Abstract
Background
Chemokine (C-X-C motif) ligand 13 (CXCL13/BLC/BCA-1) is a cytokine from C-X-C chemokine family, which is selectively chemotactic for B cells. Previous research has demonstrated that high CXCL13 expression is correlated to poor prognosis in various cancers. However, the association between CXCL13 expression and gastric cancer is still unclear.
Methods
Intratumoral CXCL13 expression was evaluated by immunohistochemistry using a semi-quantitative method (modified H-score) in a testing set of 214 and a validation set of 227 randomly selected gastric cancer patients resected in 2008 in one institution. The median value was used as the cut-off point. We performed correlative analysis of CXCL-13 expression with clinicopathological variables, Kaplan–Meier analysis for association with overall survival (OS), and multivariate modeling.
Results
High CXCL13 expression was associated with larger tumor diameter and shorter OS. By multivariate analysis, CXCL13 expression was associated with OS independently from clinicopathological factors. Within the T2–4 stage patients group, low CXCL13 expression was associated with longer survival, especially in the subgroup of patients (57.6%) who received adjuvant chemotherapy.
Conclusions
Intratumoral CXCL13 expression appears as an independent prognostic marker for patients after gastric cancer resection. In addition, CXCL13 expression may serve as a predictive biomarker of response to postoperative adjuvant chemotherapy in these patients.
Keywords: Gastric cancer, CXCL13, Prognosis, Biomarker, Postoperative adjuvant chemotherapy
Introduction
Gastric cancer is the second most common cause of death within cancer-related diseases and the fourth most common malignancy worldwide [1]. Moreover, gastric cancer accounts for roughly 1,000,000 new cases and 628,000 deaths per year [2]. Over 70% of those cases occur in developing countries, with the highest incidence rates in Eastern Asia, particularly in Korea, Japan and China. Because of the mild and atypical symptoms in the early stages of gastric cancer, more than 80% of the patients are diagnosed in advanced stages, causing poor outcomes in most cases [3]. Since gastric cancer is a heterogeneous disease with different molecular phenotypes, it is difficult to predict the outcome. Therefore, new and valuable prognostic biomarkers are in urgent need to improve the current gastric cancer prognosis model.
In the process of tumor development, gastric cancer is closely related with cytokines and signaling pathways [4, 5], which have become a focus in the study of the pathogenesis of gastric cancer in recent years. As a family of small signaling cytokines, chemokines can interact with its receptor and regulate the infiltration and angiogenesis of a variety of malignant cells, thereby activating the host immune response and stimulating tumor cell proliferation [6, 7].
A number of recent studies have demonstrated that chemokine (C-X-C motif) ligand 13 (CXCL13), a B-cell attracting chemokine that is expressed by stromal cells [8], serves as an important factor in the process of tumor proliferation and migration. It has been found that the co-expression of CXCL13 and its receptor chemokine (C-X-C motif) receptor 5 (CXCR5) are remarkably related to the lymph node metastasis of breast cancer [9]. Moreover, CXCL13 has been defined as a predictor for poor prognosis in advanced colorectal cancer [10], prostate cancer [11], hepatocellular carcinoma [12], oral squamous cell carcinoma [13], non-small cell lung carcinoma [1, 14], lymphoma [15] as well as in neuroblastic tumor [16]. Nevertheless, the clinical importance of CXCL13 expression in gastric cancer is seldom reported and needs further examination [17–19].
In the present study, we carried out immunostaining analysis to investigate the potential prognostic role of CXCL13 expression in gastric cancer and explored the relationship between CXCL13 expression and clinicopathological characteristics. We also examined the possible correlation between CXCL13 expression and clinical outcomes, particularly with patients receiving postoperative adjuvant chemotherapy.
Materials and methods
Patients and database
The clinicopathological data of 441 gastric cancer patients from Zhongshan Hospital, Fudan University (Shanghai, China) in 2008 were retrospectively analyzed in the present study. All patients were divided randomly to the testing set or the validation set, comprising 214 and 227 patients, respectively. Patients with a history of preoperative chemotherapy or adjuvant radiation therapy were excluded. All patients were followed up until April, 2014. The Clinical Research Ethics Committee of Zhongshan Hospital sanctioned the use of human specimens, and all patients involved in the study signed informed consent. After sufficient preoperative evaluation, 210 patients in testing set and 222 patients in validation set received standard gastrectomy with D2 lymphadenectomy, while the other patients with distant metastasis received palliative resection.
The following clinicopathological variables were collected for each patient: age, gender, tumor size, tumor differentiation, Lauren’s classification, tumor lymph node metastasis stage (TNM) and receiving postoperative adjuvant chemotherapy. The reassessment of each patient’s tumor stage was in accordance with the 7th edition of the UICC (Union for International Cancer Control) and AJCC (American Joint Committee on Cancer) TNM Staging System. Overall survival (OS) was defined as the period of time between the operation date and either the most recent follow-up date or the date of disease-specific death. Patients who died of other causes were excluded. The median follow-up for the testing set was 39 months, and for the validation set was 42 months.
Immunohistochemistry and evaluation
The surgical specimens were formalin-fixed and paraffin-embedded. In the immunostaining of the tissue microarray (TMA), the primary antibody was anti-CXCL13 antibody (1:100 dilution, ab112521, Abcam, Cambridge, MA, USA). The stained sections were observed at 200× magnification by Olympus CDD camera and Nikon eclipse Ti-s microscope, and each image was taken under identical settings. Two independent pathologists, who were blinded to the patients’ outcomes, scored the staining intensity and extent using a semi-quantitative H-score: 3 × percentage of strongly staining cells + 2 × percentage of moderately staining cells + percentage of weakly staining cells, giving a range of 0–300. Median value derived from the testing set and applied to the validation set determined the cut-off point for tumoral CXCL13 high/low-level expression. The cut-off point was set at 40.
Statistical analysis
The statistical analyses were carried out by MedCalc 15.2.2 software (MedCalc, Mariakerke, Belgium). The Fisher’s exact method test, Pearson χ 2 test, and the Cochran–Mantel–Haenszel χ 2 test were applied to compare categorical variables. To assess the relationship between tumoral CXCL13 expression and clinical pathological features, continuous variables were analyzed by the Student’s t test. In both the univariate and multivariate models, Cox proportional hazard models were applied to analyze the continuous variables, and only those with statistically significant were included in the multivariate analyses. Next, OS curves were made using Kaplan–Meier analyses and the log rank test. All statistical tests were conducted at a two-sided significance level of 0.05.
Results
Patient characteristics
Patient characteristics are summarized in Table 1. In this study, there were 441 patients [316 male (71.7%), 125 female (28.3%)] with age ranging from 27 to 90. The average age of patients in the testing set was 59.0 ± 11.6 years old, and the average age of the validation set was 61.1 ± 11.4 years old. Two independent sets were with an overall similar tumour burden. Within the testing and validation set, 57.5% (testing set, n = 123) and 57.7% (validation set, n = 131) patients received postoperative adjuvant chemotherapy, respectively.
Table 1.
Correlation between CXCL13 expression and clinical pathological features in testing set (n = 214) and validation set (n = 227) of patients with gastric cancer
| Characteristics | Testing set | Validation set | ||||||
|---|---|---|---|---|---|---|---|---|
| CXCL13 expression | CXCL13 expression | |||||||
| All (n = 214) | Low (n = 100) | High (n = 114) | P* | All (n = 227) | Low (n = 102) | High (n = 125) | P* | |
| Age (years) | 0.735 | 0.110 | ||||||
| Mean ± SD | 59.0 ± 11.6 | 59.1 ± 11.4 | 58.8 ± 11.8 | 61.1 ± 11.4 | 60.8 ± 12.4 | 61.4 ± 10.6 | ||
| Gender | 0.222 | 0.684 | ||||||
| Female | 63 | 34 | 29 | 62 | 26 | 36 | ||
| Male | 151 | 66 | 85 | 165 | 76 | 89 | ||
| Tumor size (cm) | 0.031 | 0.008 | ||||||
| Mean ± SD | 3.78 ± 2.09 | 3.33 ± 1.81 | 4.16 ± 2.23 | 3.77 ± 2.18 | 3.42 ± 1.85 | 4.05 ± 2.38 | ||
| Differentiation | 0.607 | 0.911 | ||||||
| Well | 10 | 6 | 4 | 12 | 5 | 7 | ||
| Moderately | 60 | 26 | 34 | 66 | 31 | 35 | ||
| Poorly | 144 | 68 | 76 | 149 | 66 | 83 | ||
| Lauren’s classification | 0.251 | 0.007 | ||||||
| Intestinal | 138 | 69 | 69 | 144 | 75 | 69 | ||
| Diffuse | 76 | 31 | 45 | 83 | 27 | 56 | ||
| Gastrectomy | 0.093 | 0.118 | ||||||
| Proximal | 28 | 15 | 13 | 37 | 15 | 22 | ||
| Distal | 155 | 76 | 79 | 147 | 73 | 74 | ||
| Total | 31 | 9 | 22 | 43 | 14 | 29 | ||
| Depth of tumor invasion | 0.264 | 0.206 | ||||||
| T1 | 44 | 26 | 18 | 39 | 18 | 21 | ||
| T2 | 23 | 11 | 12 | 40 | 21 | 19 | ||
| T3 | 45 | 21 | 24 | 37 | 11 | 26 | ||
| T4 | 102 | 42 | 60 | 111 | 52 | 59 | ||
| Lymph node metastasis | 0.553 | 0.846 | ||||||
| N0 | 82 | 41 | 41 | 88 | 40 | 48 | ||
| N1 | 24 | 13 | 11 | 26 | 10 | 16 | ||
| N2 | 44 | 17 | 27 | 40 | 17 | 23 | ||
| N3 | 64 | 29 | 35 | 73 | 35 | 38 | ||
| Distant metastasis | 0.709 | 0.818 | ||||||
| M0 | 210 | 99 | 111 | 222 | 100 | 122 | ||
| M1 | 4 | 1 | 3 | 5 | 2 | 3 | ||
| pTNM stage | 0.278 | 0.959 | ||||||
| I | 54 | 31 | 23 | 59 | 27 | 32 | ||
| II | 48 | 21 | 27 | 46 | 22 | 24 | ||
| III | 108 | 47 | 61 | 117 | 51 | 66 | ||
| IV | 4 | 1 | 3 | 5 | 2 | 3 | ||
| Receiving postoperative adjuvant chemotherapy | 0.402 | 0.922 | ||||||
| No | 91 | 39 | 52 | 96 | 44 | 52 | ||
| Yes | 123 | 61 | 62 | 131 | 58 | 73 | ||
CXCL13 C-X-C motif ligand 13
* Wilcoxon rank-sum test; Chi-square test for all the other analyses
Correlation between CXCL13 expression and clinicopathologic features
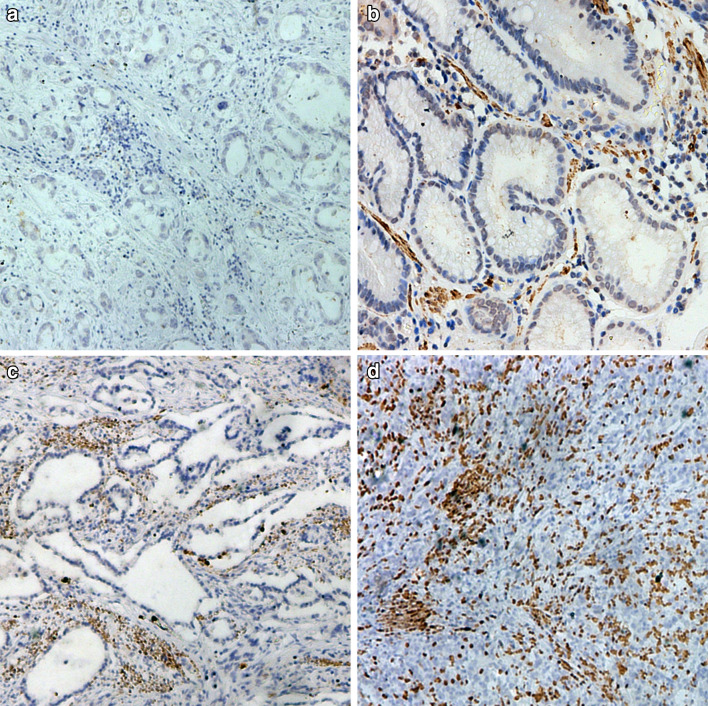
As shown in Fig. 1, CXCL13 was predominantly expressed in the tumor stroma. The staining intensity varied greatly in different specimens. High expression of CXCL13 was 53.3% (114 out of 214) and 55.1% (125 out of 227) in the training and validation sets, respectively. High CXCL13 expression was associated with large tumor size (P = 0.031 and 0.008, respectively) in the two independent sets. Additionally, solely in the validation set, CXCL13 expression was correlated with Lauren’s classification (P = 0.007). No other baseline characteristics were observed to be statistically significantly associated with CXCL13 expression level (Table 1).
Fig. 1.
CXCL13 expression in gastric cancer tissues (×200 optical magnification). Representative immunohistochemical images of low CXCL13 expression (a, b) and high CXCL13 expression (c, d). H-score was calculated by the formula: 3 × percentage of strongly staining cells + 2 × percentage of moderately staining cells + percentage of weakly staining cells, giving a range of 0–300. a H-score = 3 × 0% + 2 × 0% + 1 × 0% = 0; b H-score = 3 × 10% + 2 × 0% + 1 × 10% = 40; c H-score = 3 × 20% + 2 × 10% + 1 × 0% = 80; d H-score = 3 × 40% + 2 × 0% + 1 × 0% = 120
The relationship between CXCL13 expression and clinical outcomes of gastric cancer patients
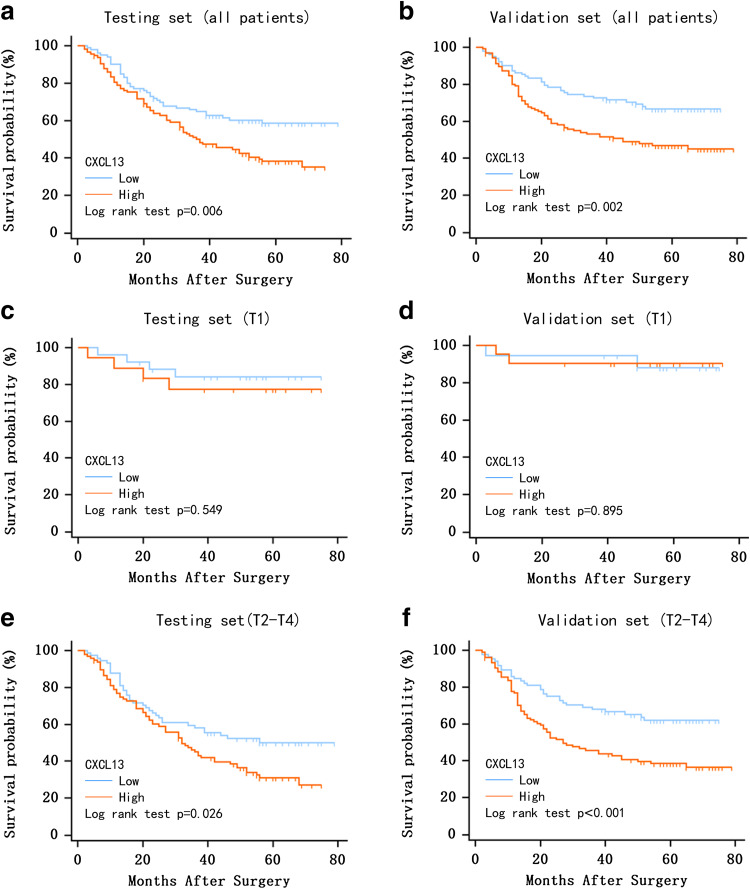
Kaplan–Meier survival analyses were conducted to evaluate the clinical outcomes according to CXCL13 expression. Patients with high CXCL13 expression were prone to worse OS in both the testing set (P = 0.006, Fig. 2a) and the validation set (P = 0.002, Fig. 2b), when compared to patients with low CXCL13 expression. Furthermore, the stratified analysis demonstrated that in patients with T2–T4 stage tumor, those with low intratumoral CXCL13 expression survived longer compared to those with high CXCL13 expression, in both the testing (P = 0.026, Fig. 2e) and validation sets (P < 0.001, Fig. 2f). For patients with a T1 stage tumor, the difference was not significant (testing set: P = 0.549, Fig. 2c; validation set: P = 0.895, Fig. 2d).
Fig. 2.
Kaplan–Meier analyses of overall survival in gastric cancer based on CXCL13 expression. Kaplan–Meier analysis for overall survival of patients with gastric cancer in testing set (n = 214) and validation set (n = 227) according to CXCL13 expression in all patients (a, b), patients with T1 stage tumor (c, d) and patients with T2–4 stage tumor (e, f). P-value was calculated by log rank test, P < 0.05 was regarded as statistically significant
CXCL13 expression as independent indicator of gastric cancer prognosis
In univariate Cox regression analysis of OS, CXCL13 expression was demonstrated to be a prognostic factor (P < 0.001). To further examine whether CXCL13 expression is an independent predictor for gastric cancer prognosis, we conducted a multivariate analysis with the parameters depth of tumor invasion, lymph node metastasis, and distant metastasis (Table 2). For both the testing and validation set, the multivariate analysis revealed that CXCL13 expression could be an independent predictor of prognosis in gastric cancer cases. (P = 0.048 and P < 0.001, respectively).
Table 2.
Cox multivariate analysis identified the independent prognostic factors for overall survival in testing and validation sets of patients with gastric cancer
| Variables | Testing set | Validation set | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Depth of invasion | 0.037 | < 0.001 | ||
| T1 | 1 (reference) | 1 (reference) | ||
| T2 + T3 + T4 | 2.292 (1.057–4.970) | 1.154 (1.067–1.247) | ||
| Lymph node metastasis | < 0.001 | < 0.001 | ||
| N0 | 1 (reference) | 1 (reference) | ||
| N1 + N2 + N3 | 2.609 (1.547–4.400) | 3.677 (2.198–6.153) | ||
| Distant metastasis | 0.872 | < 0.001 | ||
| No | 1 (reference) | 1 (reference) | ||
| Yes | 1.100 (0.348–3.478) | 6.383 (2.544–16.016) | ||
| CXCL13 | 0.048 | < 0.001 | ||
| Low | 1 (reference) | 1 (reference) | ||
| High | 1.486 (1.005–2.197) | 2.186 (1.429–3.345) | ||
CXCL13 C-X-C motif ligand 13, HR hazard ratio, CI confidence interval
Association between postoperative adjuvant chemotherapy and CXCL13 expression
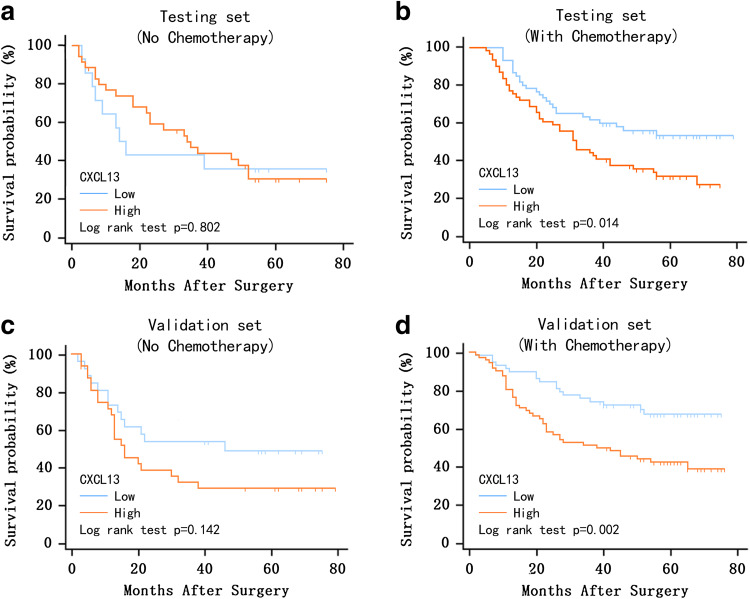
Continuing on previous research suggesting that the immune system contributed to the effect of chemotherapy in gastric cancer, we evaluated the benefit of 5-FU (5-fluorouracil) based postoperative adjuvant chemotherapy according to CXCL13 expression. Chi-square test indicated that the proportion of CXCL13 high vs low patient was not statistically different in patients who received vs did not receive postoperative adjuvant chemotherapy (testing group: P = 0.402; validation group: P = 0.922). Since adjuvant chemotherapy were recommended for patients with advanced T stage tumors (pathologically staged T2 or higher) in Asia [20], we further divided the patients into two subgroups: T1 stage disease and T2–4 stage disease. Within both the testing and validation set, it was found that for the T2–4 stage patients who did not receive postoperative adjuvant chemotherapy, CXCL13 expression was not significantly correlated with OS (testing set: P = 0.802, 3-year survival rates: 45.7%, 5-year survival rates: 32.4%, Fig. 3a; validation set: P = 0.142, 3-year survival rates: 42.1%, 5-year survival rates: 38.4%, Fig. 3c). Whereas, in both the testing and validation set, it was found that for T2–4 stage patients who received postoperative adjuvant chemotherapy, low CXCL13 expression indicated better chemotherapy response, and positively correlated with OS (testing set: P = 0.014, 3-year survival rates: 52.9%, 5-year survival rates: 42.4%, Fig. 3b; validation set: P = 0.002, 3-year survival rates: 61.5%, 5-year survival rates: 53.7%, Fig. 3d).
Fig. 3.
Relationship between CXCL13 expression and benefit from postoperative adjuvant chemotherapy. Among all T2–4 stage tumor patients, those who did not receive postoperative adjuvant chemotherapy showed no significant benefit from low CXCL13 expression in both the testing (a, P = 0.802, 3-year survival rates: 45.7%, 5-year survival rates: 32.4%) and validation set (c, P = 0.142, 3-year survival rates: 42.1%, 5-year survival rates: 38.4%). Whereas, for those received chemotherapy, low CXCL13 expression was positively, significantly correlated with OS in both the testing (b, P = 0.014, 3-year survival rates: 52.9%, 5-year survival rates: 42.4%) and validation set (d, P = 0.002, 3-year survival rates: 61.5%, 5-year survival rates: 53.7%). P-value was calculated by log rank test
Discussion
The results of our multivariate analysis confirmed that CXCL13 could be regarded as an independent, prognostic factor for gastric cancer patients. Patients with higher CXCL13 expression tended to have worse clinical outcomes in survival analyses. It was also found that CXCL13 expression could further stratify gastric cancer patients in T2–4 stage, while it failed to stratify patients with T1 stage cancer. Low expression of CXCL13 predicted better chemotherapy response in T2–4 patients who received postoperative adjuvant chemotherapy. To our knowledge, this is the first paper reporting the association between CXCL13 expression level and clinical outcomes of gastric cancer patients, particularly in those receiving postoperative adjuvant chemotherapy.
CXCL13 is a small cytokine from C-X-C chemokine family, and is selectively chemotactic for B cells. It is also known as a follicular helper T cell marker [21], and is involved in germinal center formation. Moreover, it has been reported that CXCL13 is mainly expressed in the tumor stroma, which is consistent with our study’s findings, and was up-regulated by macrophages [22, 23]. Tumor-associated macrophages (TAMs) were found to be related with poor outcomes in gastric cancer [24], and it was pointed out that TAMs promoted the progression of tumors [25].
In the incipient stage, the host provides nutrition for the tumor and supports tumor development and progression via dispersion. Then, when the tumor reaches the advanced state, this process is not sufficient anymore for the tumor to further develop, and other mechanisms promoting neoangiogenesis and lymphangiogenesis are needed. Previous studies suggest that CXCL13, along with its receptor, CXCR5, plays an important role in the interaction between tumor cells and TAMs [22, 26]. Previous research reported TAMs to have the ability of supporting neoangiogenesis and lymphangiogenesis in gastric cancer, and, thus, to promote tumor cell proliferation and invasion [27]. Considering that CXCL13 is mainly expressed in tumor stroma and up-regulated by macrophages, we hypothesize that its high expression may relate to tumor invasion in this way.
Additionally, it is reported that CXCL13 and its receptor CXCR5 are associated with the expression of Matrix Metalloproteinases (MMPs). As a group of calcium-dependent zinc-containing endopeptidasess, MMPs are known to be involved in cell proliferation, migration and neoangiogenesis [28]. CXCL13 stimulates MMP-9 expression via RANKL–Src (receptor activator of nuclear factor kappa-B ligand–sarcoma) axis in breast cancer cell lines [9]. Expressions of other MMPs, such as MMP-1, MMP-2, MMP-3, MMP-10, MMP-12 and MMP-13 [29], are also increased, indicating that CXCL13 may promote the synthesis of MMPs, and further aggravate the degradation of extracellular matrix, initiate angiogenesis. In the meantime, a large number of malignant tumor cells could be transferred through blood vessels, causing tumor growth and invasion. This may provide a possible explanation for our finding that in more advanced tumors (T2–4 stage tumor), the OS of high CXCL13 expression and low CXCL13 expression subgroups differed significantly.
In the phase III AVAGAST trial, the addition of bevacizumab (Avastin) to chemotherapy failed to improve OS rates in patients with advanced gastric cancer [30]. From these facts, we infer that gastric cancer can promote angiogenesis, besides the VEGF (vascular endothelial growth factor) signal pathway. Being an angiogenesis inhibitor, bevacizumab can reduce the blood supply and form a hypoxia microenvironment causing tumor apoptosis [31]. Ammirante et al. has demonstrated that hypoxia promotes prostate cancer progression by up-regulating myofibroblast-derived CXCL13 expression [32]. Based on this, we hypothesize that under the hypoxia microenvironment, macrophages are recruited by the tumor microenvironment to eliminate tumor necrosis tissue. At the same time, the recruited TAMs, secreting CXCL13, modulate MMPs to degrade the vascular basement membrane, and thereby increase tumor angiogenesis, leading to bevacizumab resistance. This suggests that the combined use of CXCL13-targeted antibody with bevacizumab may reduce the risk of drug resistance and even improve patient clinical outcomes, provided that attention is paid to the CXCL13 expression in tumor tissues.
Postoperative adjuvant chemotherapy has been demonstrated to prolong some gastric cancer patients’ survival periods. However, the side effects of cytotoxic chemotherapy drugs can result in a decline of life quality when the patient is not sensitive to the chemotherapy. Thus, it is crucial to distinguish patients that benefit from chemotherapy from those do not. In the present study, 254 out of 441 patients received 5-FU-based postoperative adjuvant chemotherapy, and the correlation between CXCL13 expression and OS were assessed. As shown in Fig. 3, within patients with T2–4 stage tumors, only those with low CXCL13 expression benefited from postoperative adjuvant chemotherapy. Thus, it appears that low CXCL13 expression indicates better chemotherapy response. It has been reported that CXCL13 expression was closely related to the activity of phosphatidylinositol 3-kinase/protein kinase B (PI3-K/Akt) signaling pathway [29], and it could induce phosphorylation of ERK (extracellular regulated protein kinase), JNK (c-Jun N-terminal kinase) and Akt [33], which subsequently makes CXCL13 a potent activation of Akt. In addition, activation of Akt-mediated signaling pathways could provide cells with a survival signal and help them fight against apoptosis [34]. From this information, we infer that inactivation of Akt expression could effectively increase the drug-induced apoptosis of tumor cells [35], and could cooperate with 5-FU to inhibit tumor cell proliferation as well as to enhance the tumor sensitivity to chemotherapy [36]. Thus, low expression of CXCL13 might be associated with down-regulation of Akt expression in PI3K/Akt signaling pathway and, therefore, enhance the tumor sensitivity to chemotherapy. This might form an explanation for why T2–4 stage gastric cancer patients with low CXCL13 expression could benefit from 5-FU-based postoperative adjuvant chemotherapy.
Conclusions
In brief, our study suggests that increased CXCL13 expression might be regarded as a prognostic factor for the gastric cancer patients. Higher CXCL13 expression is often associated with poorer clinical outcomes. Furthermore, CXCL13 expression could further stratify gastric cancer patients in the T2–4 stage. More importantly, it is promising to explore CXCL13 as a chemotherapeutic-sensitive marker for patients with gastric cancer, in particular for patients with advanced tumor invasion.
Acknowledgements
This study was funded by Grants from National Natural Science Foundation of China (81471621, 81472227, 81501999, 81671628 and 31770851) and Shanghai Sailing Program (17YF1402200). All these study sponsors have no roles in the study design, in the collection, analysis, and interpretation of data.
Abbreviations
- Akt
Protein kinase B, PKB
- BCA-1
B cell attracting chemokine 1
- CXCL13
Chemokine (C-X-C motif) ligand 13
- CXCR5
Chemokine (C-X-C motif) receptor 5
- IOD
Integrated optical density
- MMP
Matrix metalloproteinase
- PI3-K
Phosphatidylinositol 3-kinase
- TAMs
Tumor-associated macrophages
- TMA
Tissue microarray
Author contributions
Y. Wei, C. Lin and H. Li: acquisition of data, analysis and interpretation of data, statistical analysis and drafting of the manuscript. Z. Xu, J. Wang, R. Li, H. Liu and H. Zhang: technical and material support. H. He and J. Xu: study concept and design, analysis and interpretation of data, drafting of the manuscript, obtained funding and study supervision. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Yichou Wei, Chao Lin and He Li contributed equally to this work.
Contributor Information
Hongyong He, Phone: +86 21 15021383022, Email: he.hongyong@zs-hospital.sh.cn.
Jiejie Xu, Phone: +86 21 54237332, Email: jjxufdu@fudan.edu.cn.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49(1):33. doi: 10.3322/canjclin.49.1.33. [DOI] [PubMed] [Google Scholar]
- 3.Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, Xue Y, Suo J, Tao K, He X. Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol. 2016;34(12):1350. doi: 10.1200/JCO.2015.63.7215. [DOI] [PubMed] [Google Scholar]
- 4.Balkwill FR. The chemokine system and cancer. J Pathol. 2012;226(2):148–157. doi: 10.1002/path.3029. [DOI] [PubMed] [Google Scholar]
- 5.Sarvaiya PJ, Guo D, Ulasov I, Gabikian P, Lesniak MS. Chemokines in tumor progression and metastasis. Oncotarget. 2013;4(12):2171–2185. doi: 10.18632/oncotarget.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franciszkiewicz K, Boissonnas A, Boutet M, Combadière C, Mami-Chouaib F. Role of chemokines and chemokine receptors in shaping the effector phase of the antitumor immune response. Cancer Res. 2012;72(24):6325–6332. doi: 10.1158/0008-5472.CAN-12-2027. [DOI] [PubMed] [Google Scholar]
- 7.Raman D, Sobolikdelmaire T, Richmond A. Chemokines in health and disease. Exp Cell Res. 2011;317(5):575–589. doi: 10.1016/j.yexcr.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature. 1998;391(6669):799. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- 9.Biswas S, Sengupta S, Roy CS, Jana S, Mandal G, Mandal PK, Saha N, Malhotra V, Gupta A, Kuprash DV. CXCL13–CXCR5 co-expression regulates epithelial to mesenchymal transition of breast cancer cells during lymph node metastasis. Breast Cancer Res Treat. 2016;155:615. doi: 10.1007/s10549-016-3713-3. [DOI] [PubMed] [Google Scholar]
- 10.Qi XW, Xia SH, Yin Y, Jin LF, Pu Y, Hua D, Wu HR. Expression features of CXCR5 and its ligand, CXCL13 associated with poor prognosis of advanced colorectal cancer. Eur Rev Med Pharmacol Sci. 2014;18(13):1916. [PubMed] [Google Scholar]
- 11.Singh S, Singh R, Sharma PK, Singh UP, Rai SN, Chung LW, Cooper CR, Novakovic KR, Grizzle WE, Jr LJ. Serum CXCL13 positively correlates with prostatic disease, prostate-specific antigen and mediates prostate cancer cell invasion, integrin clustering and cell adhesion. Cancer Lett. 2009;283(1):29–35. doi: 10.1016/j.canlet.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan Z, Gao J, Zhang L, Liang H, Huang X, Xu Q, Zhang Y, Shen T, Lu F. Phenotype and function of CXCR5 + CD45RA − CD4 + T cells were altered in HBV-related hepatocellular carcinoma and elevated serum CXCL13 predicted better prognosis. Oncotarget. 2015;6(42):44239–44253. doi: 10.18632/oncotarget.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sambandam Y, Sundaram K, Liu A, Kirkwood KL, Ries WL, Reddy SV. CXCL13 activation of c-Myc induces RANK ligand expression in stromal/preosteoblast cells in the oral squamous cell carcinoma tumor-bone microenvironment. Oncogene. 2012;32(1):97. doi: 10.1038/onc.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh R, Gupta P, Kloecker GH, Singh S, Jr LJ. Expression and clinical significance of CXCR5/CXCL13 in human non-small cell lung carcinoma. Int J Oncol. 2014;45(6):2232–2240. doi: 10.3892/ijo.2014.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SJ, Ryu KJ, Hong M, Ko YH, Kim WS. The serum CXCL13 level is associated with the Glasgow Prognostic Score in extranodal NK/T cell lymphoma patients. J Hematol Oncol. 2015;8(1):49. doi: 10.1186/s13045-015-0142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grosso FD, Coco S, Scaruffi P, Stigliani S, Valdora F, Benelli R, Salvi S, Boccardo S, Truini M, Croce M. Role of CXCL13-CXCR5 crosstalk between malignant neuroblastoma cells and schwannian stromal cells in neuroblastic tumors. Mol Cancer Res. 2011;9(7):815. doi: 10.1158/1541-7786.MCR-10-0367. [DOI] [PubMed] [Google Scholar]
- 17.Ding Y, Shen J, Zhang G, Chen X, Wu JM, Chen W. CD40 controls CXCR5-induced recruitment of myeloid-derived suppressor cells to gastric cancer. Oncotarget. 2015;6(36):38901–38911. doi: 10.18632/oncotarget.5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofman VJ, Moreilhon C, Brest PD, et al. Gene expression profiling in human gastric mucosa infected with Helicobacter pylori . Mod Pathol. 2007;20(9):974–989. doi: 10.1038/modpathol.3800930. [DOI] [PubMed] [Google Scholar]
- 19.Galamb O, Gyõrffy B, Sipos F, et al. Helicobacter pylori and antrum erosion-specific gene expression patterns: the discriminative role of CXCL13 and VCAM1 transcripts. Helicobacter. 2008;13(2):112–126. doi: 10.1111/j.1523-5378.2008.00584.x. [DOI] [PubMed] [Google Scholar]
- 20.Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–2664. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 21.Ebert LM, Schaerli P, Moser B. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol Immunol. 2005;42(7):799. doi: 10.1016/j.molimm.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 22.Carlsen HS, Baekkevold ES, Morton HC, Haraldsen G, Brandtzaeg P. Monocyte-like and mature macrophages produce CXCL13 (B cell-attracting chemokine 1) in inflammatory lesions with lymphoid neogenesis. Blood. 2004;104(10):3021–3027. doi: 10.1182/blood-2004-02-0701. [DOI] [PubMed] [Google Scholar]
- 23.Bürkle A, Niedermeier M, Schmittgräff A, Wierda WG, Keating MJ, Burger JA. Overexpression of the CXCR5 chemokine receptor, and its ligand, CXCL13 in B-cell chronic lymphocytic leukemia. Blood. 2007;110(110):3316–3325. doi: 10.1182/blood-2007-05-089409. [DOI] [PubMed] [Google Scholar]
- 24.Fallarino F, Grohmann U, You S, Mcgrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176(11):6752. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 25.Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T cell tolerance and tumoral immune escape. Immunol Rev. 2008;222(1):206. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 26.Widney DP, Olafsen T, Wu AM, Kitchen CM, Said JW, Smith JB, Peña G, Magpantay LI, Penichet ML, Martinez-Maza O. Levels of murine, but not human, CXCL13 are greatly elevated in NOD-SCID mice bearing the AIDS-associated Burkitt lymphoma cell line, 2F7. PLoS One. 2013;8(8):e72414. doi: 10.1371/journal.pone.0072414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Astigiano S, Morandi B, Costa R, Mastracci L, D’Agostino A, Ratto GB, Melioli G, Frumento G. Eosinophil granulocytes account for indoleamine 2,3-dioxygenase-mediated immune escape in human non-small cell lung cancer. Neoplasia. 2005;7(4):390–396. doi: 10.1593/neo.04658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lint PV, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol. 2007;82(82):1375–1381. doi: 10.1189/jlb.0607338. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Z, Zhang X, Guo H, Fu L, Pan G, Sun Y. CXCL13-CXCR5 axis promotes the growth and invasion of colon cancer cells via PI3K/AKT pathway. Mol Cell Biochem. 2015;400(1):287–295. doi: 10.1007/s11010-014-2285-y. [DOI] [PubMed] [Google Scholar]
- 30.Ohtsu A, Shah MA, Van CE, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29(30):3968–3976. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 31.Selvakumaran M, Yao KS, Feldman MD, O’Dwyer PJ. Antitumor effect of the angiogenesis inhibitor bevacizumab is dependent on susceptibility of tumors to hypoxia-induced apoptosis. Biochem Pharmacol. 2008;75(3):627. doi: 10.1016/j.bcp.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ammirante M, Shalapour S, Kang Y, Jamieson CA, Karin M. Tissue injury and hypoxia promote malignant progression of prostate cancer by inducing CXCL13 expression in tumor myofibroblasts. PNAS. 2014;111(41):14776. doi: 10.1073/pnas.1416498111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durand C, Westendorf J, Tse K, Gold M. The Rap GTPases mediate CXCL13- and sphingosine1-phosphate-induced chemotaxis, adhesion, and Pyk2 tyrosine phosphorylation in B lymphocytes. Eur J Immunol. 2006;36(8):2235–2249. doi: 10.1002/eji.200535004. [DOI] [PubMed] [Google Scholar]
- 34.Song G, Ouyang G, Bao S. The activation of AKT/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9(1):59. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Page C, Lin HJ, Jin Y, Castle VP, Nunez G, Huang M, Lin J. Overexpression of Akt/AKT can modulate chemotherapy-induced apoptosis. Anticancer Res. 2000;20(1A):407. [PubMed] [Google Scholar]
- 36.Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61(10):3986. [PubMed] [Google Scholar]