Abstract
l-arginine depletion by regulatory cells and cancer cells expressing arginase-1 (Arg-1) is a vital contributor to the immunosuppressive tumor microenvironment in patients with cancer. We have recently described the existence of pro-inflammatory effector T cells that recognize Arg-1. Hence, Arg-1-specific self-reactive T cells are a naturally occurring part of the memory T-cell repertoire of the human immune system. Here, we further characterize a highly immunogenic epitope from Arg-1. We describe frequent T-cell-based immune responses against this epitope in patients with cancer, as well as in healthy donors. Furthermore, we show that Arg-1-specific T cells expand in response to the TH2 cytokine interleukin (IL)-4 without any specific stimulation. Arg-1-specific memory TH1 cells that respond to increased IL-4 concentration may, therefore, drive the immune response back into the TH1 pathway. Arg-1-specific T cells thus appear to have an important function in immune regulation. Because Arg-1 plays an important role in the immunosuppressive microenvironment in most cancers, an immune modulatory vaccination approach can readily be employed to tilt the balance away from immune suppression in these settings.
Keywords: Arginase, Anti-regulatory T cells, Immune-modulating vaccines, IO112, PIVAC 18
Introduction
l-arginine-depleting cells are targets for naturally occurring anti-regulatory T cells
Activated T cells require large amounts of l-arginine [1], creating an opportunity for immune suppression through l-arginine-depleting enzymes, such as arginase-1 (Arg-1). Arg-1 is an enzyme that catalyzes the first step in arginine metabolism, and its expression by various immune-suppressive cells (M2 macrophages, myeloid-derived suppressor cells, and different dendritic cell [DC] subtypes) is involved in the immune inhibitory pathways that suppress T-cell activation. Arg-1-associated l-arginine deprivation downregulates expression of the TCR ζ chain [2] and decreases T-cell cytokine production and proliferation [3]. Cancer development and progression are associated with multiple mechanisms of immune inhibition at the tumor site. Increased Arg-1 activity is seen in many cancers, such as breast [4], head and neck [5], renal cell carcinoma [6], and non-small cell lung cancer [7]. In general, arginase-expressing myeloid cells play a significant role in preventing effector lymphocyte proliferation and thereby promoting the immune-suppressive tumor microenvironment [8].
Pro-inflammatory T cells that specifically target immune-suppressive cells and counteract a range of regulatory immune-feedback signals have been described previously [9, 10]. These T cells (termed anti-regulatory T cells (anti-Tregs) due to their role in targeting regulatory immune mechanisms) recognize human leukocyte antigen (HLA)-restricted epitopes, generated from degraded intracellular self-antigens derived from immune inhibitory proteins, such as Arg-1 [11]. We have previously described the existence of Arg-1-specific T cells and demonstrated that arginase-specific T cells recognize and react against DCs and B cells expressing Arg-1 [12, 13]. We identified a 50-amino-acid hot-spot region in the protein sequence of Arg-1 that contains multiple epitopes spontaneously recognized by CD4+ and CD8+ T cells in healthy donors and cancer patients, as well as by tumor-infiltrating lymphocytes. Recently, we have also shown that these pre-existing T-cell responses against Arg-1 are part of the T-cell memory repertoire [14]. A phase I vaccination trial with arginase peptides was recently initiated at Copenhagen University Hospital, Herlev, Denmark (NCT03689192).
Materials and methods
Patient material
Peripheral blood mononuclear cells (PBMCs) (obtained at Copenhagen University Hospital, Herlev, Denmark or Blood Bank, Copenhagen University Hospital, Copenhagen, Denmark) were isolated using density gradient separation over Lymphoprep™ (STEMCELL Technologies) and cryopreserved at − 140 °C in fetal bovine serum supplemented with 10% dimethyl sulfoxide. PBMCs from cancer patients were isolated a minimum of 4 weeks after the termination of any anti-cancer therapy.
Peptides
Arginase peptides were synthesized by Schafer-N ApS (Denmark) and dissolved in dimethyl sulfoxide to yield a stock concentration of 10 mM. Peptide sequences were as follows: ArgLong, ISAKDIVYIGLRDVDPGEHYILKTLGIKYFSMTEVDRLGIGK; ArgLong2, ISAKDIVYIGLRDVDPGEHYILKTLGIKYFSMTEVDRL; and ArgLong3, ISAKDIVYIGLRDVDPGEHYILKTLGIKYFSM; 20-mer peptides: Arg171–190, AKDIVYIGLRDVDPGEHYIL; Arg181–200, DVDPGEHYILKTLGIKYFSM; and Arg191–210: KTLGIKYFSMTEVDRLGIGK.
ELISPOT assay
In vitro ELISPOT were performed as described previously [12].
For IL-4-induced Arg-1 responses, PBMCs were stimulated with IL-4 (100 or 50 U/ml) (PeproTech) and/or IL-2 (120 or 60 U/ml) (Proleukin, Novartis) for 1 week before being set up in the ELISPOT assay as described previously [12].
Statistical analysis
Statistical analysis of ELISPOT responses was performed as described by Moodie et al. [15]. Statistical analysis was performed with RStudio (RStudio Team 2016; RStudio: Integrated Development for R. RStudio, Inc., Boston, MA; http://www.rstudio.com/) and GraphPad for Windows Version 5.01.
Results
Peptide length determines the efficiency of Arg-1-specific T-cell stimulation
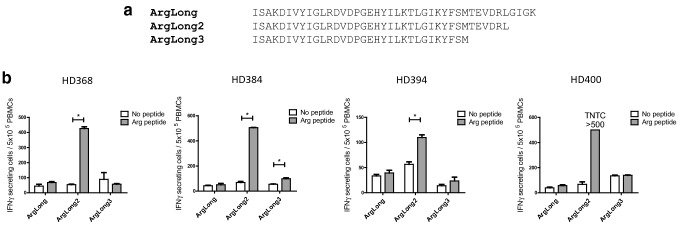
The most efficient activation of naturally occurring Arg-1-specific T cells across diverse HLA types in a population of patients is likely to require a peptide that contains multiple epitopes and can also be efficiently processed. We tested this using three different peptides that cover the majority of the previously identified Arg-1 hot-spot region (positions 161–210) [12]: 42-mer ArgLong (positions 169–210), 38-mer ArgLong2 (positions 169–206), and 32-mer ArgLong3 (positions 169–200).
The ability of each of these peptides to stimulate naturally occurring Arg-1-specific T-cell responses was tested by screening PBMCs from six healthy donors for responses in IFNγ ELISPOT. Despite sequence similarities, the ArgLong2 peptide appeared to be superior at stimulating T-cell responses, as shown in Fig. 1: high responses against ArgLong2 peptide were seen in four of six donors, while low or no responses were present against ArgLong and ArgLong3 peptides. This shows that a small difference in the length of Arg-1-derived peptides has a notable effect on processing and presentation of Arg-1-derived epitopes.
Fig. 1.
Responses against long Arg-1-derived peptides. a Aligned peptide sequences of ArgLong, ArgLong2 and ArgLong3. b IFNγ ELISPOT responses against ArgLong, ArgLong2, and ArgLong3 peptides in PBMCs from four healthy donors (HDs). Bars represent the mean number of spots per well + standard error of the mean. Experiments performed with 5 × 105 PBMCs/well in triplicate. TNTC, too numerous to count. *p ≤ 0.05 according to the distribution free resampling rule
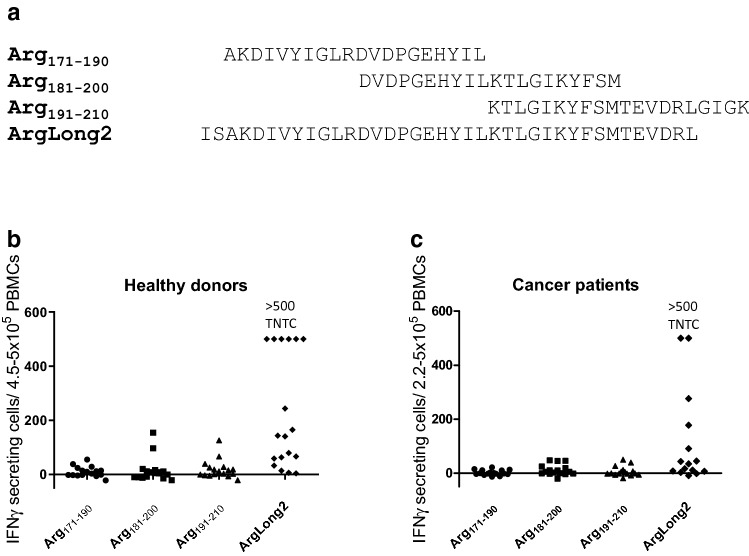
To determine whether the ArgLong2 peptide is comparable to previously described individual 20-mer peptides covering the same sequence, PBMCs from 19 healthy donors and 16 cancer patients (8 melanoma, 6 multiple myeloma, 1 breast cancer, and 1 renal cell carcinoma) were screened for responses against three 20-mer peptides and the 38-mer ArgLong2. In cancer patients and in healthy donors, ArgLong2 stimulation showed the most responses compared to all three 20-mer peptides (Fig. 2): strong responses against ArgLong2 were seen in 14 of 19 healthy donors (Fig. 2b) and 8 of 16 cancer patients (Fig. 2c). The strong immune response detected against ArgLong2 highlights the potential of this Arg-1 peptide for anti-cancer immunotherapy. Since Arg-1 expression is seen in non-terminally differentiated myeloid cells, it may be possible to achieve functional re-programming of these cells under a pro-inflammatory stimulus of ArgLong2-activated CD4+ T cells. On the other hand, suppressive Arg-1+ myeloid cells may also be directly depleted by ArgLong2-activated CD8+ T cells. This dual approach is contrary to other current clinical strategies targeting tumor-associated macrophages [16] and can be realized using the 38-amino-acid ArgLong2 peptide described in this paper. Our data have demonstrated that Arglong2 can be used to stimulate both Arg-1-specific CD4+ and CD8+ T-cell responses [14] making it an excellent vaccination candidate for Arg-1-targeted anti-cancer immunotherapy.
Fig. 2.
a Aligned peptide sequences of Arg171–190, Arg181–200, Arg191–210, and ArgLong2. b IFNγ ELISPOT responses against Arg171–190, Arg181–200, Arg191–210, and ArgLong2 in 19 healthy donors (left) and 16 cancer patients (right). Each dot represents an average number of peptide-specific spots for a single patient/donor. Responses were calculated by subtracting the average count of IFNγ-producing cells in control wells from the average count in peptide-stimulated wells. Experiments were performed with 4.5–5 × 105 PBMCs/well for healthy donors and 2.2–5 × 105 PBMCs/well for cancer patients, in triplicate or in duplicate. Responses against ArgLong2 peptide were too numerous to count (TNTC) in six healthy donors and two cancer patients and set as > 500 spots
Arg-1-specific T cells expand in an IL-4-abundant microenvironment
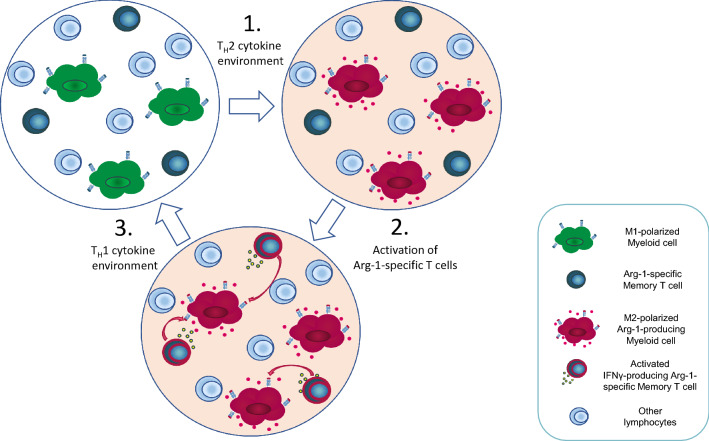
The presence of Arg-1-specific CD4+ and CD8+ memory T cells in both cancer patients and healthy donors suggests that these cells may play a role in regulating immune inhibition in the conditions that are known to induce Arg-1 expression by immune cells. Upregulated Arg-1 expression has been described previously in myeloid cells in response to stimulations with TH2 cytokines, such as IL-4 [17]. We tested whether Arg-1-specific T cells in PBMCs from a patient with melanoma would be activated in such conditions without added external peptide stimulation. Indeed, PBMCs stimulated with IL-4, IL-2, or a combination of both showed strong significant responses against Arg-1, with the highest IFNγ responses seen in IL-4-stimulated groups compared to no stimulation or stimulation with IL-2 alone (Fig. 3a). A similar increase in the pre-existing Arg-1-specific T-cell response was seen in IL-4-stimulated PBMCs from two cancer patients (breast cancer and malignant melanoma) and six healthy donors after 7 days in culture: seven out of eight cultures showed increased responses against ArgLong2 in IL-4-stimulated cultures compared to controls (p = 0.039, two tailed t test) (Fig. 3b). These data suggest a possible common mechanism for the in vivo activation of Arg-1-specific T cells. Based on these findings, we propose a model mechanism of interplay between Arg-1-specific memory T cells and Arg-1+ myeloid cells in vivo, as depicted in Fig. 4. IL-4, the prototypical inducer of the macrophage M2 phenotype, induces Arg-1 upregulation in the myeloid cells, resulting in an increase in the presentation of Arg-1-derived peptides on the surface of these cells in the context of HLA molecules [Fig. 4(1)]. Increased presentation of such peptides is recognized by Arg-1-specific memory T cells, which secrete pro-inflammatory cytokines, such as IFNγ [Fig. 4(2)], and thus drive the immune response back towards the TH1 pathway, re-polarizing the myeloid cells into the M1 phenotype [Fig. 4(3)]. The pro-inflammatory activity of Arg-1-specific T cells in turn is likely to be naturally suppressed by M2 phenotype cells expressing high levels of Arg-1. Such reciprocal suppressive effect of Arg-1-specific T cells and M2 macrophages is a likely explanation for the apparent lack of autoimmunity in healthy donors despite the strong ex vivo T-cell responses against Arg-1. Therefore, it is likely that the primary function of these self-reactive T cells is directed towards very immunosuppressed microenvironments.
Fig. 3.
IL-4 stimulation activates ArgLong2 peptide-specific T cells. a IFNγ ELISPOT for ArgLong2 peptide responses in melanoma patient PBMCs stimulated with IL-4 and/or IL-2 for 1 week. Non-stimulated PBMCs used as control. Superimposed bars show the mean counts of IFNγ-producing cells in control (white bars) and peptide-stimulated (grey bars) wells + standard error of the mean. Experiment performed in triplicate with 3 × 105 cells/well. b ArgLong2 peptide-specific responses in PBMCs from six healthy donors (HDs) and two cancer patients (BC, breast cancer; MM, malignant melanoma) after stimulation with IL-4 (100 U/ml) for 1 week. Cells without cytokine stimulation were used as control. Responses calculated as the difference in mean counts of IFNγ-secreting cells between peptide-stimulated and control wells in ELISPOT. Experiment performed in triplicate or in duplicate with 3 × 105 cells/well
Fig. 4.
Arg-1-specific T cells help redirect the immune microenvironment towards TH1. TH2 cytokines cause upregulated Arg-1 expression in M2-polarized myeloid cells, such as macrophages. Arg-1-expressing cells present Arg-1-derived peptide epitopes in the context of HLA class I and II molecules (1). Arg-1-specific memory T cells recognize presented Arg-1-derived peptides on the surface of myeloid cells, and in response produce pro-inflammatory cytokines (e.g., IFNγ) (2). Pro-inflammatory cytokines produced by Arg-1-specific memory T cells create a TH1 cytokine microenvironment and drive the polarization of myeloid cells to M1 phenotype (3)
Discussion
General targeting of inhibitory immune cells by anti-Tregs
Contrary to common belief, self-antigen reactive T cells in the blood are present at frequencies similar to non-self-antigen-specific T cells, thus suggesting that self-reactive T cells indeed avoid clonal deletion in the thymus [18]. However, despite the presence of self-reactive T cells in the periphery, it is likely that activation of these cells is tightly regulated and requires a more robust activation signal compared to non-self-specific T cells. Self-reactive T cells specific against immune-regulatory protein can be effective at selectively targeting immune inhibitory cells as shown by specific targeting of regulatory T cells through activation of Foxp3-reactive T cells using DC vaccines in mice [19, 20]. Anti-Tregs that recognize other immune-regulatory proteins, such as programmed death-ligand 1 (PD-L1) [21–24] and indoleamine-pyrrole 2,3-dioxygenase (IDO) [25, 26], have also been described as targets for T cells in humans. Interestingly, the pro-inflammatory cytokines IL-2 and IFN-γ, which induce expression of IDO and PD-L1, expand populations of IDO- or PD-L1-specific T cells among human PBMCs without external peptide stimulation [25] (Ahmad et al., in preparation). Immune-suppressive feedback signals such as upregulated expression of PD-L1 and IDO are evoked by professional antigen-presenting cells during early stages of an immune reaction. Elevated expression of these proteins by the potent professional antigen-presenting cells, therefore, leads to activation of IDO-specific and PD-L1-specific anti-Tregs under inflammation. Thus, in immune regulation, the primary role of IDO- and/or PD-L1-specific anti-Tregs may, therefore, be as specific first-responder helper cells at the site of inflammation. However, whereas IDO- and PD-L1-specific anti-Tregs expand in response to a TH1 inflammatory stimulus, Arg-1-specific T cells are activated in response to a TH2 cytokine, such as IL-4 (Fig. 3). It is important to emphasize that IFN-γ does not induce Arg-1; thus, the normal presence of these cells in the memory pool of healthy donors suggests different roles for Arg-1-specific and IDO-/PD-L1-specific T cells in immune homeostasis. Taken together, this suggests that different classes of anti-Tregs exist, each potentially having a different function in the regulation of immune responses. The differing modulation of IDO/PDL1- and Arg-1-driven immune suppression could be utilized in a vaccination setting, where the combination of epitopes from different anti-Treg target antigens could give an additive effect. In this scenario, an arginase vaccination could induce TH1 inflammation at tumor sites, where TAMs otherwise prevent lymphocyte infiltration. In turn, this effect would induce IDO and/or PD-L1 activation, which could enable further targeting by anti-Tregs that recognize epitopes derived from these targets.
Unlike current cancer vaccine strategies that tend to focus on cancer-specific CD8 cytotoxic T cells, therapeutic immunomodulatory vaccine based on anti-Tregs targets would involve both CD8+ and CD4+ T-cell responses. Thus, as previously described, anti-Tregs could directly eliminate regulatory immune cells [23, 25, 27, 28] as well as indirectly boost pre-existing anti-tumor immune responses through the secretion of effector cytokines [25]. Subsequently, involvement of CD4+ anti-Tregs may be as important in a therapeutic setting as CD8+ T-cell responses, since helper T cells are one of the most capable cytokine-producing cells. In the context of our current findings targeting Arg-1-producing cells by activation of Arg-1-specific anti-Tregs combines both TAM re-programming (through pro-inflammatory cytokines secreted by CD4+ T cells) and TAM depletion (through direct killing by cytotoxic T cells) (Fig. 4). Both may be vital to achieve rebalancing of the tumor microenvironment and can be used to enhance the effect of other therapeutic agents, such as checkpoint blockers and thus potentially increase the number of patients who respond to therapy.
Developing new immune-therapeutic approaches in cancer treatment is likely to be most efficient and versatile when common suppressive mechanisms are targeted. As shown in the present review, Arg-1-specific anti-Tregs exist as a natural part of the immune system and can be readily employed to tilt the balance away from immune suppression in cancer.
Abbreviations
- Arg1
Arginase 1
- BC
Breast cancer
- HD
Healthy donor
- MM
Malignant melanoma
- TAM
Tumor-associated macrophages
- TNTC
Too numerous to count
Author contributions
MHA designed and supervised the study. EM designed the experiments and analyzed the data. EM, SMA, SKB, MAJ, and SEWB performed experiments. IMS provided the relevant clinical material. All authors contributed to drafting the manuscript.
Funding
This work has been supported by grants from the Danish Cancer Society (Grant number R146-A9440-16-S2), Herlev Hospital (CCIT-Dk funding) and Innovation Fund Denmark (Grant number 8054-00058B).
Compliance with the ethical standards
Conflict of interest
Mads Hald Andersen has filed several patent applications based on the use of arginase for vaccinations. The rights of the patent applications have been transferred to Copenhagen University Hospital, Herlev, in accordance with the Danish Law of Public Inventions at Public Research Institutions. The capital region has licensed these patents to the company IO Biotech ApS, whose purpose is to develop immune-modulating vaccines for cancer treatments. Mads Hald Andersen is a shareholder and board member of IO Biotech ApS. Evelina Martinenaite is employed by IO Biotech ApS. Other authors declare no conflict of interest.
Ethical approval and ethical standards
The protocol was approved by the Scientific Ethics Committee for the Capital Region of Denmark (H-A-2009-013) and conducted in accordance with the provisions of the Declaration of Helsinki.
Informed consent
Written informed consent for the use of the PBMCs for research purposes was obtained from the patients and healthy donors prior to inclusion in the study.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Eighteenth International Conference on Progress in Vaccination against Cancer (PIVAC 18), held in Oslo, Norway, 3rd–5th October, 2018. It is part of a Cancer Immunology, Immunotherapy series of PIVAC 18 papers.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Geiger R, et al. l-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. 2016;167(3):829–842. doi: 10.1016/j.cell.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zea AH, et al. L-Arginine modulates CD3ζ expression and T cell function in activated human T lymphocytes. Cell Immunol. 2004;232(1–2):21–31. doi: 10.1016/j.cellimm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109(4):1568–1574. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Boniface J, Mao Y, Schmidt-mende J, Kiessling R, Poschke I. Expression patterns of the immunomodulatory enzyme arginase 1 in blood, lymph nodes and tumor tissue of early-stage breast cancer patients. Oncoimmunology. 2012;1(8):1305–1312. doi: 10.4161/onci.21678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang S, et al. Clinical relevance and suppressive capacity of human MDSC subsets. Clin Cancer Res. 2018;24(19):4834–4844. doi: 10.1158/1078-0432.CCR-17-3726. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez PC, et al. Arginase I—producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69(4):1553–1561. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rotondo R, et al. IL-8 induces exocytosis of arginase 1 by neutrophil polymorphonuclears in nonsmall cell lung cancer. Int J Cancer. 2009;125:887–893. doi: 10.1002/ijc.24448. [DOI] [PubMed] [Google Scholar]
- 8.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen MH. Anti-regulatory T cells. Semin Immunopathol. 2016 doi: 10.1007/s00281-016-0593-x. [DOI] [PubMed] [Google Scholar]
- 10.Andersen MH. Immune regulation by self-recognition: novel possibilities for anticancer immunotherapy. J Natl Cancer Inst. 2015;107(9):1–8. doi: 10.1093/jnci/djv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen MH. The balance players of the adaptive immune system. Cancer Res. 2018;15:1379–1383. doi: 10.1158/0008-5472.CAN-17-3607. [DOI] [PubMed] [Google Scholar]
- 12.Martinenaite E, et al. Frequent adaptive immune responses against arginase-1. Oncoimmunology. 2018;7(3):1–9. doi: 10.1080/2162402X.2017.1404215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jørgensen MA, et al. Spontaneous T-cell responses against Arginase-1 in the chronic myeloproliferative neoplasms relative to disease stage and type of driver mutation. Oncoimmunology. 2018 doi: 10.1080/2162402X.2018.1468957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinenaite E, Ahmad SM, Svane IM, Andersen MH. Peripheral memory T cells specific for Arginase-1. Cell Mol Immunol. 2019 doi: 10.1038/s41423-019-0231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moodie Z, Price L, Janetzki S, Cedrik B. Response determination criteria for ELISPOT: toward a standard that can be applied across laboratories. Methods Mol Biol. 2012;792:185–196. doi: 10.1007/978-1-62703-239-1_1. [DOI] [PubMed] [Google Scholar]
- 16.Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17(12):887–904. doi: 10.1038/nrd.2018.169. [DOI] [PubMed] [Google Scholar]
- 17.Munder M, Eichmann K, Morán JM, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999;163(7):3771–3777. [PubMed] [Google Scholar]
- 18.Yu W, Jiang N, Quake SR, Davis MM. Clonal deletion prunes but does not eliminate self-specific alphabeta CD8(+) T lymphocytes. Immunity. 2015;42:929–941. doi: 10.1016/j.immuni.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Namdar A, et al. Prophylactic DNA vaccine targeting Foxp3 + regulatory T cells depletes myeloid-derived suppressor cells and improves anti-melanoma immune responses in a murine model. Cancer Immunol Immunother. 2018;67(3):367–379. doi: 10.1007/s00262-017-2088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair S, Boczkowski D, Fassnacht M, Pisetsky D, Gilboa E. Vaccination against the forkhead family transcription factor Foxp3 enhances tumor immunity. Cancer Res. 2007;67(1):371–380. doi: 10.1158/0008-5472.CAN-06-2903. [DOI] [PubMed] [Google Scholar]
- 21.Munir S, et al. HLA-restricted CTL that are specific for the immune checkpoint ligand PD-L1 occur with high frequency in cancer patients. Cancer Res. 2013;73(6):1764–1776. doi: 10.1158/0008-5472.CAN-12-3507. [DOI] [PubMed] [Google Scholar]
- 22.Munir S, Andersen GH, Svane IM, Andersen MH. The immune checkpoint regulator PD-L1 is a specific target for naturally occurring CD4(+) T cells. Oncoimmunology. 2013;2(4):e23991. doi: 10.4161/onci.23991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munir S, Andersen GH, Woetmann A, Ødum N, Becker JC, Andersen MH. Cutaneous T cell lymphoma cells are targets for immune checkpoint ligand PD-L1-specific, cytotoxic T cells. Leukemia. 2013;27(11):2251–2253. doi: 10.1038/leu.2013.118. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad SM, Larsen SK, Svane IM, Andersen MH. Harnessing PD-L1-specific cytotoxic T cells for anti-leukemia immunotherapy to defeat mechanisms of immune escape mediated by the PD-1 pathway. Leukemia. 2014;28(1):236–238. doi: 10.1038/leu.2013.261. [DOI] [PubMed] [Google Scholar]
- 25.Soerensen RB, Hadrup SR, Svane IM, Hjortso MC, Straten PT, Andersen MH. Indoleamine 2,3-dioxygenase specific, cytotoxic T cells as immune regulators. Blood. 2011;117(7):2200–2210. doi: 10.1182/blood-2010-06-288498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersen MH. CD4 responses against IDO. Oncoimmunology. 2012;1(7):1211–1212. doi: 10.4161/onci.20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen SK, et al. Functional characterization of Foxp3-specific spontaneous immune responses. Leukemia. 2013;27(12):2332–2340. doi: 10.1038/leu.2013.196. [DOI] [PubMed] [Google Scholar]
- 28.Martinenaite E, et al. CCL22-specific T Cells: modulating the immunosuppressive tumor microenvironment. Oncoimmunology. 2016;5(11):e1238541. doi: 10.1080/2162402X.2016.1238541. [DOI] [PMC free article] [PubMed] [Google Scholar]