the positive margin status [12, 13]. Furthermore, the spread of primary EMPD into underlying adnexal structures is a very common feature that occurs in the majority of the cases [5], thus complicating topical therapy or the development of novel local treatment modalities for EMPD.
A locally applied immunomodulator, imiquimod, was evaluated in a few patients with recurrent vulvar Paget disease, which showed promising results [7]. Furthermore, it has been proposed that this neoplasm harbors an immune-suppressive micro-environment, which is possibly driven by the RANK/RANKL pathway [9]. In this present study, we showed that the majority of Paget diseases are characterized by an intense T-lymphocytic response in the underlying stroma and a mild T-cell exocytosis. PD-L1 is not a feature of this neoplasm in either the tumor cells or the lymphocytes, while CTLA-4 is expressed by few lymphocytes or tumor cells in about one-fifth of the cases. CD3 expression was almost always found in these lesions, suggesting that lymphocytic response is actually a unifying feature of Paget disease. The absence of any PD-L1 expression characterizes a neoplasm that is well-controlled by the immune surveillance. This is consistent with the clinical course of this disease as it often recurs, but it seldom transforms into a frankly malignant disease in the form of metastasis. The elimination of cancer cells by the immune system occurs when neoplastic cells are highly immunogenic before any clinically apparent tumor arises, while the equilibrium between cancer cells and immune system results in no elimination of the non-immunogenic cells. These cancer cells finally escape from the immune surveillance to give rise to a truly malignant tumor. These are the three Es of the cancer immune-editing hypothesis [14]. It seems that Paget disease is in equilibrium with the immune system. The tumor cells are not eliminated despite the intense lymphocytic reaction, which is because of the immune-suppressive environment that they achieve through activating the RANK/RANKL pathway [9] and the presence of FoxP3+ cells [15]. More advanced tools used by tumor cells to achieve escape, such as the PD-L1 pathway, are not employed by Paget tumor cells.
To the best of our knowledge, the role of the factors studied here has not been evaluated before in Paget disease and in other forms of vulvar intra-epithelial neoplasia. Vulvar invasive squamous cell carcinomas that are negative for p16 are more often infiltrated by immune cells than p16 positive tumors [16]. A study examining the presence of FoxP3 positive regulatory cells, which are immune-suppressive cells, showed that they are abundant in the epithelial–stromal junction in vulvar Paget disease [15]. These T regulatory cells are thought to be recruited by RANK+ macrophages, which respond to RANKL secreted by Paget cells [9]. Regarding other forms of in situ disease, the immune micro-environment of ductal carcinomas in situ (DCIS) of the breast has been recently studied which showed that fewer CD8 positive cells are found in invasive than in situ carcinomas. Furthermore, they found that the amplification of CD274, encoding PD-L1, was only detected in triple negative invasive carcinomas [17]. The median score of the lymphocytes in the stroma around the DCIS is only 5 and 89% of the in situ neoplasms are PD-L1 negative, whereas these factors are more elevated in DCIS with higher grade features [18]. Similarly, a study of 27 cases of DCIS showed that none of them expressed PD-L1 in tumor cells [19]. Thus, it is consistent with our findings that this protein is not found in MPD, which most often arises in the context of an underlying DCIS. Interestingly, according to our results, MPD seems to be characterized by a more intense lymphocytic reaction than the reported DCIS. Further studies will be needed to explain the difference between the intra-epidermal and the intra-ductal components.
We have shown (work under submission) in the lesions of the uterine cervix that PD-L1 expression is found in almost two-thirds of invasive squamous cell carcinomas, while it is rare in squamous intra-epithelial lesions and in adenocarcinomas whether they are in situ or invasive. Previous studies have shown using flow cytometry that cervical cells from patients harboring a cervical intra-epithelial neoplasia (CIN) contain more PD-1+ T cells and more PD-L1+ dendritic cells compared to those from patients without CIN, which suggests a possible role of this pathway in immune tolerance against HPV infection and progression to dysplasia [20]. We also found that CTLA-4 is more often expressed in squamous cell carcinomas of the uterine cervix than in adenocarcinomas, while it is also frequently present in squamous intra-epithelial lesions of this organ. These findings are consistent with the only occasional CTLA-4 expression in Paget disease, which is a glandular-type neoplasia.
Immunotherapy has been shown to be more effective in patients with highly mutated tumors [21], such as squamous cell carcinomas of the lung, in patients with solid tumors that show microsatellite instability for which an immunotherapy has been recently approved by the FDA independent of other tumor characteristics [22]; in virus-induced tumors [23]; and in PD-L1 expressing tumors [24]. The absence of these features in Paget disease could explain the absence of PD-L1 involvement as it is a pathway that is not yet “needed” by the tumor to overcome the immune system with which it is in equilibrium. On the other hand, given the intense lymphocytic reaction constantly found in Paget disease, other types of immunomodulators, such as imiquimod or denosumab, could be beneficial.
Our study has certain limitations. First, it is a retrospective study that examines a limited number of cases. Furthermore, the studied immunohistochemical markers do not cover all micro-environment factors. However, the current study does provide preliminary data showing that this rare neoplasm is characterized by an intense sub-epithelial lymphocytic response, which is not affected by the immune-suppressive PD-L1 pathway, but which shows occasional CTLA-4 expression. As such, the immune modulators could be of use in the treatment of this disease.
Acknowledgements
The authors would like to thank Mr Philippe Cosmo from the Tumorothèque/Centre de Ressources Biologiques de CHU Saint-Etienne (BRIF no. BB-0033-00041) for his assistance.
Abbreviations
- CIN
Cervical intra-epithelial neoplasia
- DCIS
Ductal carcinoma in situ
- EMPD
Extra-mammary Paget disease
- FDA
Food and Drug Administration
- MPD
Mammary Paget disease
- PD
Paget disease
- RANK/RANKL
Receptor activator of nuclear factor kappa B ligand
Author contributions
GK conceived the study. GK and MP designed the study. GK, CC, SH and CH reviewed the clinical files. GK and SH executed the laboratory techniques. All authors were involved in data analysis and interpretation. GK wrote the manuscript. CC, SH, CH and MP critically revised the manuscript. All authors approved the final form.
Funding
No relevant funding.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Local Ethics Committee of the University Hospital of Saint-Etienne approved the study (IRBN132018/CHUSTE).
Informed consent
The acquisition of written informed consent was waived by the institutional review board given the retrospective nature of the study and the anonymization of all data.
References
- 1.Lloyd J, Flanagan AM. Mammary and extramammary Paget’s disease. J Clin Pathol. 2000;53(10):742–749. doi: 10.1136/jcp.53.10.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duan X, Sneige N, Gullett AE, Prieto VG, Resetkova E, Andino LM, et al. Invasive paget disease of the breast: clinicopathologic study of an underrecognized entity in the breast. Am J Surg Pathol. 2012;36(9):1353–1358. doi: 10.1097/PAS.0b013e318259ef7f. [DOI] [PubMed] [Google Scholar]
- 3.Lam C, Funaro D. Extramammary Paget’s disease: summary of current knowledge. Dermatol Clin. 2010;28(4):807–826. doi: 10.1016/j.det.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Kazakov DV, Spagnolo DV, Kacerovska D, Michal M. Lesions of anogenital mammary-like glands. Adv Anat Pathol. 2011;18(1):1–28. doi: 10.1097/PAP.0b013e318202eba5. [DOI] [PubMed] [Google Scholar]
- 5.Konstantinova AM, Shelekhova KV, Stewart CJ, Spagnolo DV, Kutzner H, Kacerovska D, et al. Depth and patterns of adnexal involvement in primary extramammary (anogenital) Paget disease: a study of 178 lesions from 146 patients. Am J Dermatopathol. 2016;38(11):802–808. doi: 10.1097/DAD.0000000000000552. [DOI] [PubMed] [Google Scholar]
- 6.Edey KA, Allan E, Murdoch JB, Cooper S, Bryant A (2013) Interventions for the treatment of Paget’s disease of the vulva. Cochrane Database Syst Rev (Wiley, Chichester) [DOI] [PubMed]
- 7.Cowan RA, Black DR, Hoang LN, Park KJ, Soslow RA, Backes FJ, et al. A pilot study of topical imiquimod therapy for the treatment of recurrent extramammary Paget’s disease. Gynecol Oncol. 2016;142(1):139–143. doi: 10.1016/j.ygyno.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fletcher J, Haniffa M. Mechanisms of immune evasion in extramammary Paget disease. Br J Dermatol. 2017;176(2):293–294. doi: 10.1111/bjd.15253. [DOI] [PubMed] [Google Scholar]
- 9.Fujimura T, Kambayashi Y, Furudate S, Kakizaki A, Hidaka T, Aiba S. Possible mechanisms of the crosstalk between Langerhans cells and regulatory T cells in extramammary Paget disease by receptor activator of nuclear factor kappa B (RANK) ligand/RANK pathways. Br J Dermatol. 2017;176(2):387–394. doi: 10.1111/bjd.14864. [DOI] [PubMed] [Google Scholar]
- 10.Karpathiou G, Casteillo F, Giroult JB, Forest F, Fournel P, Monaya A, et al. Prognostic impact of immune microenvironment in laryngeal and pharyngeal squamous cell carcinoma: immune cell subtypes, immuno-suppressive pathways and clinicopathologic characteristics. Oncotarget. 2017;8(12):19310–19322. doi: 10.18632/oncotarget.14242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padrnos L, Karlin N, Halfdanarson TR. Mayo Clinic Cancer Center experience of metastatic extramammary Paget disease 1998–2012. Rare Tumors. 2016;8(4):6804. doi: 10.4081/rt.2016.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onaiwu CO, Salcedo MP, Pessini SA, Munsell MF, Euscher EE, Reed KE, et al. Paget’s disease of the vulva: a review of 89 cases. Gynecol Oncol Rep. 2017;19:46–49. doi: 10.1016/j.gore.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones ISC, Crandon A, Sanday K. Paget’s disease of the vulva: diagnosis and follow-up key to management; a retrospective study of 50 cases from Queensland. Gynecol Oncol. 2011;122(1):42–44. doi: 10.1016/j.ygyno.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 14.Budhu S, Wolchok J, Merghoub T. The importance of animal models in tumor immunity and immunotherapy. Curr Opin Genet Dev. 2014;24:46–51. doi: 10.1016/j.gde.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Press JZ, Allison KH, Garcia R, Everett EN, Pizer E, Swensen RE, et al. FOXP3+ regulatory T-cells are abundant in vulvar Paget’s disease and are associated with recurrence. Gynecol Oncol. 2011;120(2):296–299. doi: 10.1016/j.ygyno.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Sznurkowski JJ, Żawrocki A, Biernat W. Local immune response depends on p16INK4a status of primary tumor in vulvar squamous cell carcinoma. Oncotarget. 2017;8(28):46204–46210. doi: 10.18632/oncotarget.17581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gil Del Alcazar CR, Huh SJ, Ekram MB, Trinh A, Liu LL, Beca F, et al. Immune escape in breast cancer during in situ to invasive carcinoma transition. Cancer Discov. 2017;7(10):1099–1115. doi: 10.1158/2159-8290.CD-17-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendry S, Pang JMB, Byrne DJ, Lakhani SR, Cummings MC, Campbell IG, et al. Relationship of the breast ductal carcinoma in situ immune microenvironment with clinicopathological and genetic features. Clin Cancer Res. 2017;23(17):5210–5217. doi: 10.1158/1078-0432.CCR-17-0743. [DOI] [PubMed] [Google Scholar]
- 19.Thompson E, Taube JM, Elwood H, Sharma R, Meeker A, Warzecha HN, et al. The immune microenvironment of breast ductal carcinoma in situ. Mod Pathol. 2016;29(3):249–258. doi: 10.1038/modpathol.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang W, Song Y, Lu Y-L, Sun J-Z, Wang H-W. Increased expression of programmed death (PD)-1 and its ligand PD-L1 correlates with impaired cell-mediated immunity in high-risk human papillomavirus-related cervical intraepithelial neoplasia. Immunology. 2013;139(4):513–522. doi: 10.1111/imm.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm560167.htm
- 23.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med. 2016;374(26):2542–2552. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]